Nitrogen Rate, Irrigation Frequency and Volume Differentially Influence Growth, Flowering, and Nutrient Uptake of Container-Grown Rhododendron during the Following Growing Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Year 1 N Rate Treatments
2.3. Year 1 Irrigation Treatments
2.4. Year 2 Experimental Conditions
2.5. Plant Growth and Flowering Measurements
2.6. Nutrient Analyses
2.7. Substrate EC and Moisture
2.8. Leaf Stomatal Conductance
2.9. Calculations
2.10. Data Analyses
3. Results
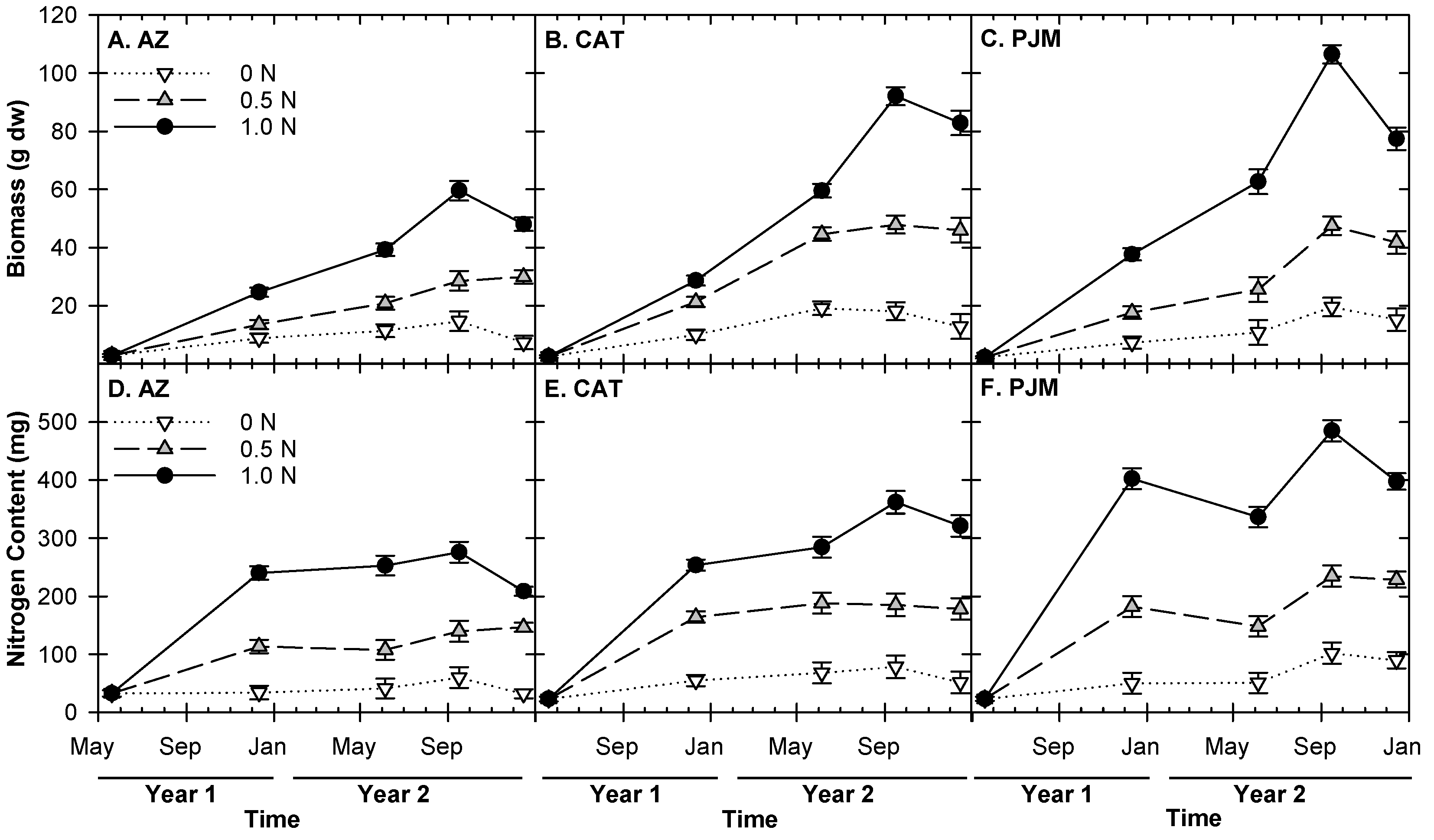
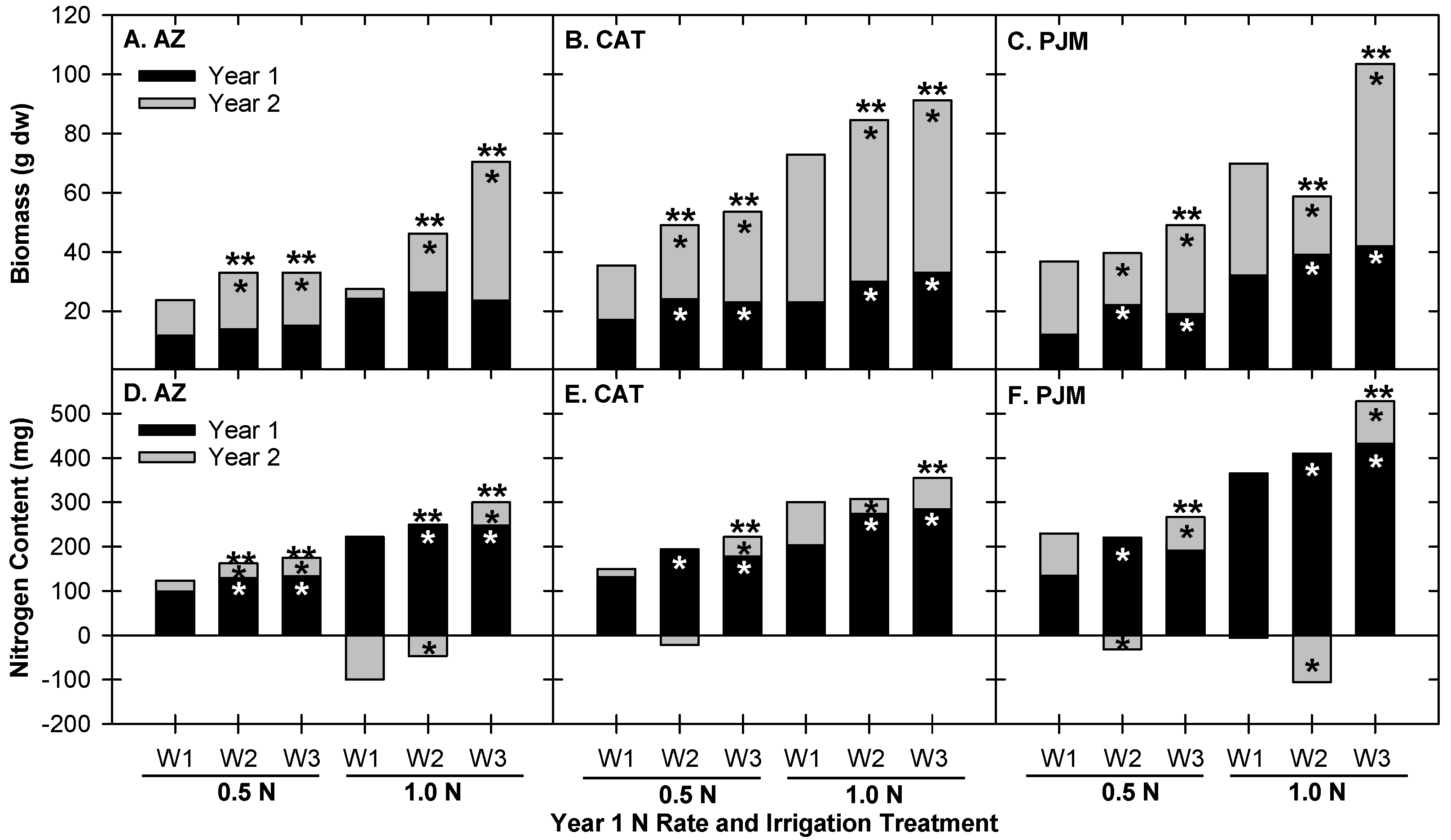
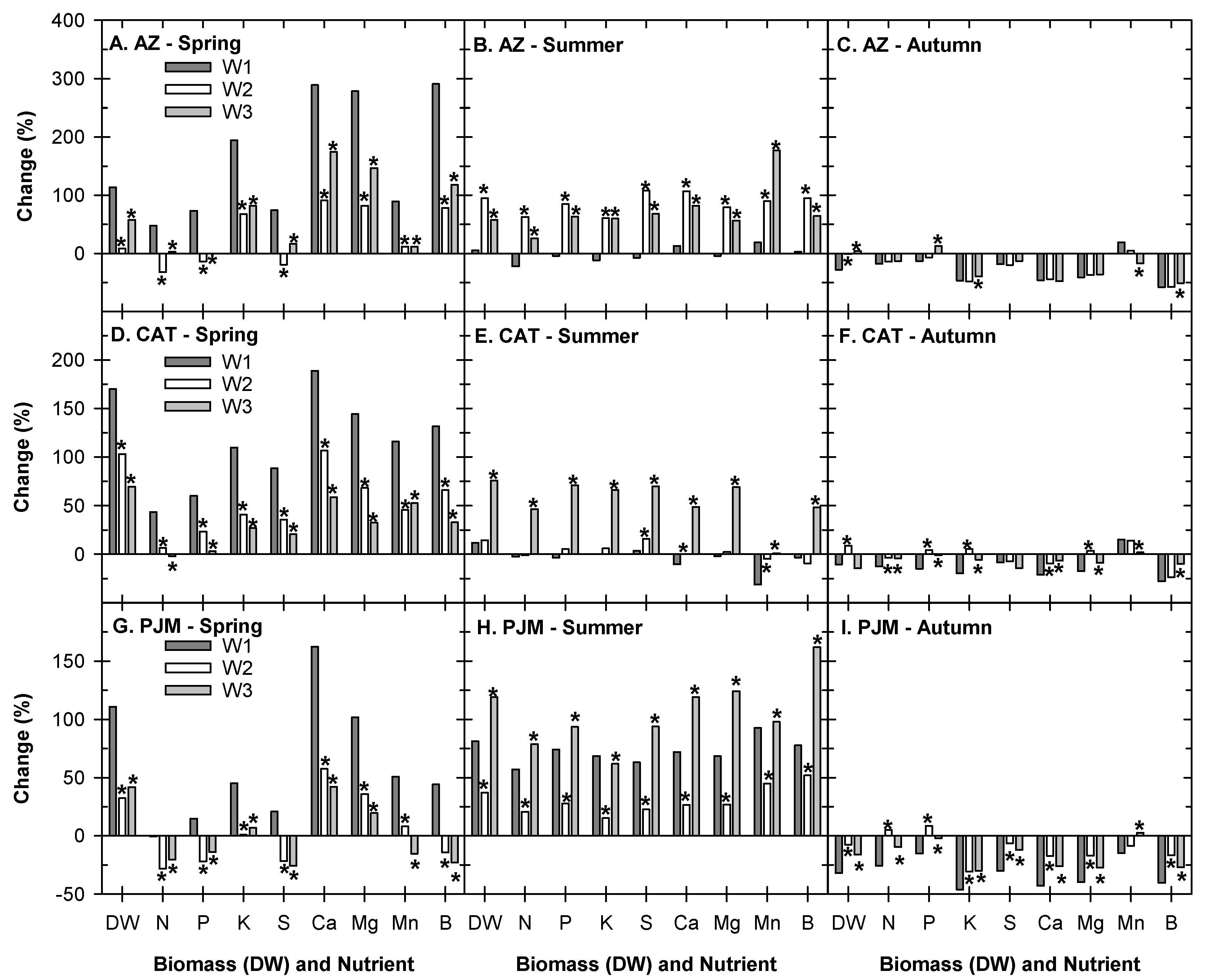
3.1. Effects of Different Nitrogen Rates on Plant Growth and Nutrient Uptake
3.2. Effects of Irrigation Volume and Frequency on Plant Growth and Nutrient Uptake
3.3. Allocation of Plant N and Biomass
3.4. Flowering
3.5. Electrical Conductivity and Moisture Content of the Substrate
3.6. Stomatal Conductance
4. Discussion
4.1. Rate of N Application
4.2. Irrigation Frequency
4.3. Irrigation Volume
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Aghai, M.M.; Pinto, J.R.; Davis, A.S. Container volume and growing density influence western larch (Larix occidentalis Nutt.) seedling development during nursery culture and establishment. New For. 2014, 45, 199–213. [Google Scholar] [CrossRef]
- Landis, T.D.; Pinto, J.R.; Dumroese, R.K. Light-emitting diodes (LED): Applications in forest and native plant nurseries. For. Nurs. Notes 2013, 33, 5–13. [Google Scholar]
- Oliet, J.A.; Tejada, M.; Salifu, K.F.; Collazos, A.; Jacobs, D.F. Performance and nutrient dynamics of holm oak (Quercus ilex L.) seedlings in relation to nursery nutrient loading and post-transplant fertility. Eur. J. For. Res. 2009, 128, 253–263. [Google Scholar] [CrossRef]
- Riikonen, J.; Luoranen, J. Seedling production and the field performance of seedlings. Forests 2018, 9, 740. [Google Scholar] [CrossRef]
- Currey, C.J.; Lopez, R.G. Cuttings of Impatiens, Pelargonium, and Petunia propagated under light-emitting diodes and high-pressure sodium lamps have comparable growth, morphology, gas exchange, and post-transplant performance. HortScience 2013, 48, 428–434. [Google Scholar] [CrossRef]
- Franco, J.A.; Cros, V.; Bañon, S.; Gonzalex, A.; Abrisqueta, J.M. Effects of nursery irrigation on post-planting root dynamics of Lotus creticus in semiarid field conditions. HortScience 2002, 37, 525–528. [Google Scholar] [CrossRef]
