Pectin-Based Edible Coating Combined with Chemical Dips Containing Antimicrobials and Antibrowning Agents to Maintain Quality of Fresh-Cut Pears
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Edible Coatings and Crosslinking Solutions
2.4. Fruit Preparation and Treatment
2.5. Weight Loss
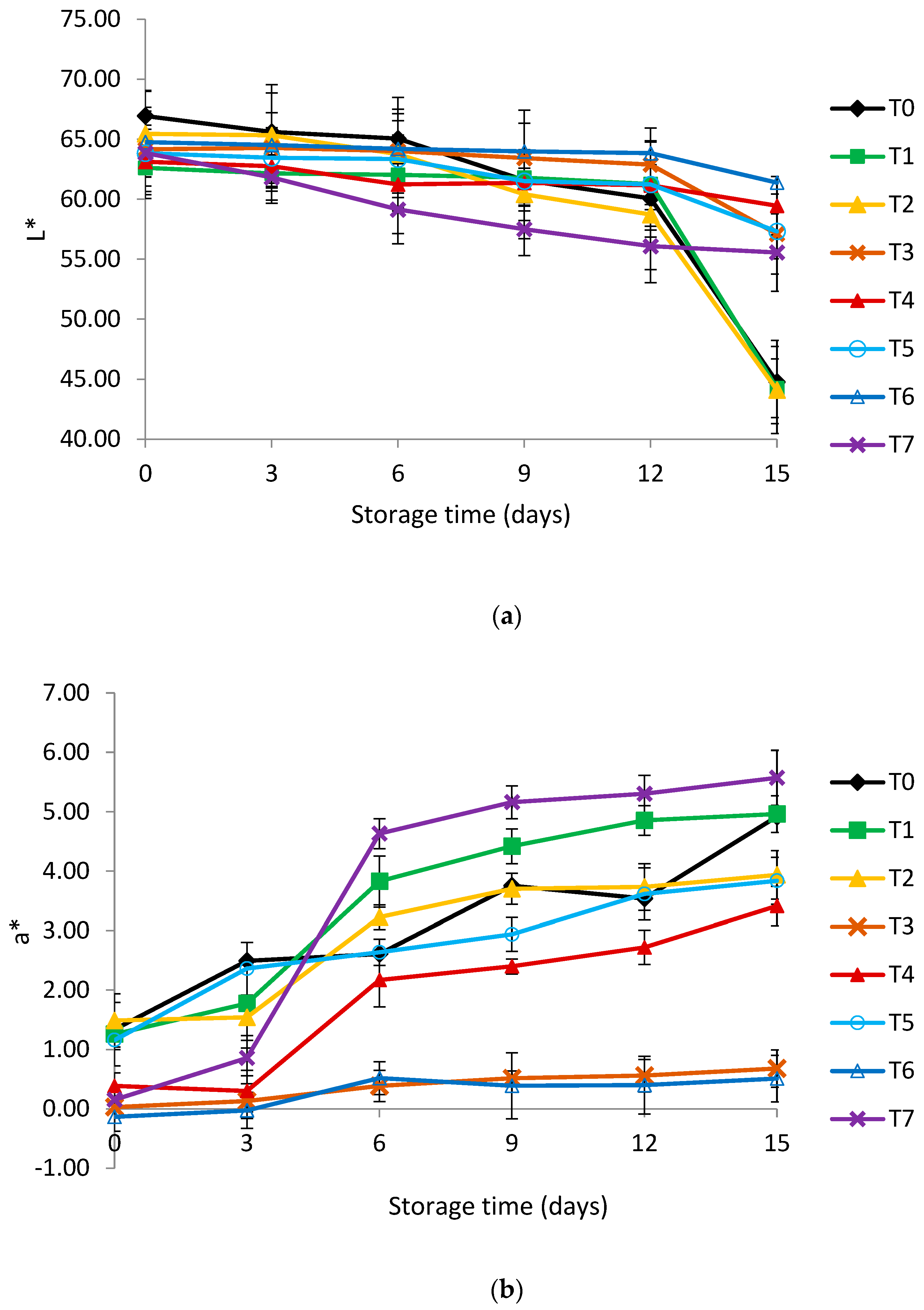
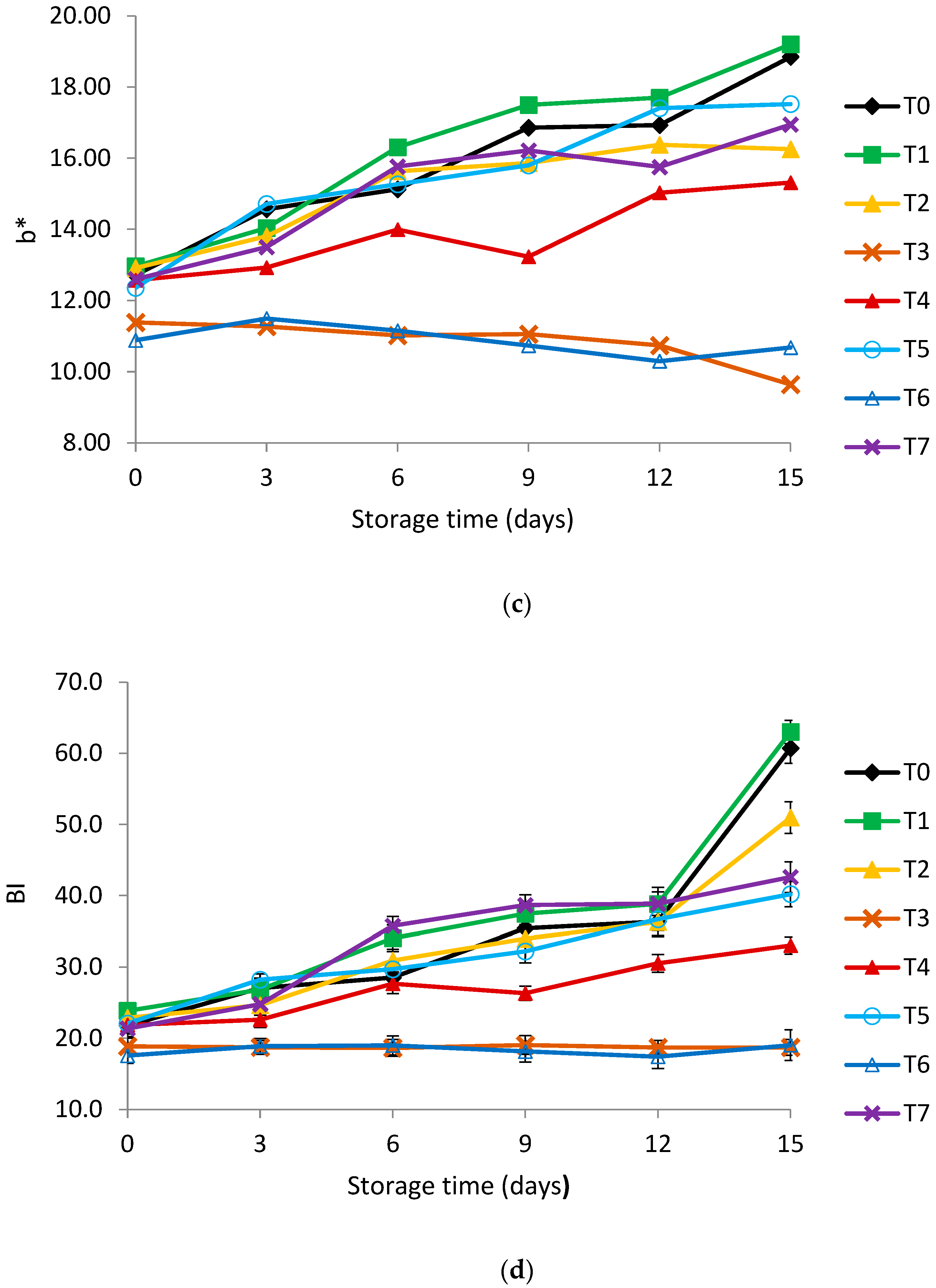
2.6. Color Measurements
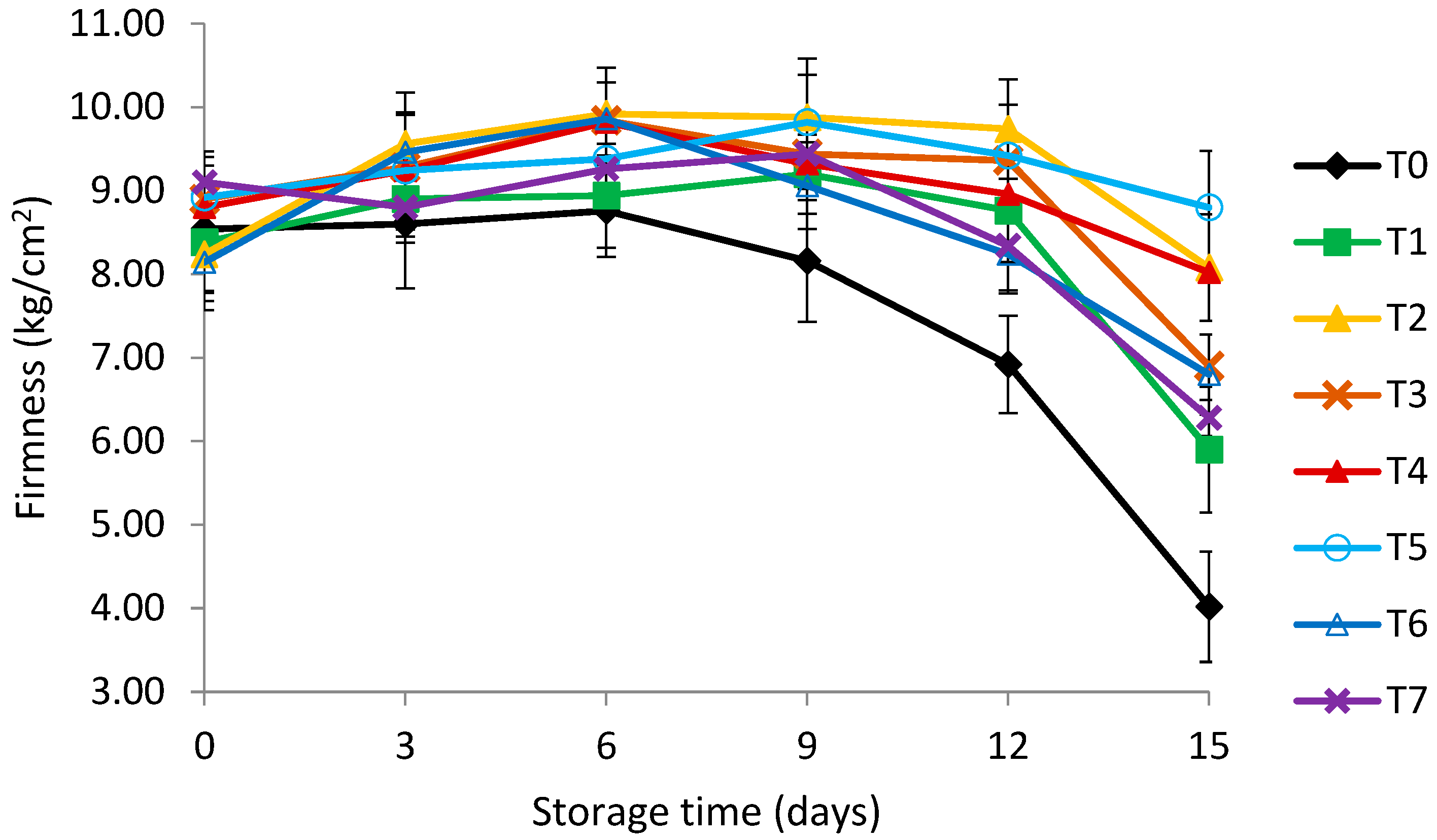
2.7. Firmness
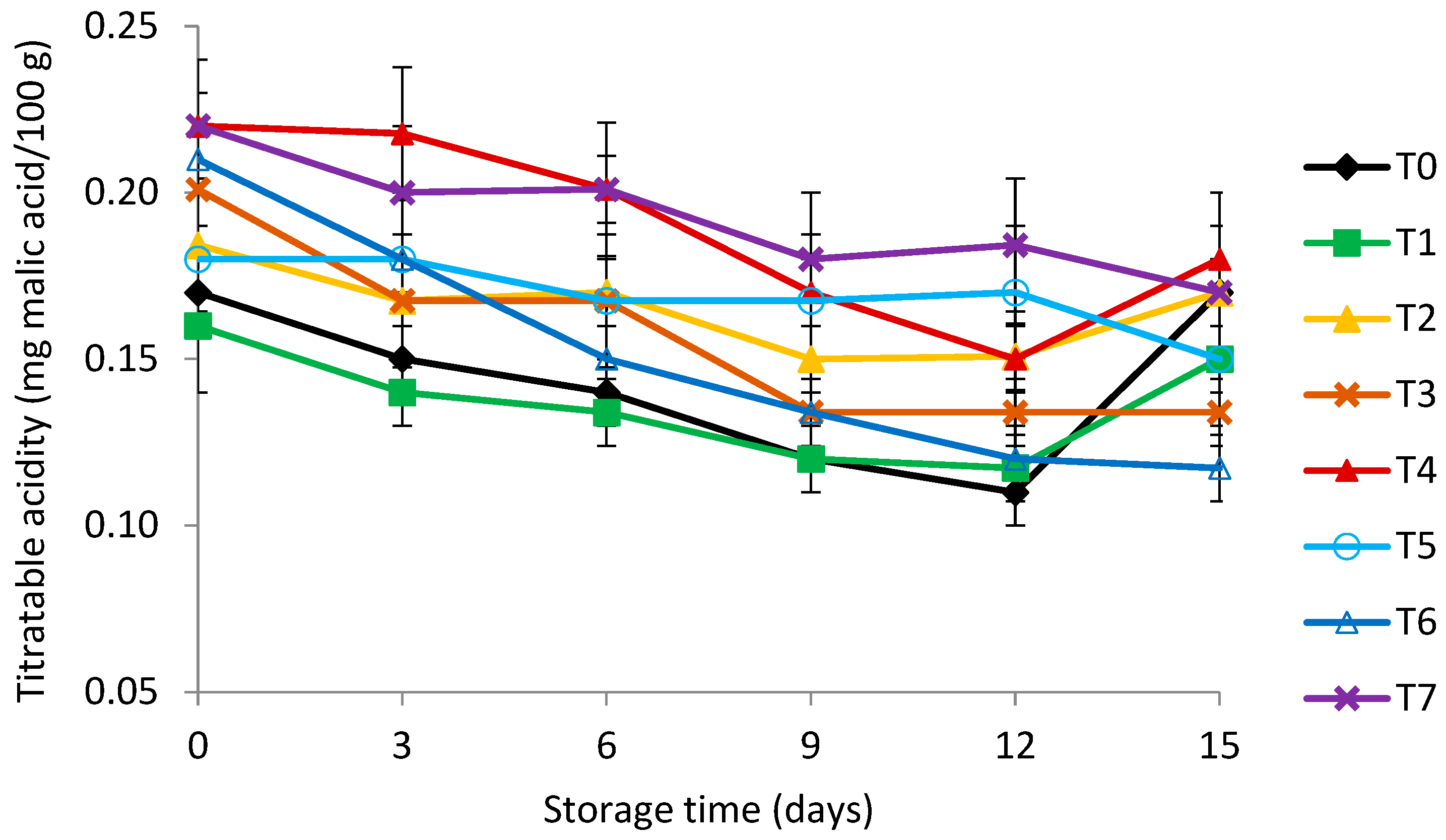
2.8. Titratable Acidity and Soluble Solids Content
2.9. Total Phenolic Content
2.10. DPPH Free-Radical-Scavenging Activity
2.11. Sensory Analysis
2.12. Statistical Analysis
3. Results and Discussion
3.1. Weight Loss
3.2. Color
3.3. Firmness
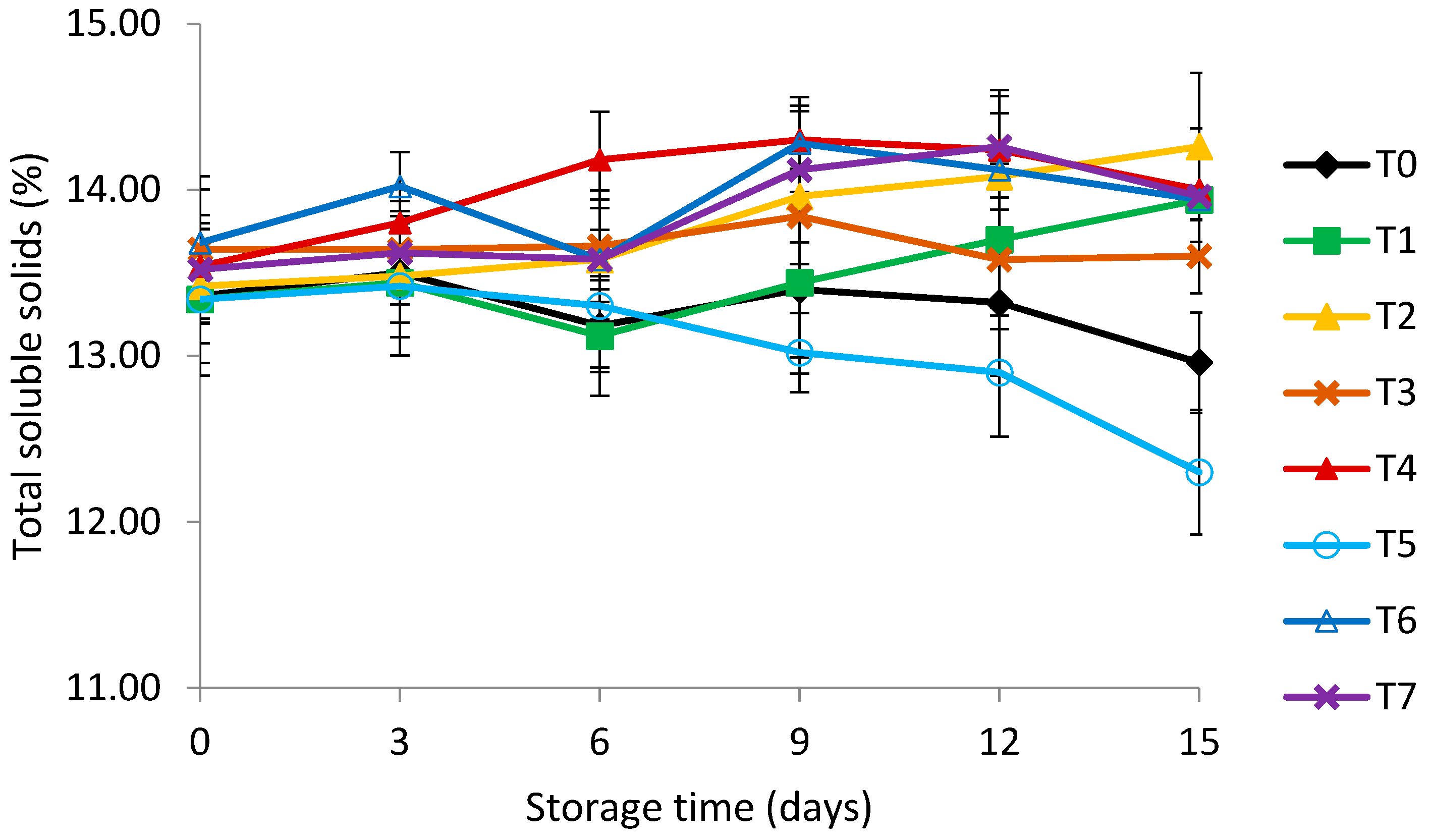
3.4. Titratable Acidity (TA) and Total Soluble Solids (TSS)
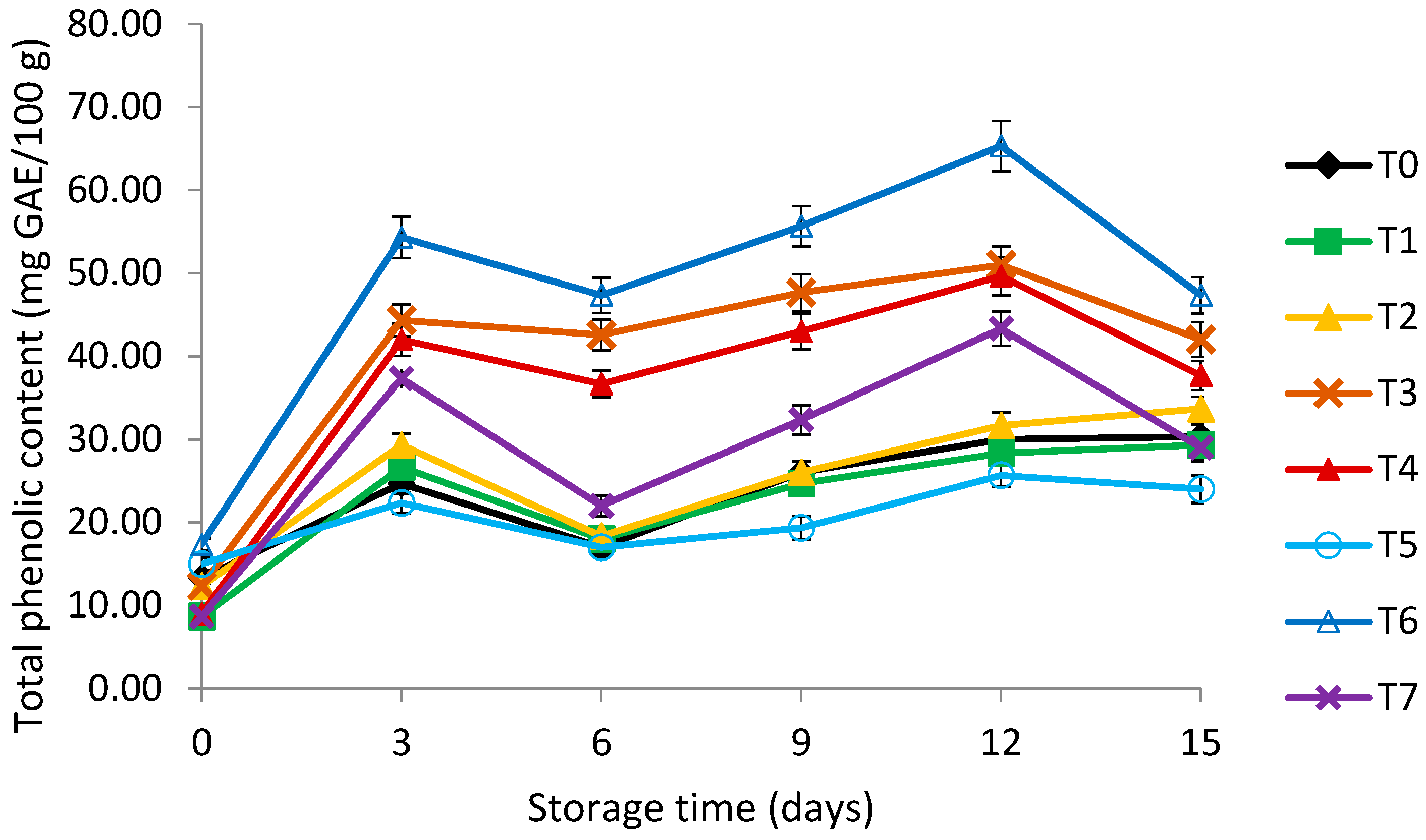
3.5. Total Phenolic Content
3.6. DPPH Radical-Scavenging Activity
3.7. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yousuf, B.; Qadri, O.S.; Srivastava, A.K. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review. LWT-Food Sci. Technol. 2018, 89, 198–209. [Google Scholar] [CrossRef]
- Passafiume, R.; Gaglio, R.; Sortino, G.; Farina, V. Effect of three different aloe vera gel-based edible coatings on the quality of fresh-cut “Hayward” kiwifruits. Foods 2020, 9, 939. [Google Scholar] [CrossRef] [PubMed]
- Olivas, G.I.; Barbosa-Cánovas, G.V. Edible coatings for fresh-cut fruits. Crit. Rev. Food Sci. 2005, 45, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Tapia, M.S. Edible coatings as carriers of food additives on fresh-cut fruits and vegetables. Stewart Postharvest Rev. 2010, 449, 1–7. [Google Scholar] [CrossRef]
