Abstract
Mango fruits have a high nutritional value and are beneficial to health. However, losses frequently occur after harvest, because they are perishable. Salicylic acid (SA) can be used to preserve fruit quality and maintain their nutritional contents. Therefore, this study was conducted to investigate the effects of applications of 2 mM SA on the physicochemical properties, bioactive compounds, and antioxidant and anti-inflammatory activities of mango fruit. For this purpose, mango fruits received preharvest (Pre SA) or postharvest applications of SA (Post SA), or their combination (Pre + Post SA); the fruits were stored at 13 °C for 20 days. Weight loss, decay, and 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging activity were maintained in SA-treated fruit. The Pre + Post SA treatment was superior in delaying fruit ripening, and maintaining lower soluble solids contents and higher total acidity. In addition, total phenolic compounds, ferric reducing antioxidant power, and free radical scavenging activity of anti-inflammatory substances (such as nitric oxide), as well as hyaluronidase inhibition, were higher in the Pre + Post SA treatment throughout storage. Therefore, both pre- and postharvest SA treatments are recommended for preserving the quality of mango fruit, such as Nam Dok Mai Si Thong, and for maintaining their nutritional properties for human health.
1. Introduction
Mango is a popular tropical fruit that is highly valued for its delicate taste, pleasant aroma, and economic benefits. Moreover, mango has a high nutritional value due to its phytochemical composition [1]. It is grown in 85 countries worldwide, including Asian countries. Among them, Thailand was the 10th highest producer in the world in 2019–2020, and the Nam Dok Mai Si Thong mango is one of the leading export products of Thailand [2,3]. The skin of mango is initially green and becomes golden-yellow as it ripens. This variety is fragrant, sweet, and juicy, and has little fibrous tissue [4]. Although mango has many advantages, its quality and nutritional value are difficult to maintain, because the fruit can ripen within 7 to 9 days after harvest at ambient temperatures. During ripening, biochemical changes, including those associated with texture, conversion of starch to sugar, and pigmentation, occur [5]. In addition, ripening imparts a variety of qualities and nutritional properties to fruits that are beneficial for human health.
Salicylic acid (SA), also known as ortho-hydroxybenzoic acid, is an endogenous signaling molecule that modulates stress responses and plant physiological processes, such as photosynthesis, heat production, stomatal conductance, transpiration, disease resistance, seed germination, crop yield, and glycolysis [6]. In recent years, exogenous applications of SA have been used as pre- and postharvest treatments. Preharvest practices not only influence fruit development, but also affect postharvest performance and final quality [7]. Fruit firmness, total phenolic content (TPC), and the antioxidant capacity of peach fruits can be improved by postharvest application of 2 mM SA, with no negative effects on flavor or appearance [8]. This concentration also had the greatest effect on reducing weight loss, fruit softening, disease incidence of Chausa mango, and increased concentrations of bioactive compounds and antioxidant capacity [9]. Preharvest SA applications have also maintained the TPC and total antioxidant activity of tomatoes [10]. In addition, preharvest applications of SA also helped reduce weight loss and firmness of grapes [11] and lemons [12], and maintained postharvest quality by enhancing the preservation of soluble solids concentration (SSC), titratable acidity (TA), and ascorbic acid and total antioxidant contents of Amrapali mango fruit [13]. In addition, pre- and postharvest applications of SA reduced internal browning and maintained the quality of winter pineapple fruit [14], and have also maintained the quality of tomatoes [15]. However, the effect of both pre- and postharvest applications of SA have been little studied in mango fruit.
A variety of compounds contribute to the maintenance of human health. Antioxidants in fruits help the human body’s defense systems by lowering the steady production of prooxidants, such as reactive oxygen species and their products. Cardiovascular disease, cerebrovascular illness, and cancer can all be caused by oxidative stress [16]. Nitric oxide (NO) damages membranes by causing lipid peroxidation and lipoprotein oxidation, both of which can contribute to inflammatory illnesses, atherosclerosis, cancer, cardiovascular disease, and aging [17]; NO scavenging assay are often used to measure anti-inflammatory activity of fruit. In addition, NO scavengers and hyaluronidase inhibitors are effective in reducing allergic reactions and inflammation [18], and work by inhibiting hyaluronidase, an enzyme that breaks down hyaluronic acid and chondroitin sulfate. To date, the effect of SA applications on anti-inflammatory activities, such as nitric oxide scavenging activity and hyaluronidase inhibitory activity (HA), have never been reported in mango fruit.
Therefore, the present study aimed to evaluate the effects of pre- and postharvest treatments of SA, and their combination, on Nam Dok Mai Si Thong mango fruit with respect to physiochemical quality, bioactive compounds, and antioxidant and anti-inflammatory activities, during storage at 13 °C.
