Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits
Abstract
:1. Introduction
2. Materials and Methods
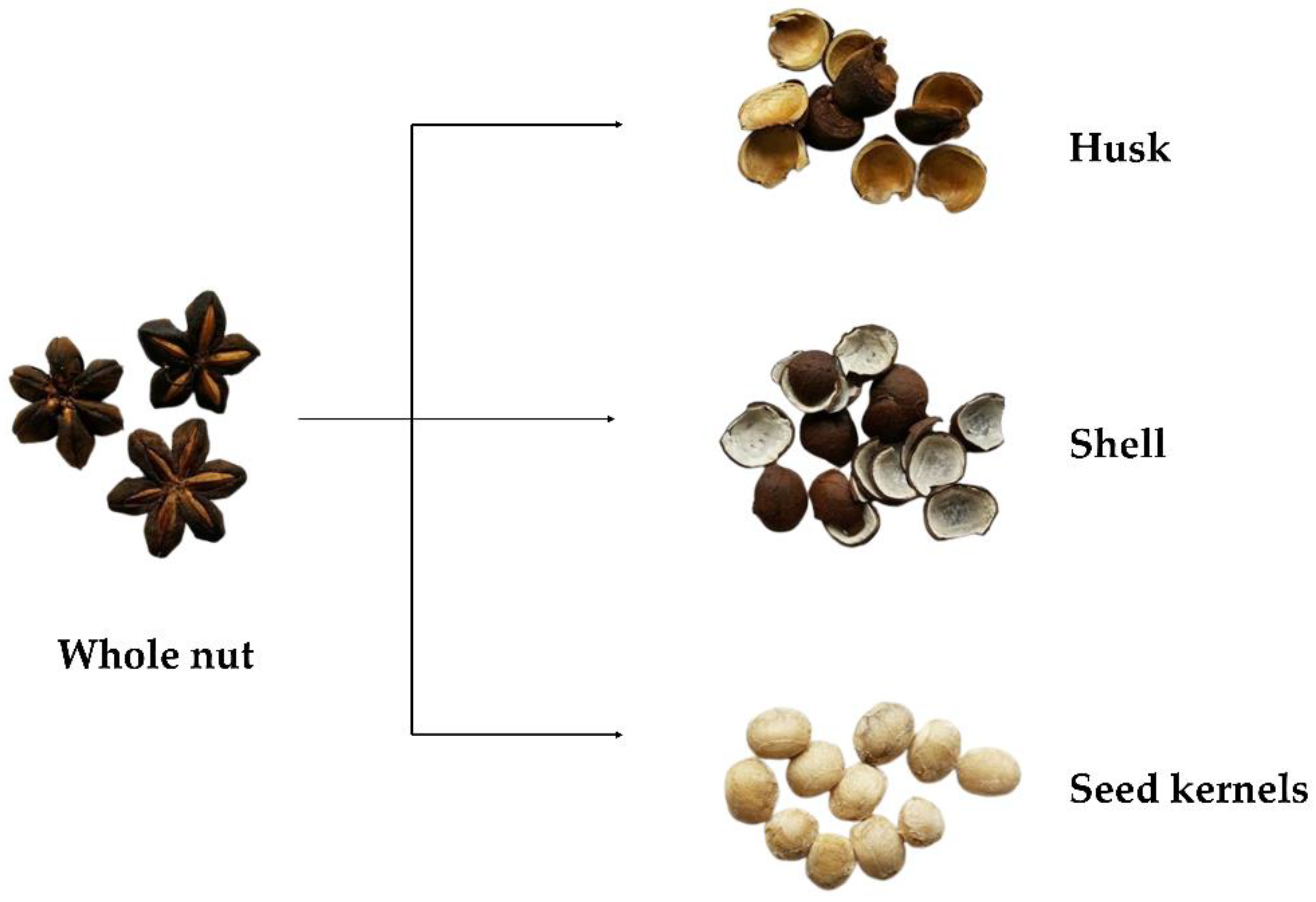
2.1. Sample Collection, Preparation, and Extraction
2.2. Determination of Nutritive Values
2.3. Determination of Fatty Acid Profile
2.4. Determination of Phenolic Profiles
2.5. Determination of Antioxidant Activities
2.6. Determination of Enzyme Inhibitory Activities
2.7. Statistical Analysis
3. Results
3.1. Nutritional Compositions
3.2. Fatty Acid Profiles
3.3. Phenolic Profiles
3.4. Antioxidant Activities
3.5. Enzyme Inhibitory Activities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kodahl, N.; Sørensen, M. Sacha inchi (Plukenetia volubilis L.) is an underutilized crop with a great potential. Agronomy 2021, 11, 1066. [Google Scholar] [CrossRef]
- Chirinos, R.; Necochea, O.; Pedreschi, R.; Campos, D. Sacha inchi (Plukenetia volubilis L.) shell: An alternative source of phenolic compounds and antioxidants. Int. J. Food Sci. Technol. 2016, 51, 986–993. [Google Scholar] [CrossRef]
- Kumar, B.; Smita, K.; Sánchez, E.; Stael, C.; Cumbal, L. Andean Sacha inchi (Plukenetia volubilis L.) shell biomass as new biosorbents for Pb2+ and Cu2+ ions. Ecol. Eng. 2016, 93, 152–158. [Google Scholar] [CrossRef]
- Rueda-Ordóñez, Y.J.; Mariño-Bohórquez, M.A.; Rueda-Ordóñez, D.A. Thermal upgrading of sacha inchi shell: Kinetics and combustion characteristics. Bioresour. Technol. Rep. 2021, 15, 100807. [Google Scholar] [CrossRef]
- Benítez, R.; Coronell, C.; Martin, J. Chemical characterizaction sacha inchi (Plukenetia volubilis) seed: Oleaginosa promising from the Colombian Amazon. Int. J. Curr. Sci. Res. Rev. 2018, 1, 11–22. [Google Scholar]
- Goyal, A.; Tanwar, B.; Sihag, M.K.; Sharma, V. Sacha inchi (Plukenetia volubilis L.): An emerging source of nutrients, omega-3 fatty acid and phytochemicals. Food Chem. 2022, 373, 131459. [Google Scholar] [CrossRef]
- de Souza, A.H.P.; Gohara, A.K.; Rodrigues, A.C.; de Souza, N.E.; Visentainer, J.V.; Matsushita, M. Sacha inchi as potential source of essential fatty acids and tocopherols: Multivariate study of nut and shell. Acta Sci. Technol. 2013, 35, 757–763. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Cai, X.; Xiao, N.; Ma, X.; Zeng, L.; Zhang, L.H.; Xie, L.; Du, B. Sacha inchi (Plukenetia volubilis L.) shell extract alleviates hypertension in association with regulation of gut microbiota. Food Funct. 2020, 11, 8051–8067. [Google Scholar] [CrossRef]
