Irrigation Levels and Fertilization Rates as Pre-Harvest Factors Affecting the Growth and Quality of Hippeastrum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment 1: Effect of Fertilization Rates on Growth and Water Use Efficiency of Hippeastrum
- 1.
- Evapotranspiration rate (ET)
- 2.
- Crop evapotranspiration (ETc)
- 3.
- Water use efficiency (WUE)
- 4
- Crop coefficient (Kc)
2.2. Experiment 2: Effect of Irrigation Level and Fertilizer Rate on Growth and Yield of Hippeastrum
2.3. Statistical Analysis
3. Results
3.1. Experiment 1: Effect of Fertilization Rates on Growth and Water Use Efficiency of Hippeastrum
3.2. Experiment 2: Effect of Irrigation Level and Fertilizer Rate on Growth and Yield of Hippeastrum
4. Discussion
4.1. Experiment 1: Effect of Fertilization Rates on Growth and Water Use Efficiency of Hippeastrum
4.2. Experiment 2: Effect of Irrigation Level and Fertilization Rate on Growth and Yield of Hippeastrum
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bayer, A.; Whitaker, K.; Chappell, M.; Ruter, J.; van Iersel, M.W. Effect of irrigation duration and fertilizer rate on plant growth, substrate solution EC and leaching volume. Acta Hortic. 2014, 1034, 477–484. [Google Scholar] [CrossRef]
- Scherer, T.F.; Franzen, D.; Cihacek, L. Soil, Water and Plant Characteristics Important to Irrigation. NDSU Extension Service. Available online: https://www.ag.ndsu.edu/publications/crops/soil-water-and-plant-characteristics-important-to-irrigation (accessed on 13 October 2021).
- Edwards, C.E.; Ewers, B.E.; McClung, C.R.; Lou, P.; Weinig, C. Quantitative Variation in water-use efficiency across water regimes and its relationship with circadian, vegetative, reproductive, and leaf gas-exchange traits. Mol. Plant 2012, 5, 653–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chen, D.; He, X.; Shen, J.; Xiong, M.; Wang, X.; Zhou, D.; Wei, Z. Revealing the complex genetic structure cultivated amaryllis (Hippeastrum hybridum) using transcriptome-derive microsatellite markers. Sci. Rep. 2018, 8, 10645. Available online: https://www.nature.com/articles/s41598-018-28809-9 (accessed on 13 October 2021). [CrossRef] [PubMed] [Green Version]
