Effects of 1−MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’ at Low Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Experimental Design
2.3. Sensory Evaluation
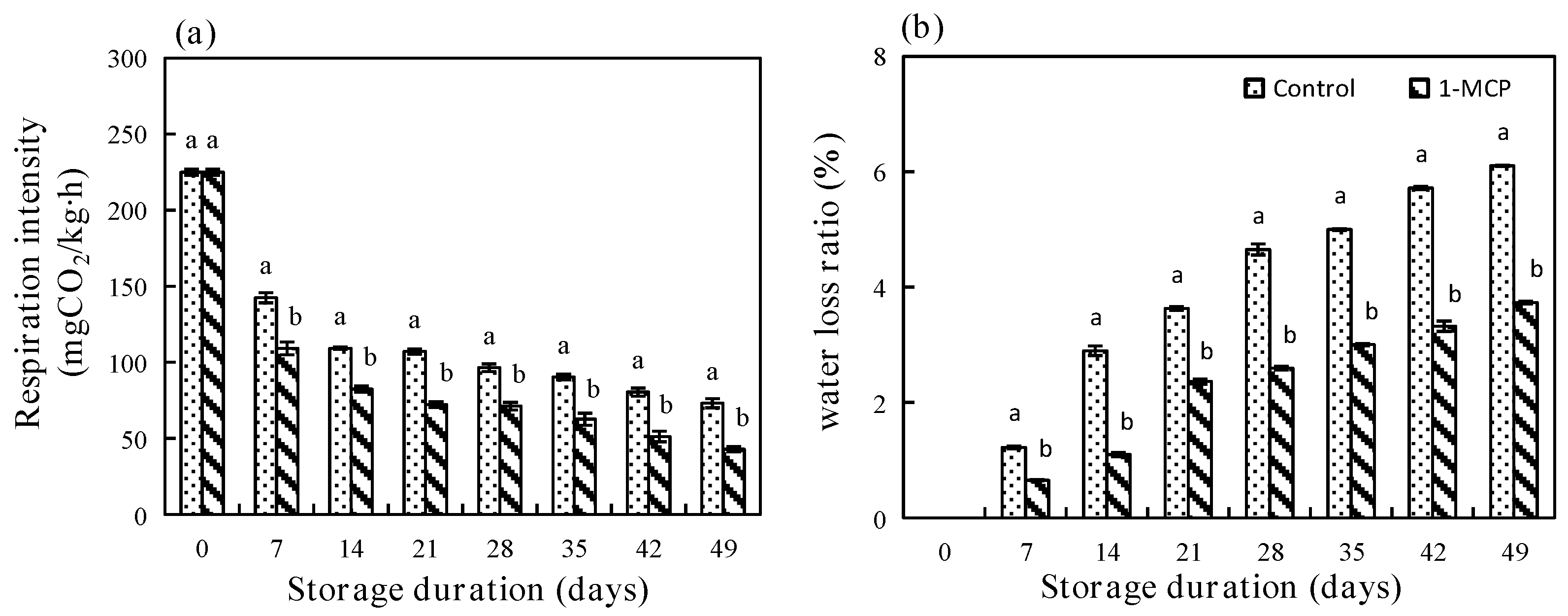
2.4. Respiratory Intensity
2.5. Determination of Water Loss
2.6. Enzyme Activity Assays
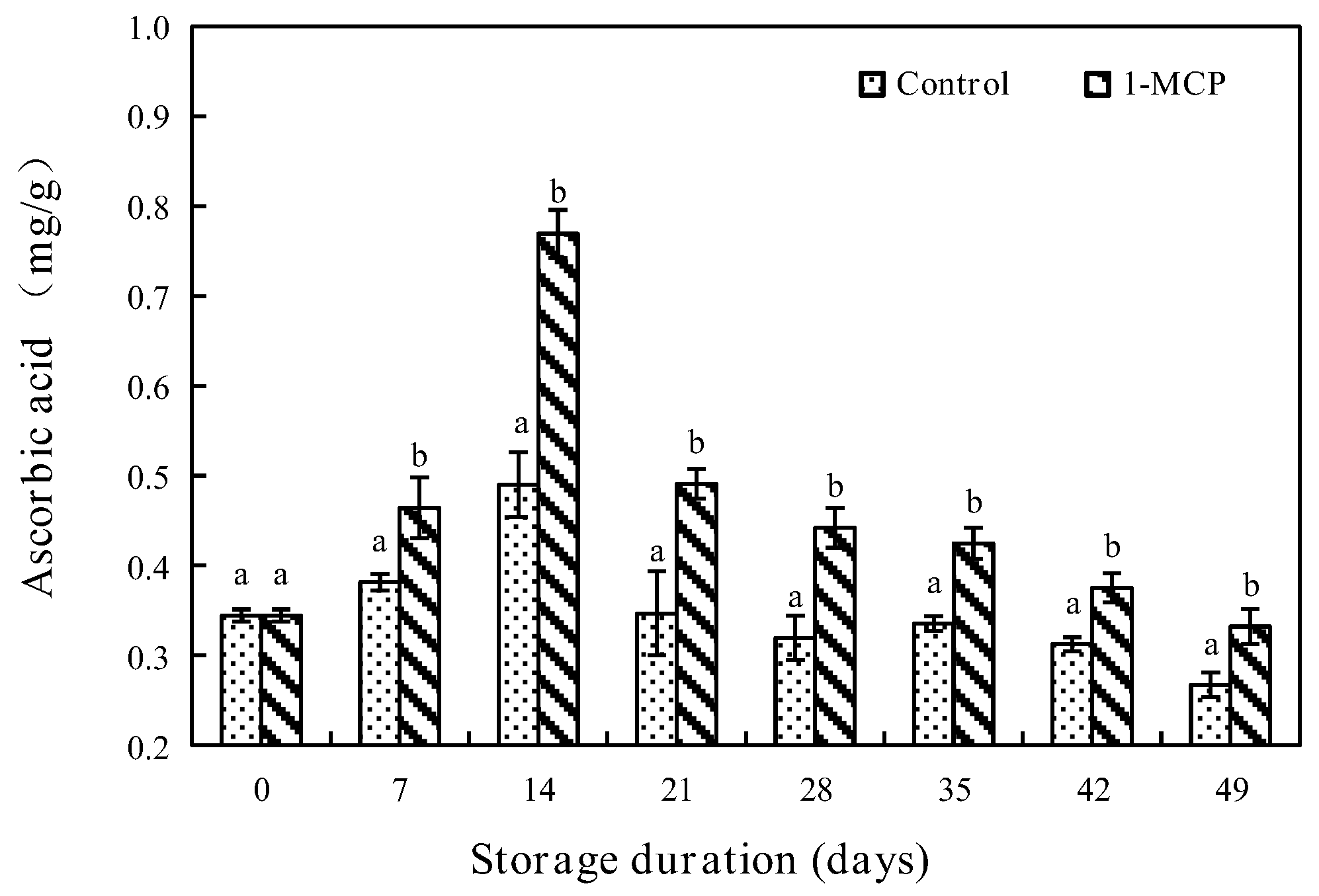
2.7. Determination of AsA, Total Phenols and Anthocyanin Contents
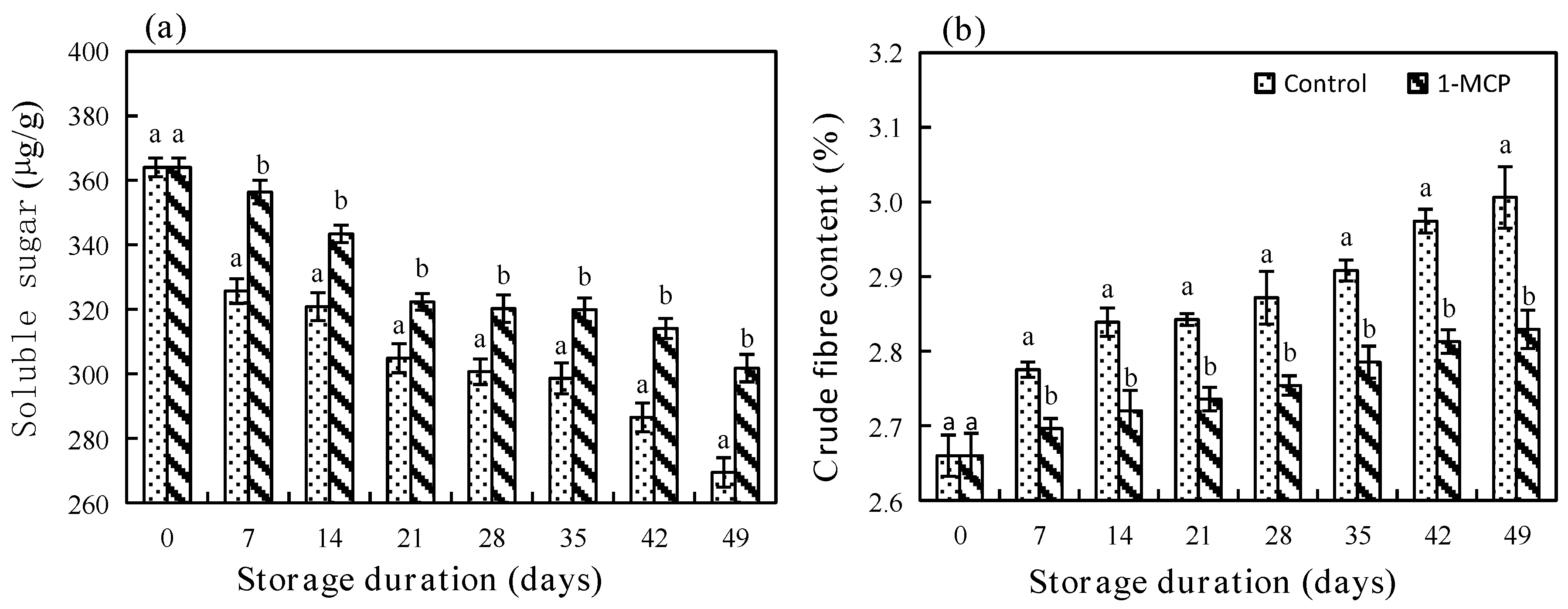
2.8. Analysis of Soluble Sugar, Crude Fibre and MDA Contents
2.9. Statistical Analysis
3. Results
3.1. Evaluation of Sensory Quality
3.2. Effects of 1−MCP on Physiological Metabolism of Rose Petals
3.2.1. Respiration Intensity and Water Loss Rate
3.2.2. Soluble Sugar and Crude Fibre Contents
3.2.3. AsA Content
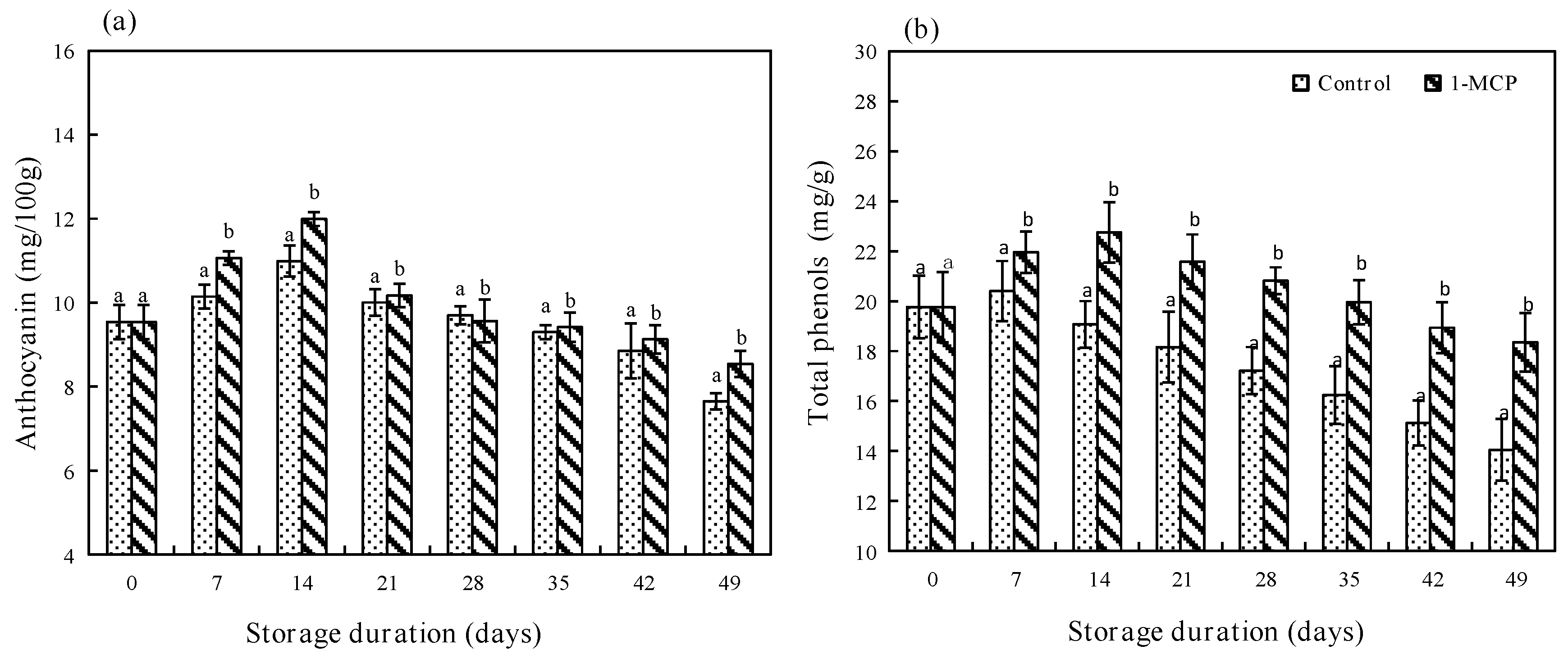
3.2.4. Anthocyanin and Total Phenols Contents
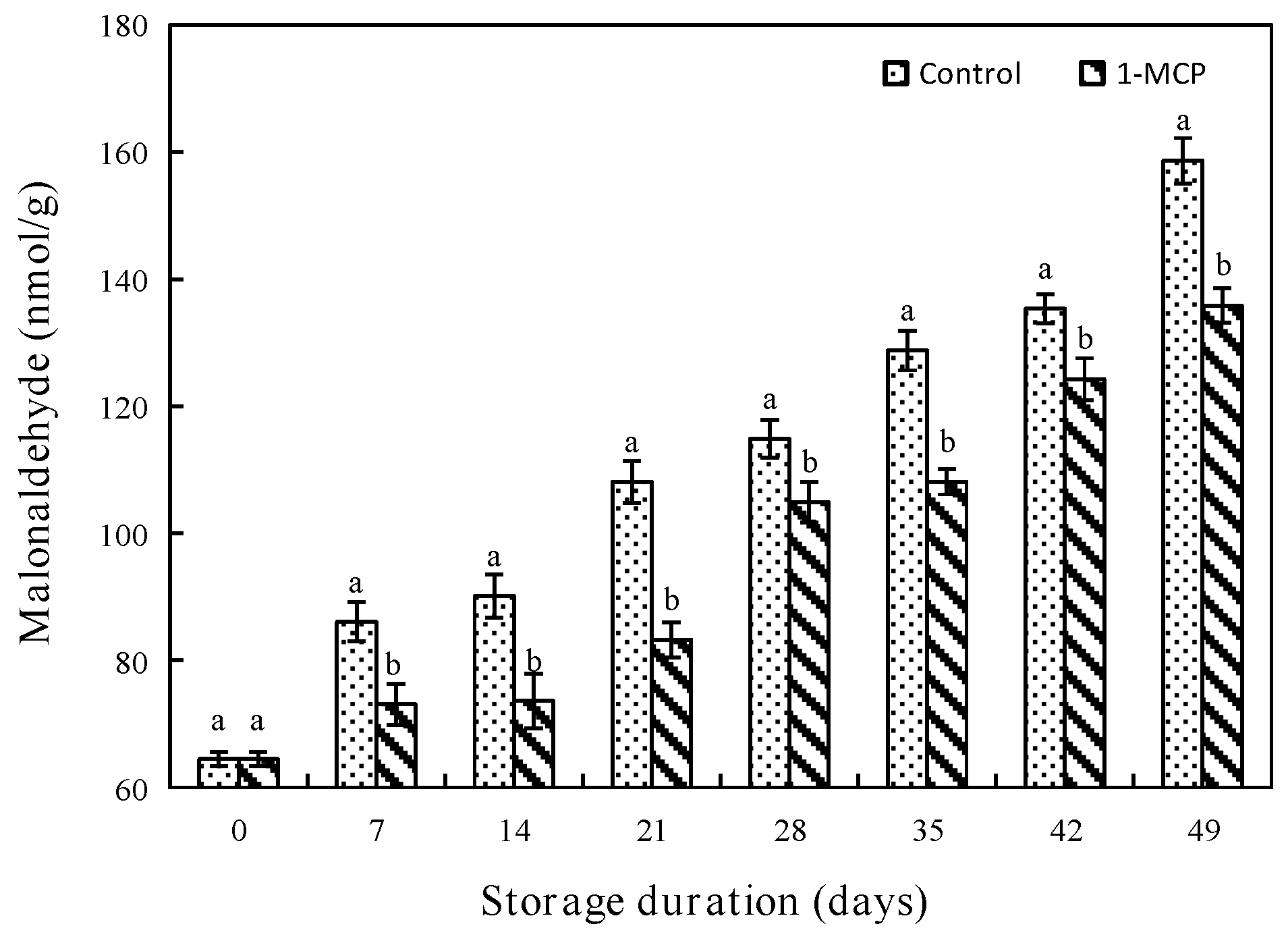
3.2.5. MDA Content
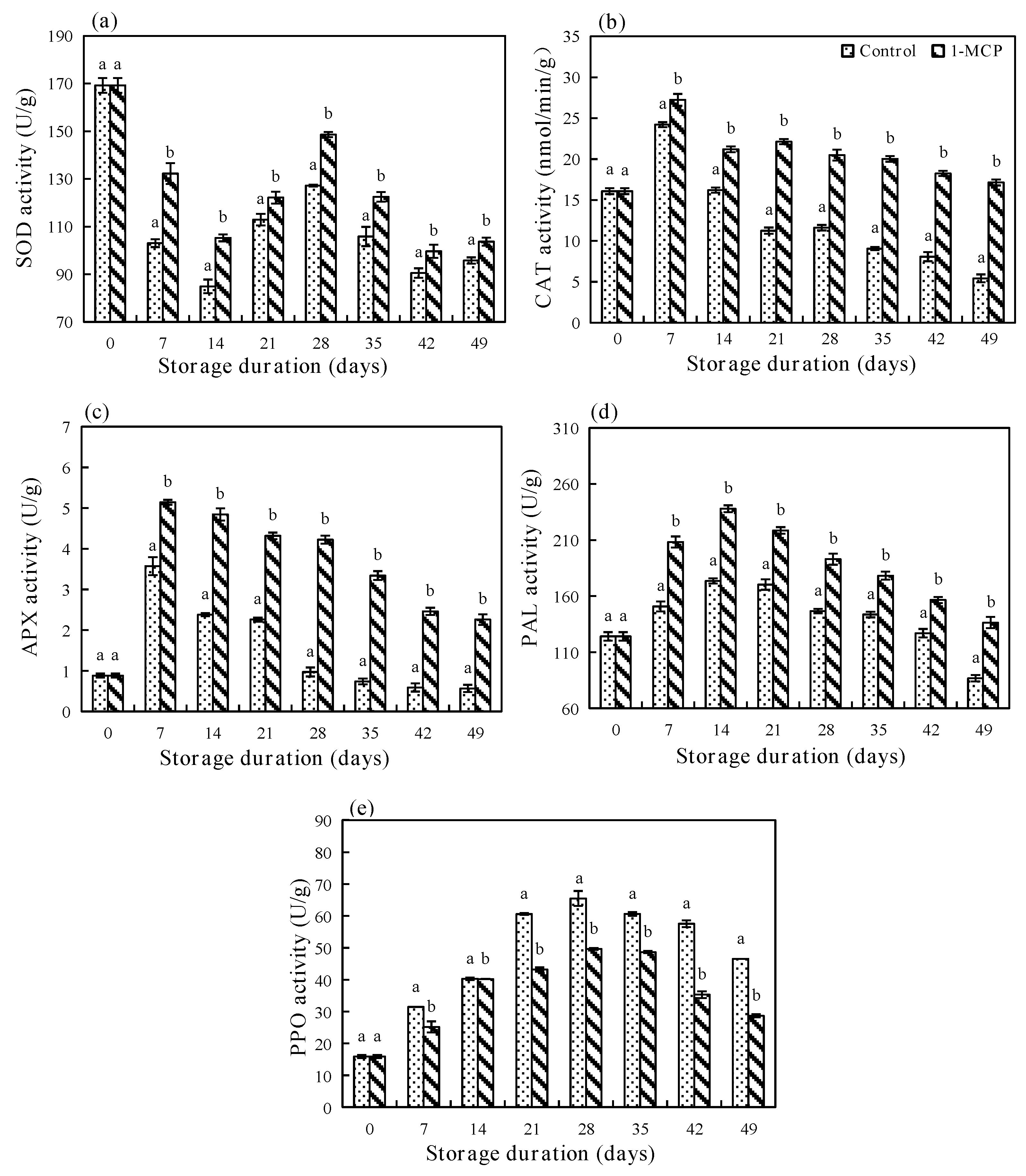
3.3. Effects of 1−MCP on Enzyme Activities in Rose Petals during Different Storage Periods
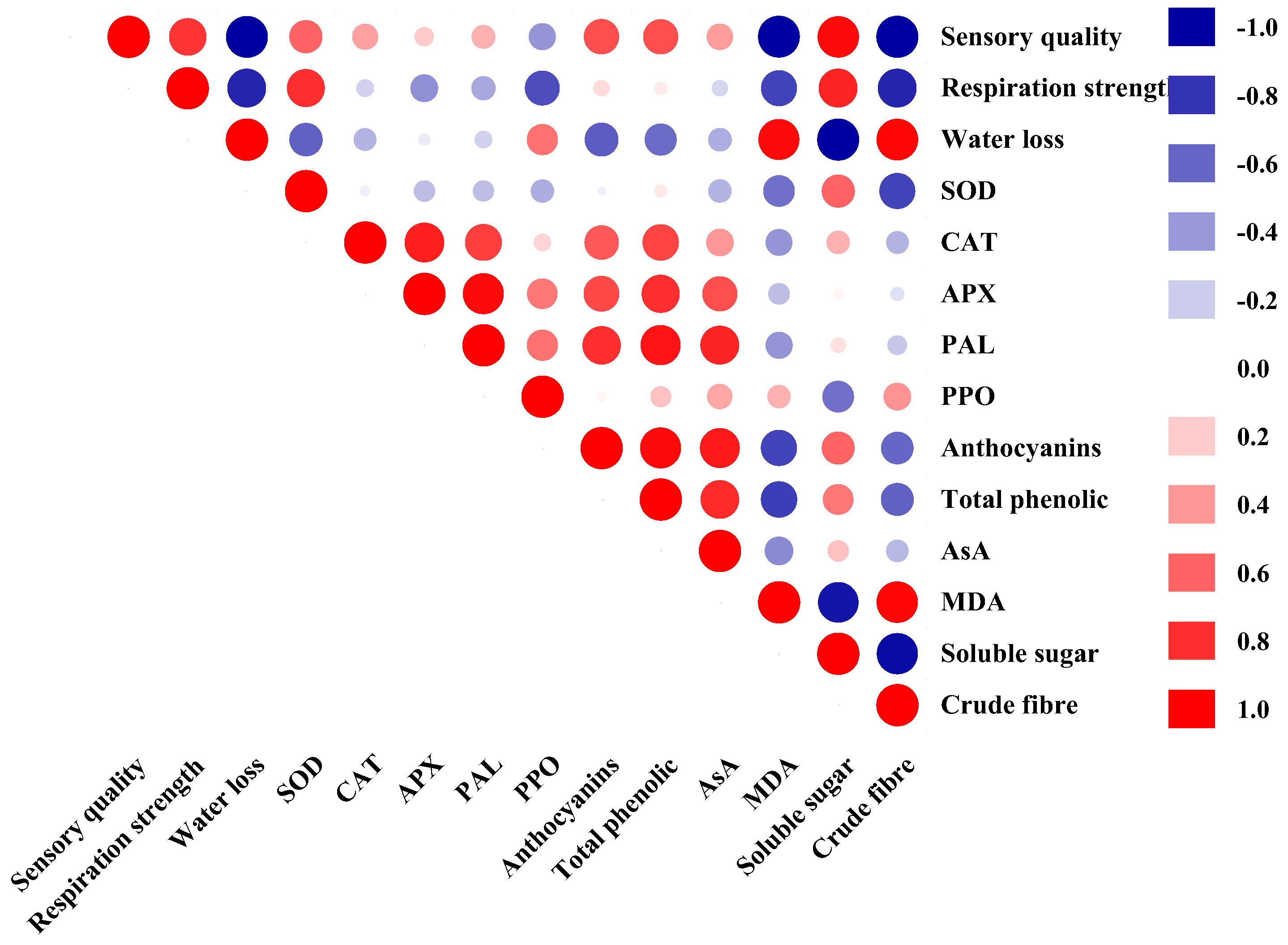
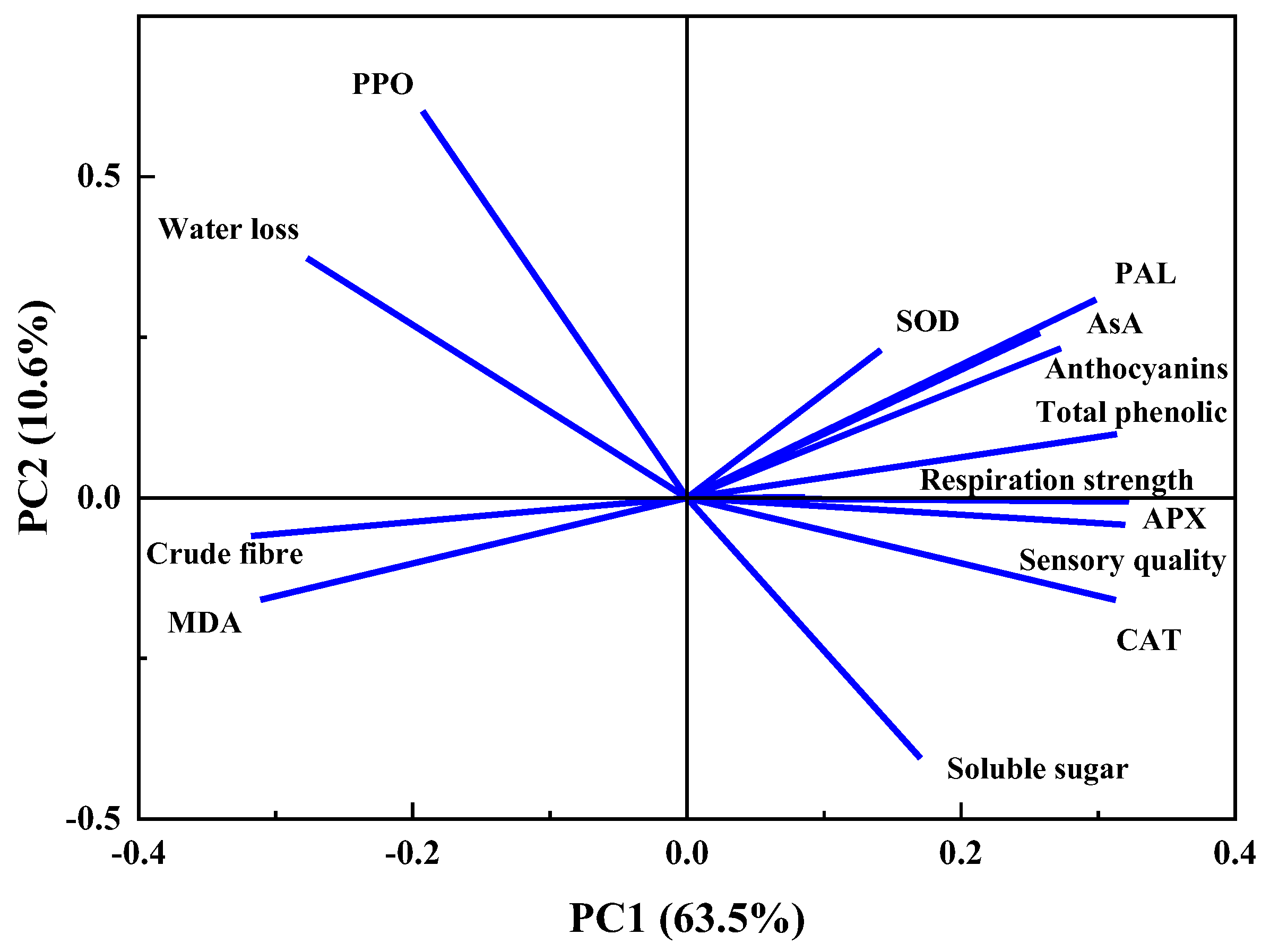
3.4. Correlation Analysis
3.5. Evaluation of Storage Quality in Rose Petals
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, H.; Shin, Y. Antioxidant compounds and activities of edible roses (Rosa hybrida spp.) from different cultivars grown in Korea. Appl. Biol. Chem. 2017, 60, 129–136. [Google Scholar] [CrossRef]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Fernandes, L.; Casal, S.; Pereira, J.A.; Saraiva, J.A.; Ramalhosa, E. Edible flowers: A review of the nutritional, antioxidant, antimicrobial properties and effects on human health. J. Food Compos. Anal. 2017, 60, 38–50. