Abstract
Culture medium and light are important factors that affect the process of in vitro propagation of plants. Particularly for orchids, diverse culture media have been evaluated for micropropagation of many species and hybrids. More recently, light-emitting diodes (LEDs) have become widely used in agriculture, including micropropagation commercial operations, resulting in increased production and reduced costs compared to traditional fluorescent lights. Brassavola nodosa (L.) Lindl. is an orchid, with great potential for commercialization as a potted flowering plant due to the beauty and fragrance of its inflorescences. In this study, we evaluated the effects of culture media (VW, MS, and ½ MS) and light sources (three LED sources and one fluorescent light source) on the micropropagation of B. nodosa orchids. VW medium resulted in the best growth and development of in vitro shoots compared to MS and ½ MS media. Light sources with lower intensity, such as LED-3 (80 μmol m−2 s−1 PPFD) resulted in the best plant performance in vitro, while LED-2 (1015 μmol m−2 s−1 PPFD) showed the best plant performance ex vitro. Rooting was obtained in vitro without the need for a rooting phase. Survival ex vitro was 100%, with the successful growth and development of in vitro-derived plantlets during acclimatization.
1. Introduction
The Orchidaceae is the largest family of flowering plants, consisting of over 25,000 species within over 850 genera [1]. Their specialized floral morphology, structure, and physiology have always fascinated people, making them among the most popular flowering plants worldwide [2]. Brassavola nodosa (L.) Lindl. is an epiphytic orchid found in the West Indies, Mexico through Central America to Venezuela and Peru [3,4]. The inflorescences show strong fragrance at night, rendering its common name as the “Lady of the Night”. Some characteristics of this plant, such as its fragrant flowers, low maintenance, year-round blooming, and unique flower shape, make it a candidate of high value for ornamental use and commercial production. Brassavola orchids are propagated by branching, pseudobulbs, and seeds. However, seed germination is very low and vegetative propagation is lengthy [5]. Micropropagation or in vitro propagation offers a potential alternative for commercial large-scale clonal propagation of plants [6,7,8,9]. Several orchid species within the genera, such as Cymbidium [10], Dendrobium [11], Phalaenopsis [12], and Vanilla [13], among others, have been successfully propagated in vitro.
Micropropagation involves the in vitro propagation of plant material under aseptic conditions in a culture medium, which contains minerals, vitamins, and plant growth regulators, as well as the supplements necessary for the proper multiplication, growth, and development of in vitro plants. The culture medium requires adjustments according to the species or genotype used, as well as for the different developmental steps involved in micropropagation, such as shoot multiplication or rooting [9]. For in vitro propagation of orchids, several culture media have been successfully employed, including the MS medium [14], one of the most widely used culture media for different micropropagation stages [15,16]. The VW medium [17] has also been widely used, showing advantages for the production of protocorm-like bodies (PLBs), which provide a means for large-scale in vitro clonal multiplication of orchids [18]. This culture medium is also commonly used for epiphytic orchids that require low nitrate [19].
Another important factor affecting micropropagation is light; both light intensity and light quality [20]. Light intensity measured as photosynthetic photon flux density (PPFD; μmol·m−2·s−1), is considered one of the primary factors that affect in vitro plant morphogenesis, including photosynthetic activity, is also affected by chlorophyll content [21,22,23]. Responses to light intensity are species-specific and vary according to the different micropropagation stages. Light quality is also important for in vitro plant propagation, as defined by the relative intensity and quantity of the different wavelengths emitted by a light source and perceived by photoreceptors within the plant [24]. Cool-white fluorescent lamps have been the most common light source used in micropropagation [25,26,27]. However, more recently, the utilization of light-emitting diodes (LEDs) has become widespread in agriculture, showing high potential for commercial application due to increased production by using selective light, and the reduced cost of LEDs compared to fluorescent lights, as well as for its applications for in vitro plant growth and morphogenesis [28,29]. The advantages of using LEDs for either shoot culture or embryogenesis have been reported for several plant species, including vanilla [30] and sugarcane [31]. In recent years, the effects of light intensity and quality on micropropagation of orchids have received considerable interest. Different spectra can alter organogenesis in slow-growing orchids, such as Cattleya hybrids, and it can alter the morphology and anatomy of orchid plantlets obtained through in vitro adventitious shoot multiplication [32]. For example, blue LEDs can stimulate shoot elongation, the number of roots formed, and the number of leaves per shoot in vanilla during the in vitro rooting phase [33].