- Lloyd, J.; Herms, D.A.; Rose, M.A.; Van Wagoner, J. Fertilization rate and irrigation scheduling in the nursery influence growth, insect performance, and stress tolerance of ‘Sutyzam’ Crabapple in the landscape. HortScience 2006, 51, 442–445. [Google Scholar] [CrossRef]
- Marler, T.E. Repetitive pruning of Serianthes nursery plants improves transplant quality and post-transplant survival. Plant Signal. Behav. 2019, 14, 8. [Google Scholar] [CrossRef]
- McGrath, D.; Henry, J.; Munroe, R.; Williams, C. From propagation to field: Influence of tray design on tree seedling quality and performance. J. Environ. Hortic. 2021, 39, 33–40. [Google Scholar] [CrossRef]
- Wright, A.N.; Warren, S.L.; Blazich, F.A.; Blum, U. Root and shoot growth periodicity of Kalmia latifolia ‘Sarah’ and Ilex crenata ‘Compacta’. HortScience 2004, 39, 243–247. [Google Scholar] [CrossRef]
- Goyette, B.; Piché, M.; Brownbridge, M.; McGrath, D. Impact of handling practices on the quality of bare-root plants: A review. J. Environ. Hort. 2014, 32, 103–113. [Google Scholar] [CrossRef]
- Bilderback, T.E. Water management is key in reducing nutrient runoff from container nurseries. Horttechnology 2002, 12, 541–544. [Google Scholar] [CrossRef]
- Lea-Cox, J.D.; Bauerle, W.L.; van Iersel, M.W.; Kantor, G.F.; Bauerle, T.L.; Lichtenberg, E.; King, D.M.; Crawford, L. Advancing wireless sensor networks for irrigation management of ornamental crops: An overview. Horttechnology 2013, 23, 717–724. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Fernandez, R.T.; Fisher, P.R.; Hitchcock, D.R.; Lea-Cox, J.; Owen, J.S.; Oki, L.R.; White, S.A. Water use and treatment in container-grown specialty crop production: A review. Water Air Soil Pollut. 2017, 228, 1–27. [Google Scholar] [CrossRef]
- Majsztrik, J.C.; Ristvey, A.G.; Ross, D.S.; Lea-Cox, J.D. Comparative water and nutrient application rates among ornamental operations in Maryland. HortScience 2018, 53, 1364–1371. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Nutrient uptake and loss by container-grown deciduous and evergreen Rhododendron nursery plants. HortScience 2011, 46, 296–305. [Google Scholar] [CrossRef]
- Cabrera, R.I. Nitrogen balance for two contain-grown woody ornamental plants. Scientia Hort. 2002, 97, 297–308. [Google Scholar] [CrossRef]