- Sainakham, M.; Mungmai, L. In vitro anti-oxidative activity and tyrosinase inhibition of Inca peanut (Plukenetia volubilis L.) shell extracts from different preparation methods. TJST 2020, 9, 407–417. [Google Scholar]
- Prasongsub, W.; Pimsan, N.; Buranapattarachote, C.; Punturee, K. Anti-HMG-CoA reductase and antioxidant activities of Sacha inchi (Plukenetia volubilis L.) nutshell extract. J. Assoc. Med. Sci. 2021, 54, 18–26. [Google Scholar]
- Latimer, G.W. Official Method of Analysis of AOAC International, 21st ed.; AOAC International: Rockville, ML, USA, 2019. [Google Scholar]
- Temviriyanukul, P.; Sritalahareuthai, V.; Promyos, N.; Thangsiri, S.; Pruesapan, K.; Srinuanchai, W.; Nuchuchua, O.; Siriwan, D.; On-nom, N.; Suttisansanee, U. The effect of sacred lotus (Nelumbo nucifera) and its mixtures on phenolic profiles, antioxidant activities, and inhibitions of the key enzymes relevant to Alzheimer’s disease. Molecules 2020, 25, 3713. [Google Scholar] [CrossRef] [PubMed]
- Hinkaew, J.; Sahasakul, Y.; Tangsuphoom, N.; Suttisansanee, U. The effect of cultivar variation on total phenolic contents and antioxidant activities of date palm fruit (Phoenix dactylifera L.). Curr. Res. Nutr. Food Sci. 2020, 8, 155–163. [Google Scholar] [CrossRef]
- Sripum, C.; Kukreja, R.K.; Charoenkiatkul, S.; Kriengsinyos, W.; Suttisansanee, U. The effect of extraction conditions on antioxidant activities and total phenolic contents of different processed Thai Jasmine rice. Int. Food Res. J. 2017, 24, 1644–1650. [Google Scholar]
- Sirichai, P.; Kittibunchakul, S.; Thangsiri, S.; On-Nom, N.; Chupeerach, C.; Temviriyanukul, P.; Inthachat, W.; Nuchuchua, O.; Aursalung, A.; Sahasakul, Y.; et al. Impact of drying processes on phenolics and in vitro health-related activities of indigenous plants in Thailand. Plants 2022, 11, 294. [Google Scholar] [CrossRef]
- Chupeerach, C.; Aursalung, A.; Watcharachaisoponsiri, T.; Whanmek, K.; Thiyajai, P.; Yosphan, K.; Sritalahareuthai, V.; Sahasakul, Y.; Santivarangkna, C.; Suttisansanee, U. The effect of steaming and fermentation on nutritive values, antioxidant activities, and inhibitory properties of tea leaves. Foods 2021, 10, 117. [Google Scholar] [CrossRef]
- FAO. Food energy: Methods of analysis and conversion factors. In Report of a Technical Workshop, Food and Nutrition; Agricultural Research Service, US Department of Agriculture: Beltsville, MD, USA, 2003; p. 77. [Google Scholar]