To date, no study has been reported on the effects of culture medium or light sources on the micropropagation of Brassavola nodosa orchids. Therefore, the objective of this study was to evaluate the effects of culture media and light sources on the micropropagation of B. nodosa orchids. The elucidation of such parameters may contribute to the development of an efficient protocol for the commercial production of this desirable ornamental orchid species.
2. Materials and Methods
2.1. Plant Material and Culture Establishment
Young shoot tips (0.3–0.5 cm) collected from in vitro seedlings of Brassavola nodosa ‘Remar’ × ‘Mas Mejor’ hybrid were used as explants for shoot multiplication. Explants were cultured in 125-mL Erlenmeyer flasks containing 25 mL of liquid MS [14] medium supplemented with 2 mg L−1 of Glycine, 0.1 mg L−1 naphthalene acetic acid (NAA), 10% coconut water, 2.0 mg L−1 benzyladenine (BA), 30.0 mg L−1 adenine sulfate, and 30 g L−1 sucrose. Flasks containing liquid media were placed on an orbital shaker at 90 rpm. After 45 days, adventitious shoots developed from shoot multiplication were collected and transferred to MS basal medium supplemented with 30 g L−1 sucrose and solidified with 7 g L−1 agar in TP1600 Microboxes (Sac O2, Belgium) containing 500 mL of semi-solid agar-based medium. The pH of the medium was adjusted to 5.2 before autoclaving at 121 °C and 20 psi for 20 min. The cultures were maintained under a controlled environment with a 16-h photoperiod at a light intensity of 50 μmol m−2 s−1 PPFD and a temperature of 26 ± 4 °C. After 30 days of culture establishment, adventitious shoots (0.3–0.5 cm) were selected for the experiments.
2.2. Light Sources
Four light sources were evaluated in this experiment:
- a
- LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 PPFD consisting of 17% blue light (B) at 400–500 nm, 38% green light (G) at 500–600 nm, and 45% red light (R) at 600–700 nm (VOLT® VL-1, VOLT® Lighting, Lutz, FL, USA).
- b
- LED-2: LED light with 1018 ± 50 μmol m−2 s−1 PPFD consisting of 17% B, 38% G, and 45% R (VOLT® VL-1, VOLT® Lighting, Lutz, FL, USA).
- c
- LED-3: Red LED light with 77 ± 5 μmol m−2 s−1 PPFD consisting of 13% B, 26% G, and 61% R (Valoya Model L35 AP67, Helsinki, Finland).
- d
- WF: White fluorescent light with 45 ± 5 μmol m−2 s−1 PPFD consisting of 17% B, 45% G, and 38% R (GE Lighting F96T8/XL/SPP35, East Cleveland, OH, USA).
The intensity and composition of all light sources were measured using an LI-180 Li-Cor spectrometer (Li-Cor, Lincoln, NE, USA) (Figure 1).
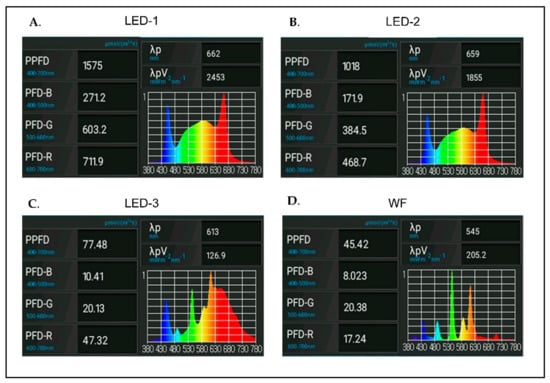
Figure 1.
Intensity and composition of light-emitting diodes (LED) and white fluorescent (WF) light sources evaluated for the micropropagation of Brassavola nodosa in this experiment: (A) LED-1 = 1575 ± 50 μmol m−2 s−1 PPFD consisting of 17% blue light (B) at 400–500 nm, 38% green light (G) at 500–600 nm, and 45% red light (R) at 600–700 nm; (B) LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD consisting of 17% B, 38% G, and 45% R; (C) LED-3: Red LED light with 80 ± 5 μmol m−2 s−1 PPFD consisting of 13% B, 26% G, and 61% R; and (D) WF: white fluorescent with 50 ± 5 μmol m−2 s−1 PPFD consisting of 17% B, 45% G, and 38% R. Measurements were obtained with an LI-180 Li-Cor spectrometer.
2.3. Culture Media
Three culture media were evaluated in this experiment:
- a
- MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar.
- b
- ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1 agar.
- c
- VW: VW [17] basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1 agar.