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, H.; Zhang, M.; Chitrakar, B.; Bhandari, B.; Wang, B. Edible flowers: Review of flower processing and extraction of bioactive compounds by novel technologies. Food Res. Int. 2019, 12, 108660. [Google Scholar] [CrossRef]
- Demasi, S.; Mellano, M.G.; Falla, N.M.; Caser, M.; Scariot, V. Sensory profifile, shelf life, and dynamics of bioactive compounds during cold storage of 17 edible flowers. Horticulturae 2021, 7, 166. [Google Scholar] [CrossRef]
- Nicolau, A.I.; Gostin, A.I. Safety of edible flowers. In Regulating Safety of Traditional and Ethnic Foods; Academic Press: Cambridge, MA, USA, 2016; pp. 395–419. [Google Scholar] [CrossRef]
- Serek, M.; Reid, M. Ethylene and postharvest performance of potted kalanchoe. Postharvest Biol. Technol. 2000, 18, 43. [Google Scholar] [CrossRef]
- Aquino-Bolaños, E.N.; Urrutia-Hernández, T.A.; López Del Castillo-Lozano, M.; Chavéz-Servia, J.L.; Verdalet-Guzmán, I. Physicochemical parameters and antioxidant compounds in edible squash (Cucurbita pepo) flower stored under controlled atmospheres. J. Food Qual. 2013, 36, 302–308. [Google Scholar] [CrossRef]
- Kou, L.; Turner, E.R.; Luo, Y. Extending the shelf life of edible flowers with controlled release of 1-methylcyclopropene and modified atmosphere packaging. J. Food Sci. 2012, 77, 188–193. [Google Scholar] [CrossRef]
- Kelley, K.M.; Cameron, A.C.; Biernbaum, J.A.; Poff, K.L. Effect of storage temperature on the quality of edible flowers. Postharvest Biol. Technol. 2003, 27, 341–344. [Google Scholar] [CrossRef]
- Cheema, M.U.A.; Rees, D.; Colgan, R.J.; Taylor, M.; Westby, A. The effects of ethylene, 1−MCP and AVG on sprouting in sweetpotato roots. Postharvest Biol. Technol. 2013, 85, 89–93. [Google Scholar] [CrossRef]