2. Materials and Methods
2.1. Plant Material and Experimental Design
Eight-year-old mango trees (Mangifera indica L. cv. Nam Dok Mai Si Thong) from a farm in the Mae Kon Sub District, Chiang Rai Province, Thailand, were selected for this study. Approximately 100 mango fruits, formed ~80 days after flowering, were chosen based on their size and lack of physical damage. These mango fruits were randomly selected from different parts of each tree. The fruits were divided into four groups and subjected to the treatments shown in Table 1. According to our preliminary study, 2 mM SA induced a superior delay in ripening, and reduction in decay, than treatment with 1.0 and 1.5 mM SA. Therefore, 2 mM SA was used in this study. For preharvest (Pre SA) and postharvest (Post SA) SA treatments, and their combination (Pre + Post SA), aliquots of 25 fruit were treated. For the Pre SA treatment, fruits were sprayed with 2 mM SA using a hand-held sprayer until the solution was thoroughly applied to the entire fruit. Three weeks after the Pre SA treatment, the fruits were harvested and immediately transferred to the postharvest laboratory within 1 h.

Table 1.
Pre- and postharvest SA treatments applied to Nam Dok Mai Si Thong mango fruit.
The fruits were cleaned with 200 mg L−1 sodium hypochlorite, then immersed in hot water (49 °C, 10 min) to reduce disease infection. For the Post SA treatment, the fruits were dipped for 10 min in a 2 mM SA solution immediately after being immersed in the hot water. Then, all fruits were dried at room temperature, and stored at 13 °C with 80–85% relative humidity; one aliquot of fruit was sprayed and dipped in water to act as a control. Five replications of each treatment were made for each assay, and one fruit was used per replication. The fruits were sampled every 5 days until the 20th day of storage.
2.2. Determination of Decay and Physio-Chemical Properties
The percentage of decayed fruit was calculated by counting the number of infected fruits out of the total.
To determine the weight loss of stored mango, both the treated and control fruits were weighed at different sampling intervals. The weight loss was expressed as a percentage (%).
The color of the mango peel was measured on both cheeks of the fruit using a colorimeter (Model: CM-600d, Konica Minolta, Tokyo, Japan). The color changes were assessed using L* (lightness), a* (red to green), and b* (yellow to blue) values; these values were then used to calculate total color differences (ΔE) [19], using the following equation:
ΔE* = [(ΔL*)2 + (Δa*)2 + (Δb*)2]0.5
Fruit firmness was measured using a texture analyzer (TA-XT plus stable micro system Co., Ltd., Surrey, UK) fitted with a P/6 (6 mm) probe. After removal of the peel, each fruit was penetrated at the equator at a speed of 1.5 mm s−1 to a depth of 10 mm.
SSC was measured using a hand-held digital refractometer (Model: PR-a, ATAGO, Tokyo, Japan). TA was measured with a digital pocket acidity meter (Model: PAL-Easy ACID F5, ATAGO, Japan), and expressed as percentage of citric acid.
2.3. Bioactive Compounds
Mesocarp tissue (5 g) was mixed with 2.5 mL of distilled water and 25 mL of 95% hexane. The total carotenoid content was measured using the method of Desobry et al. [20], with some modifications. The mixture was homogenized by vortexing for 1 min, then centrifuged at 5000× g at 4 °C for 10 min, following which, the reaction mixture was assayed at 454 nm using a spectrophotometer (Model: GENESYS 10S, Thermo Scientific, Waltham, MA, USA). The total carotenoid content of mango was expressed as μg g−1 dry weight (DW).
Sample extraction for measurement of TPC, and other biochemical analyses, followed the method of Sogi et al. [21], with some modification. Briefly, crushed cubes of mango pulp (1 g) were mixed with 20 mL of 95% methanol, kept in a water bath shaker at 20 °C for 1 h, and then centrifuged at 10,000× g for 10 min. The supernatant was collected and residues were re-extracted using 10 mL of 95% methanol by vortexing for 1 min, followed by centrifugation at 10,000× g for 10 min. For final analysis, both supernatants were combined.
Total phenolic content was determined by the Folin–Ciocalteu method [22]. For this, 0.25 mL of the supernatant was mixed with 1.25 mL of 7.5% w/v sodium carbonate and 1.25 mL of 10% Folin–Ciocalteu reagent. The mixture was placed at room temperature for 1 h, and the reaction mixture was assayed at 765 nm using a spectrophotometer. A standard curve was constructed with gallic acid (GA), and the total phenolic content was expressed as mg GA g−1 DW.
2.4. Antioxidant Capacity
Two methods were used to assess antioxidant capacity. In the first, 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical scavenging activity was determined according to the method of Molyneux [23], with slight modifications. First, 50 µL of crude extract was combined with 1.95 mL methanolic solution of DPPH. Then, the mixture was placed in the dark at room temperature (~25 °C) for 30 min, following which, the absorbance was measured at 517 nm. The DPPH radical scavenging activity was expressed as micromoles of Trolox equivalent (TE) per gram of dry sample (µM TE g−1 DW).