- Raviv, M.; Wallach, R.; Silber, A.; Medina, S.; Krasnovsky, A. The effect of hydraulic characteristics of volcanic materials on yield of roses grown in soilless culture. J. Amer. Soc. Hort. Sci. 1999, 124, 205–209. [Google Scholar] [CrossRef]
- Mack, R.; Owen, J.S., Jr.; Niemiera, A.X.; Sample, D.J. Validation of nursery and greenhouse best management practices through scientific evidence. HortTechnology 2019, 29, 700–715. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Irrigation frequency alters nutrient uptake in container-grown Rhododendron plants grown with different rates of nitrogen. HortScience 2012, 47, 189–197. [Google Scholar] [CrossRef]
- Cameron, R.; Harrison-Murray, R.; Fordham, M.; Wildinson, S.; Davies, W.; Atkinson, C.; Else, M. Regulated irrigation of woody ornamentals to improve plant quality and precondition against drought stress. Ann. Appl. Biol. 2008, 153, 49–61. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ortuño, M.F.; Bañon, S.; Álvarez, S. Deficit irrigation as a strategy to control growth in ornamental plants and enhance their ability to adapt to drought conditions. J. Hortic. Sci. Biotechnol. 2019, 94, 137–150. [Google Scholar] [CrossRef]
- Timmer, V.R.; Miller, B.D. Effects of contrasting fertilization and moisture regimes on biomass, nutrients, and water relations of container grown red pine seedlings. New For. 1991, 5, 335–348. [Google Scholar] [CrossRef]
- Beeson, R.C., Jr. Relationship of plant growth and actual evapotranspiration to irrigation frequency based on management allowed deficits for container nursery stock. J. Amer. Soc. Hort. Sci. 2006, 131, 140–148. [Google Scholar] [CrossRef]
- Koniarski, M.; Matysiak, B. Growth and development of potted Rhododendron cultivars ‘Catawbiense Boursault’ and ‘Old Port’ in response to regulated deficit irrigation. J. Hort. Res. 2013, 21, 29–37. [Google Scholar] [CrossRef][Green Version]
- Welsh, D.F.; Zajicek, J.M. A model for irrigation scheduling in contain-grown nursery crops utilizing management allowed deficits (MAD). J. Environ. Hort. 1993, 11, 115–118. [Google Scholar]
- Cabrera, R.I.; Devereaux, D. Crape myrtle post-transplant growth as affected by nitrogen nutrition during nursery production. J. Amer. Soc. Hort. Sci. 1999, 124, 94–98. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Bryla, D.R.; Fuchigami, L.H.; Regan, R.P. Irrigation frequency during container production alters Rhododendron growth, nutrient uptake, and flowering after transplanting into a landscape. HortScience 2014, 49, 955–960. [Google Scholar] [CrossRef]
