Larvicidal Activity and Phytochemical Profiling of Sweet Basil (Ocimum basilicum L.) Leaf Extract against Asian Tiger Mosquito (Aedes albopictus)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction of Plant Material
2.3. Mosquito Sampling and Larvae Culturing
2.4. Larvicidal Bioassay
2.5. Column Chromatography
2.6. Thin-Layer Chromatography
2.7. Phytochemical Profiling Using Gas Chromatography–Mass Spectrometry (GC-MS)
2.8. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Misbah, S.; Low, V.L.; Mohd Rahim, N.F.; Jaba, R.; Basari, N.; Ya’cob, Z.; Abu Bakar, S. Mitochondrial diversity of the Asian tiger mosquito Aedes albopictus (Diptera: Culicidae) in Peninsular Malaysia. J. Med. Entomol. 2022, 59, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, R.F.; Shakeel, M.; Rizvi, S.A.H.; Zafar, J.; Zhang, Y.; Freed, S.; Xu, X.; Jin, F. Larvicidal, ovicidal, synergistic, and repellent activities of Sophora alopecuroides and its dominant constituents against Aedes albopictus. Insects 2020, 11, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, P.S.; Li, M.Z.; Chong, C.S.; Ng, L.C.; Tan, C.H. Aedes (Stegomyia) albopictus (Skuse): A potential vector of Zika virus in Singapore. PLoS Negl. Trop. Dis. 2013, 7, e2348. [Google Scholar] [CrossRef] [PubMed]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Ondo, S.M.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (Central Africa)–2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Kweka, E.J.; Baraka, V.; Mathias, L.; Mwang’onde, B.; Baraka, G.; Lyaruu, L.; Mahande, A.M. Ecology of Aedes mosquitoes, the major vectors of arboviruses in human population. In Dengue Fever. A Resilient Threat in the Face of Innovation; Falcón-Lezama, J.A., Betancourt-Cravioto, M., Tapia-Conyer, R., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Zahratulhayat, M.A.; Kinta District Declared Dengue Epidemic Area. The Star. Available online: https://www.nst.com.my/news/nation/2020/01/553440/kinta-district-declared-dengue-epidemic-area (accessed on 7 May 2022).
- Ho, L.Y.; Loh, T.S.; Yam, L.Y. Surveillance and resistance status of Aedes population in two suburban residential areas in Kampar town, Perak, Malaysia. Trop. Biomed. 2014, 31, 441–448. [Google Scholar]
- Rajendran, D.; Najwa, F.; Ummi, A.; Besar, A.; Yusoff, M.; Zuharah, W.F. Status of insecticide resistance on Aedes aegypti (L.) and Aedes albopictus (Skuse) in Kampar, Perak, Malaysia. Serangga 2021, 26, 245–254. [Google Scholar]
- National Pesticides Information Center. Pesticides Used in Mosquitoes Control. Available online: http://npic.orst.edu/pest/mosquito/mosqcides.html (accessed on 19 July 2019).
- Park, Y.L.; Tak, J.H. Essential oils for arthropod pest management in agricultural production systems. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Elsevier: San Diego, CA, USA, 2015; pp. 61–70. [Google Scholar]
- Khan, B.A.; Freed, S.; Zafar, J.; Farooq, M.; Shoukat, R.F.; Ahmad, K.W.; Li, S.; Zhang, Y.; Hua, Y.; Shoukat, R.F. Efficacy of different entomopathogenic fungi on biological parameters of pulse beetle Callosobruchus chinensis L. (Coleoptera: Bruchidae). J. Entomol. Zool. Stud. 2018, 6, 1972–1976. [Google Scholar]
- Mdoe, F.P.; Cheng, S.S.; Lyaruu, L.; Nkwengulila, G.; Chang, S.T.; Kweka, E.J. Larvicidal efficacy of Cryptomeria japonica leaf essential oils against Anopheles gambiae. Parasites Vectors 2014, 7, 426. [Google Scholar] [CrossRef] [Green Version]
- Shoukat, R.F.; Freed, S.; Ahmad, K.W. Evaluation of binary mixtures of entomogenous fungus and botanicals on biological parameters of Culex pipiens (Diptera: Culicidae) under laboratory and field conditions. Int. J. Mosq. Res. 2016, 3, 17–24. [Google Scholar]
- Makri, O.; Kintzios, S. Ocimum sp. (Basil): Botany, cultivation, pharmaceutical properties, and biotechnology. J. Herbs Spices Med. Plants 2008, 13, 123–150. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complement Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef] [Green Version]