In the second assessment, ferric reducing antioxidant power (FRAP) was evaluated according to the methodology of Benzie and Szeto [24], with modifications. To start, 400 μL of crude extract was mixed into 2.6 mL of FRAP reagent (consisting of acetate buffer, 2,4,6-tris (2,4-pyridyl)-s-triazine (TPTZ) solution, and ferric chloride, in a 10:1:1 ratio). Then, the solution was incubated at 37 °C for 30 min, and measured at an absorbance of 595 nm. The FRAP content was expressed as mM FeSO4 g−1 DW.
2.5. Anti-Inflammatory Activity
The nitric oxide radical scavenging activity was analyzed according to the method described by Hazra et al. [25], with slight modification. For this, 40 µL of diluted extract was mixed with 800 μL of 10 mM sodium nitroprusside. The reaction solution was then incubated for 2 h and 30 min at room temperature. Then, 200 μL of reactive solution was transferred to a new tube, and 400 µL of 0.33% sulfanilamide in 20% glacial acetic acid was added. The sample tubes were kept at room temperature for 5 min. Subsequently, 400 µL of 0.1% N-(1-naphthyl) ethylene-diamine dihydrochloride was added to the test tube, and then incubated at room temperature for 30 min. The mixture was measured at 540 nm using a spectrophotometer. A dilution series containing Trolox, ranging from 0.2 to 0.5 mg mL−1, was used to determine a standard curve, and the results are represented in millimoles of TE per gram of dry weight (mM TE g−1 DW).
Hyaluronidase inhibitory activity was performed following the method described by Bralley et al. [26], with slight modifications. Aliquots of the crude extract (15 µL) were mixed with 147 μL of 0.2 M sodium acetate (pH 6) and 120 μL of 0.5 mg mL−1 sodium hyaluronate, and the mixture stirred for 30 s. Then, 18 mL of 1 mg mL−1 hyaluronidase was added and incubated at 37 °C for 15 min. The reaction was stopped by adding 1.2 mL of 2.3% hexadecyltrimethyl ammonium bromide in NaOH at 2% (pH 12). The samples were then incubated at room temperature for 10 min. The hyaluronidase inhibitory activity was measured at 400 nm and expressed as the percentage (%) of inhibition.
2.6. Statistical Analysis
Experiments were analyzed using five replications in a completely randomized design. The mean and standard error were used to express the data, using the Statistical Analysis System (SPSS, Version 25) for analysis of variance (ANOVA). The effect of treatment on dependent variables was determined using Tukey’s multiple range tests (p < 0.05).
3. Results and Discussion
3.1. Decay and Physio-Chemical Quality in Mango Fruit
The visual appearance of the mango fruit can be seen in Figure 1. Control fruit showed decay at the end of the storage period. Fruit that received the Pre + Post treatment maintained good appearance compared to the control and other treated fruits. Fruit decay is one of the most important parameters affecting postharvest shelf life. No fungal infection was observed on the fruit during the first 10 days of storage (Figure 2A). After that, the control fruit quickly decayed, and the percentage of decayed fruit was higher than that of all the SA-treated fruit. There were no significant differences among the SA-treated fruit, with all treatments having around 20% decay on days 15 and 20. SA promotes the expression of pathogenesis-related genes, thereby conferring resistance to pathogens [27]. The combination of pre- and postharvest SA treatments also had the most beneficial effects on storage life and fruit quality of tomato fruit, including reductions in decay [15].

Figure 1.
The visual appearance of control and SA-treated fruits during storage at 13 °C.
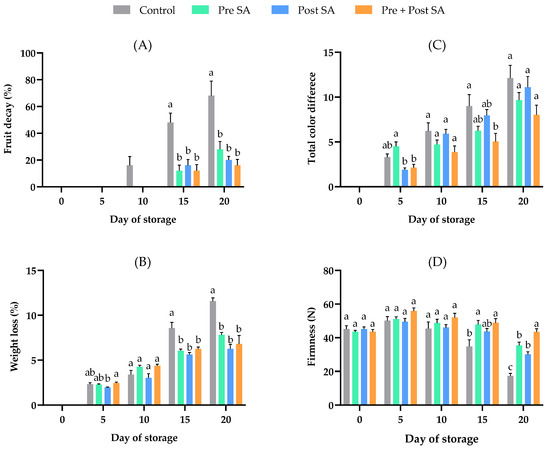
Figure 2.
Effect of SA application on (A) fruit decay, (B) weight loss, (C) total color difference, and (D) firmness of Nam Dok Mai Si Thong mango fruit during storage at 13 °C. Vertical bars represent the standard errors of the means (n = 5). Bars annotated with different letters indicate statistically significant differences among treatments at each storage time.