- Watkins, C.B. The use of 1-methylcyclopropene (1−MCP) on fruits and vegetables. Biotechnol. Adv. 2006, 24, 389–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, K.; Xiao, X.; Cao, S.; Chen, W.; Yang, Z.; Shi, L. Effect of 1−MCP on the regulation processes involved in ascorbate metabolism in kiwifruit. Postharvest Biol. Technol. 2021, 179, 111563. [Google Scholar] [CrossRef]
- Xia, Y.; Zhuo, R.; Li, B.; Tian, S. Effects of 1-methylcyclopropene on disease resistance of red-flfleshed kiwifruit during long-term cold storage and the possible mechanisms. N. Z. J. Crop Hortic. 2021, 49, 182–195. [Google Scholar] [CrossRef]
- Zhang, F.; Shi, J.; Xie, Y.; Jiang, L. Effects of 1-methylcyclopropene on the antioxidant system of Gynura bicolor DC. Food Sci. 2022, 43, 164–170. [Google Scholar] [CrossRef]
- Lv, J.; Bai, L.; Han, X.; Xu, D.; Ding, S.; Li, C.; Ge, Y.; Li, J. Effects of 1−MCP treatment on sprouting and preservation of ginger rhizomes during storage at room temperature. Food Chem. 2021, 349, 129004. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.M.K.E.; Mousa, R.M.A. Nutritional assessment of low-calorie baladi rose petals jam. Food Public Health 2012, 2, 197–201. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, P.; Gao, X.; Xu, H. Antioxidation of edible rose from different producing areas and varieties. Southwest. China J. Agric. Sci. 2020, 33, 2215–2219. [Google Scholar] [CrossRef]
- Zhang, N.; Yuan, R.; Guan, W.; Wang, C. Effects of red light-emitting diode (LED) on the postharvest yellowing change of broccoli. Spectrosc. Spectral. Anal. 2016, 36, 955–959. [Google Scholar] [CrossRef]