- Yamg, Q.; Li, F.; Zhang, F.; Lui, X. Interactive effects of irrigation frequency and nitrogen addition on growth and water use of Jatropha curcas. Biomass Bioenergy 2013, 59, 234–242. [Google Scholar] [CrossRef]
- Fare, D.C.; Gilliam, C.H.; Keever, G.J.; Olive, J.W. Cyclic irrigation reduces container leachate nitrate-nitrogen concentration. HortScience 1994, 29, 1514–1517. [Google Scholar] [CrossRef]
- Perdue, S.; Hamer, H. 2019 Census of Horticultural Specialties; United States Department of Agriculture: Washington, DC, USA, 2020; Volume 3, p. 3. [Google Scholar]
- Gavlak, R.G.; Horneck, D.A.; Miller, R.O. The Soil, Plant and Water Reference Methods for the Western Region, 3rd ed.; Western Regional Extension Publication 125; University of Alaska: Fairbanks, AK, USA, 2005; p. 204. [Google Scholar]
- Jones, J.B.; Case, V.W. Sample, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 389–427. [Google Scholar]
- Scoggins, H.L.; VanIersal, M.W. In situ probes for measurement of electrical conductivity of soilless substrates: Effects of temperature and substrate moisture content. HortScience 2006, 41, 210–214. [Google Scholar] [CrossRef]
- Chapin, F.S.; Van Cleve, K. Approaches to Studying Nutrient Uptake, Use and Loss in Plants. In Plant Physiological Ecology; Springer: Dordrecht, The Netherlands, 2000; pp. 185–207. [Google Scholar]
- Chapin, F.S.; Bloom, A.J.; Field, C.B.; Waring, R.H. Plant responses to multiple environmental factors. BioScience 1987, 37, 49–57. [Google Scholar] [CrossRef]
- Bi, G.; Scagel, C.F.; Fuchigami, L.H.; Regan, R.P. Differences in growth, and nitrogen uptake and storage between two container-grown cultivars of Rhododendron. J. Environ. Hort. 2007, 25, 13–20. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients, and water: A quantitative review. Funct. Plant Biol. 2000, 27, 595–607. [Google Scholar] [CrossRef]
- Agren, G.I.; Ingestad, T. Root:shoot ratio as a balance between nitrogen productivity and photosynthesis. Plant Cell Environ. 1987, 10, 579–586. [Google Scholar]
- Villar-Salvador, P.; Planelles, R.; Enríquez, E.; Peñuelas Rubira, J.L. Nursery cultivation regimes, plant functional attributes, and field performance relationships in the Mediterranean oak Quercus ilex L. Forest Ecol. Manag. 2004, 196, 257–266. [Google Scholar] [CrossRef]