- AIPH. International Statistics Flowers and Plants; International Association of Horticultural Producers: Oxford, UK, 2020; Volume 68, Available online: www.aiph.org/statistical-yearbook (accessed on 13 October 2021).
- Okubo, H. Hippeastrum (Amaryllis). In The Physiology of Flower Bulb; Hertogh, A.D., Le Nard, M., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 321–334. [Google Scholar]
- Jerry, L.; Christian, D. Water-Use Efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Pereira, J.S.; Kozlowski, T.T. Leaf anatomy and water relations of Eucalyptus camaldulensis and E. globulus seedlings. Can. J. Bot. 1976, 54, 2868–2880. [Google Scholar] [CrossRef]
- Turner, N.C. Crop Water Deficits: A Decade of Progress. Adv. Agron. 1986, 39, 1–51. [Google Scholar] [CrossRef]
- Allen, R. Penman–Moteith Equation. In Encyclopedia of Soils in the Environment; Elsevier B.V.: Amsterdam, The Netherlands, 2005; pp. 180–188. [Google Scholar]
- Vudhivanich, V. Monthly potential evapotranspiration of Thailand. Kasetsart J. (Nat. Sci.) 1996, 30, 392–399. [Google Scholar]
- Roberts, A.N.; Blaney, L.T. Growth and development of the Easter lily bulb, Lilium longiflorum Thunb. ‘Croft’. Proc. Am. Soc. Hortic. Sci. 1996, 89, 643–650. [Google Scholar]
- Anuwong, C.; Ohyama, T.; Sueyoshi, K.; Ohtake, N.; Sato, T.; Ruamrungsri, S. Uptake and translocation of nitrogen in patumma (Curcuma alismatifolia) by leaves or root. J. Plant Nutr. 2017, 40, 1204–1212. [Google Scholar] [CrossRef]
- Slangen, J.H.G.; Krook, G.J.; Hendriks, C.H.M.; Hof, N.A.A. Nitrogen dressing and nutrient absorption of lilies (Asiatic hybrids) on sandy soils. Neth. J. Agric. Sci. 1989, 37, 269–272. [Google Scholar] [CrossRef]
- Batal, K.M.; Bondari, K.; Granberry, D.M.; Mullinix, B.G. Effects of source, rate, and frequency of N application on yield, marketable grades and rot incidence of sweet onion (Allium cepa L. cv. Granex-33). J. Hortic. Sci. 1994, 69, 1043–1051. [Google Scholar] [CrossRef]
- Diaz-perez, P.J.; Purvis, A.C.; Paulk, J.T. Bolting, yield, and bulb decay of sweet onion as affected by nitrogen fertilization. J. Am. Soc. Hortic. Sci. 2003, 128, 144–149. [Google Scholar] [CrossRef] [Green Version]
- Tombolato, A.F.C. Cultivo Comercial de Plantas Ornamentais; Campinas Instituto Agronômico: Sao Paulo, Brazil, 2004; p. 211. [Google Scholar]