- Sanchez-Reinoso, Z.; Mora-Adames, W.I.; Fuenmayor, C.A.; Darghan-Contreras, A.E.; Gardana, C.; Gutiérrez, L.F. Microwave-assisted extraction of phenolic compounds from Sacha Inchi shell: Optimization, physicochemical properties and evaluation of their antioxidant activity. Chem. Eng. Process. Process Intensif. 2020, 153, 107922. [Google Scholar] [CrossRef]
- Boateng, J.; Verghese, M.; Walker, L.T.; Ogutu, S. Effect of processing on antioxidant contents in selected dry beans (Phaseolus spp. L.). LWT-Food Sci. Technol. 2008, 41, 1541–1547. [Google Scholar] [CrossRef]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids-ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef]
- Suttisansanee, U.; Thiyajai, P.; Chalermchaiwat, P.; Wongwathanarat, K.; Pruesapan, K.; Charoenkiatkul, S.; Temviriyanukul, P. Phytochemicals and in vitro bioactivities of aqueous ethanolic extracts from common vegetables in Thai food. Plants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Cao, X.; Xia, Y.; Zeng, M.; Wang, W.; He, Y.; Liu, J. Caffeic acid inhibits the formation of advanced glycation end products (AGEs) and mitigates the AGEs-induced oxidative stress and inflammation reaction in human umbilical vein endothelial cells (HUVECs). Chem. Biodivers. 2019, 16, e1900174. [Google Scholar] [CrossRef]
- Chen, H.; Virk, M.S.; Chen, F. Phenolic acids inhibit the formation of advanced glycation end products in food simulation systems depending on their reducing powers and structures. Int. J. Food. Sci. Nutr. 2016, 67, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, S.; Yerneni, K.K.; Scott, S.; Gonzales, N.; Lalezari, I. Novel inhibitors of advanced glycation endproducts (part II). Mol. Cell Biol. Res. Commun. 2000, 3, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Thilavech, T.; Sompong, W.; Pasukamonset, P. Interaction between ascorbic acid and gallic acid in a model of fructose-mediated protein glycation and oxidation. Electron. J. Biotechnol. 2017, 27, 32–36. [Google Scholar] [CrossRef]
- Istvan, E.S. Structural mechanism for statin inhibition of 3-hydroxy-3-methylglutaryl coenzyme A reductase. Am. Heart J. 2002, 144, S27–S32. [Google Scholar] [CrossRef]
- Buchholz, T.; Melzig, M.F. Polyphenolic compounds as pancreatic lipase inhibitors. Planta Med. 2015, 81, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Chauhan, S. Pancreatic lipase inhibitors: The road voyaged and successes. Life Sci. 2021, 271, 119115. [Google Scholar] [CrossRef]