- Jirovetz, L.; Buchbauer, G.; Shafi, M.P.; Kaniampady, M.M. Chemotaxonomical analysis of the essential oil aroma compounds of four different Ocimum species from southern India. Eur. Food Res. Technol. 2003, 217, 120–124. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. Chemical components and pharmacological benefits of basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020, 23, 1961–1970. [Google Scholar] [CrossRef]
- Bączek, K.; Kosakowska, O.; Gniewosz, M.; Gientka, I.; Węglarz, Z. Sweet basil (Ocimum basilicum L.) productivity and raw material quality from organic cultivation. Agronomy 2019, 9, 279. [Google Scholar] [CrossRef] [Green Version]
- Filip, S. Basil (Ocimum basilicum L.) a source of valuable phytonutrients. Int. J. Clin. Nutr. Diet. 2017, 3, 118. [Google Scholar] [CrossRef] [Green Version]
- Adam, A.A.; Ahmed, A.S.; Mohamed, T.A.; Azrag, R.A.; Mustfa, S.E.; Hamdi, O.A.A. Evaluation of repellent activities of the essential oil of Ocimum basilicum against Anopheles mosquito and formulation of mosquitoes repellent cream. Biomed. Res. Clin. Pract. 2019, 4, 2–5. [Google Scholar] [CrossRef] [Green Version]
- Abo-Elseoud, M.A.; Sorhan, M.M.; Omar, A.E.; Helal, M.M. Biocides formulation of essential oils having antimicrobial activity. Arch. Phytopathol. Plant Prot. 2005, 38, 175–184. [Google Scholar] [CrossRef]
- de la Parte, E.M.; Pérez-Vicente, L.; Bernal, B.; García, D. First report of Peronospora sp. on sweet basil (Ocimum basilicum) in Cuba. Plant Pathol. 2010, 59, 800. [Google Scholar] [CrossRef]
- Erler, F.; Ulug, I.; Yalcinkaya, B. Repellent activity of five essential oils against Culex pipiens. Fitoterapia 2006, 77, 491–494. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Formulation and characterization of plant essential oil based nanoemulsion: Evaluation of its larvicidal activity against Aedes aegypti. Asian J. Chem. 2013, 25, S321–S323. [Google Scholar]
- Ikhsanudin, A.; Lolita, L.; Ramadani, Z.S. Larvicidal activity of granulated pharmaceutical products using Indonesian holy basil leaf extract. Int. J. Public Health 2021, 10, 934–941. [Google Scholar] [CrossRef]
- Krüger, H.; Wetzel, S.B.; Zeiger, B. The chemical variability of Ocimum species. J. Herbs Spices Med. Plants 2002, 9, 335–344. [Google Scholar] [CrossRef]
- Klimankova, E.; Holadová, K.; Hajšlová, J.; Cajka, T.; Poustka, J.; Koudela, M. Aroma profile of five basil (Ocimum basilicum L.) cultivars grown under conventional and organic conditions. Food Chem. 2008, 107, 464–472. [Google Scholar] [CrossRef]
- Chokechaijaroenporn, O.; Bunyapraphatsara, N.; Kongchuensin, S. Mosquito repellent activities of Ocimum volatile oils. Phytomedicine 1994, 1, 135–139. [Google Scholar] [CrossRef]
- Kumar, S.; Mishra, M.; Wahab, N.; Warikoo, R. Larvicidal, repellent, and irritant potential of the seed-derived essential oil of Apium graveolens against dengue vector, Aedes aegypti L. (Diptera: Culicidae). Front. Public Health 2014, 2, 147. [Google Scholar] [CrossRef] [Green Version]
- Thomas, A.; Mazigo, H.D.; Manjurano, A.; Morona, D.; Kweka, E.J. Evaluation of active ingredients and larvicidal activity of clove and cinnamon essential oils against Anopheles gambiae (sensu lato). Parasites Vectors 2017, 10, 411. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Ochoa, S.; Sánchez-Aldana, D.; Chacón-Vargas, K.F.; Rivera-Chavira, B.E.; Sánchez-Torres, L.E.; Camacho, A.D.; Nogueda-Torres, B.; Nevárez-Moorillón, G.V. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism–antagonism effects. Insects 2018, 9, 25. [Google Scholar] [CrossRef] [Green Version]
- Tuetun, B.; Choochote, W.; Kanjanapothi, D.; Rattanachanpichai, E.; Chaithong, U.; Chaiwong, P.; Jitpakdi, A.; Tippawangkosol, P.; Riyong, D.; Pitasawat, B. Repellent properties of celery, Apium graveolens L., compared with commercial repellents, against mosquitoes under laboratory and field conditions. Trop. Med. Int. Health 2005, 10, 1190–1198. [Google Scholar] [CrossRef]
- Rueda, L. Pictorial Keys for the Identification of Mosquitoes (Diptera: Culicidae) Associated with Dengue Virus Transmission. Available online: http://www.mosquitocatalog.org/files/pdfs/wr385.pdf (accessed on 20 March 2015).
- World Health Organization. Guidelines for Laboratory and Field Testing of Mosquito Larvicides. Available online: https://apps.who.int/iris/handle/10665/69101 (accessed on 19 August 2014).