- Cuesta, B.; Villar-Salvador, P.; Puértolas, J.; Jacobs, D.F.; Rey Benayas, J.M. Why do large, nitrogen rich seedlings better resist stressful transplanting conditions? A physiological analysis in two functionally contrasting Mediterranean forest species. For. Ecol. Manag. 2010, 260, 71–78. [Google Scholar] [CrossRef]
- Villar-Salvador, P.; Puértolas, J.; Cuesta, B.; Peñuelas, J.L.; Uscola, M.; Heredia-Guerrero, N.; Rey Benayas, J.M. Increase in size and nitrogen concentration enhances seedling survival in Mediterranean plantations. Insights from an ecophysiological conceptual model of plant survival. New For. 2012, 43, 755–770. [Google Scholar] [CrossRef]
- Li, T.; Bi, G.; Harkess, R.L.; Denny, G.C.; Scagel, C.F. Nitrogen fertilization and irrigation frequency affect hydrangea growth and nutrient uptake in two container types. HortScience 2019, 54, 167–174. [Google Scholar] [CrossRef]
- Scagel, C.F.; Bi, G.; Fuchigami, L.H.; Regan, R.P. Seasonal variation in growth, nitrogen uptake and allocation by container-grown evergreen and deciduous Rhododendron cultivars. HortScience 2007, 42, 1440–1449. [Google Scholar] [CrossRef]
- Klute, A. Water retention: Laboratory methods. In Methods or Soil Analysis: Part 1. Physical and Mineralogical Methods, 2nd ed.; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 635–662. [Google Scholar]
- White, J.W.; Mastalerz, J.W. Soil moisture as related to container capacity. Proc. Amer. Horticult. Sci. 1966, 89, 758–765. [Google Scholar]
- Wong, S.C.; Cowan, I.R.; Farquhar, G.D. Stomatal conductance correlates with photosynthetic capacity. Nature 1979, 282, 424–426. [Google Scholar] [CrossRef]
- Pershey, N.A.; Cregg, B.M.; Anresen, J.A.; Fernandez, R.T. Irrigation based on dialy water use reduces nursery runoff volume and nutrient load without reducing growth of four conifers. HortScience 2015, 50, 1553–1561. [Google Scholar] [CrossRef]
- Warsaw, A.L.; Fernandez, R.T.; Cregg, B.M.; Andresen, J.A. Water conservation, growth, and water use efficiency of container-grown woody ornamentals irrigation based on daily water use. HortScience 2009, 44, 1308–1318. [Google Scholar] [CrossRef]
- Karam, N.S.; Niemiera, A.X. Cyclic sprinkler irrigation and pre-irrigation substrate water content affect water and N leaching from containers. J. Environ. Hort. 1994, 12, 198–202. [Google Scholar] [CrossRef]
