Dof Transcription Factors Are Involved in High CO2 Induced Persimmon Fruit Deastringency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Gene Isolation and Sequence Analysis
2.3. Soluble Tannins Printing Assay
2.4. RNA Extraction and cDNA Synthesis
2.5. Real-Time PCR Analysis
2.6. Dual Luciferase Assay
2.7. EMSA
2.8. Statistical Analysis
3. Results
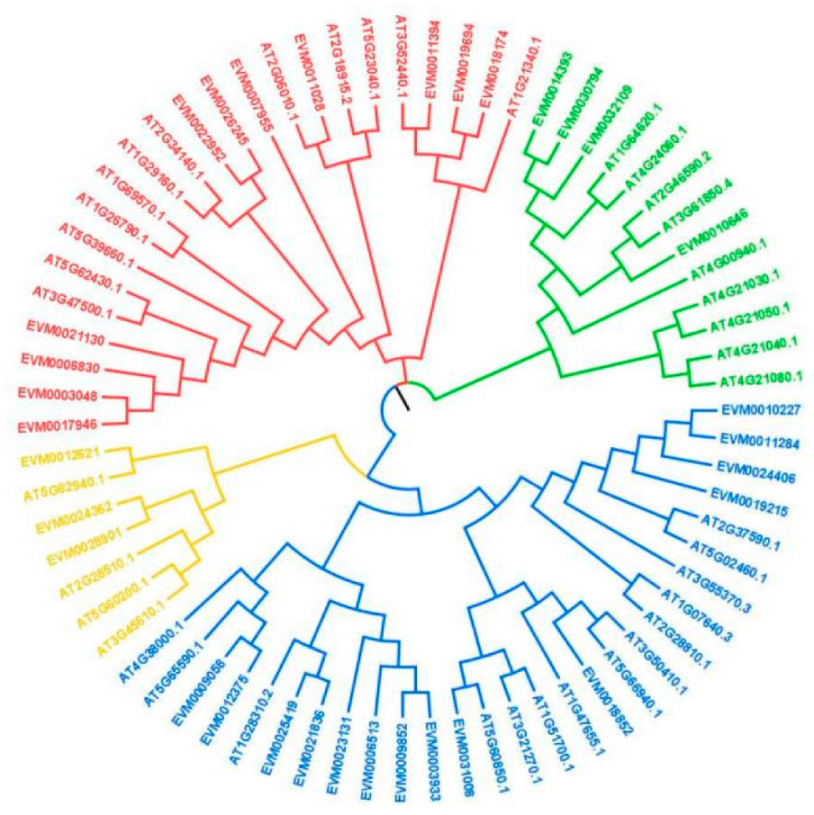
3.1. Analysis of DkDof Genes
3.2. Effects of High CO2 Treatment on ‘Gongcheng-Shuishi’ Persimmon Fruit
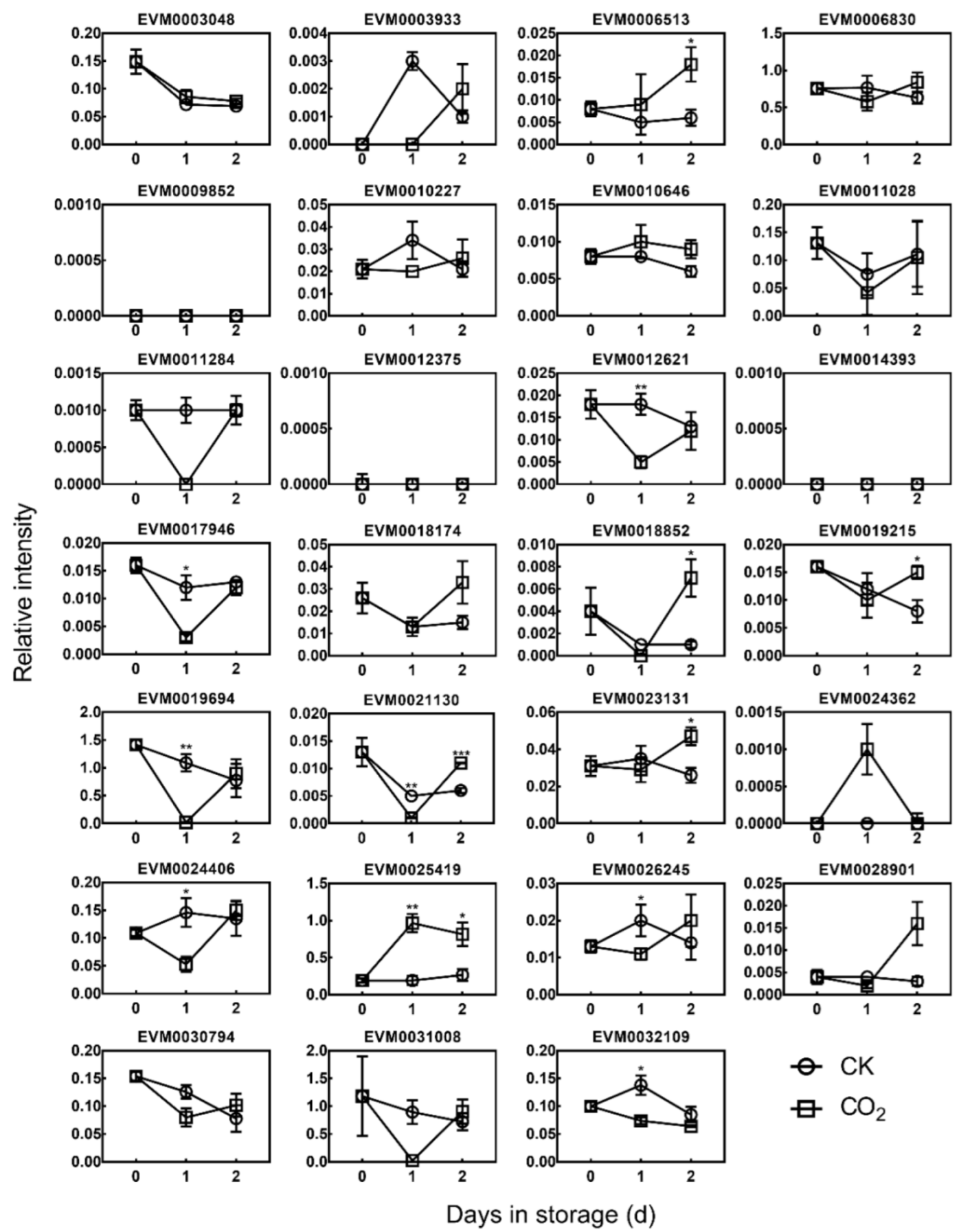
3.3. Expression of DkDofs in Response to High CO2 Treatment
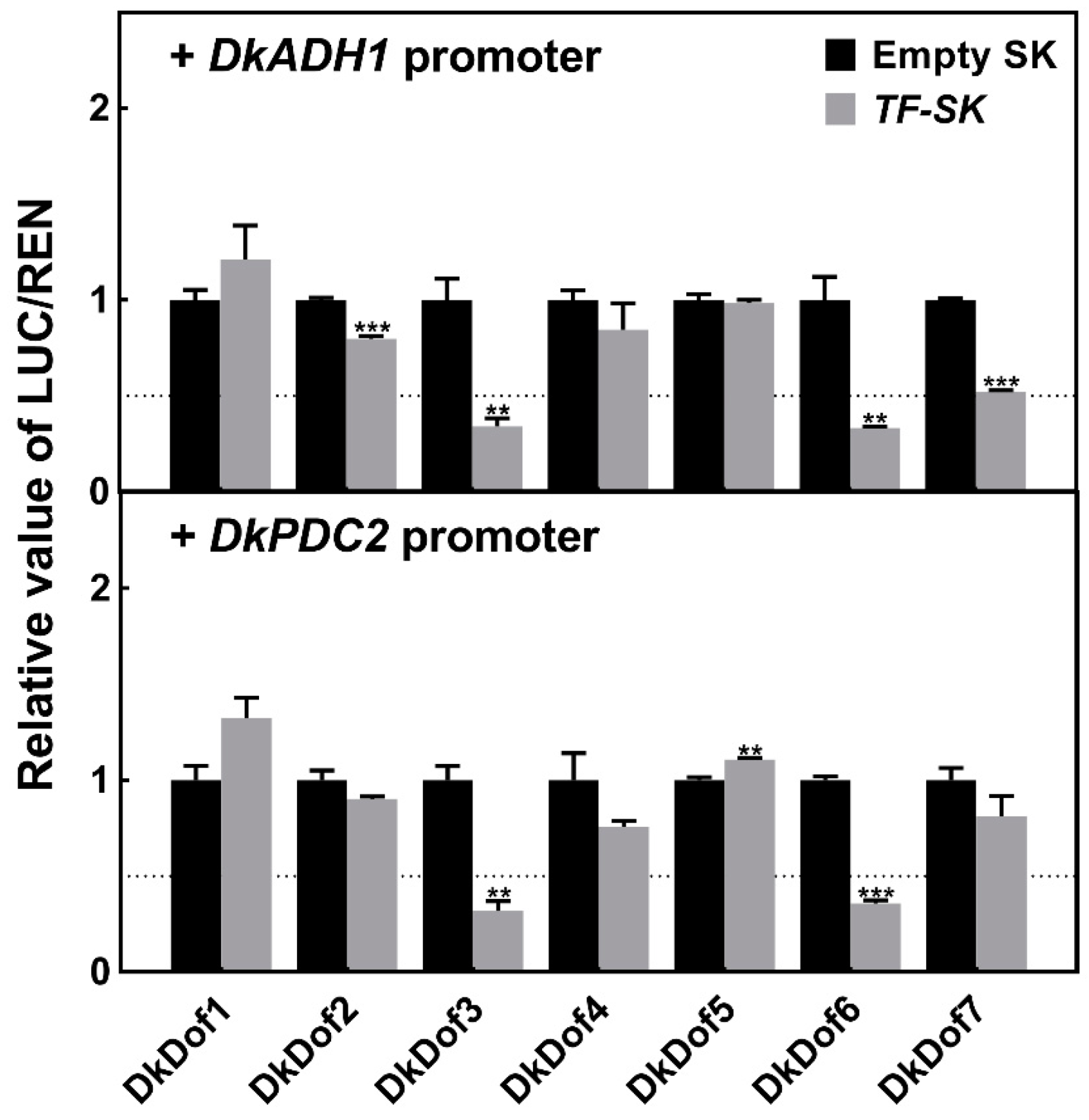
3.4. Regulatory Effects of DkDof1-7 on DkADH1 and DkPDC2 Promoters
3.5. Binding Abilities of DkDof3 and DkDof6 on DkADH1 and DkPDC2 Promoters
3.6. Expression of DkDof3 and DkDof6 in ‘Jingmianshi’ Fruit
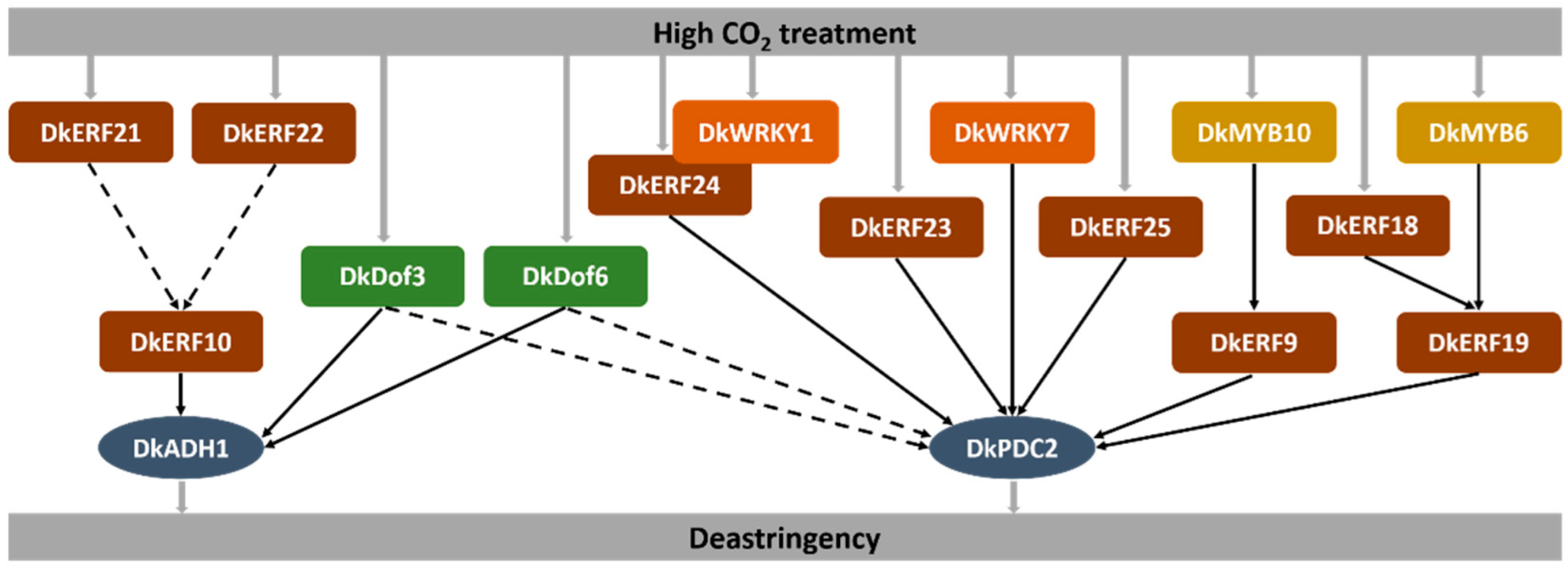
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yonemori, K.; Sugiura, A.; Yamada, M. Persimmon genetics and breeding. Plant Breed. Rev. 2000, 19, 191–225. [Google Scholar]
- Yonemorin, K.; Suzuki, Y. Differences in three-dimensional distribution of tannin cells in flesh tissue between astringent and non-astringent type persimmon. In Proceedings of the IV International Symposium on Persimmon, Firenze, Italy, 16 June 2009; pp. 119–124. [Google Scholar]