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Rajamma, A.J.; Dubey, S.; Sateesha, S.B.; Tiwari, S.N.; Ghosh, S.K. Comparative larvicidal activity of different species of Ocimum against Culex quinquefasciatus. Nat. Prod. Res. 2011, 25, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Manzoor, F.; Samreen, K.B.; Parveen, Z. Larvicidal activity of essential oils against Aedes aegypti and Culex quinquefasciatus larvae (Diptera: Culicidae). J Anim. Plant Sci. 2013, 23, 420–424. [Google Scholar]
- Dris, D.; Tine-Djebbar, F.; Bouabida, H.; Soltani, N. Chemical composition and activity of an Ocimum basilicum essential oil on Culex pipiens larvae: Toxicological, biometrical and biochemical aspects. S. Afr. J. Bot. 2017, 113, 362–369. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.M.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against three plant pathogenic fungi and mosquito larvae: GC/MS analysis of bioactive compounds. BioResources 2019, 14, 4489–4511. [Google Scholar] [CrossRef]
- Govindarajan, M.; Sivakumar, R.; Rajeswary, M.; Yogalakshmi, K. Chemical composition and larvicidal activity of essential oil from Ocimum basilicum (L.) against Culex tritaeniorhynchus, Aedes albopictus and Anopheles subpictus (Diptera: Culicidae). Exp. Parasitol. 2013, 134, 7–11. [Google Scholar] [CrossRef]
- Murugan, K.; Aarthi, N.; Kovendan, K.; Pannerselvam, C.; Chandramohan, B.; Kumar, P.M.; Amerasan, D.; Paulpandi, M.; Chandirasekar, R.; Dinesh, D.; et al. Mosquitocidal and antiplasmodial activity of Senna occidentalis (Cassiae) and Ocimum basilicum (Lamiaceae) from Maruthamalai hills against Anopheles stephensi and Plasmodium falciparum. Parasitol. Res. 2015, 114, 3657–3664. [Google Scholar] [CrossRef]
- Pandey, A.; Pandey, U.K.; Shrivastava, P.K. Studies on mosquito larvicidal efficacy of indigenous plant: Extracts. J. Ecophysiol. Occup. Health 2010, 10, 223–227. [Google Scholar]
- Murugan, K.; Murugan, P.; Noortheen, A. Larvicidal and repellent potential of Albizzia amara Boivin and Ocimum basilicum Linn against dengue vector, Aedes aegypti (Insecta:Diptera:Culicidae). Bioresour. Technol. 2007, 98, 198–201. [Google Scholar] [CrossRef]
- Nasir, S.; Batool, M.; Hussain, S.M.; Nasir, I.; Hafeez, F.; Debboun, M. Bioactivity of oils from medicinal plants against immature stages of dengue mosquito Aedes aegypti (Diptera: Culicidae). Int. J. Agric. Biol. 2015, 17, 843–847. [Google Scholar] [CrossRef]
- Giatropoulos, A.; Kimbaris, A.; Michaelakis, A.; Papachristos, D.P.; Polissiou, M.G.; Emmanouel, N. Chemical composition and assessment of larvicidal and repellent capacity of 14 Lamiaceae essential oils against Aedes albopictus. Parasitol. Res. 2018, 117, 1953–1964. [Google Scholar] [CrossRef]
- Ahbirami, R.; Zuharah, W.F.; Thiagaletchumi, M.; Subramaniam, S.; Sundarasekar, J. Larvicidal efficacy of different plant parts of railway creeper, Ipomoea cairica extract against dengue vector mosquitoes, Aedes albopictus (Diptera: Culicidae) and Aedes aegypti (Diptera: Culicidae). J. Insect Sci. 2014, 14, 180. [Google Scholar] [CrossRef] [Green Version]
- Aziz, E.I.A.; Rahim, N.A.A.; Raduan, S.Z.; Safii, R. A preliminary study on larvicidal efficacy of Piper nigrum L. (Piperaceae) extracts against dengue vector, Aedes albopictus (Diptera: Culicidae). Serangga 2021, 26, 80–94. [Google Scholar]
- Soonwera, M.; Phasomkusolsil, S. Effect of Cymbopogon citratus (lemongrass) and Syzygium aromaticum (clove) oils on the morphology and mortality of Aedes aegypti and Anopheles dirus larvae. Parasitol. Res. 2016, 115, 1691–1703. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Hassani, A.; Vojodi, L.; Farsad-Akhtar, N. Drying method affects essential oil content and composition of basil (Ocimum basilicum L.). J. Essent. Oil-Bear. Plants 2010, 13, 759–766. [Google Scholar] [CrossRef]
- Ilić, A.S.; Antić, M.P.; Jelačić, S.C.; Šolević Knudsen, T.M. Chemical composition of the essential oils of three Ocimum basilicum L. cultivars from Serbia. Not. Bot. Horti Agrobot. Cluj Napoca 2019, 47, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Anwar, F.; Alkharfy, K.M.; Mehmood, T.; Bakht, M.A.; Najeeb-ur-Rehman. Variation in chemical composition and effective antibacterial potential of Ocimum basilicum L. essential oil harvested from different regions of Saudi Arabia. Pharm. Chem. J. 2021, 55, 187–193. [Google Scholar] [CrossRef]
- Govindarajan, M. Chemical composition and larvicidal activity of leaf essential oil from Clausena anisata (Willd.) Hook. f. ex Benth (Rutaceae) against three mosquito species. Asian Pac. J. Trop. Med. 2010, 3, 874–877. [Google Scholar] [CrossRef] [Green Version]
- Perumalsamy, H.; Kim, N.J.; Ahn, Y.J. Larvicidal activity of compounds isolated from Asarum heterotropoides against Culex pipiens pallens, Aedes aegypti, and Ochlerotatus togoi (Diptera: Culicidae). J. Med. Entomol. 2009, 46, 1420–1423. [Google Scholar] [CrossRef]
- Perumalsamy, H.; Chang, K.S.; Park, C.; Ahn, Y.J. Larvicidal activity of Asarum heterotropoides root constituents against insecticide-susceptible and-resistant Culex pipiens pallens and Aedes aegypti and Ochlerotatus togoi. J. Agric. Food Chem. 2010, 58, 10001–10006. [Google Scholar] [CrossRef]