Weight loss increased with storage time, and reached its highest value of 11.57 ± 0.37% in control fruits, but was significantly (p < 0.05) lower in fruit the received the Post SA, Pre + Post SA, and Pre SA treatments, being 6.26 ± 0.49, 6.81 ± 0.93, and 7.8 ± 0.28%, respectively (Figure 2B). However, there were no significant differences among the SA-treated fruit. The main cause of weight loss in fresh fruits and vegetables is the loss of water due to transpiration and respiration. Respiration using oxygen to break down glucose, and release carbon dioxide and water, reduces the weight of fresh produce [28]. SA applications reduce respiration rates in horticultural crops, thereby reducing weight loss [29]; treatment with 2 mM SA has been shown to reduce weight loss in Chausa mango fruits [9]. The fruits treated with Pre + Post SA maintained their visual appearance at the end of the storage period more than the controls (Figure 1) as a result of reducing shriveling and wilting.
The total color difference (ΔE) of the fruits gradually increased during the storage period (Figure 2C). There are two trends in the data. Firstly, the ΔE values of fruit in the control tend to be higher than those that underwent the SA treatments. Secondly, fruit in the Pre + Post SA treatment tended to have the lowest ΔE values at each assessment time. The changes in peel color during the ripening process are due to a loss of chlorophyll and the biosynthesis of other pigments [30], and treatment with SA appears to slow the ripening process and these changes in pigmentation. A similar effect of SA on color changes was previously found in sweet cherries [31] and peaches [32].
Fruit firmness is one of the most important quality factors for consumer acceptance. The firmness of the control fruit increased slightly by day 5 (50.11 ± 2.48 N), but then decreased, particularly on days 15 and 20. All treatments with SA maintained fruit firmness. Preharvest SA treatments have been shown to significantly lessen reductions in firmness in lemons [12], grapes [11], and Zil mangoes [33]. In our experiment, the Pre SA treatment also showed potential for maintaining the firmness of mango fruit. However, Pre + Post SA maintained the fruit firmness better than the other treatments. SA can beneficially maintain firmness by inhibiting cell wall- and membrane-degrading enzyme activities, and by decreasing ethylene production [34,35].
The effect of SA application on changes in the soluble solids content (SSC) of the mango fruit is shown in Figure 3A. The SSC gradually increased in both control and treated fruit. On day 10 and onwards, the SSC contents of SA-treated fruit were significantly lower (p < 0.05) compared to those of control fruit. At the end of the storage period, the SSC of fruit in the Pre + Post SA (11.82 ± 0.51%) was significantly (p < 0.05) lower than in the Pre SA (14.7 ± 0.47%) and Post SA (13.79 ± 0.15%) treatments. The SSC of mangoes increases during ripening due to starch breakdown [36] and the actions of sucrose-phosphate synthase [37], and the lower SSC in SA-treated mango fruits could be related to their ability to reduce the activity of this enzyme [38].
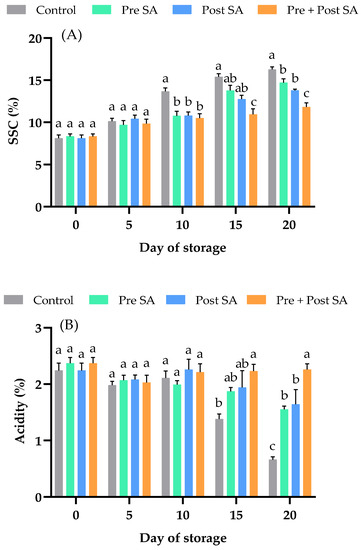
Figure 3.
Effect of SA application on (A) soluble solids content and (B) acidity of Nam Dok Mai Si Thong mango fruits during storage at 13 °C. Vertical bars represent the standard errors of the means (n = 5). Bars annotated with different letters indicate statistically significant differences among treatments at each storage time.
Figure 3B shows the effect of SA on acidity. A significant difference (p < 0.05) between the control and Pre + Post SA can be seen on days 15 and 20. Moreover, on day 20, the acidity of fruit that received the Pre + Post SA treatment was also significantly (p < 0.05) higher than that of fruit in the other SA treatments. On day 20, the percent acidity of the control was 0.66 ± 0.05%, while those of fruit in the Pre SA, Post SA, and Pre + Post SA treatment groups were 1.55 ± 0.06%, 1.64 ± 0.26%, and 2.26 ± 0.1%, respectively. The decrease in acidity during the storage period could be due to the use of organic acids as a substrate for respiration. Preharvest SA treatment has been shown to lower reductions in TA of Amrapali mangoes [13], and a combination of pre- and postharvest SA treatments had a similar effect in tomato fruit [15].
3.2. Bioactive Compounds
SA has been considered as an elicitor, as it increases concentrations of bioactive compounds such as phenolics, glucosinolates, carotenoids, betalains, and vitamins that promote defense responses [38]. In this study, carotenoid and total phenolic contents were analyzed to determine the effects of SA on such bioactive compounds. Carotenoids can be considered as an important factor protecting organisms against photooxidative processes [39]. The carotenoid content of the mango fruits gradually increased from day 0 to day 15 for all fruit (Figure 4A). There was also a trend for fruit that underwent a postharvest dip in SA to have higher carotenoid contents from day 15 onwards, and there was no significant difference between the Post SA and Pre + Post SA fruits during the storage period. Postharvest applications of 2 mM SA have been shown to maintain carotenoid contents in Chausa mango fruit [9].