The pH of all media was adjusted to 5.2 before autoclaving at 121 °C and 20 psi for 20 min. Explants were cultured on these treatments under a controlled environment with a 16-h photoperiod and temperature of 26 ± 4 °C in 220-mL baby-food jars containing 25 mL semi-solid medium. The experiment was comprised of 5 replicates per treatment, each replicate containing 4 explants, with a total of 20 repetitions. The entire experiment was repeated.
2.4. Growth and Development Parameters
Leaf number, leaf length, leaf width, rooting percentage, root number, root length, relative chlorophyll content, and fresh and dry weight were evaluated 60 days after culture establishment. For relative chlorophyll content, fully expanded leaves were selected from 5 plants per treatment. Relative chlorophyll content was evaluated as a SPAD value by placing the third expanded leaf of each plantlet, counted from top downwards, into a portable SPAD-502 chlorophyll meter (SPAD-502, Minolta Co., Ltd., Tokyo, Japan).
2.5. Acclimatization
Ten rooted plants were selected from each treatment for acclimatization. Rooted shoots were transferred to 40-cell plastic trays with soilless media containing orchid bark (Sequoia Bark Sales, Reedley, CA, USA). Plants were maintained in a greenhouse with a mist system running for 2 min every 6 h. Plants were fertilized weekly with 300 ppm of 11-35-15 (N-P2O5-K2O) orchid fertilizer (Better-Gro® Orchid Better-Bloom®, Arcadia, FL, USA). Plant survival, leaf number, leaf length, leaf width, rooting percentage, root number, root length, shoot length, and fresh and dry weight were evaluated for all treatments after 30 days.
2.6. Experimental Design and Statistical Analysis
A completely randomized experimental design was applied for all experiments. Data were collected and submitted to analysis of variance (ANOVA) using the OriginPro® 2021b software (OriginLab, Northampton, MA, USA). Tukey’s post hoc multiple comparison adjustment (α = 0.05) was used for all pairwise comparisons of means.
3. Results
3.1. Light Sources and Culture Media
The effects of the different light sources and culture media are illustrated in Figure 2. Significant differences were observed for leaf number (p < 0.008), leaf length (p < 0.001), and leaf width (p < 0.001) during micropropagation of B. nodosa as affected by the light sources (Figure 2 and Figure 3A–C). The highest number of leaves was obtained under LED-1 (6.6/explant), followed by LED-2 (6.2/explant), LED-3 (5.4/explant), and WF (5.4/explant) (Figure 3A). The average leaf length under LED-3 (2.00 cm) and under WF (1.79 cm) were not significantly different, but significantly higher than LED-2 (1.60 cm), and LED-1 (1.19 cm) (Figure 3B). The leaf width under WF (0.42 cm) and under LED-3 (0.38 cm) was not significantly different. The leaf width under LED-2 (0.34 cm) was similar to LED-1 (0.29 cm) (Figure 3C).
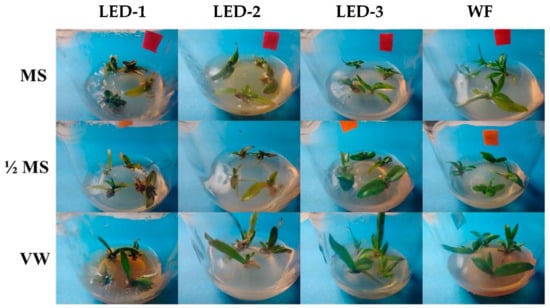
Figure 2.
Effects of light sources and culture media on micropropagation of B. nodosa after 60 days of culture establishment. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1.
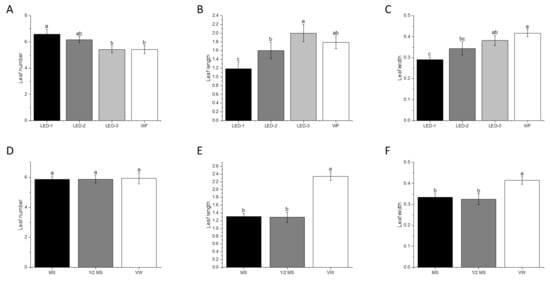
Figure 3.
Effects of light sources and culture media on leaf number (A,D), leaf length (cm) (B,E), and leaf width (cm) (C,F) on B. nodosa in vitro plantlets after 60 days of culture establishment. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Bars indicate mean ± SE. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
Regarding culture media, no significant differences were observed between culture media for leaf number (Figure 3D). Leaf length (p < 0.001) (2.34 cm) and leaf width (p < 0.001) (0.41 cm) of explants cultured in VW medium were significantly higher than for the explants cultured in MS medium (1.31 cm and 0.33 cm) and ½ MS medium (1.29 cm and 0.33 cm) (Figure 3E,F).