- Ghidelli, C.; Pérez-Gago, M.B. Recent advances in modified atmosphere packaging and edible coatings to maintain quality of fresh-cut fruits and vegetables. Crit. Rev. Food Sci. 2018, 58, 662–679. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Soliva-Fortuny, R.; MartínBelloso, O. Edible coating to incorporate active ingredients to fresh-cut fruits: A review. Trends Food Sci. Technol. 2009, 20, 438–447. [Google Scholar] [CrossRef]
- Alves, M.M.; Gonçalves, M.P.; Rocha, C.M.R. Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT Food Sci. Technol. 2017, 80, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Radi, M.; Firouzi, E.; Akhavan, H.; Amiri, S. Effect of gelatin-based edible coatings incorporated with Aloe vera and black and green tea extracts on the shelf life of fresh-cut oranges. J. Food Qual. 2017, 2017, 9764650. [Google Scholar] [CrossRef] [Green Version]
- Pizato, S.; Chevalier, R.C.; Dos Santos, M.F.; Da Costa, T.S.; Arévalo Pinedo, R.; Cortez Vega, W.R. Evaluation of the shelf-life extension of fresh-cut pineapple (Smooth Cayenne) by application of different edible coatings. Br. Food J. 2019, 121, 1592–1604. [Google Scholar] [CrossRef]
- Maringgal, B.; Hashim, N.; Tawakkal, I.S.M.A.; Mohamed, M.T.M. Recent advance in edible coating and its effect on fresh/fresh-cut fruits quality. Trends Food Sci. Technol. 2020, 96, 253–267. [Google Scholar] [CrossRef]
- Castro-Rosas, J.; Cruz-Galvez, A.M.; Gomez-Aldapa, C.A.; FalfanCortes, R.N.; Guzman-Ortiz, F.A.; Rodríguez-Marín, M.L. Biopolymer films and the effects of added lipids, nanoparticles and antimicrobials on their mechanical and barrier properties: A review. Int. J. Food Sci. Technol. 2016, 51, 1967–1978. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martín Belloso, O. Edible coatings with antibrowning agents to maintain sensory quality and antioxidant properties of fresh-cut pears. Postharvest Biol. Technol. 2008, 50, 87–94. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and applications of pectin in food, biomedical, and pharmaceutical industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Siroli, L.; Patrignani, F.; Lanciotti, R.; Rocculi, P.; Dalla Rosa, M.; Romani, S. Study on the efficacy of edible coatings on quality of blueberry fruits during shelf-life. LWT- Food Sci. Technol. 2017, 85, 440–444. [Google Scholar] [CrossRef]
- Bico, S.L.S.; Raposo, M.F.J.; Morais, R.M.S.C.; Morais, A.M.M.B. Chemical dips and edible coatings to retard softening and browning of fresh-cut banana. Int. J. Postharvest Technol. Innov. 2010, 2, 13–24. [Google Scholar] [CrossRef]
- Sanchís, E.; Gonzalez, S.; Ghidelli, C.; Sheth, C.C.; Mateos, M.; Palou, L.; Perez-Gago, M.B. Browning inhibition and microbial control in fresh-cut persimmon (Diospyros kaki Thunb. cv. Rojo Brillante), by apple pectin-based edible coatings. Postharvest Biol. Technol. 2016, 112, 186–193. [Google Scholar] [CrossRef]
- Martín-Diana, A.B.; Rico, D.; Frias, J.M.; Barat, J.M.; Henehan, G.T.M.; Barry-Ryan, C. Calcium for extending the shelf life of fresh whole and minimally processed fruits and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 210–218. [Google Scholar] [CrossRef]
- He, Q.; Luo, Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 2007, 3, 1–7. [Google Scholar] [CrossRef]
- Kuwar, U.; Sharma, S.; Tadapaneni, V.R.R. Aloe vera gel and honey-based edible coatings combined with chemical dip as a safe means for quality maintenance and shelf life extension of fresh-cut papaya. J. Food Qual. 2015, 38, 347–358. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, W.; Chen, Y.; Peng, Y. Browning inhibition of plant extracts on fresh-cut fruits and vegetables—A review. J. Food Process. Preserv. 2022, 46, e16532. [Google Scholar] [CrossRef]