- Zhou, R.; Gao, H.; Fan, X.; Yin, C.; Chen, Z.; Yao, F.; Cheng, W.; Shi, D. Preliminary comparison of preservation effect of fresh Lentinus edodes by 60Co-γ ray and electron beam irradiation. Acta Agric. Nucl. Sin. 2019, 33, 490–497. [Google Scholar] [CrossRef]
- Fukuoka, N.; Suzuki, T.; Yamada, Y. Changes in polyphenol biosynthesis induced in Gynura bicolor DC. leaves by infrared irradiation. J. Hortic. Sci. Biotechnol. 2012, 87, 130–136. [Google Scholar] [CrossRef]
- Hu, T.; Ye, J.; Tao, P.; Li, H.; Zhang, J.; Zhang, Y.; Ye, Z. The tomato HD-Zip I transcription factor SlHZ24 modulates ascorbate accumulation through positive regulation of the D-mannose/L-galactose pathway. Plant J. 2016, 85, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Ruffoni, B.; Combournac, L.; Guidi, L. Nutraceutical value of edible flowers upon cold storage. Ital. J. Food Sci. 2018, 30, 336–347. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Zhang, Y.; Cao, J.; Jiang, W. Synergistic effects of 1−MCP and hot air treatments on delaying softening and promoting anthocyanin biosynthesis in nectarines. Postharvest Biol. Technol. 2021, 180, 111598. [Google Scholar] [CrossRef]
- Shi, L.; Li, M.; Cui, X.; Li, M. Comprehensive quality appraisal of flower buds of Tussilago farfara from 39 populations in Gansu province and analysis of affecting factors. Chin. Tradit. Herb. Drugs. 2022, 53, 3784–3792. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Zhang, W.; Wang, J.; Li, K. Effects of 1-methylcyclopropene fumigation combined with tea polyphenol coating treatment on quality preservation of Pteridium aquilinum var. Latiusculum. Food Sci. 2021, 42, 218–225. [Google Scholar] [CrossRef]