For rooting performance, 100% rooting was achieved in all treatments. Light sources did not have a significant effect on the root number or root length of B. nodosa in vitro plantlets (Figure 4A,B). However, the explants cultured in VW media had a higher root number (p < 0.038) (4.06) than the explants in MS medium (2.50), and a higher root length (p < 0.001) (1.64 cm) compared to the explants in ½ MS medium (0.84 cm) and MS medium (0.59 cm) (Figure 4C,D).
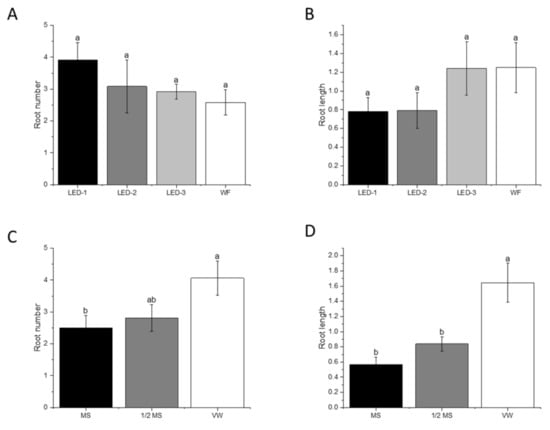
Figure 4.
Effects of light sources and culture media on root number (A,C) and root length (cm) (B,D) on B. nodosa in vitro plantlets after 60 days of culture establishment. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Bars indicate mean ± SE. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
Light sources did not affect the fresh and dry weight of explants in the experiment, but they did affect the relative chlorophyll content (p < 0.001) (Figure 5A–C). WF had the highest relative chlorophyll content, followed by LED-3, LED-1, and LED-2 (Figure 5A). The culture media had significant effects on fresh weight (p < 0.001), dry weight (p < 0.001), and relative chlorophyll content (p < 0.034) (Figure 5D–F). Explants cultured in VW media had higher fresh weight, dry weight, and relative chlorophyll content as compared to the MS medium and ½ MS medium (Figure 5D–F).

Figure 5.
Effects of light sources and culture media on relative chlorophyll content (A,D), fresh weight (g) (B,E), and dry weight (g) (C,F) of Stage III B. nodosa in vitro plantlets after 60 days of culture establishment. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Bars indicate mean ± SE. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
3.2. Acclimatization
During acclimatization, 100% ex vitro survival was observed after 30 days of the establishment for plantlets originating from all in vitro treatments (Figure 6A). Light sources had no effect on leaf number in plants transferred to the greenhouse for acclimatization, however, leaf length (p < 0.001) and leaf width (p < 0.023) showed significant differences (Figure 7A–C). In vitro-derived plantlets that were previously cultured under LED-3, LED-2, and WF showed significantly higher leaf length compared to LED-1 (Figure 6B and Figure 7A–C). The highest leaf width was observed for WF, although not significantly different from LED-2 and LED-3, but was significantly higher than LED-1.

Figure 6.
Effects of light sources and culture media on ex vitro plant survival (A) and plant performance (B) of B. nodosa during acclimatization for 30 days. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Scale bar = 1 cm.
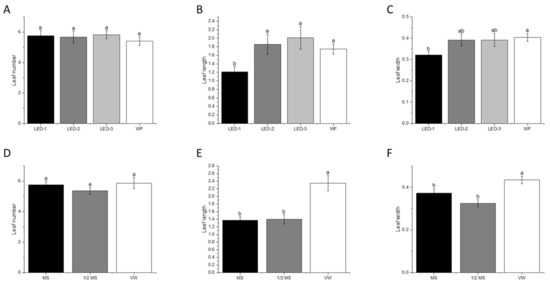
Figure 7.
Effects of light sources and culture media on leaf number (A,D), leaf length (cm) (B,E), and leaf width (cm) (C,F) of B. nodosa plantlets during acclimatization for 30 days. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Bars indicate mean ± SE. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
Plantlets under acclimatization also showed differences in leaf length (p < 0.001) and leaf width (p < 0.001) as affected by in vitro culture media (Figure 6B and Figure 7E,F) from the previous experiment, although no differences were observed for leaf number (Figure 7D). Plantlets derived from the VW medium had higher leaf length and width compared to plantlets derived from MS and ½ MS media (Figure 7E,F).