- Ashok, P.K.; Upadhyaya, K. Tannins are astringent. J. Pharmacogn. Phytochem. 2012, 1, 45–50. [Google Scholar]
- Yin, X.R.; Shi, Y.N.; Min, T.; Luo, Z.R.; Yao, Y.C.; Xu, Q.; Ferguson, I.; Chen, K.S. Expression of ethylene response genes during persimmon fruit astringency removal. Planta 2012, 235, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.F.; Aguila, J.S.; Pessoa, C.O.; Kluge, R.A. Vacuum packaging is efficient to remove astringency and to maintain the firmness of ‘Giombo’ persimmon. Rev. Bras. Frutic. 2017, 39, e358. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.G.; Gong, Z.Y.; Huang, J.W.; Grierson, D.; Chen, K.S.; Yin, X.R. High-CO2/hypoxia-responsive transcription factors DkERF24 and DkWRKY1 interact and activate DkPDC2 promoter. Plant Physiol. 2019, 180, 621–633. [Google Scholar] [CrossRef]
- Guan, C.F.; Chen, L.; Chen, W.X.; Mo, R.; Zhang, Q.; Du, X.; Liu, J.; Luo, Z. SSAP analysis reveals candidate genes associated with deastringency in persimmon (Diospyros kaki Thunb.) treated with 40 °C water. Tree Genet. Genomes 2015, 11, 20. [Google Scholar] [CrossRef]