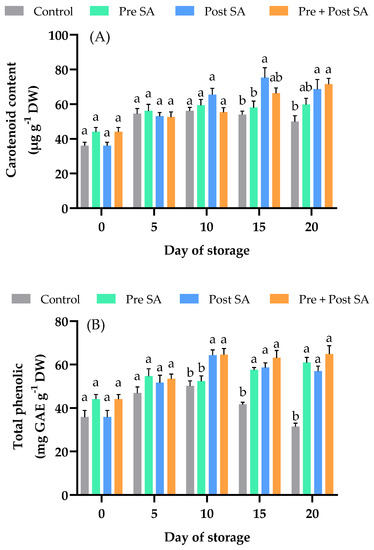
Figure 4.
Effect of SA application on contents of (A) carotenoids and (B) total phenolic compounds of Nam Dok Mai Si Thong mango fruits during storage at 13 °C. Vertical bars represent the standard errors of the means (n = 5). Bars annotated with different letters indicate statistically significant differences among treatments at each storage time.
Phenolic compounds have antioxidant, anti-inflammatory, antimutagenic, antitumor, anticancer, antimicrobial, and cytotoxic properties [40]. Phenolic compounds also have high antioxidant properties. Their structure is built around one or more aromatic rings bonded to at least one hydroxyl group [41]. These hydroxyl groups are able to donate hydrogen atoms, which gives phenolic compounds their ability to neutralize free radicals, making them more stable and less toxic [40]. The effect of the SA treatments on TPC can be seen in Figure 4B. During the storage period, the TPC varied from 31.42 to 64.85 mg g−1 DW. A significant difference (p < 0.05) between control and treated fruits occurred at days 15 and 20. There was no difference between SA-treated fruit in all storage periods; however, there was a trend for the fruit in the Pre + Post SA treatment to have the highest TPC. Beneficial effects of postharvest SA applications on TPC have been found for cornelian cherries [42] and papaya fruits [43].
3.3. Antioxidant Activity
The DPPH radical scavenging activity (Figure 5A) of the control fruits gradually reduced after 10 days of storage, particularly after 20 days (43.45 ± 0.7 μM g−1 DW to 21.23 ± 1.64 μM g−1 DW). After 10 days of storage, the scavenging activity of all SA-treated fruit was significantly (p < 0.05) higher than that in control fruit. However, there were no significant differences among these treatments. Our results are in line with those of Dokhanieh et al. [42], who reported that treatment with SA significantly increased the DPPH scavenging activity of cornelian cherry fruits by enhancing concentrations of bioactive compounds such as phenolics.
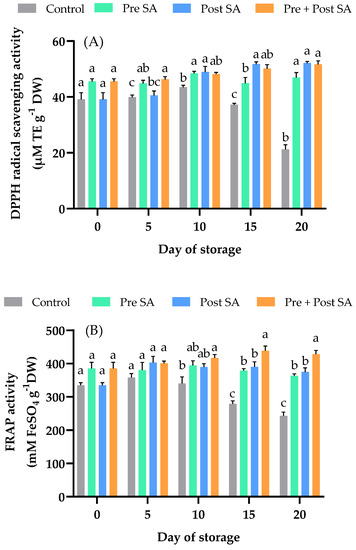
Figure 5.
Effect of SA application on (A) DPPH radical scavenging activity and (B) FRAP activity of Nam Dok Mai Si Thong mango fruits during storage at 13 °C. Vertical bars represent the standard errors of the means (n = 5). Bars annotated with different letters indicate statistically significant differences among treatments at each storage time.
FRAP activity of all samples gradually increased up to day 15, but decreased slightly on day 20 (Figure 5B). A significant difference (p < 0.05) between control fruit and those in the Pre + Post SA treatment group was found after day 10. At days 15 and 20, FRAP activity was significantly (p < 0.05) higher in fruit that received the Pre + Post SA treatment than in fruit in the other two SA treatment groups. In a previous study, Erogul and Özsoydan [44] showed that a preharvest application of 2 mM SA increased FRAP activity in the Cresthaven peach.
3.4. Anti-Inflammatory Activity
Exogenous application of SA increases anti-inflammatory activity and the production of secondary metabolites in Ajuga integrifolia Buch. Ham. ex D. Don, a plant grown as a source of medicine and for its essential oil [45], and in Centella asiatica (L.) Urban, a plant also used as a medicinal herb as well as a culinary vegetable [46]. In this study, SA treatments were applied to mango fruits to promote NO scavenging and hyaluronidase inhibition. The NO radical scavenging activity in the fruit of the control treatment gradually decreased during storage (Figure 6A). A significant difference (p < 0.05) between control and all SA-treated mango fruits was observed after day 5. On day 20, NO radical scavenging activity in fruit of the Pre + Post SA treatment was significantly higher than those in other two SA treatments.