The root number (p < 0.001) in plantlets from LED-2 (5.6/explant) was significantly higher than those from LED-1 (3.7/explant), LED-3 (4.1/explant), and WF (3.8/explant). The root length (p < 0.001) from LED-1 was significantly lower than those from LED-2, LED-3, and WF (Figure 6B and Figure 8A,B). The culture media did not affect root number during acclimatization, but it showed significant differences in root length (p < 0.001). Plantlets from the VW medium produced the highest root length, followed by the ½ MS medium and MS medium (Figure 8C,D).
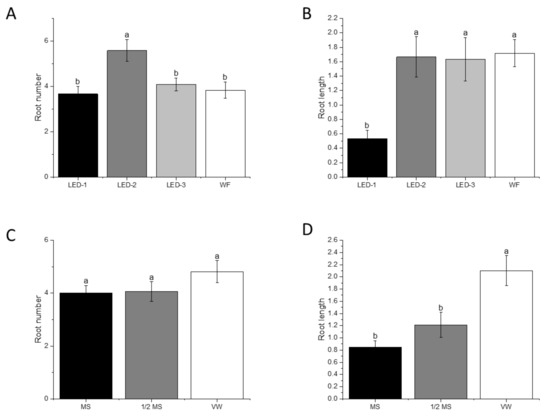
Figure 8.
Effects of light sources and culture media on root number (A,C), and root length (cm) (B,D) of B. nodosa plantlets during acclimatization for 30 days. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Bars indicate mean ± SE. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
Plantlets from LED-1 had the lowest shoot length (p < 0.001), fresh weight (p < 0.001), and dry weight (p < 0.001) compared to plantlets from other treatments (Figure 6B and Figure 9A–C). Plantlets from LED-2 showed the highest fresh and dry weight, followed by WF, LED-3, and LED-1 (Figure 6B and Figure 9A). Plantlets previously cultured in VW medium showed the highest shoot length (p < 0.001), fresh weight (p < 0.001), and dry weight (p < 0.001) compared to the plantlets derived from other media treatments (Figure 9D–F).
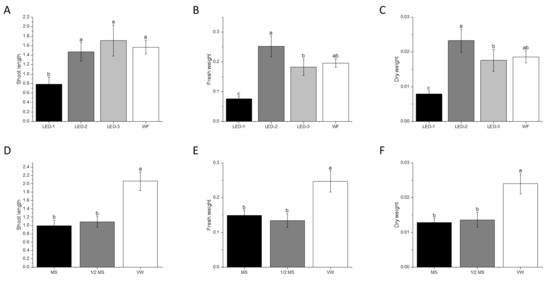
Figure 9.
Effects of light sources and culture media on shoot length (g) (A,D), fresh weight (g) (B,E), and dry weight (g) (C,F) of B. nodosa during acclimatization for 30 days. LED-1: Light-emitting diode (LED) light with 1575 ± 50 μmol m−2 s−1 Photosynthetic Photon Flux Density (PPFD); LED-2: LED light with 1015 ± 50 μmol m−2 s−1 PPFD; LED-3: LED light with 80 ± 5 μmol m−2 s−1 PPFD; and WF: white fluorescent light with 50 ± 5 μmol m−2 s−1 PPFD. MS: MS basal medium supplemented with 30 g L−1 sucrose and 7 g L−1 agar; ½ MS: half-strength MS basal medium supplemented with 15 g L−1 sucrose and 7 g L−1; VW: VW basal medium supplemented with 10% coconut water, 20 g L−1 sucrose and 7 g L−1. Bars indicate mean ± SE. Different letters indicate significant differences by Tukey’s test at p ≤ 0.05.
4. Discussion
Light intensity and quality play an important role in micropropagation, with some studies have indicated significant effects of light on Cattleya and Vanilla orchids [32,33]. It has also been reported that the red spectrum emitted from LEDs promotes leaf growth in Cymbidium orchids in vitro, although it decreases chlorophyll content, and that this effect can be reversed by the blue spectrum emitted from LEDs [34]. Similarly, in our study, LED-3, which contained a high percentage of red LEDs, yielded higher leaf length, but lower chlorophyll content compared to WF. This contrasted with values of lower leaf length, leaf width, and chlorophyll content for plants under LED-1 (1575 μmol m−2 s−1) and LED-2 (1018 μmol m−2 s−1), which had higher light intensity than LED-3 (77 μmol m−2 s−1) and WF (45 μmol m−2 s−1). The chlorophyll content is related to the ability of plants to photosynthesize, whereas higher chlorophyll content improves plant performance [35]. However, light intensities higher than 90 μmol m−2 s−1 result in a negative impact on plantlet performance, as chlorophyll content can be reduced by photo-inhibition or photo-oxidation [35]. In our study, LED-1 and LED-2 resulted in higher leaf numbers, which conflicted with previous findings [36,37]. This could be explained by the spectrum distribution, whereas both LED-1 and LED-2 had a ratio of about 3:1 red:blue light, providing a proper balance for plant growth and development.