- Nabubuya, A.; Namutebi, A.; Byaruhanga, Y.; Narvhus, J.; Wicklund, T. Influence of development, postharvest handling, and storage conditions on the carbohydrate components of sweetpotato (Ipomea batatas Lam.) roots. Food Sci. Nut. 2017, 5, 1088–1097. [Google Scholar] [CrossRef]
- Xie, G.; Fei, Y.; Chai, Y.; Zhang, M. Effect of 1−MCP on lignocellulose of three kinds of fresh common bean in Postharvest. J. Chin. Inst. Food Sci. Technol. 2020, 20, 206–214. [Google Scholar] [CrossRef]
- Chen, K.; Gong, H.; Wang, S. Biosynthesis, transport and function of ascorbate in plants. Acta Bot. Boreal. Occident. Sin. 2004, 24, 329–336. [Google Scholar] [CrossRef]
- Dong, C.; Li, L.; Cao, N.; Shang, Q.; Zhang, Z. Roles of phenylalanine ammonia-lyase in low temperature tolerance in cucumber seedlings. Chin. J. Appl. Ecol. 2015, 26, 2041–2049. [Google Scholar] [CrossRef]
- Rogers, H.J. From models to ornamentals: How is flower senescence regulated? Plant Mol. Biol. 2013, 82, 563–574. [Google Scholar] [CrossRef]
- Trivellini, A.; Cocetta, G.; Hunter, D.A.; Vernieri, P.; Ferrante, A. Spatial and temporal transcriptome changes occurring during flower opening and senescence of the ephemeral hibiscus flower, Hibiscus rosa-sinensis. J. Exp. Bot. 2016, 67, 5919–5931. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.S.; Tahir, I. How and why of flower senescence: Understanding from models to ornamentals. Ind. J. Plant Physiol. 2016, 21, 446–456. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Z.; Feng, M.; Chen, J.; Qin, M.; Wang, W.; Bao, Y.; Xu, Q.; Ye, Y.; Ma, C.; et al. The circadian-controlled PIF8–BBX28 module regulates petal senescence in rose flowers by governing mitochondrial ROS homeostasis at night. Plant Cell 2021, 33, 2716–2735. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Zhang, M.; Ren, G.; Zhou, S.; Zhao, L.; Xu, Y. Drying models and quality changes of rose subjected to infrared assisted spouted bed drying. Trans. Chin. Soc. Agric. Eng. 2020, 36, 238–245. [Google Scholar] [CrossRef]