- Olivas, G.I.; Rodriguez, J.J.; Barbosa-Cánovas, G.V. Edible coatings composed of methylcellulose, stearic acid, and additives to preserve quality of pear wedges. J. Food Process. Preserv. 2003, 27, 299–320. [Google Scholar] [CrossRef]
- Gorny, J.R.; Hess-Pierce, B.; Cifuentes, R.A.; Kader, A.A. Quality changes in freshcut pear slices as affected by controlled atmospheres and chemical preservatives. Postharvest Biol. Technol. 2002, 24, 271–278. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.Y.; Park, H.J.; Lee, C.Y.; Choi, W.Y. Extending shelf-life of minimally processed apples with edible coatings and antibrowning agents. LWT-Food Sci.Technol. 2003, 36, 323–329. [Google Scholar] [CrossRef]
- Rojas-Graü, M.A.; Tapia, M.S.; Rodriguez, F.J.; Carmona, A.J.; Martin-Belloso, O. Alginate and gellan-based edible coatings as carriers of antibrowning agents applied on fresh-cut Fuji apples. Food Hydrocoll. 2007, 21, 118–127. [Google Scholar] [CrossRef]
- Wu, X.; Li, G.; He, F. Nondestructive analysis of internal quality in pears with a self-made near-infrared spectrum detector combined with multivariate data processing. Foods 2021, 10, 1315. [Google Scholar] [CrossRef] [PubMed]
- Pintó, E.; Lentheric, I.; Vendrell, M.; Larrigaudière, C. Role of fermentative and antioxidant metabolisms in the induction of core browning in controlled-atmosphere stored pears. J. Sci. Food. Agric. 2001, 81, 364–370. [Google Scholar] [CrossRef]
- Moreira, M.R.; Válvarez, M.; Martín-Belloso, O.; Soliva-Fortuny, R. Effects of pulsed light treatments and pectin edible coatings on the quality of fresh-cut apples: A hurdle technology approach. J. Sci. Food Agric. 2017, 97, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Varghese, E. Improving the shelf life of fresh-cut ‘Royal Delicious’ apple with edible coatings and anti-browning agents. J. Food Sci. Technol. 2018, 55, 3767–3778. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Oliveira, I.; Sousa, A.; Ferreira, I.C.F.R.; Bento, A.; Estevinho, L.; Pereira, J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008, 46, 2326–2331. [Google Scholar] [CrossRef]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of edible coatings on the quality of fresh blueberries (Duke and Elliott) under commercial storage conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. Edible coatings enriched with essential oils for extending the shelf-life of ‘Bravo de Esmolfe’ fresh-cut apples. Int. J. Food Sci. Technol. 2016, 51, 87–95. [Google Scholar] [CrossRef]
- Chiabrando, V.; Giacalone, G. Effect of chitosan and sodium alginate edible coatings on the postharvest quality of fresh-cut nectarines during storage. Fruits 2016, 71, 79–85. [Google Scholar] [CrossRef]
- Guerreiro, A.C.; Gago, C.M.L.; Faleiro, M.L.; Miguel, M.G.C.; Antunes, M.D.C. The effect of edible coatings on the nutritional quality of ‘Bravo de Esmolfe’ fresh-cut apple through shelf-life. LWT 2017, 75, 210–219. [Google Scholar] [CrossRef]
- Robles-Sánchez, R.; Rojas-Graü, A.; Odriozola-Serrano, I.; González-Aguilar, G.; Martín-Belloso, O. Influence of alginate-based edible coating as carrier of antibrowning agents on bioactive compounds and antioxidant activity in fresh-cut Kent mangoes. LWT Food Sci. Technol. 2013, 50, 240–246. [Google Scholar] [CrossRef]
- Cortellino, G.; Gobbi, S.; Bianchi, G.; Rizzolo, A. Modified atmosphere packaging for shelf life extension of fresh-cut apples. Trends Food Sci. Technol. 2015, 46, 320–330. [Google Scholar] [CrossRef]
- Qi, H.; Hu, W.; Jiang, A.; Tian, M.; Li, Y. Extending shelf-life of fresh-cut ‘Fuji’ apples with chitosan-coatings. Innov. Food Sci. Emerg. Technol. 2011, 12, 62–66. [Google Scholar] [CrossRef]
- Saba, M.K.; Sogvar, O.B. Combination of carboxymethyl cellulose-based coatings with calcium and ascorbic acid impacts in browning and quality of fresh-cut apples. Food Sci. Technol. 2016, 66, 165–171. [Google Scholar] [CrossRef]