The culture medium also influenced some parameters of in vitro plant growth and development. Although no differences were observed for leaf number among the different culture media, the VW medium showed higher values for leaf length and width, root number and length, chlorophyll content, and fresh and dry weight. The low nitrate content of the WV medium is considered more suitable for epiphytic orchids, such as B. nodosa [19].
Rooting of in vitro shoots was observed under all treatments without the use of plant growth regulators. This implies time and cost savings for the commercial micropropagation of B. nodosa, whereas transfer to a rooting medium is not required, thus increasing the protocol efficiency. Although limited studies exist, a similar response has been reported, showing that adventitious roots formed during the in vitro multiplication of Guarianthe skinneri, the orchid considered the national flower of Costa Rica [38].
Although all treatments resulted in 100% ex vitro survival during acclimatization, a significant difference in plant growth and development was still observed between treatments, likely resulting from carry-over effects from the culture media and/or light source used during in vitro multiplication. Plantlets regenerated from the VW medium showed increased values for most growth and development parameters, including leaf length, root length, fresh weight, and dry weight, but not for root number. This adds confirmation to the fact the WV medium seems to be more suitable for epiphytic orchids, as reported previously [19].
The quality of light could also affect the plant’s acclimatization process [39]. Plantlets that were cultured in vitro under the highest light intensity (LED-1) showed the lowest growth and development parameters ex vitro, except for leaf number, where no differences were observed. However, plantlets that were previously cultured under LED-2 showed higher values for growth and development, including root number, fresh weight, and dry weight as compared to plantlets from other light sources after being transferred to an ex vitro environment. The increased light level in the greenhouse could also have accounted for this effect, as reported previously [40]. Although we did not evaluate the light levels of the greenhouse, plantlets that were previously in vitro under a high light intensity showed better performance and acclimatization in the greenhouse. Plantlets that were previously under LED-3, which contained a high percentage of red light, did not show significant growth and development during acclimatization. Therefore, future evaluations of high light intensity combined with a high percentage of red light might be necessary for elucidating the effects of selective lighting for micropropagation as well as for the acclimatization of B. nodosa orchids.
5. Conclusions
This is the first study reporting the effects of culture media and light sources on the micropropagation of B. nodosa hybrid orchids. We demonstrated that the VW medium is more suitable for micropropagation of B. nodosa compared to the MS medium and ½ MS medium. Light sources with lower intensity, such as LED-3 (80 μmol m−2 s−1 PPFD) resulted in the best plant performance in vitro, while LED-2 (1015 μmol m−2 s−1 PPFD) showed the best plant performance ex vitro. Furthermore, this study also revealed that during micropropagation of B. nodosa, a rooting phase is not required, resulting in time and cost savings when considering the potential commercial micropropagation of this species and its hybrids. Therefore, our study provided important information for developing an efficient protocol for the micropropagation of B. nodosa toward efficient commercial production.