- Taye, A.; Tilahun, S.; Seo, M.; Park, D.; Jeong, C. Effects of 1−MCP on quality and storability of cherry tomato (Solanum lycopersicum L.). Horticulturae 2019, 5, 29. [Google Scholar] [CrossRef]
- Villalta, A.M.; Ergun, M.; Berry, A.D.; Shaw, N.; Sargent, S.A. Quality changes of yellow summer squash blossoms (Cucurbita pepo) during storage. Acta Hortic. 2004, 659, 831–834. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Woltering, E.J. Physiology and molecular biology of petal senescence. J. Exp. Bot. 2008, 59, 453–480. [Google Scholar] [CrossRef]
- Horibe, T.; Yamada, K. Petal growth physiology of cut rose flowers: Progress and future prospects. J. Hortic. Res. 2017, 25, 5–18. [Google Scholar] [CrossRef]
- Win, N.M.; Yoo, J.; Naing, A.H.; Kwon, J.G.; Kang, I. 1-Methylcyclopropene (1−MCP) treatment delays modification of cell wall pectin and fruit softening in “Hwangok” and “Picnic” apples during cold storage. Postharvest Biol. Technol. 2021, 180, E111599. [Google Scholar] [CrossRef]
- Ge, Q.; Ma, X. Composition and antioxidant activity of anthocyanins isolated from Yunnan edible rose (An ning). Food Sci. Hum. Wellness 2013, 2, 68–74. [Google Scholar] [CrossRef]
- Li, C.; Li, J.; Yan, S.; Wang, Q. Effects of ascorbic acid treatment on enzymatic browning and quality characteristics of fresh lotus rhizome juice. J. Chin. Inst. Food Sci. Technol. 2021, 21, 151–158. [Google Scholar] [CrossRef]
- Zhang, Z.; Jiang, S. Causes and control measures of the fading of edible roses. Food Sci. 2017, 38, 322–328. [Google Scholar] [CrossRef]