- Christianson, J.A.; Wilson, I.W.; Llewellyn, D.J.; Dennis, E.S. The low-oxygen-induced NAC domain transcription factor ANAC102 affects viability of Arabidopsis seeds following low-oxygen treatment. Plant Physiol. 2009, 149, 1724–1738. [Google Scholar] [CrossRef] [Green Version]
- Min, T.; Fang, F.; Ge, H.; Shi, Y.N.; Luo, Z.R.; Yao, Y.C.; Grierson, D.; Yin, X.R.; Chen, K.S. Two novel anoxia-induced ethylene response factors that interact with promoters of deastringency-related genes from persimmon. PLoS ONE 2014, 9, e97043. [Google Scholar] [CrossRef]
- Zhu, Q.G.; Gong, Z.Y.; Wang, M.M.; Li, X.; Grierson, D.; Yin, X.R.; Chen, K.S. A transcription factor network responsive to high CO2/hypoxia is involved in deastringency in persimmon fruit. J. Exp. Bot. 2018, 69, 2061–2070. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Zhu, Q.G.; Wang, W.Q.; Grierson, D.; Yin, X.R. Molecular basis of the formation and removal of fruit astringency. Food Chem. 2022, 372, 131234. [Google Scholar] [CrossRef]
- Min, T.; Yin, X.R.; Shi, Y.N.; Luo, Z.R.; Yao, Y.C.; Grierson, D.; Ferguson, I.B.; Chen, K.S. Ethylene-responsive transcription factors interact with promoters of ADH and PDC involved in