- Hongpakdee, P.; Ruamrungsri, S. Water use efficiency, nutrient leaching, and growth in potted marigolds affected by coconut coir dust amended in substrate media. Hortic. Environ. Biotechnol. 2015, 56, 27–35. [Google Scholar] [CrossRef]
- Drechsel, P.; Heffer, P.; Magen, H.; Mikkelsen, R.; Wichelns, D. Managing water and fertilizer for sustainable agricultural intensification. In Water Use Efficiency in Agriculture: Measurement, Current Situation and Trends; Sharma, B., Molden, D., Cook, S., Eds.; International Fertilizer Industry Association (IFA);: Paris, France; International Water Management Institute (IWMI): Colombo, Sri Lanka; International Plant Nutrition Institute (IPNI): Peachtree Corners, GA, USA; International Potash Institute (IPI): Zug, Switzerland, 2015; pp. 39–64. [Google Scholar]
- Tesfay, T.; Berhane, A.; Gebremariam, M. Optimizing irrigation water and nitrogen fertilizer levels for tomato production. Open Agric. J. 2019, 13, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Xu, H.; Mu, X.; Zhao, G.; Gao, P.; Sun, W. Effects of Different Fertilization Regimes on Crop Yield and Soil Water Use Efficiency of Millet and Soybean. Sustainability 2020, 12, 4125. [Google Scholar] [CrossRef]
- Brueck, H.; Senbayram, M. Low nitrogen supply decreases water-use efficiency of oriental tobacco. J. Plant Nutr. Soil Sci. 2009, 172, 216–223. [Google Scholar] [CrossRef]
- Eid, M.A.M.; Abdel-Salam, A.A.; Salem, H.M.; Mahrous, S.E.; Seleiman, M.F.; Alsadon, A.A.; Solieman, T.H.I.; Ibrahim, A.A. Interaction Effects of Nitrogen Source and Irrigation Regime on Tuber Quality, Yield, and Water Use Efficiency of Solanum tuberosum L. Plants 2020, 9, 110. [Google Scholar] [CrossRef] [Green Version]
- Inkham, C.; Hongpakdee, P.; Kajornrungsilp, I.; Thanamatee, C.; Ruamrungsri, S. Root-zone cooling by cold energy from LNG regasification process for quality improvement of flower and bulb of Hippeastrum. Hortic. Environ. Biotechnol. 2020, 61, 643–650. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef]