- Ma, X.; Zhu, D. Functional roles of the plant superoxide dismutase. Hereditas 2003, 25, 225–231. [Google Scholar] [CrossRef] [PubMed]
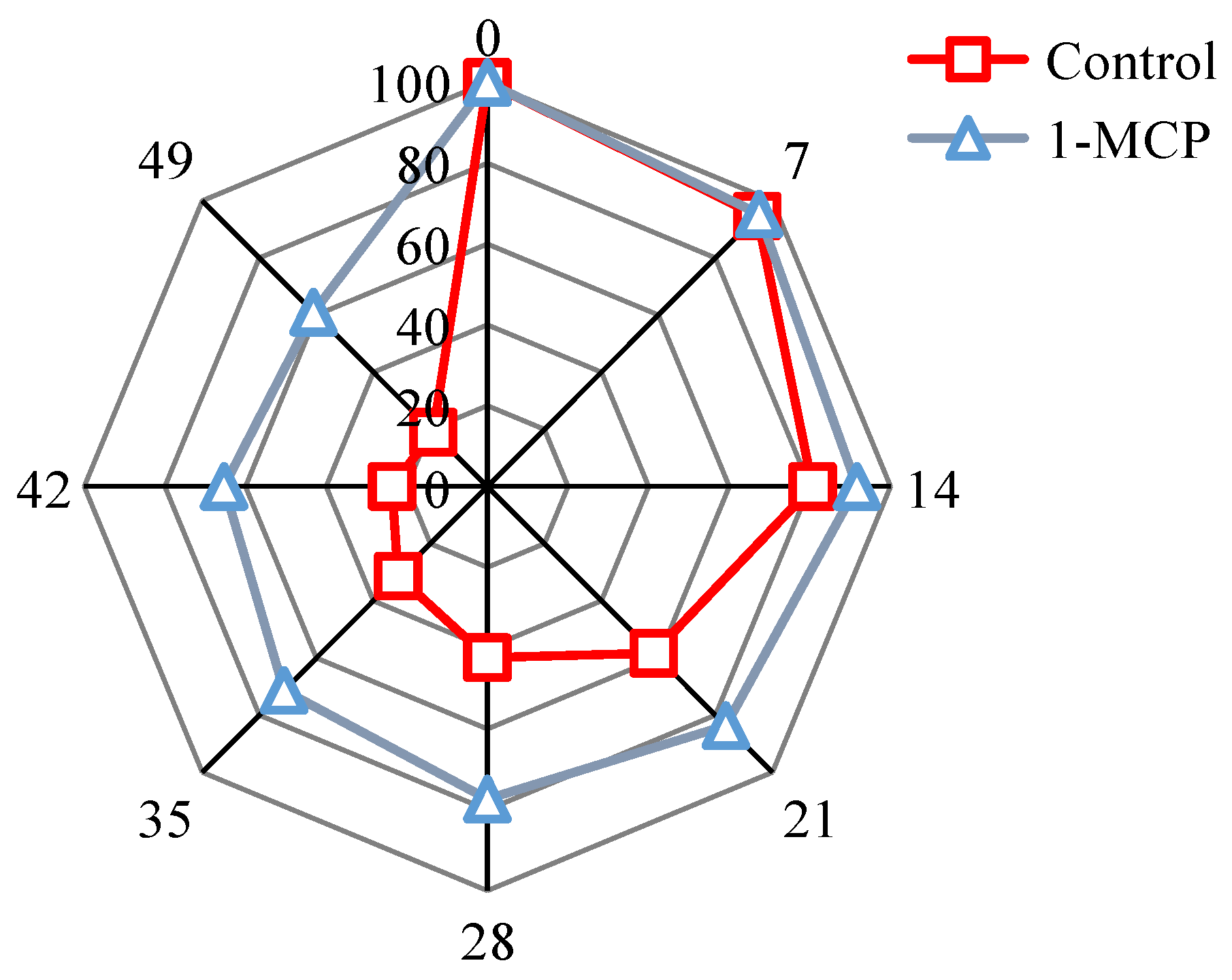

| Scores | Colour | Tissue State | Surface Morphology | Odour |
|---|---|---|---|---|
| 20–25 | bright rose red | The petals are fresh and full. | The petals have a smooth surface. | very strong aroma |
| 15–20 | deep rose | The petals are full. | No more than 10% of the petals lose water slightly. | strong aroma |
| 10–15 | light rose red | The petals are soft and collapsed. | 10–30% of the petals were slightly dehydrated and veins were clear. | light aroma, no peculiar smell |
| 5–10 | lighter rose red | The petals are soft, thin and wilting. | 30–50% of the petals have obvious water loss, and the veins were clearer. | light aroma, slight peculiar smell |
| 0–5 | pink | The petals are thin, wilting and brown | More than 50% of the petals lose water, and the veins bulged | no aroma, obvious peculiar smell |
| Sensory Quality | Respiration Strength | Water Loss | SOD | CAT | APX | PAL | PPO | Anthocyanins | Total Phenolic | AsA | MDA | Soluble Sugar | Crude Fibre | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sensory quality | 1.000 | |||||||||||||
| Respiration strength | 0.785 * | 1.000 | ||||||||||||
| Water loss | −0.980 * | −0.846 * | 1.000 | |||||||||||
| SOD | 0.616 | 0.806 * | −0.618 | 1.000 | ||||||||||
| CAT | 0.376 | −0.172 | −0.287 | −0.059 | 1.000 | |||||||||
| APX | 0.204 | −0.429 | −0.078 | −0.251 | 0.878 * | 1.000 | ||||||||
| PAL | 0.316 | −0.326 | −0.174 | −0.249 | 0.759 * | 0.942 * | 1.000 | |||||||
| PPO | −0.409 | −0.682 | 0.541 | −0.303 | 0.174 | 0.522 | 0.545 | 1.000 | ||||||