| Cultivar, Time, and Structure | Allocation (% Total Plant) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Treatment | Irrigation Treatment | |||||||||||||
| 0 N | 0.5 N | 1.0 N | W1 | W2 | W3 | |||||||||
| AZ | December Year 1 | Roots | 70 | a 1 | 71 | a | 58 | b | 67 | a | 65 | a | 61 | b |
| Stems | 30 | b | 29 | b | 42 | a | 33 | b | 35 | b | 39 | a | ||
| December Year 2 | Roots | 69 | a | 58 | b | 50 | c | 58 | a | 54 | b | 52 | b | |
| Stems | 31 | c | 42 | b | 50 | a | 42 | b | 46 | a | 48 | a | ||
| CAT | December Year 1 | Roots | 32 | a | 25 | b | 21 | c | 24 | a | 23 | a | 24 | a |
| Stems | 11 | b | 13 | ab | 14 | a | 13 | a | 14 | a | 15 | a | ||
| Leaves | 56 | b | 62 | a | 65 | a | 63 | a | 63 | a | 61 | a | ||
| December Year 2 | Roots | 48 | a | 42 | b | 40 | b | 41 | a | 41 | a | 40 | a | |
| Stems | 8 | b | 17 | a | 19 | a | 16 | b | 20 | a | 18 | b | ||
| Leaves | 43 | a | 41 | a | 41 | a | 43 | a | 39 | b | 42 | a | ||
| PJM | December Year 1 | Roots | 34 | a | 28 | b | 26 | b | 27 | a | 26 | a | 27 | a |
| Stems | 28 | a | 23 | b | 23 | b | 24 | a | 25 | a | 22 | a | ||
| Leaves | 38 | b | 49 | a | 51 | a | 49 | a | 49 | a | 52 | a | ||
| December Year 2 | Roots | 34 | b | 38 | a | 38 | a | 41 | a | 31 | b | 40 | a | |
| Stems | 19 | c | 29 | b | 35 | a | 35 | a | 30 | a | 33 | a | ||
| Leaves | 47 | a | 33 | b | 27 | c | 24 | b | 39 | a | 27 | b | ||
| Cultivar | Variable | N treatment | Irrigation Treatment | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 N | 0.5 N | 1.0 N | W1 | W2 | W3 | ||||||||
| AZ | Plants with flowers (%) | 21 | c 1 | 56 | b | 86 | a | 59 | b | 72 | a | 84 | a |
| Flowers/plant | 6 | c | 13 | b | 30 | a | 17 | b | 23 | a | 25 | a | |
| Flower dry wt (mg) | 13 | b | 17 | b | 31 | a | 19 | a | 16 | a | 20 | a | |
| Inflorescences/plant | 1 | a | 1 | a | 3 | a | 2 | a | 2 | a | 2 | a | |
| Flowers/inflorescence | 5 | b | 11 | a | 10 | a | 9 | b | 12 | a | 12 | a | |
| CAT | Plants with flowers (%) | 0 | c | 39 | b | 86 | a | 67 | a | 67 | a | 55 | a |
| Flowers/plant | 0 | c | 10 | b | 25 | a | 10 | b | 19 | a | 24 | a | |
| Flower dry wt (mg) | 0 | c | 86 | b | 99 | a | 91 | b | 122 | a | 65 | b | |
| Inflorescences/plant | 0 | b | 2 | a | 5 | a | 2 | a | 3 | a | 3 | a | |
| Flowers/inflorescence | 0 | b | 7 | a | 5 | a | 6 | a | 7 | a | 7 | a | |