persimmon (Diospyros kaki) fruit de-astringency. J. Exp. Bot. 2012, 63, 6393–6405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mo, R.L.; Yang, S.C.; Huang, Y.M.; Chen, W.; Zhang, Q.; Luo, Z. ADH and PDC genes involved in tannins coagulation leading to natural de-astringency in Chinese pollination constant and non-astringency persimmon (Diospyros kaki Thunb.). Tree Genet. Genomes 2016, 12, 17. [Google Scholar] [CrossRef]
- Jamil, W.; Wu, W.; Ahmad, M.; Zhu, Q.G.; Liu, X.F.; Jin, R.; Yin, X.R. High-CO2/hypoxia-modulated NAC transcription factors involved in de-astringency of persimmon fruit. Sci. Hortic. 2019, 252, 201–207. [Google Scholar] [CrossRef]
- Jamil, W.; Wu, W.; Gong, H.; Huang, J.W.; Ahmad, M.; Zhu, Q.G.; Jin, R.; Liu, X.F.; Yin, X.R. C2H2-type zinc finger proteins (DkZF1/2) synergistically control persimmon fruit deastringency. Int. J. Mol. Sci. 2019, 20, 5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Malviya, N.; Kushwaha, H.; Nasim, J.; Bisht, N.C.; Singh, V.K.; Yadav, D. Insights into structural and functional diversity of dof (DNA binding with one finger) transcription factor. Planta 2015, 241, 549–562. [Google Scholar] [CrossRef]