| Anthocyanins | 0.697 | 0.146 | −0.635 | −0.042 | 0.660 | 0.714 * | 0.820 * | 0.056 | 1.000 | |||||
| Total phenolic | 0.686 | 0.100 | −0.572 | 0.096 | 0.739 * | 0.816 * | 0.904 * | 0.241 | 0.941 * | 1.000 | ||||
| AsA | 0.386 | −0.148 | −0.307 | −0.300 | 0.419 | 0.687 | 0.845 * | 0.357 | 0.886 * | 0.830 * | 1.000 | |||
| MDA | −0.986 * | −0.727 * | 0.942 * | −0.560 | −0.401 | −0.259 | −0.404 | 0.302 | −0.736 * | −0.742 * | −0.455 | 1.000 | ||
| Soluble sugar | 0.956 * | 0.846 * | −0.990 * | 0.612 | 0.301 | 0.053 | 0.132 | −0.556 | 0.604 | 0.526 | 0.252 | −0.915 * | 1.000 | |
| Crude fibre | −0.987 * | −0.847 * | 0.971 * | −0.726 * | −0.285 | −0.106 | −0.214 | 0.424 | −0.590 | −0.604 | −0.277 | 0.962 * | −0.946 * | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, P.; Fu, J.; Du, W.; Li, H.; Cui, G. Effects of 1−MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’ at Low Temperatures. Horticulturae 2022, 8, 954. https://doi.org/10.3390/horticulturae8100954
Jin P, Fu J, Du W, Li H, Cui G. Effects of 1−MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’ at Low Temperatures. Horticulturae. 2022; 8(10):954. https://doi.org/10.3390/horticulturae8100954
Chicago/Turabian StyleJin, Pengcheng, Jian Fu, Wenwen Du, Hong Li, and Guangfen Cui. 2022. "Effects of 1−MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’ at Low Temperatures" Horticulturae 8, no. 10: 954. https://doi.org/10.3390/horticulturae8100954
APA StyleJin, P., Fu, J., Du, W., Li, H., & Cui, G. (2022). Effects of 1−MCP on Storage Quality and Enzyme Activity of Petals of Edible Rose Cultivar ‘Dianhong’ at Low Temperatures. Horticulturae, 8(10), 954. https://doi.org/10.3390/horticulturae8100954