- Jancikova, S.; Dordevic, D.; Tesikova, K.; Antonic, B.; Tremlova, B. Active edible films fortified with natural extracts: Case study with fresh-cut apple pieces. Membranes 2021, 11, 684. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Singh, Z.; Khan, A.S. Postharvest Aloe vera gel-coating modulates fruit ripening and quality of ‘Arctic Snow’ nectarine kept in ambient and cold storage. Int. J. Food Sci. Technol. 2009, 44, 1024–1033. [Google Scholar] [CrossRef]
- Maftoonazad, N.; Ramaswamy, H.S.; Marcotte, M. Shelflife extension of peaches through sodium alginate and methyl cellulose edible coatings. Int. J. Food Sci. Technol. 2008, 43, 951–957. [Google Scholar] [CrossRef]
- Benítez, S.; Achaerandio, I.; Sepulcre, F.; Pujola, M. Aloe vera based coatings improve the quality of minimally processed ‘Hayward’ kiwifruit. Postharvest Biol. Technol. 2013, 81, 29–36. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Soliva-Fortuny, R.; Martin-Belloso, O. Using polysaccharide-based edible coatings to enhance quality and antioxidant properties of fresh-cut melon. LWT-Food Sci. Technol. 2008, 41, 1862–1870. [Google Scholar] [CrossRef]
- Gonzalez-Aguilar, G.A.; Ruiz-Cruz, S.; Soto-Valdez, H.; Vazquez-Ortiz, F.; Pacheco-Aguilar, R.; Wang, C.Y. Biochemical changes of fresh-cut pineapple slices treated with antibrowning agents. Int. J. Food Sci. Technol. 2005, 40, 377–383. [Google Scholar] [CrossRef]
- Moreira, M.; Cassani, L.; Martin-Belloso, O.; Soliva-Fortuny, R. Effects of polysaccharide-based edible coatings enriched with dietary fiber on quality attributes of fresh-cut apple. J. Food Sci. Technol. 2015, 52, 7795–7805. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Treatment | Components |
|---|---|
| T0 | Distilled water (control) |