- Giuberti, G.; Rocchetti, G.; Lucini, L. Interactions between phenolic compounds, amylolytic enzymes and starch: An updated overview. Curr. Opin. Food Sci. 2020, 31, 102–113. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic. Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Taslimi, P.; Gulçin, İ. Antidiabetic potential: In vitro inhibition effects of some natural phenolic compounds on α-glycosidase and α-amylase enzymes. J. Biochem. Mol. Toxicol. 2017, 31, e21956. [Google Scholar] [CrossRef]
- Tadera, K.; Minami, Y.; Takamatsu, K.; Matsuoka, T. Inhibition of α-glucosidase and α-amylase by flavonoids. J. Nutr. Sci. Vitaminol. 2006, 52, 149–153. [Google Scholar] [CrossRef] [Green Version]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Lee, S.; Hwang, E.S.; Maeng, S.; Park, J.H. p-Coumaric acid enhances long-term potentiation and recovers scopolamine-induced learning and memory impairments. Biochem. Biophys. Res. Commun. 2017, 492, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Moussa-Pacha, N.M.; Abdin, S.M.; Omar, H.A.; Alniss, H.; Al-Tel, T.H. BACE1 inhibitors: Current status and future directions in treating Alzheimer’s disease. Med. Res. Rev. 2020, 40, 339–384. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.H.; Sheng, R.; Hu, Y.Z. Progress in the development of nonpeptidomimetic BACE 1 inhibitors for Alzheimer’s disease. Curr. Med. Chem. 2009, 16, 1806–1820. [Google Scholar] [CrossRef]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. Biophenols: Enzymes (β-secretase, Cholinesterases, histone deacetylase and tyrosinase) inhibitors from olive (Olea europaea L.). Fitoterapia 2018, 128, 118–129. [Google Scholar] [CrossRef]
- Takahashi, T.; Miyazawa, M. Serotonin derivatives as inhibitors of β-secretase (BACE 1). Pharmazie 2011, 66, 301–305. [Google Scholar]
- Bhullar, K.S.; Lassalle-Claux, G.; Touaibia, M.; Rupasinghe, H.P.V. Antihypertensive effect of caffeic acid and its analogs through dual renin–angiotensin–aldosterone system inhibition. Eur. J. Pharmacol. 2014, 5, 125–132. [Google Scholar] [CrossRef]
- Al Shukor, N.; Van Camp, J.; Gonzales, G.B.; Staljanssens, D.; Struijs, K.; Zotti, M.J.; Raes, K.; Smagghe, G. Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: A study of structure activity relationships. J. Agric. Food Chem. 2013, 61, 11832–11839. [Google Scholar] [CrossRef]
- Murray, B.A.; FitzGerald, R.J. Angiotensin converting enzyme inhibitory peptides derived from food proteins: Biochemistry, bioactivity and production. Curr. Pharm. Des. 2007, 13, 773–791. [Google Scholar] [CrossRef]