| PJM | Plants with flowers (%) | 9 | b | 97 | a | 95 | a | 96 | a | 92 | a | 100 | a |
| Flowers/plant | 3 | b | 4 | b | 18 | a | 12 | b | 23 | a | 12 | b | |
| Flower dry wt (mg) | 38 | c | 54 | b | 93 | a | 38 | b | 77 | a | 61 | a | |
| Inflorescences/plant | 1 | b | 2 | b | 6 | a | 6 | a | 5a | 6 | a | ||
| Flowers/inflorescence | 3 | a | 2 | a | 3 | a | 2 | a | 4a | 2 | a | ||
| Variable and Irrigation Treatment | AZ | CAT | PJM | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 N | 0.5 N | 1.0 N | 0 N | 0.5 N | 1.0 N | 0 N | 0.5 N | 1.0 N | |||||||||||
| Substrate Moisture (%CC) | |||||||||||||||||||
| Early August | W1 | 69 | a 1 | 70 | a | 65 | b | 69 | a | 65 | ab | 61 | b | 75 | a | 73 | a | 64 | b |
| W2 | 66 | ab | 68 | a | 63 | b | 71 | a | 67 | a | 62 | b | 74 | a | 71 | a | 62 | b | |
| W3 | 71 | ab | 74 | a * | 68 | b * | 72 | a | 72 | a * | 68 | b * | 77 | a * | 74 | a | 65 | b | |
| Late August | W1 | 63 | a | 64 | a | 62 | a | 65 | a | 62 | a | 64 | a | 68 | a | 66 | a | 60 | b |
| W2 | 57 | a * | 54 | a * | 55 | a * | 66 | a | 65 | a | 64 | a | 62 | a * | 60 | b * | 58 | c | |
| W3 | 76 | a * | 73 | a * | 68 | b * | 73 | a * | 75 | a * | 73 | a * | 76 | a * | 71 | b * | 64 | c | |
| Substrate EC (µS cm−1) | |||||||||||||||||||
| 2.54 × 10−2 m | W1 | 600 | b | 536 | b | 853 | a | 587 | b | 608 | b | 833 | a | 538 | b | 542 | b | 864 | a |
| W2 | 702 | c | 1013 | b * | 1327 | a * | 645 | b | 1189 | a * | 1243 | a * | 633 | c | 865 | b * | 1682 | a * | |
| W3 | 620 | b | 865 | a * | 1068 | a * | 682 | c | 854 | b * | 1139 | a * | 591 | b | 718 | b | 1038 | a * | |
| 7.62 × 10−2 m | W1 | 862 | c | 1331 | a | 1165 | b | 1042 | b | 1147 | b | 1344 | a | 765 | c | 1112 | b | 1645 | a |
| W2 | 889 | b | 1321 | a | 1483 | a * | 1027 | c | 1854 | b * | 2335 | a * | 907 | c | 1348 | b * | 2188 | a * | |
| W3 | 927 | b | 1154 | a * | 1180 | a | 1013 | b | 1644 | a * | 1748 | a * | 882 | c | 1194 | b | 1941 | a * | |
| Time and Irrigation Treatment 1 | gs (mmol m−2 s−1) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AZ | CATy | PJMy | ||||||||||||||||
| 0 N | 0.5 N | 1.0 N | 0 N | 0.5 N | 1.0 N | 0 N | 0.5 N | 1.0 N | ||||||||||
| Early August Year 1 | ||||||||||||||||||
| W1 | 69 | b 1 | 143 | a | 144 | a | 173 | b | 284 | a | 252 | a | 161 | b | 334 | a | 309 | a |
| W2 | 75 | c | 246 | a * | 199 | b | 198 | b | 342 | a * | 312 | a * | 172 | b | 337 | a | 374 | a * |