- Stefan, M.; Zamfirache, M.M.; Padurariu, C.; Trutǎ, E.; Gostin, I. The composition and antibacterial activity of essential oils in three Ocimum species growing in Romania. Cent. Eur. J. Biol. 2013, 8, 600–608. [Google Scholar] [CrossRef]
- Ricarte, L.P.; Bezerra, G.P.; Romero, N.R.; Da Silva, H.C.; Lemos, T.L.G.; Arriaga, A.M.C.; Alves, P.B.; Santos, M.B.D.; Millitao, G.C.G.; Silva, T.D.S.; et al. Chemical composition and biological activities of the essential oils from Vitex-agnus castus, Ocimum campechianum and Ocimum carnosum. An. Acad. Bras. Cienc. 2020, 92, e20180569. [Google Scholar] [CrossRef]
- Braglia, R.; Costa, P.; Di Marco, G.; D’Agostino, A.; Redi, E.L.; Scuderi, F.; Gismondi, A.; Canini, A. Phytochemicals and quality level of food plants grown in an aquaponics system. J. Sci. Food Agric. 2022, 102, 844–850. [Google Scholar] [CrossRef]
- Dawson, B.S.W.; Franich, R.A.; Meder, R. Essential oil of Melissa officinalis L. subsp. altissima (Sibthr. et Smith) Arcang. Flavour Fragr. J. 1988, 3, 167–170. [Google Scholar] [CrossRef]
- Bueno, J.; Escobar, P.; Martínez, J.R.; Leal, S.M.; Stashenko, E.E. Composition of three essential oils, and their mammalian cell toxicity and antimycobacterial activity against drug resistant-tuberculosis and nontuberculous mycobacteria strains. Nat. Prod. Commun. 2011, 6, 1743–1748. [Google Scholar] [CrossRef] [Green Version]
- Trendafilova, A.; Todorova, M.; Ivanova, V.; Zhelev, P.; Aneva, I. Essential oil composition of five Thymus species from Bulgaria. Chem. Biodivers. 2021, 18, e2100498. [Google Scholar] [CrossRef]
- Claeson, P.; Andersson, R.; Samuelsson, G. T-cadinol: A pharmacologically active constituent of scented myrrh: Introductory pharmacological characterization and high field 1H- and 13C-NMR data. Planta Med. 1991, 57, 352–356. [Google Scholar] [CrossRef]
- Takei, M.; Umeyama, A.; Arihara, S. T-cadinol and calamenene induce dendritic cells from human monocytes and drive Th1 polarization. Eur. J. Pharmacol. 2006, 537, 190–199. [Google Scholar] [CrossRef]
- Su, Y.C.; Hsu, K.P.; Wang, E.I.C.; Ho, C.L. Composition, in vitro cytotoxic, and antimicrobial activities of the flower essential oil of Diospyros discolor from Taiwan. Nat. Prod. Commun. 2015, 10, 1311–1314. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.L.; Amaral, M.; Hasegawa, F.R.; Lago, J.H.G.; Tempone, A.G.; Sartorelli, P. (-)-T-Cadinol—A sesquiterpene isolated from Casearia sylvestris (Salicaceae) displayed in vitro activity and causes hyperpolarization of the membrane potential of Trypanosoma cruzi. Front. Pharmacol. 2021, 12, 734127. [Google Scholar] [CrossRef]
- Rojas, R.; Bustamante, B.; Ventosilla, P.; Fernadez, I.; Caviedes, L.; Gilman, R.H.; Lock, O.; Hammond, G.B. Larvicidal, antimycobacterial and antifungal compounds from the bark of the Peruvian plant Swartzia polyphylla DC. Chem. Pharm. Bull. 2006, 54, 278–279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Wang, P.; Lucardi, R.D.; Su, Z.; Li, S. Natural sources and bioactivities of 2,4-di-tert-butylphenol and its analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.Q.; Perumalsamy, H.; Wang, M.; Shu, S.; Ahn, Y.J. Larvicidal activity of Magnolia denudata seed hydrodistillate constituents and related compounds and liquid formulations towards two susceptible and two wild mosquito species. Pest Manag. Sci. 2016, 72, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Gutbrod, K.; Romer, J.; Dörmann, P. Phytol metabolism in plants. Prog. Lipid Res. 2019, 74, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Araújo, S.G.; Alves, L.F.; Pinto, M.E.A.; Oliveira, G.T.; Siquera, E.P.; Ribeiro, R.I.M.A.; Ferreira, J.M.S.; Lima, L.A.R.S. Volatile compounds of Lamiaceae exhibit a synergistic antibacterial activity with streptomycin. Braz. J. Microbiol. 2014, 45, 1341–1347. [Google Scholar] [CrossRef] [Green Version]
- Chogo, J.B.; Crank, G. Chemical composition and biological activity of the Tanzanian plant Ocimum suave. J. Nat. Prod. 1981, 44, 308–311. [Google Scholar] [CrossRef]
- Naidoo, Y.; Sadashiva, C.T.; Kasim, N.; Nicholas, A.; Naidoo, G. Chemical composition and antimicrobial activity of the essential oil of Ocimum obovatum E. Mey. Ex Benth. (Lamiaceae). J. Essent. Oil-Bear. Plants 2014, 17, 142–147. [Google Scholar] [CrossRef]
- Selvaraju, R.; Sakuntala, P.; Jaleeli, K.A. Comparative analysis of chemical compounds and trace elements in the leaves of few Ocimum species plants by using different spectroscopic methods. Rom. J. Biophys. 2021, 31, 65–78. [Google Scholar]
- Islam, M.T.; De Alencar, M.V.O.B.; Da Conceição Machado, K.; Da Conceição Machado, K.; De Carvalho Melo-Cavalcante, A.A.; De Sousa, D.P.; De Freitas, R.M. Phytol in a pharma-medico-stance. Chem.-Biol. Interact. 2015, 240, 60–73. [Google Scholar] [CrossRef]
- European Chemicals Agency. 2-(2-Butoxyethoxy)Ethanol. Available online: https://echa.europa.eu/substance-information/-/substanceinfo/100.003.601 (accessed on 28 February 2022).