- Barbosa-Filho, J.M.; Martins, V.K.M.; Rabelo, L.A.; Moura, M.D.; Silva, M.S.; Cunha, E.V.L.; Souza, M.F.V.; Almeida, R.N.; Medeiros, I.A. Natural products inhibitors of the angiotensin converting enzyme (ACE). A review between 1980–2000. Rev. Bras. Farmacogn. 2006, 16, 421–446. [Google Scholar] [CrossRef]
| Assay | Enzyme (Effectiveness, Source) | Substrate | Indicator | Detecting Wavelength |
|---|---|---|---|---|
| Lipase | ≥700 unit/mg (type VII), Candida rugosa | DNPDB | DTNB | 412 nm |
| AChE | 1000 units/mg, Electrophorus electricus | acetylthiocholine | ||
| BChE | ≥10 units/mg, equine serum | butyrylthiocholine | ||
| BACE-1 | a BACE-1 FRET assay kit | λex = 320 nm, λem = 405 nm | ||
| ACE | ≥2 unit/mg, rabbit lung | hippuryl-histidyl-leucine | PDA | λex = 360 nm, λem = 485 nm |
| α-Glucosidase | ≥10 U/mg protein (type I), Saccharomyces cerevisiae | p-nitrophenyl-α-d-glucopyranoside | 405 nm | |
| α-Amylase | ≥10 unit/mg (type VII), porcine pancreatic | p-nitrophenyl-α-d-maltopentaoside | ||
| Nutritive Values (Per 100 g Fresh Weight) | Parts of Sacha Inchi | |
|---|---|---|
| Husk | Shell | |
| Energy (kcal) | 421.47 ± 0.42 * | 552.89 ± 0.98 |
| Moisture (g) | 3.01 ± 0.01 * | 4.66 ± 0.04 |
| Protein (g) | 59.97 ± 0.06 * | 43.12 ± 0.36 |
| Total fat (g) | 11.25 ± 0.05 * | 37.87 ± 0.16 |
| Total carbohydrates (g) | 20.10 ± 0.07 * | 9.90 ± 0.47 |
| Dietary fiber (g) | 16.14 ± 0.16 * | 6.47 ± 0.27 |
| Ash (g) | 5.68 ± 0.03 * | 4.46 ± 0.01 |
| Fatty Acids (Per 100 g Fresh Weight) | Parts of Sacha Inchi | |||
|---|---|---|---|---|
| Husk | Shell | |||
| Amt (g) | % of TFC | Amt (g) | % of TFC | |
| Caprylic acid (C8:0) | 0.02 | 0.16 | ND | ND |
| Capric acid (C10:0) | 0.02 | 0.18 | ND | ND |
| Lauric acid (C12:0) | 0.15 | 1.43 | 0.07 | 0.20 |
| Myristic acid (C14:0) | 0.10 | 0.90 | 0.05 | 0.14 |
| Palmitic acid (C16:0) | 0.71 | 6.58 | 1.74 | 4.79 |
| Stearic acid (C18:0) | 0.45 | 4.14 | 1.18 | 3.26 |
| Oleic acid (C18:1) | 1.03 | 9.58 | 3.08 | 8.50 |
| Linoleic acid (C18:2n-6) | 4.11 | 38.19 | 14.17 | 39.15 |
| γ-Linoleic acid (C18:3n-6) | 0.002 | 0.02 | ND | ND |
| Linolenic acid (C18:3n-3) | 4.17 | 38.80 | 15.91 | 43.96 |
| Phenolics (mg/100 g DW) | Parts of Sacha Inchi | |
|---|---|---|
| Husk | Shell | |
| Flavonoids | ||
| Quercetin | 1.72 ± 0.06 | ND |
| Naringenin | ND | 29.21 ± 0.17 |
| Hesperidin | ND | 23.92 ± 1.55 |
| Kaempferol | 0.27 ± 0.01 * | 12.63 ± 0.45 |
| Isorhamnetin | 0.27 ± 0.01 | ND |
| Phenolic acids | ||