- Wallace, J.G.; Zhang, X.C.; Beyene, Y.; Semagn, K.; Olsen, M.; Prasanna, B.M.; Buckler, E.S. Genome-wide association for plant height and flowering time across 15 tropical maize populations under managed drought stress and well-watered conditions in sub-Saharan Africa. Crop Sci. 2016, 56, 2365–2378. [Google Scholar] [CrossRef] [Green Version]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Shirke, P.A.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef] [PubMed]
- Laza, R.C.; Bergman, B.; Vergara, B.S. Cultivar differences in growth and chloroplast ultrastructure in rice as affected by nitrogen. J. Exp. Bot. 1993, 44, 1643–1648. [Google Scholar] [CrossRef]
- McDowell, N.G.; Beerling, D.J.; Breshears, D.D.; Fisher, R.A.; Raffa, K.F.; Stitt, M. The interdependence of mechanisms underlying climate-driven vegetation mortality. Trends Ecol. Evol. 2011, 26, 523–532. [Google Scholar] [CrossRef]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate Forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, D.J.; Lemaire, G.; Gosse, G.; Cruz, P.; Draycott, A.; Neeteson, J.J. Decline in percentage N of C3 and C4 crops with in-creasing plant mass. Ann. Bot. 1990, 66, 425–436. [Google Scholar] [CrossRef] [Green Version]
- Bradford, K.J.; Hsiao, T.C. Physiological responses to moderate water stress. In Physiological Plant Ecology II. Water Relations and Carbon Assimilation; Lange, O.L., Nobel, P.S., Osmond, C.B., Ziegler, H., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 263–324. [Google Scholar]
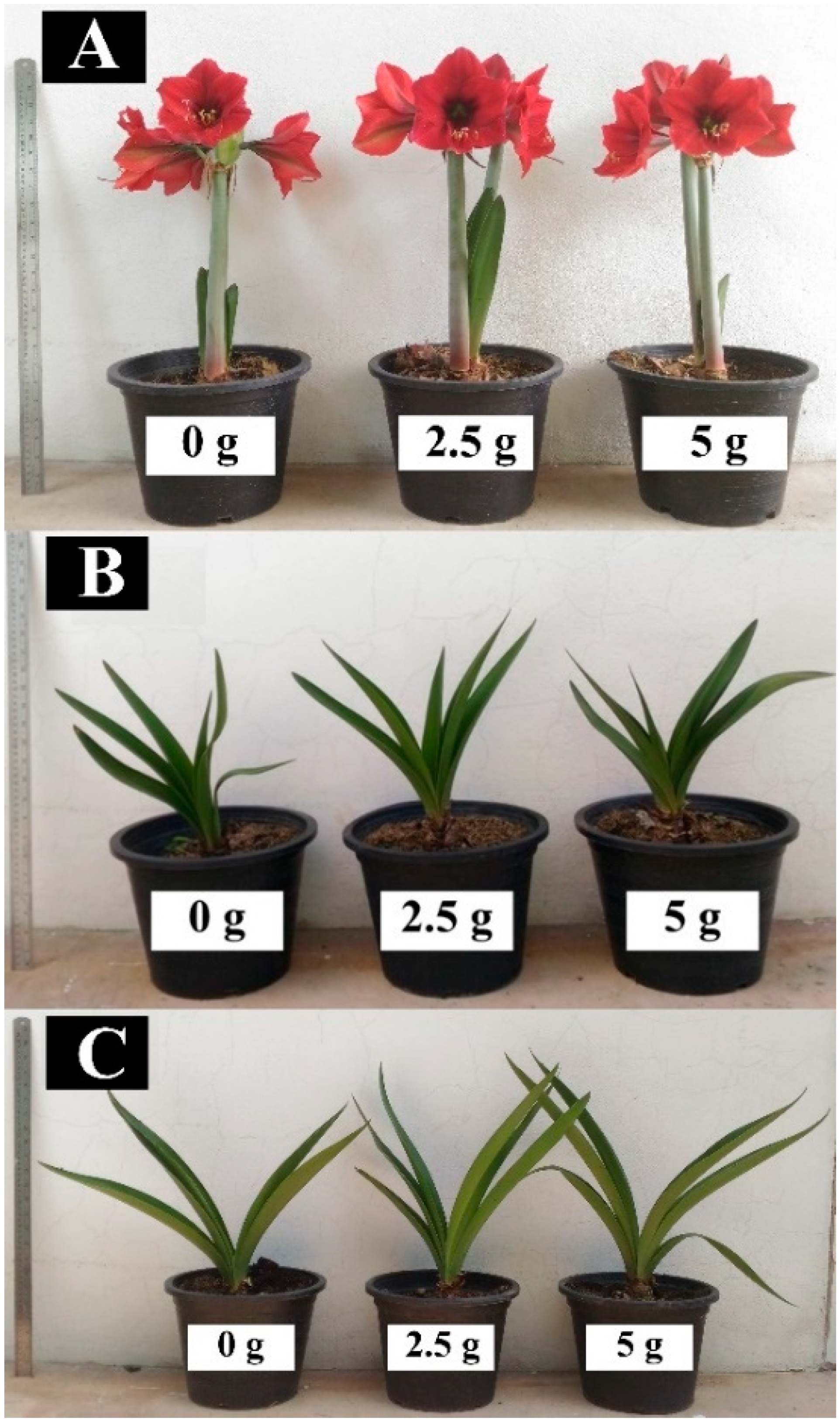



| Fertilization Rate (g per Plant per Month) | Plant Height (cm) | Number of Leaves per Plant | ||||
|---|---|---|---|---|---|---|
| 45 DAP | 60 DAP | 90 DAP | 45 DAP | 60 DAP | 90 DAP | |
| 0 g | NL | 31.5 | 42.6 b | NL | 4.1 | 4.8 b |
| 2.5 g | NL | 31.8 | 46.1 a | NL | 4.3 | 6.1 a |
| 5.0 g | NL | 30.8 | 47.1 a | NL | 4.1 | 6.6 a |
| LSD0.05 | - | NS | * | - | NS | * |
| Fertilizer Rate (g per Plant per Month) | Flower Quality (45 DAP) | Bulb Quality (180 DAP) | |||
|---|---|---|---|---|---|
| Flower Stalk Length (cm) | Flower Diameter (cm) | Bulb Circumference (cm) | Number of New Bulbs | Bulb Firmness (Newtons) | |
| 0 g | 31.8 | 12.8 | 24.0 b | 0.0 b | 2.3 b |
| 2.5 g | 32.2 | 13.1 | 28.0 a | 0.5 ab | 2.7 ab |
| 5.0 g | 32.3 | 12.7 | 28.5 a | 0.8 a | 2.9 a |
| LSD0.05 | NS | NS | * | * | * |
| Fertilizer Rate (g per Plant per Month) | Evapotranspiration Rate (ET; mm) | Water Use Efficiency (WUE; mg DW mL−1) | Crop Evapotranspiration (ETc; mm) | Crop Coefficient (Kc) |