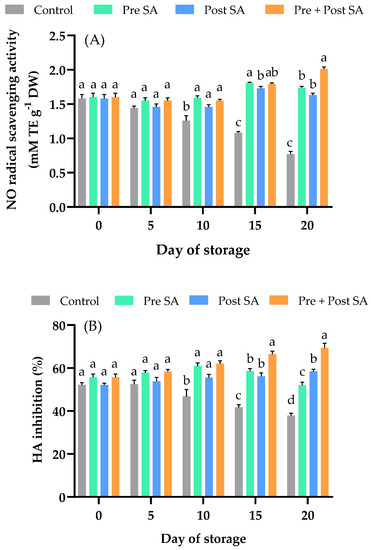
Figure 6.
Effect of SA application on (A) NO radical scavenging activity (mM TE g−1) DW and (B) HA inhibition (%) of Nam Dok Mai Si Thong mango fruits during storage at 13 °C. Vertical bars represent the standard errors of the means (n = 5). Bars annotated with different letters indicate statistically significant differences among treatments at each storage time.
The effects of SA treatment on hyaluronidase inhibition are shown in Figure 6B. No significant difference was seen between control and treated samples in the early storage period, up to day 5. After day 5, the HA inhibition of control fruit declined remarkably rapidly, and a significant difference (p < 0.05) was found between the control and all SA-treated fruit. On days 15 and 20, HA inhibition was higher in fruit in the Pre + Post SA treatment group than those in the other two SA treatment groups. Inflammatory responses occur when tissues are injured or infected by microbes, leading to a complex process involving immune cells and pro-inflammatory mediators [47]. SA is an elicitor produced in plants that mediates signal transmission in plant defense responses [48]; in our study, SA maintained the anti-inflammatory properties of mango fruits. However, clarification of the mechanism by which SA impacts anti-inflammatory activity needs further study.
4. Conclusions
The present study concludes that SA is an effective way of slowing the ripening process, thereby maintaining the quality of mango fruit during storage, resulting in reduced weight loss and fruit decay, while delaying changes in total color differences, firmness, SSC, and TA. It also enhanced the TPC, antioxidant activity, and anti-inflammatory properties of the fruit. Among the SA treatments, the Pre + Post SA treatment showed the greatest effect on firmness, TSS, TA, FRAP, NO radical scavenging activity, and HA inhibition. Thus, this treatment is recommended for use on mango fruits to maintain postharvest quality and their biochemical contents, and to extend storage life, thereby providing fruits to consumers with better health benefits.
Author Contributions
S.S. conceptualized, designed experiments, wrote-reviewed and edited the manuscript. S.T.W. performed experiments, analyzed the data and wrote a draft manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Mae Fah Luang University, Chiang Rai, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request corresponding author.
Acknowledgments
The authors thank Paul Holford for kind suggestions and English proofreading of the manuscript and Thamarath Pranamornkith for advisory on experimental details.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional composition and bioactive compounds in three different parts of mango fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Noiwan, D.; Suppakul, P.; Joomwong, A.; Uthaibutra, J.; Rachtanapun, P. Kinetics of mango fruits (Mangifera indica cv.‘Nam Dok Mai Si Thong’) quality changes during storage at various temperatures. J. Agric. Sci. 2017, 9, 199–212. [Google Scholar] [CrossRef][Green Version]
- Chimvaree, C.; Cumsingnok, T.; Wongs-Aree, C.; Supapvanich, S.; Charoenrat, T.; Tepsorn, R.; Boonyaritthongchai, P. Substrate reactivity of polyphenol oxidase and browning inhibition of fresh-cut ‘Nam Dok Mai Si-Thong’ mangoes by protein-based sericin coating. Hortic. J. 2020, 89, 537–544. [Google Scholar] [CrossRef]
- Singh, Z.; Singh, R.K.; Sane, V.A.; Nath, P. Mango-postharvest biology and biotechnology. Crit. Rev. Plant Sci. 2013, 32, 217–236. [Google Scholar] [CrossRef]
- Klessig, D.F.; Malamy, J. The salicylic acid signal in plants. Plant Mol. Biol. 1994, 26, 1439–1458. [Google Scholar] [CrossRef]
- Hewett, E.W. An overview of preharvest factors influencing postharvest quality of horticultural products. Int. J. Postharvest Technol. Innov. 2006, 1, 4–15. [Google Scholar] [CrossRef]
- Khademi, Z.; Ershadi, A. Postharvest application of salicylic acid improves storability of peach (Prunus persica cv. Elberta) fruits. International. J. Agric. Crop Sci. 2013, 5, 651. [Google Scholar]
- Barman, K.; Asrey, R. Salicylic acid pre-treatment alleviates chilling injury, preserves bioactive compounds and enhances shelf life of mango fruit during cold storage. J. Sci. Ind. Res. 2014, 73, 713–718. [Google Scholar]