- Aburjai, T.A.; Mansi, K.; Azzam, H.; Alqudah, D.A.; Alshaer, W.; Abuirjei, M. Chemical compositions and anticancer potential of essential oil from greenhouse-cultivated Ocimum basilicum leaves. Indian J. Pharm. Sci. 2020, 82, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Ravi Kiran, S.; Bhavani, K.; Sita Devi, P.; Rajeswara Rao, B.R.; Janardhan Reddy, K. Composition and larvicidal activity of leaves and stem essential oils of Chloroxylon swietenia DC against Aedes aegypti and Anopheles stephensi. Bioresour. Technol. 2006, 97, 2481–2484. [Google Scholar] [CrossRef]
- Govindarajan, M.; Benelli, G. α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: Highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae). Parasitol. Res. 2016, 115, 2771–2778. [Google Scholar] [CrossRef]
- França, L.P.; Amaral, A.C.F.; Ramos, A.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S., Jr.; Branches, A.D.S.; Silva, J.N.; Barros, G.A.; et al. Piper capitarianum essential oil: A promising insecticidal agent for the management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. 2021, 28, 9760–9776. [Google Scholar] [CrossRef]
- Tong, H.; Liu, Y.; Jiang, L.; Wang, J. Multi-targeting by β-elemene and its anticancer properties: A good choice for oncotherapy and radiochemotherapy sensitization. Nutr. Cancer 2020, 72, 554–567. [Google Scholar] [CrossRef]

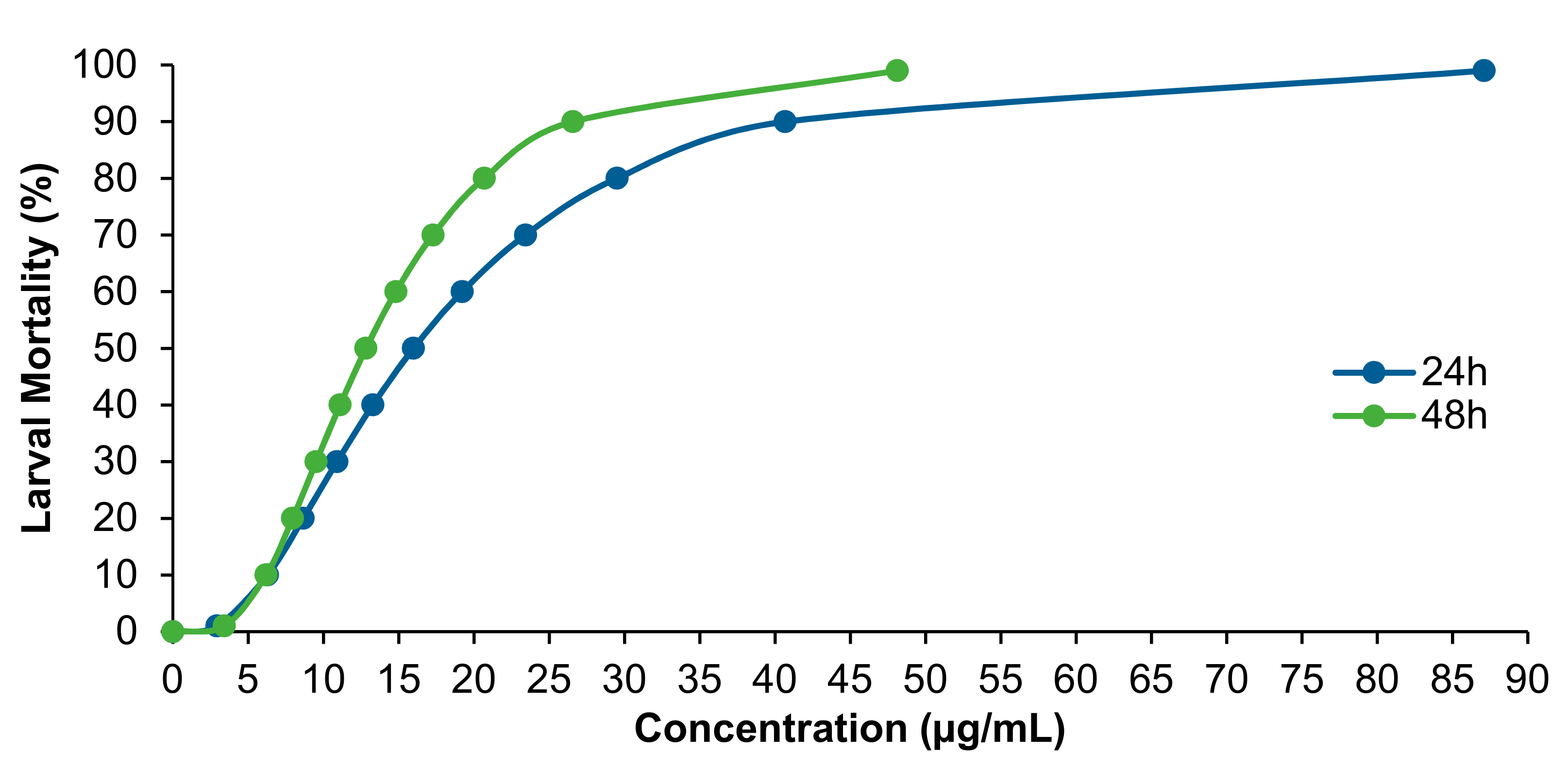
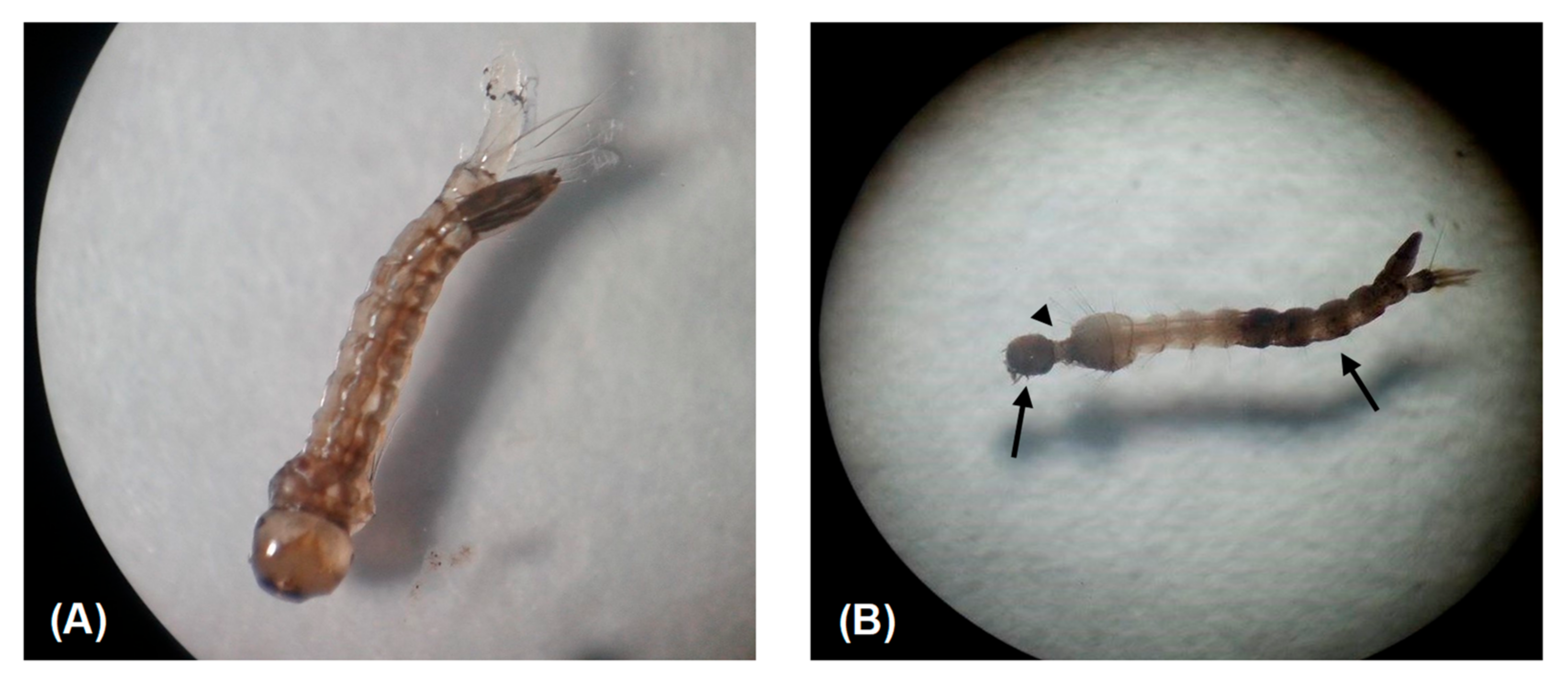
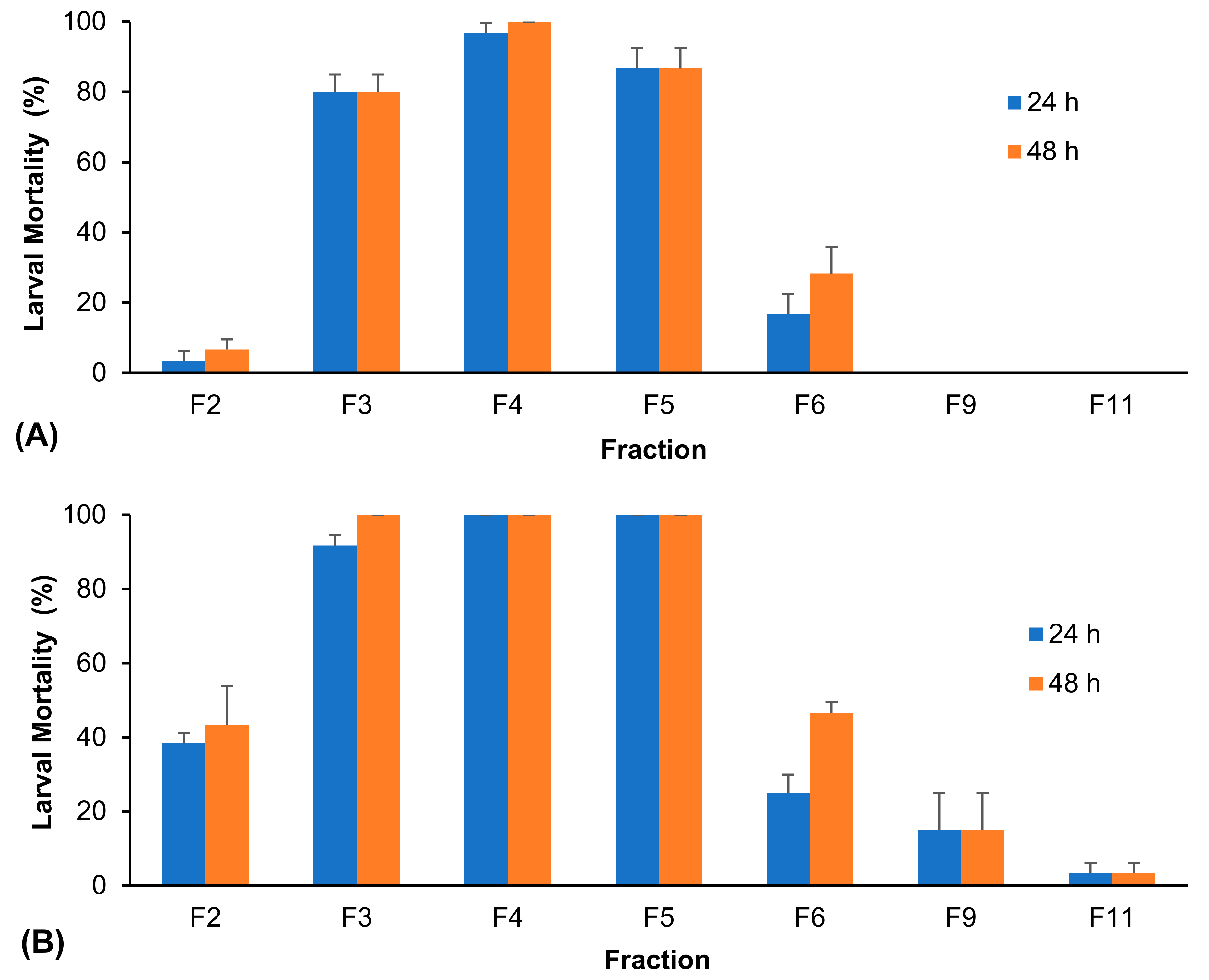