| W3 | 95 | c | 210 | a * | 204 | b | 196 | b | 351 | a * | 304 | a * | 188 | b | 380 | a * | 401 | a * |
| Late August Year 1 | ||||||||||||||||||
| W1 | 65 | b | 147 | a | 157 | a | 184 | b | 276 | a | 232 | ab | 173 | b | 270 | a | 281 | a |
| W2 | 70 | c | 252 | a * | 219 | b * | 212 | c | 332 | a * | 288 | b * | 190 | c | 279 | b | 326 | a * |
| W3 | 97 | b | 216 | a * | 207 | a * | 210 | b | 319 | a * | 297 | a * | 185 | c | 259 | b | 350 | a * |
| July Year 2 | ||||||||||||||||||
| W1 | 106 | b | 112 | ab | 128 | a | 116 | b | 263 | a | 246 | a | 152 | a | 161 | a | 86 | b |
| W2 | 109 | b | 130 | a | 134 | a | 193 | c * | 247 | b | 244 | a | 270 | a * | 129 | b | 101 | b |
| W3 | 102 | b | 129 | ab | 142 | a | 241 | a * | 260 | a | 184 | b * | 238 | a * | 143 | b | 110 | c |
| August Year 2 | ||||||||||||||||||
| W1 | 122 | a | 95 | b | 121 | a | 189 | c | 369 | a | 297 | b | 222 | a | 180 | b | 101 | c |
| W2 | 102 | a | 74 | b | 106 | a | 120 | b * | 216 | a * | 187 | a * | 173 | a * | 97 | b * | 92 | b |
| W3 | 115 | a | 69 | b | 134 | a | 396 | a * | 245 | b * | 214 | b * | 324 | a * | 139 | b * | 118 | b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bi, G.; Scagel, C.F.; Bryla, D.R. Nitrogen Rate, Irrigation Frequency and Volume Differentially Influence Growth, Flowering, and Nutrient Uptake of Container-Grown Rhododendron during the Following Growing Season. Horticulturae 2022, 8, 647. https://doi.org/10.3390/horticulturae8070647
Bi G, Scagel CF, Bryla DR. Nitrogen Rate, Irrigation Frequency and Volume Differentially Influence Growth, Flowering, and Nutrient Uptake of Container-Grown Rhododendron during the Following Growing Season. Horticulturae. 2022; 8(7):647. https://doi.org/10.3390/horticulturae8070647
Chicago/Turabian StyleBi, Guihong, Carolyn F. Scagel, and David R. Bryla. 2022. "Nitrogen Rate, Irrigation Frequency and Volume Differentially Influence Growth, Flowering, and Nutrient Uptake of Container-Grown Rhododendron during the Following Growing Season" Horticulturae 8, no. 7: 647. https://doi.org/10.3390/horticulturae8070647
APA StyleBi, G., Scagel, C. F., & Bryla, D. R. (2022). Nitrogen Rate, Irrigation Frequency and Volume Differentially Influence Growth, Flowering, and Nutrient Uptake of Container-Grown Rhododendron during the Following Growing Season. Horticulturae, 8(7), 647. https://doi.org/10.3390/horticulturae8070647