- Yanagisawa, S. The Dof family of plant transcription factors. Trends Plant Sci. 2002, 7, 555–560. [Google Scholar] [CrossRef]
- Enkatesh, J.; Park, S.W. Genome-wide analysis and expression profiling of DNA-binding with one zinc finger (Dof) transcription factor family in potato. Plant Physiol. Biochem. 2015, 94, 73–85. [Google Scholar] [CrossRef]
- Noguero, M.; Atif, R.M.; Ochatt, S.; Thompson, R.D. The role of the DNA-binding one zinc finger (DOF) transcription factor family in plants. Plant Sci. 2013, 209, 32–45. [Google Scholar] [CrossRef]
- Wang, M.M.; Zhu, Q.G.; Deng, C.L.; Luo, Z.R.; Sun, N.J.; Grierson, D.; Yin, X.R.; Chen, K.S. Hypoxia-responsive ERFs involved in postdeastringency softening of persimmon fruit. Plant Biotechnol. J. 2017, 15, 1409–1419. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Q.G.; Xu, Y.; Yang, Y.; Guan, C.F.; Zhang, Q.Y.; Huang, J.W.; Grierson, D.; Chen, K.S.; Gong, B.C.; Yin, X.R. The persimmon (Diospyros oleifera Cheng) genome provides new insights into the inheritance of astringency and ancestral evolution. Hortic. Res. 2019, 6, 138. [Google Scholar] [CrossRef] [Green Version]
- The amino acid sequences of AtDofs. Available online: https://www.arabidopsis.org/ (accessed on 30 January 2022).