| T1 | 2% PE + 1% CaCl2 |
| T2 | 2% PE + 1% CaCl2 + 0.2% PS |
| T3 | 2% PE + 1% CaCl2 + 0.2% PS + 1% N-AC |
| T4 | 2% PE + 1% CaCl2 + 0.2% PS + 1% CA + 1% AA |
| T5 | 2% PE + 1% CaCl2 + 0.2% SB |
| T6 | 2% PE + 1% CaCl2 + 0.2% SB + 1% N-AC |
| T7 | 2% PE + 1% CaCl2 + 0.2% SB + 1% CA + 1% AA |
| Treatment | Storage Time (Days) | Appearance | Texture | Taste | Flavor | Overall Acceptability |
|---|---|---|---|---|---|---|
| T0 | 0 | 4.25 ± 0.45 ab | 4.50 ± 0.52 abc | 4.83 ± 0.39 e | 4.67 ± 0.49 d | 4.58 ± 0.51 ab |
| 6 | 3.75 ± 0.45 c | 3.08 ± 0.29 a | 3.83 ± 0.39 d | 3.42 ± 0.51 ab | 3.42 ± 0.51 b | |
| 12 | 2.42 ± 0.51 b | 2.17 ± 0.39 a | 2.75 ± 0.45 ab | 2.83 ± 0.49 ab | 2.17 ± 0.39 b | |
| T1 | 0 | 4.08 ± 0.29 a | 4.25 ± 0.45 a | 4.50 ± 0.52 de | 4.42 ± 0.51 bcd | 4.50 ± 0.52 ab |
| 6 | 3.58 ± 0.51 bc | 3.67 ± 0.49 bc | 3.67 ± 0.49 bcd | 3.67 ± 0.49 ab | 3.25 ± 0.45 b | |
| 12 | 2.58 ± 0.39 b | 2.75 ± 0.45 cd | 2.83 ± 0.39 abc | 2.75 ± 0.51 ab | 2.33 ± 0.49 b | |
| T2 | 0 | 4.33 ± 0.49 ab | 4.42 ± 0.51 abc | 4.33 ± 0.49 cd | 4.33 ± 0.49 bcd | 4.33 ± 0.49 ab |
| 6 | 3.50 ± 0.52 bc | 3.83 ± 0.39 c | 3.42 ± 0.51 abc | 3.58 ± 0.51 ab | 3.17 ± 0.39 b | |
| 12 | 2.67 ± 0.49 b | 2.83 ± 0.39 d | 2.67 ± 0.49 a | 2.92 ± 0.51 ab | 2.42 ± 0.51 b | |
| T3 | 0 | 4.83 ± 0.39 c | 4.67 ± 0.49 bc | 4.17 ± 0.39 bcd | 4.50 ± 0.52 cd | 4.67 ± 0.49 b |
| 6 | 4.58 ± 0.51 d | 4.42 ± 0.51 d | 3.92 ± 0.67 d | 3.75 ± 0.45 bc | 4.25 ± 0.62 c | |
| 12 | 3.92 ± 0.29 c | 3.58 ± 0.51 e | 3.17 ± 0.39 d | 3.08 ± 0.51 b | 3.42 ± 0.51 c | |
| T4 | 0 | 4.58 ± 0.51 bc | 4.58 ± 0.51 abc | 4.08 ± 0.67 bc | 4.58 ± 0.51 d | 4.58 ± 0.51 ab |
| 6 | 3.33 ± 0.49 b | 3.83 ± 0.39 c | 3.42 ± 0.51 abc | 4.08 ± 0.29 c | 3.08 ± 0.29 b | |
| 12 | 2.33 ± 0.49 b | 2.67 ± 0.49 bcd | 2.83 ± 0.39 abc | 2.87 ± 0.52 ab | 2.42 ± 0.51 b | |
| T5 | 0 | 4.08 ± 0.29 a | 4.33 ± 0.49 ab | 3.92 ± 0.29 ab | 3.92 ± 0.29 a | 4.25 ± 0.45 a |