- Baek, M.W.; Choi, H.R.; Yun Jae, L.; Kang, H.-M.; Lee, O.-H.; Jeong, C.S.; Tilahun, S. Preharvest treatment of methyl jasmonate and salicylic acid increase the yield, antioxidant activity and GABA content of tomato. Agronomy 2021, 11, 2293. [Google Scholar] [CrossRef]
- Lo’ay, A. Preharvest salicylic acid and delay ripening of ‘superior seedless’ grapes. Egypt. J. Basic Appl. Sci. 2017, 4, 227–230. [Google Scholar] [CrossRef][Green Version]
- Serna-Escolano, V.; Martínez-Romero, D.; Giménez, M.J.; Serrano, M.; García-Martínez, S.; Valero, D.; Valverde, J.M.; Zapata, P.J. Enhancing antioxidant systems by preharvest treatments with methyl jasmonate and salicylic acid leads to maintain lemon quality during cold storage. Food Chem. 2021, 338, 128044. [Google Scholar] [CrossRef]
- Reddy, S.; Sharma, R. Effect of pre-harvest application of salicylic acid on the postharvest fruit quality of the Amrapali mango (Mangifera indica). Indian J. Agric. Sci. 2016, 86, 727–731. [Google Scholar]
- Lu, X.; Sun, D.; Li, Y.; Shi, W.; Sun, G. Pre-and post-harvest salicylic acid treatments alleviate internal browning and maintain quality of winter pineapple fruit. Sci. Hortic. 2011, 130, 97–101. [Google Scholar] [CrossRef]
- Baninaiem, E.; Mirzaaliandastjerdi, A.; Rastegar, S.; Abbaszade, K. Effect of pre-and postharvest salicylic acid treatment on quality characteristics of tomato during cold storage. Adv. Hortic. Sci. 2016, 30, 183–192. [Google Scholar]
- Tesoriere, L.; Butera, D.; Pintaudi, A.M.; Allegra, M.; Livrea, M.A. Supplementation with cactus pear (Opuntia ficus-indica) fruit decreases oxidative stress in healthy humans: A comparative study with vitamin C. Am. J. Clin. Nutr. 2004, 80, 391–395. [Google Scholar] [CrossRef]
- Boora, F.; Chirisa, E.; Mukanganyama, S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Process. 2014, 2014, 918018. [Google Scholar] [CrossRef]
- Furusawa, M.; Narita, Y.; Iwai, K.; Fukunaga, T.; Nakagiri, O. Inhibitory effect of a hot water extract of coffee “silverskin” on hyaluronidase. Biosci. Biotechnol. Biochem. 2011, 75, 1205–1207. [Google Scholar] [CrossRef]
- Chen, C.; Ramaswamy, H. Color and texture change kinetics in ripening bananas. LWT-Food Sci. Technol. 2002, 35, 415–419. [Google Scholar] [CrossRef]
- Desobry, S.A.; Netto, F.M.; Labuza, T.P. Comparison of spray-drying, drum-drying and freeze-drying for β-carotene encapsulation and preservation. J. Food Sci. 1997, 62, 1158–1162. [Google Scholar] [CrossRef]
- Sogi, D.; Siddiq, M.; Roidoung, S.; Dolan, K. Total phenolics, carotenoids, ascorbic acid, and antioxidant properties of fresh-cut mango (Mangifera indica L., cv. Tommy Atkin) as affected by infrared heat treatment. J. Food Sci. 2012, 77, C1197–C1202. [Google Scholar] [CrossRef] [PubMed]
- ISO 14502-1; Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea-Colorimetric Method Using Folin-Ciocalteu Reagent. International Organization for Standardization: Geneva, Switzerland, 2005; p. 10.
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Benzie, I.F.; Szeto, Y. Total antioxidant capacity of teas by the ferric reducing/antioxidant power assay. J. Agric. Food Chem. 1999, 47, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Hazra, B.; Biswas, S.; Mandal, N. Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complementary Altern. Med. 2008, 8, 63. [Google Scholar] [CrossRef]
- Bralley, E.; Greenspan, P.; Hargrove, J.L.; Hartle, D.K. Inhibition of hyaluronidase activity by Vitis rotundifolia (Muscadine) berry seeds and skins. Pharm. Biol. 2007, 45, 667–673. [Google Scholar] [CrossRef]
- Malamy, J.; Klessig, D.F. Salicylic acid and plant disease resistance. Plant J. 1992, 2, 643–654. [Google Scholar] [CrossRef]
- Xanthopoulos, G.T.; Templalexis, C.G.; Aleiferis, N.P.; Lentzou, D.I. The contribution of transpiration and respiration in water loss of perishable agricultural products: The case of pears. Biosyst. Eng. 2017, 158, 76–85. [Google Scholar] [CrossRef]
- Asghari, M.; Aghdam, M.S. Impact of salicylic acid on post-harvest physiology of horticultural crops. Trends Food Sci. Technol. 2010, 21, 502–509. [Google Scholar] [CrossRef]