Author Contributions
Conceptualization, W.A.V.; methodology, W.A.V. and J.X.; validation, W.A.V., J.X., and D.B.; formal analysis, J.X.; investigation, J.X.; resources, W.A.V. and D.B.; data curation, J.X.; writing—original draft preparation, J.X.; writing—review and editing, W.A.V. and D.B.; visualization, J.X.; supervision, W.A.V. and D.B.; project administration, W.A.V. and D.B.; funding acquisition, W.A.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work is supported by the USDA National Institute of Food and Agriculture, Hatch project 1012202.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roberts, D.L.; Dixon, K.W. Orchids. Curr. Biol. 2008, 18, 325–329. [Google Scholar] [CrossRef] [Green Version]
- Arditti, J. Fundamentals of Orchid Biology; John Wiley & Sons: New York, NY, USA, 1992; pp. 1–50. [Google Scholar]
- Jones, H.G. Nomenclatural revision of the genus Brassavola R. Br. of the Orchidaceae. Ann. Nat. Mus. Wien. 1975, 79, 9–22. [Google Scholar]
- Mata-Rosas, M.; Lastre-Puertos, E. Long-term conservation of protocorms of Brassavola nodosa (L) Lind. (Orchidaceae): Effect of ABA and a range of cryoconservation techniques. Cryoletters 2015, 36, 289–298. [Google Scholar] [PubMed]
- Mengarda, L.H.G.; Cola, G.P.A.; Oliveira, S.C.; Freitas, A.R. Multiplication, rooting in vitro, and acclimatization of Brassavola tuberculata Hook. (Orchidaceae), an orchid endemic to the Brazilian Atlantic rainforest. Biosci. J. 2017, 33, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hortic. 2009, 122, 507–520. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, M. In vitro plant propagation: A review. J. For. Environ. Sci. 2011, 27, 61–72. [Google Scholar]
- Bhoite, H.A.; Palshikar, G.S. Plant tissue culture: A review. World J. Pharm. Sci. 2014, 2, 565–572. [Google Scholar]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. In Vitro Cell. Dev. Biol. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Devi, J.; Borthakur, B.; Deka, P. Clonal propagation of Dendrobium moschatum and Cymbidium aloifolium through shoot tip culture. J. Orchid. Soc. India 1997, 11, 19–21. [Google Scholar]
- Sharon, M.; Vasundhara, G. Micropropagation of Dendrobium Joannie Osterholt. J. Orchid. Soc. India 1990, 4, 145–148. [Google Scholar]
- Tokuhara, K.; Mii, M. Micropropagation of Phalaenopsis and Doritaenopsis by culturing shoot tips of flower stalk buds. Plant Cell Rep. 1993, 13, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, K.; Senthilkumar, R.; Murugalatha, N. Regeneration and mass multiplication of Vanilla planifolia Andr.—A tropical orchid. Curr. Sci. 2006, 91, 1401–1403. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Faria, R.T.; Santiago, D.C.; Saridakis, D.P.; Albino, U.B.; Araújo, R. Preservation of the Brazilian orchid Cattleya walkeriana Gardner using in vitro propagation. Crop Breed. App. Biotechnol. 2002, 2, 489–492. [Google Scholar] [CrossRef]
- Moraes, L.; Faria, R.T.; Cuquel, F.L. Activated charcoal for in vitro propagation of Brazilian orchids. Acta Hortic. 2003, 683, 383–390. [Google Scholar] [CrossRef]
- Vacin, E.F.; Went, F. Some pH changes in nutrient solutions. Bot. Gaz. 1949, 110, 605–613. [Google Scholar] [CrossRef]
- Young, P.; Murthy, H.N.; Kee, Y.P. Mass multiplication of protocorm-like bodies using bioreactor system and subsequent plant regeneration in Phalaenopsis. Plant Cell Tissue Org. Cult. 2000, 63, 67–72. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Chan, C.; Stahl, C.; Yeung, E.C. Recent advances in orchid seed germination and micropropagation. In Orchid Propagation: From Laboratories to Greenhouses—Methods and Protocols; Lee, Y.-I., Yeung, E.C.-T., Eds.; Springer: New York, NY, USA, 2018; pp. 497–520. [Google Scholar]
- Sivakumar, G.; Heo, J.W.; Kozai, T.; Paek, K.Y. Effect of continuous or intermittent radiation on sweet potato plantlets in vitro. J. Hortic. Sci. Biotechnol. 2006, 81, 546–548. [Google Scholar] [CrossRef]
- Fujiwara, K.; Kozai, T. Physical microenvironment and its effects. In Automation and Environmental Control in Plant Tissue Culture; Aitken-Christie, J., Kozai, T., Smith, M.A.L., Eds.; Springer: Dordrecht, The Netherlands, 1995; pp. 319–369. [Google Scholar]
- Rajapakse, N.C.; Shahak, Y. Light-quality manipulation by horticulture industry. Ann. Plant Rev. Light Plant Dev. 2008, 30, 290. [Google Scholar]