| 6 | 3.42 ± 0.51 bc | 3.50 ± 0.52 bc | 3.33 ± 0.49 ab | 3.33 ± 0.49 a | 3.17 ± 0.39 b | |
| 12 | 2.42 ± 0.51 b | 2.42 ± 0.51 abc | 2.67 ± 0.49 a | 2.67 ± 0.39 a | 2.33 ± 0.49 b | |
| T6 | 0 | 4.75 ± 0.45 c | 4.75 ± 0.45 c | 4.08 ± 0.67 bc | 4.17 ± 0.58 abc | 4.58 ± 0.51 ab |
| 6 | 4.67 ± 0.49 d | 4.33 ± 0.49 d | 3.75 ± 0.45 cd | 3.50 ± 0.52 ab | 4.17 ± 0.58 c | |
| 12 | 3.67 ± 0.49 c | 3.33 ± 0.49 e | 3.08 ± 0.51 cd | 3.00 ± 0.52 ab | 3.33 ± 0.49 c | |
| T7 | 0 | 4.33 ± 0.49 ab | 4.42 ± 0.51 abc | 3.67 ± 0.49 a | 4.08 ± 0.29 ab | 4.33 ± 0.49 ab |
| 6 | 2.83 ± 0.39 a | 3.42 ± 0.51 ab | 3.25 ± 0.45 a | 3.42 ± 0.51 ab | 2.58 ± 0.51 a | |
| 12 | 1.83 ± 0.39 a | 2.33 ± 0.49 ab | 2.58 ± 0.51 a | 2.75 ± 0.45 ab | 1.67 ± 0.49 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleșoianu, A.M.; Nour, V. Pectin-Based Edible Coating Combined with Chemical Dips Containing Antimicrobials and Antibrowning Agents to Maintain Quality of Fresh-Cut Pears. Horticulturae 2022, 8, 449. https://doi.org/10.3390/horticulturae8050449
Pleșoianu AM, Nour V. Pectin-Based Edible Coating Combined with Chemical Dips Containing Antimicrobials and Antibrowning Agents to Maintain Quality of Fresh-Cut Pears. Horticulturae. 2022; 8(5):449. https://doi.org/10.3390/horticulturae8050449
Chicago/Turabian StylePleșoianu, Alina Mădălina, and Violeta Nour. 2022. "Pectin-Based Edible Coating Combined with Chemical Dips Containing Antimicrobials and Antibrowning Agents to Maintain Quality of Fresh-Cut Pears" Horticulturae 8, no. 5: 449. https://doi.org/10.3390/horticulturae8050449
APA StylePleșoianu, A. M., & Nour, V. (2022). Pectin-Based Edible Coating Combined with Chemical Dips Containing Antimicrobials and Antibrowning Agents to Maintain Quality of Fresh-Cut Pears. Horticulturae, 8(5), 449. https://doi.org/10.3390/horticulturae8050449