- Jabbar, A.; Malik, A.U.; Saeed, M.; Malik, O.H.; Amin, M.; Khan, A.S.; Rajwana, I.A.; Saleem, B.A.; Hameed, R.; Mazhar, M.S. Performance of hot water phytosanitary treated mangoes for intended export from Pakistan to Iran and China. Int. J. Agric. Biol. 2011, 13, 645–651. [Google Scholar]
- Valero, D.; Diaz-Mula, H.M.; Zapata, P.J.; Castillo, S.; Guillen, F.; Martinez-Romero, D.; Serrano, M. Postharvest treatments with salicylic acid, acetylsalicylic acid or oxalic acid delayed ripening and enhanced bioactive compounds and antioxidant capacity in sweet cherry. J. Agric. Food Chem. 2011, 59, 5483–5489. [Google Scholar] [CrossRef]
- Tareen, M.J.; Abbasi, N.A.; Hafiz, I.A. Postharvest application of salicylic acid enhanced antioxidant enzyme activity and maintained quality of peach cv. ‘Flordaking’ fruit during storage. Sci. Hortic. 2012, 142, 221–228. [Google Scholar] [CrossRef]
- Hong, K.; Gong, D.; Xu, H.; Wang, S.; Jia, Z.; Chen, J.; Zhang, L. Effects of salicylic acid and nitric oxide pretreatment on the expression of genes involved in the ethylene signalling pathway and the quality of postharvest mango fruit. N. Z. J. Crop Hortic. Sci. 2014, 42, 205–216. [Google Scholar] [CrossRef]
- Srivastava, M.K.; Dwivedi, U.N. Delayed ripening of banana fruit by salicylic acid. Plant Sci. 2000, 158, 87–96. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Zhang, S.; Ferguson, I. The role of salicylic acid in postharvest ripening of kiwifruit. Postharvest Biol. Technol. 2003, 28, 67–74. [Google Scholar] [CrossRef]
- Peroni, F.H.G.; Koike, C.; Louro, R.P.; Purgatto, E.; do Nascimento, J.R.O.; Lajolo, F.M.; Cordenunsi, B.R. Mango starch degradation. II. The binding of α-amylase and β-amylase to the starch granule. J. Agric. Food Chem. 2008, 56, 7416–7421. [Google Scholar] [CrossRef]
- Castrillo, M.; Kruger, N.J.; Whatley, F. Sucrose metabolism in mango fruit during ripening. Plant Sci. 1992, 84, 45–51. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef]
- Tapiero, H.; Townsend, D.M.; Tew, K.D. The role of carotenoids in the prevention of human pathologies. Biomed. Pharmacother. 2004, 58, 100–110. [Google Scholar] [CrossRef]
- Brito, T.; Ferreira, M.L.; Fai, A.E. Utilization of agricultural by-products: Bioactive properties and technological applications. Food Rev. Int. 2020, 21, 1305–1329. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Dokhanieh, A.Y.; Aghdam, M.S.; Fard, J.R.; Hassanpour, H. Postharvest salicylic acid treatment enhances antioxidant potential of cornelian cherry fruit. Sci. Hortic. 2013, 154, 31–36. [Google Scholar] [CrossRef]
- Hanif, A.; Ahmad, S.; Shahzad, S.; Liaquat, M.; Anwar, R. Postharvest application of salicylic acid reduced decay and enhanced storage life of papaya fruit during cold storage. J. Food Meas. Charact. 2020, 14, 3078–3088. [Google Scholar] [CrossRef]
- Erogul, D.; Özsoydan, İ. Effect of pre-harvest salicylic acid treatments on the quality and shelf life of the ‘Cresthaven’ peach cultivar. Folia Hortic. 2020, 32, 221–227. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Ullah, M.A.; Nadeem, M.; Tungmunnithum, D.; Hano, C. Exogenous application of salicylic acid and gibberellic acid on biomass accumulation, antioxidant and anti-inflammatory secondary metabolites production in multiple shoot culture of Ajuga integrifolia Buch. Ham. ex D. Don. Ind. Crops Prod. 2020, 145, 112098. [Google Scholar] [CrossRef]
- Buraphaka, H.; Putalun, W. Stimulation of health-promoting triterpenoids accumulation in Centella asiatica (L.) Urban leaves triggered by postharvest application of methyl jasmonate and salicylic acid elicitors. Ind. Crops Prod. 2020, 146, 112171. [Google Scholar] [CrossRef]
- Yun, K.-J.; Kim, J.-Y.; Kim, J.-B.; Lee, K.-W.; Jeong, S.-Y.; Park, H.-J.; Jung, H.-J.; Cho, Y.-W.; Yun, K.; Lee, K.-T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-κB inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef]
- Bulgakov, V.; Tchernoded, G.; Mischenko, N.; Khodakovskaya, M.; Glazunov, V.; Radchenko, S.; Zvereva, E.; Fedoreyev, S.; Zhuravlev, Y.N. Effect of salicylic acid, methyl jasmonate, ethephon and cantharidin on anthraquinone production by Rubia cordifolia callus cultures transformed with the rolB and rolC genes. J. Biotechnol. 2002, 97, 213–221. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).