|---|---|---|---|---|
| 45 days after planting | ||||
| 0 g | 205 | 7.88 × 10−5 | 121.3 | 2.91 |
| 2.5 g | 207.6 | 7.42 × 10−5 | 122.9 | 2.94 |
| 5.0 g | 200 | 2.11 × 10−4 | 118.4 | 2.83 |
| LSD0.05 | NS | NS | NS | NS |
| 60 days after planting | ||||
| 0 g | 100 | 6.95 × 10−4 | 59.2 | 1.2 |
| 2.5 g | 101 | 1.41 × 10−3 | 59.8 | 1.21 |
| 5.0 g | 98.4 | 6.95 × 10−4 | 58.2 | 1.18 |
| LSD0.05 | NS | NS | NS | NS |
| 90 days after planting | ||||
| 0 g | 148.2 b | 3.29 × 10−4 | 87.7 b | 1.42 b |
| 2.5 g | 163.6 ab | 3.42 × 10−4 | 96.8 ab | 1.57 ab |
| 5.0 g | 166.0 a | 5.91 × 10−4 | 98.2 a | 1.59 a |
| LSD0.05 | * | NS | * | * |
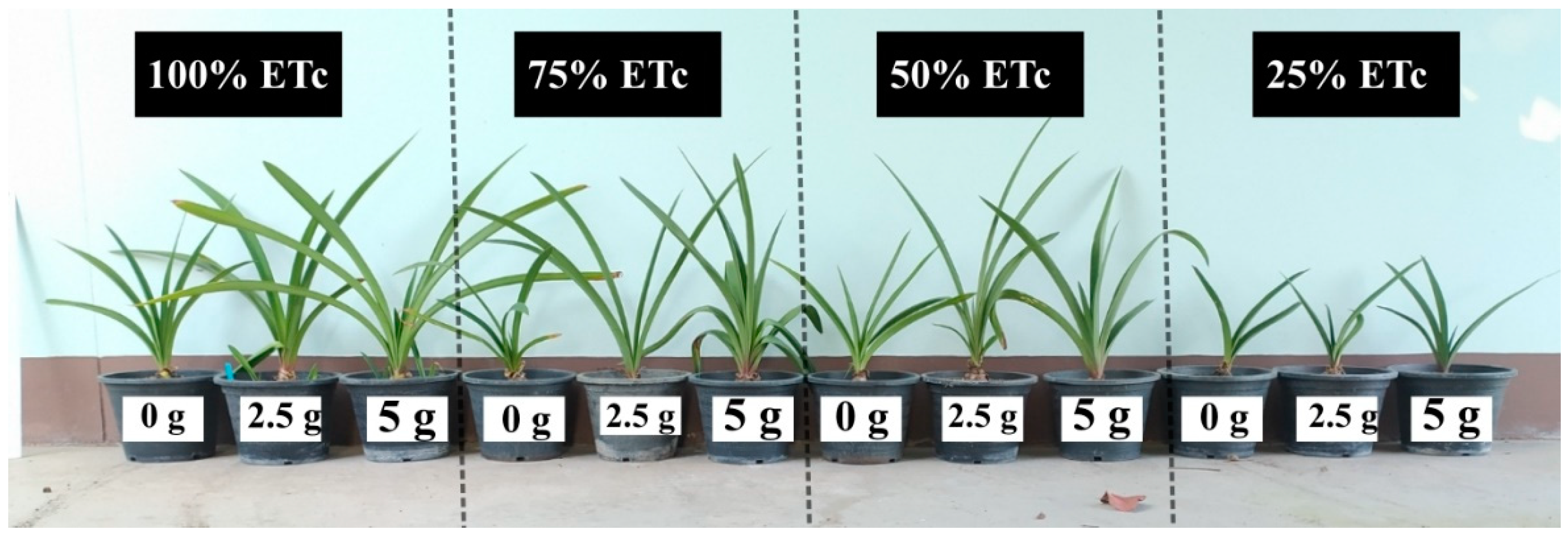
| Irrigation Levels (% ETc) | Fertilization Rates (g per Plant per Month) | Flower Quality (45 Days after Planting) | Flower Quality (45 Days after Planting) | Bulb Quality (180 Days after Planting) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Flower Diameter (cm) | Stalk Length (cm) | Plant Height (cm) | No. Shoots per Plant | No. Leaves per Plant | Leaf Color (SPAD) | Bulb Fresh Weight (g) | Bulb Circumference (cm) | Bulb Firmness (Newtons) | ||
| 100 | 0 | 11.9 | 12.7 | 50.3 b | 0.1 d | 6.7 bc | 50.1 c | 274.9 d | 25.2 bc | 2.53 b |
| 2.5 | 13 | 14.7 | 63.3 a | 1.4 ab | 8.0 ab | 58.4 ab | 426.1 b | 29.3 a | 2.60 b | |
| 5 | 12.2 | 13.7 | 63.5 a | 2.0 a | 8.2 a | 63.8 a | 511.5 a | 31.9 a | 2.70 ab | |
| 75 | 0 | 12.5 | 13 | 50.0 b | 0.1 d | 6.6 bc | 49.1 c | 220.3 f | 24.5 bc | 2.66 ab |
| 2.5 | 11.8 | 12.1 | 60.3 a | 1.1 ab | 6.8 bc | 59.1 ab | 255.4 e | 24.0 cd | 2.60 b | |
| 5 | 11.9 | 14.4 | 60.8 a | 1.2 ab | 7.0 abc | 55.6 bc | 401.5 c | 28.1 ab | 2.70 ab | |
| 50 | 0 | 12.5 | 15.5 | 40.0 c | 0.1 d | 6.4 c | 56.1 bc | 175.1 h | 20.5 d | 2.76 ab |
| 2.5 | 12.4 | 13.1 | 50.0 b | 0.1 d | 6.5 c | 62.2 ab | 220.7 f | 23.2 cd | 2.73 ab | |
| 5 | 12.3 | 13.8 | 51.0 b | 1.0 bc | 6.8 bc | 56.3 abc | 226.8 f | 23.6 cd | 2.76 ab | |
| 25 | 0 | 12.8 | 13.2 | 35.3 c | 0.1 d | 6.3 c | 58.1 ab | 170.1 h | 20.2 d | 2.76 ab |
| 2.5 | 12.6 | 14.1 | 36.0 c | 0.1 d | 6.4 c | 54.8 bc | 200.6 g | 21.8 cd | 2.90 a | |
| 5 | 12.4 | 14 | 37.0 c | 0.5 bcd | 6.6 bc | 59.5 ab | 221.3 f | 23.1 cd | 2.73 ab | |