| Extract | 24 h Exposure | 48 h Exposure |
|---|---|---|
| Median lethal concentration (LC50, µg/mL) (LCL-UCL) | 15.98 (10.95–22.12) | 12.82 (7.64–19.22) |
| 95% lethal concentration (LC95, µg/mL) (LCL-UCL) | 53.00 (34.61–136.82) | 32.66 (21.11–151.96) |
| Regression coefficient ± Standard error | 3.159 ± 0.312 | 4.051 ± 0.442 |
| Chi Square, X2 | 10.292 | 17.151 |
| Degree of freedom, df | 4 | 4 |
| p-value | 0.036 | 0.002 |
| Peak | Retention Time (min) | Peak Area (%) | Peak Height (%) | Compound | MW | Chemical Formula | Similarity (%) |
|---|---|---|---|---|---|---|---|
| 1 | 13.811 | 10.92 | 8.86 | 2-(2-Butoxyethoxy)ethanol | 162 | C8H18O3 | 95 |
| 2 | 14.544 | 57.67 | 47.12 | Methyl chavicol | 148 | C10H12O | 93 |
| 3 | 15.918 | 0.53 | 0.58 | - | |||
| 4 | 16.961 | 0.18 | 0.24 | - | |||
| 5 | 19.158 | 0.20 | 0.28 | - | |||
| 6 | 19.316 | 0.45 | 0.63 | Allyl methallyl ether | 112 | C7H12O | 91 |
| 7 | 19.864 | 0.40 | 0.64 | beta-Elemene | 204 | C15H24 | 86 |
| 8 | 19.971 | 4.35 | 6.10 | Methyl eugenol | 178 | C11H14O2 | 92 |
| 9 | 20.922 | 0.28 | 0.40 | - | |||
| 10 | 21.019 | 0.49 | 0.66 | - | |||
| 11 | 22.001 | 0.42 | 0.62 | Humulene | 204 | C15H24 | 86 |
| 12 | 22.668 | 2.39 | 3.16 | 2,4-Di-tert-butylphenol | 206 | C14H22O | 91 |
| 13 | 23.334 | 1.23 | 1.81 | Germacrene D | 204 | C15H24 | 88 |
| 14 | 24.443 | 0.53 | 0.91 | 1-Dodecene | 168 | C12H24 | 90 |
| 15 | 24.851 | 0.28 | 0.41 | - | |||
| 16 | 25.035 | 0.25 | 0.37 | - | |||