- Faria, D.V.; Correia, L.N.F.; Souza, M.V.C.; Ríos-Ríos, A.M.; Vital, C.E. Irradiance and light quality affect two annatto (Bixa orellana L.) cultivars with contrasting bixin production. J. Photochem. Photobiol. 2019, 197, 111549. [Google Scholar] [CrossRef]
- Seabrook, J.E.A. Light effects on the growth and morphogenesis of potato (Solanum tuberosum) in vitro: A review. Am. J. Potato Res. 2005, 82, 353–367. [Google Scholar] [CrossRef]
- Dooley, J. Influence of lighting spectra on plant tissue culture. Ann. Am. Soc. Agric. Eng. 1991, 8, 917530. [Google Scholar]
- Miyashita, Y.; Kitaya, Y.; Kozai, T.; Kimura, T. Effects of red and far-red light on the growth and morphology of potato plantlets in vitro: Using light emitting diode as a light source for micropropagation. Environ. Effect Control Plant Tissue Cult. 1994, 393, 189–194. [Google Scholar] [CrossRef]
- Kodym, A.; Zapata-Arias, F.J. Natural light as an alternative light source for the in vitro culture of banana (Musa acuminata cv. ‘Grande Naine’). Plant Cell Tissue Org. Cult. 1998, 55, 141–145. [Google Scholar] [CrossRef]
- Mitchell, C. Plant lighting in controlled environments for space and earth applications. Acta Hortic. 2012, 956, 23–36. [Google Scholar] [CrossRef]
- Gupta, S.D.; Jatothu, B. Fundamentals and applications of light-emitting diodes (LEDs) in in vitro plant growth and morphogenesis. Plant Biotechnol. Rep. 2013, 7, 211–220. [Google Scholar] [CrossRef]
- Bello-Bello, J.J.; Martínez-Estrada, E.; Caamal-Velázquez, J.H.; Morales-Ramos, V. Effect of LED light quality on in vitro shoot proliferation and growth of vanilla (Vanilla planifolia Andrews). Afr. J. Biotechnol. 2016, 15, 272–277. [Google Scholar]
- Ferreira, L.T.; Silva, M.M.A.; Ulisses, C.; Camara, T.R.; Willadino, L. Using LED lighting in somatic embryogenesis and micropropagation of an elite sugarcane variety and its effect on redox metabolism during acclimatization. Plant Cell Tissue Org. Cult. 2017, 128, 211–221. [Google Scholar] [CrossRef]
- Cybularz-Urban, T.; Hanus-Fajerska, E.; Swiderski, A. Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Cracov. 2007, 49, 113–118. [Google Scholar]
- Ramírez-Mosqueda, M.A.; Iglesias-Andreu, L.G.; Luna-Sánchez, I.J. Light quality affects growth and development of in vitro plantlet of Vanilla planifolia Jacks. S. Afr. J. Bot. 2017, 109, 288–293. [Google Scholar] [CrossRef]
- Tanaka, M.; Takamura, T.; Watanabe, H.; Endo, M.; Yanagi, T.; Okamoto, K. In vitro growth of Cymbidium plantlets cultured under superbright red and blue light-emitting diodes (LEDs). J. Hortic. Sci. Biotechnol. 1998, 73, 39–44. [Google Scholar] [CrossRef]
- Streit, N.M.; Canterle, L.P.; Canto, M.W.D.; Hecktheuer, L.H.H. The Chlorophylls. Ciênc. Rural 2005, 35, 748–755. [Google Scholar] [CrossRef]
- Dewir, Y.H.; El-Mahrouk, M.E.; Murthy, H.N.; Paek, K.Y. Micropropagation of Cattleya: Improved in vitro rooting and acclimatization. Hortic. Environ. Biotechnol. 2015, 56, 89–93. [Google Scholar] [CrossRef]
- Soontornchainaksaeng, P.; Chaicharoen, S.; Sirijuntarut, M.; Kruatrachue, M. In vitro studies on the effect of light intensity on plant growth of Phaius tankervilliae (Banks ex L’Herit.) Bl. and Vanda coerulea Griff. Sci. Asia 2001, 27, 233–237. [Google Scholar] [CrossRef]
- Leyva-Ovalle, O.R.; Bello-Bello, J.J.; Murguía-González, J.; Núñez-Pastrana, R.; Ramírez-Mosqueda, M.A. Micropropagation of Guarianthe skinneri (Bateman) Dressler et W. E. Higging in temporary immersion systems. 3 Biotech 2020, 10, 26. [Google Scholar] [CrossRef] [PubMed]
- Tan Nhut, D.; Takamura, T.; Watanabe, H.; Tanaka, M. Artificial light source using light-emitting diodes (LEDs) in the efficient micropropagation of Spathiphyllum plantlets. In II International Symposium on Biotechnology of Tropical and Subtropical Species 692; Interenational Society for Horticultural Science: Taipei, Taiwan, 2001; pp. 137–142. [Google Scholar]
- Preece, J.E.; Sutter, E.G. Acclimatization of micropropagated plants to the greenhouse and field. In Micropropagation: Technology and Application; Debergh, P.C., Zimmerman, R.H., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 71–93. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).