- Min, T.; Wang, M.M.; Wang, H.; Liu, X.; Fang, F.; Grierson, D.; Yin, X.R.; Chen, K.S. Isolation and expression of NAC genes during persimmon fruit postharvest astringency removal. Int. J. Mol. Sci. 2015, 16, 1894–1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, X.R.; Allan, A.C.; Chen, K.S.; Ferguson, I.B. Kiwifruit EIL and ERF genes involved in regulating fruit ripening. Plant Physiol. 2010, 153, 1280–1292. [Google Scholar] [CrossRef] [Green Version]
- Lijavetzky, D.; Carbonero, P.; Vicente-Carbajosa, J. Genome-wide comparative phylogenetic analysis of the rice and Arabidopsis Dof gene families. BMC Evol. Biol. 2003, 3, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.G.; Beppu, K.; Kataoka, I. Varietal differences in phenolic content and astringency in skin and flesh of hardy kiwifruit resources in Japan. Sci. Hortic. 2009, 120, 551–554. [Google Scholar] [CrossRef]
- Konishi, M.; Yanagisawa, S. Sequential activation of two Dof transcription factor gene promoters during vascular development in Arabidopsis thaliana. Plant Physiol. Biochem. 2007, 45, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Licausi, F.; Ohme-Takagi, M.; Perata, P. APETALA2/Ethylene responsive factor (AP2/ERF) transcription factors: Mediators of stress responses and developmental programs. New Phytol. 2013, 199, 639–649. [Google Scholar] [CrossRef]
- Chen, X.; Wang, D.; Liu, C.; Wang, M.; Wang, T.; Zhao, Q.; Yu, J. Maize transcription factor Zmdof1 involves in the regulation of Zm401 gene. Plant Growth Regul. 2012, 66, 271–284. [Google Scholar] [CrossRef]
- Boccaccini, A.; Lorrai, R.; Ruta, V.; Frey, A.; Mercey-Boutet, S.; Marion-Poll, A.; Tarkowská, D.; Strnad, M.; Costantino, P.; Vittorioso, P. The DAG1 transcription factor negatively regulates the seed-to-seedling transition in Arabidopsis acting on ABA and GA levels. BMC Plant Biol. 2016, 16, 198. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Wu, W.; Liu, X.; Chen, K.; Yin, X. Dof Transcription Factors Are Involved in High CO2 Induced Persimmon Fruit Deastringency. Horticulturae 2022, 8, 643. https://doi.org/10.3390/horticulturae8070643
Jin R, Wu W, Liu X, Chen K, Yin X. Dof Transcription Factors Are Involved in High CO2 Induced Persimmon Fruit Deastringency. Horticulturae. 2022; 8(7):643. https://doi.org/10.3390/horticulturae8070643
Chicago/Turabian StyleJin, Rong, Wei Wu, Xiaofen Liu, Kunsong Chen, and Xueren Yin. 2022. "Dof Transcription Factors Are Involved in High CO2 Induced Persimmon Fruit Deastringency" Horticulturae 8, no. 7: 643. https://doi.org/10.3390/horticulturae8070643
APA StyleJin, R., Wu, W., Liu, X., Chen, K., & Yin, X. (2022). Dof Transcription Factors Are Involved in High CO2 Induced Persimmon Fruit Deastringency. Horticulturae, 8(7), 643. https://doi.org/10.3390/horticulturae8070643