| 17 | 25.176 | 0.94 | 1.37 | - | |||
| 18 | 25.408 | 0.31 | 0.45 | - | |||
| 19 | 25.908 | 0.31 | 0.49 | - | |||
| 20 | 26.004 | 1.53 | 1.89 | - | |||
| 21 | 26.589 | 9.92 | 13.54 | Cedrelanol | 222 | C15H26O | 88 |
| 22 | 26.951 | 0.46 | 0.63 | - | |||
| 23 | 27.140 | 0.63 | 0.85 | - | |||
| 24 | 27.225 | 0.55 | 0.74 | - | |||
| 25 | 27.447 | 0.55 | 0.44 | - | |||
| 26 | 29.068 | 0.22 | 0.41 | cis-3-Tridecene | 182 | C13H26 | 88 |
| 27 | 31.949 | 0.78 | 1.34 | Methyl palmitate | 270 | C17H34O2 | 90 |
| 28 | 35.569 | 0.61 | 1.01 | 9,12,15-Octadecatrienal | 262 | C18H30O | 88 |
| 29 | 35.669 | 1.63 | 2.52 | Phytol | 296 | C20H40O | 89 |
| 30 | 37.411 | 0.21 | 0.34 | - | |||
| 31 | 40.552 | 0.76 | 1.19 | - | |||
| Peak | Retention Time (min) | Peak Area (%) | Peak Height (%) | Compound | MW | Chemical Formula | Similarity (%) |
|---|---|---|---|---|---|---|---|
| 1 | 13.816 | 11.03 | 9.08 | 2-(2-Butoxyethoxy)ethanol | 162 | C8H18O3 | 95 |
| 2 | 14.537 | 64.34 | 54.66 | Methyl chavicol | 148 | C10H12O | 94 |
| 3 | 19.867 | 0.33 | 0.49 | - | |||
| 4 | 19.982 | 4.13 | 6.00 | Methyl eugenol | 178 | C11H14O2 | 91 |
| 5 | 21.038 | 0.42 | 0.57 | - | |||
| 6 | 22.677 | 2.00 | 2.81 | 2,4-Di-tert-butylphenol | 206 | C14H22O | 88 |
| 7 | 23.347 | 0.96 | 1.44 | Germacrene D | 204 | C15H24 | 86 |
| 8 | 24.456 | 0.37 | 0.67 | 1-Dodecene | 168 | C12H24 | 88 |
| 9 | 25.184 | 0.70 | 1.12 | - | |||
| 10 | 26.010 | 1.12 | 1.51 | - | |||
| 11 | 26.589 | 9.30 | 13.13 | Cedrelanol | 222 | C15H26O | 88 |