| Irrigation × Fertilizer | NS | NS | * | * | * | * | * | * | ||
| Individual factor analysis | ||||||||||
| Irrigation levels factor | NS | NS | * | * | * | NS | * | * | ||
| Fertilization rates factor | NS | NS | * | * | NS | * | * | * | ||
| Factors | Photosynthetic Rates (µmol m−2 s−1) | Stomatal Conductance (mol m−2 s−1) |
|---|---|---|
| Irrigation level (% ETC) | ||
| 100% ETc | 3.04 a | 0.031 ab |
| 75% ETc | 2.22 b | 0.037 a |
| 50% ETc | 1.37 c | 0.036 ab |
| 25% ETc | 1.30 c | 0.024 b |
| LSD0.05 | * | * |
| Fertilizer rate (g per plant per month) | ||
| 0 g | 1.43 b | 0.03 |
| 2.5 g | 2.12 a | 0.028 |
| 5.0 g | 2.41 a | 0.038 |
| LSD0.05 | * | NS |
| Irrigation level × Fertilizer rate | ||
| 100% ETc × 0.0 g | 2.14 b | 0.023 bc |
| 100% ETc × 2.5 g | 3.37 a | 0.023 bc |
| 100% ETc × 5.0 g | 3.62 a | 0.047 a |
| 75% ETc × 0.0 g | 1.23 bc | 0.033 abc |
| 75% ETc × 2.5 g | 1.73 bc | 0.037 abc |
| 75% ETc × 5.0 g | 3.71 a | 0.043 ab |
| 50% ETc × 0.0 g | 1.31 bc | 0.043 ab |
| 50% ETc × 2.5 g | 1.51 bc | 0.030 abc |
| 50% ETc × 5.0 g | 1.30 bc | 0.033 abc |
| 25% ETc × 0.0 g | 1.03 c | 0.020 c |
| 25% ETc × 2.5 g | 1.86 bc | 0.023 bc |
| 25% ETc × 5.0 g | 1.02 c | 0.030 abc |
| LSD0.05 | * | * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inkham, C.; Panjama, K.; Ruamrungsri, S. Irrigation Levels and Fertilization Rates as Pre-Harvest Factors Affecting the Growth and Quality of Hippeastrum. Horticulturae 2022, 8, 345. https://doi.org/10.3390/horticulturae8040345
Inkham C, Panjama K, Ruamrungsri S. Irrigation Levels and Fertilization Rates as Pre-Harvest Factors Affecting the Growth and Quality of Hippeastrum. Horticulturae. 2022; 8(4):345. https://doi.org/10.3390/horticulturae8040345
Chicago/Turabian StyleInkham, Chaiartid, Kanokwan Panjama, and Soraya Ruamrungsri. 2022. "Irrigation Levels and Fertilization Rates as Pre-Harvest Factors Affecting the Growth and Quality of Hippeastrum" Horticulturae 8, no. 4: 345. https://doi.org/10.3390/horticulturae8040345
APA StyleInkham, C., Panjama, K., & Ruamrungsri, S. (2022). Irrigation Levels and Fertilization Rates as Pre-Harvest Factors Affecting the Growth and Quality of Hippeastrum. Horticulturae, 8(4), 345. https://doi.org/10.3390/horticulturae8040345