| Gallic acid | 2.51 ± 0.09 * | 49.13 ± 2.67 |
| 4-Hydroxybenzoic acid | 2.28 ± 0.13 * | 54.61 ± 3.04 |
| Chlorogenic acid | 2.16 ± 0.12 | ND |
| Vanillic acid | ND | 5.20 ± 0.15 |
| Caffeic acid | 4.44 ± 0.04 * | 16.21 ± 0.10 |
| Syringic acid | ND | 1.96 ± 0.08 |
| p-Coumaric acid | 2.51 ± 0.15 * | 148.74 ± 2.46 |
| Ferulic acid | 0.56 ± 0.04 * | 5.71 ± 0.09 |
| Sinapic acid | 0.92 ± 0.03 * | 17.41 ± 0.40 |
| Total phenolic content (mg GAE/100 g DW) | 323.74 ± 2.25 * | 503.96 ± 5.16 |
| Antioxidant Activities (µmol TE/100 g DW) | Parts of Sacha Inchi | |
|---|---|---|
| Husk | Shell | |
| FRAP activity | 100.28 ± 4.75 * | 180.17 ± 6.93 |
| ORAC activity | 4238.67 ± 89.15 * | 9751.06 ± 116.58 |
| DPPH radical scavenging activity | 0.03 ± 0.00 * | 0.08 ± 0.00 |
| Reaction Types | Inhibitory Activities (% Inhibition) | Parts of Sacha Inchi | |
|---|---|---|---|
| Husk | Shell | ||
| Enzymes | 1 Lipase | 23.80 ± 2.16 | ND |
| 2 ACE | 90.85 ± 3.42 * | 32.22 ± 2.59 | |
| 3 α-Amylase | 8.97 ± 1.36 * | 14.72 ± 1.47 | |
| 3 α-Glucosidase | 61.39 ± 3.04 * | 46.46 ± 2.54 | |
| 4 AChE | 28.84 ± 0.58 * | 51.88 ± 4.67 | |
| 4 BChE | 63.61 ± 2.96 * | 40.90 ± 3.13 | |
| 5 BACE-1 | 91.17 ± 2.50 | 94.83 ± 0.89 | |
| Chemicals | 6 MG-induced glycation | 29.43 ± 0.63 | 31.53 ± 3.40 |
| 6 Glucose-induced glycation | 47.67 ± 2.85 * | 55.81 ± 1.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittibunchakul, S.; Hudthagosol, C.; Sanporkha, P.; Sapwarobol, S.; Temviriyanukul, P.; Suttisansanee, U. Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits. Horticulturae 2022, 8, 344. https://doi.org/10.3390/horticulturae8040344
Kittibunchakul S, Hudthagosol C, Sanporkha P, Sapwarobol S, Temviriyanukul P, Suttisansanee U. Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits. Horticulturae. 2022; 8(4):344. https://doi.org/10.3390/horticulturae8040344
Chicago/Turabian StyleKittibunchakul, Suwapat, Chatrapa Hudthagosol, Promluck Sanporkha, Suwimol Sapwarobol, Piya Temviriyanukul, and Uthaiwan Suttisansanee. 2022. "Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits" Horticulturae 8, no. 4: 344. https://doi.org/10.3390/horticulturae8040344
APA StyleKittibunchakul, S., Hudthagosol, C., Sanporkha, P., Sapwarobol, S., Temviriyanukul, P., & Suttisansanee, U. (2022). Evaluation of Sacha Inchi (Plukenetia volubilis L.) By-Products as Valuable and Sustainable Sources of Health Benefits. Horticulturae, 8(4), 344. https://doi.org/10.3390/horticulturae8040344