| 12 | 26.958 | 0.28 | 0.43 | - | |||
| 13 | 27.145 | 0.43 | 0.65 | - | |||
| 14 | 27.242 | 0.31 | 0.48 | - | |||
| 15 | 31.952 | 0.76 | 1.29 | Methyl palmitate | 270 | C17H34O2 | 87 |
| 16 | 35.577 | 0.36 | 0.66 | - | |||
| 17 | 35.674 | 2.27 | 3.61 | Phytol | 296 | C20H40O | 89 |
| 18 | 40.557 | 0.61 | 0.95 | - | |||
| 19 | 42.595 | 0.29 | 0.45 | - | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.A.; Ho, L.Y.; Sit, N.W. Larvicidal Activity and Phytochemical Profiling of Sweet Basil (Ocimum basilicum L.) Leaf Extract against Asian Tiger Mosquito (Aedes albopictus). Horticulturae 2022, 8, 443. https://doi.org/10.3390/horticulturae8050443
Chan CA, Ho LY, Sit NW. Larvicidal Activity and Phytochemical Profiling of Sweet Basil (Ocimum basilicum L.) Leaf Extract against Asian Tiger Mosquito (Aedes albopictus). Horticulturae. 2022; 8(5):443. https://doi.org/10.3390/horticulturae8050443
Chicago/Turabian StyleChan, Chee Aun, Lai Yee Ho, and Nam Weng Sit. 2022. "Larvicidal Activity and Phytochemical Profiling of Sweet Basil (Ocimum basilicum L.) Leaf Extract against Asian Tiger Mosquito (Aedes albopictus)" Horticulturae 8, no. 5: 443. https://doi.org/10.3390/horticulturae8050443
APA StyleChan, C. A., Ho, L. Y., & Sit, N. W. (2022). Larvicidal Activity and Phytochemical Profiling of Sweet Basil (Ocimum basilicum L.) Leaf Extract against Asian Tiger Mosquito (Aedes albopictus). Horticulturae, 8(5), 443. https://doi.org/10.3390/horticulturae8050443






