Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media
Abstract
:1. Introduction
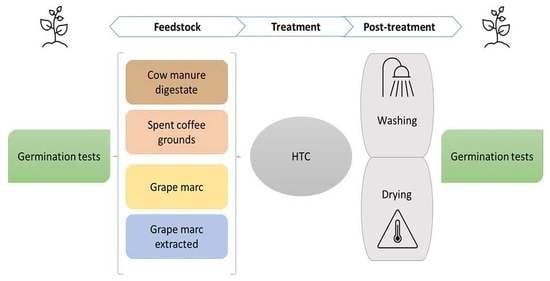
2. Materials and Methods
2.1. Feedstocks and HTC by-Products
2.2. Products Characterization
2.3. Germination Tests
2.3.1. Contact Method for Solid Substrates
2.3.2. Extraction Method for Liquid Substrates
2.3.3. Statistical Analyses
3. Results and Discussion
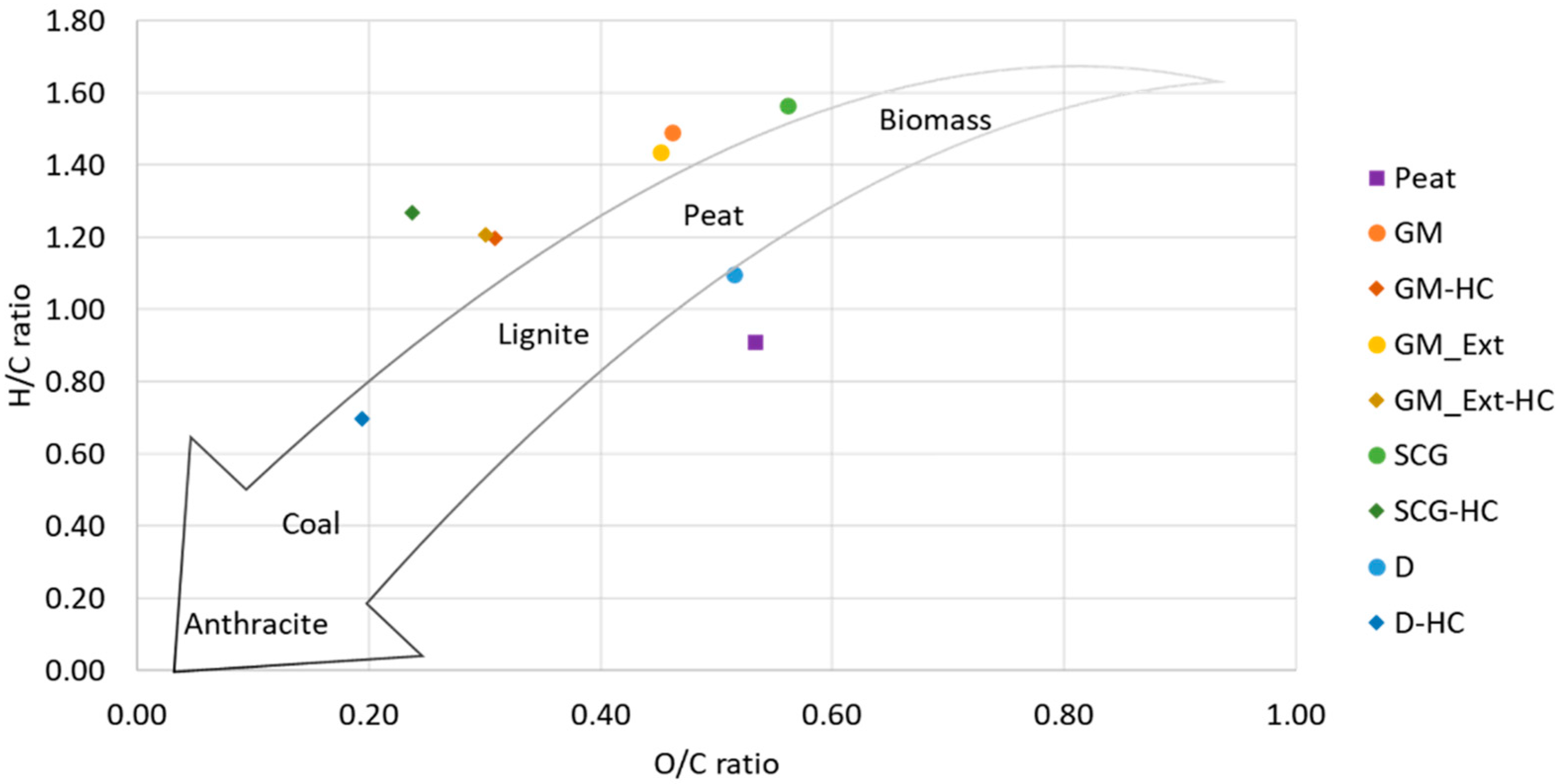
3.1. Properties of Selected Substrates: Feedstocks, Hydrochar, and Process Water
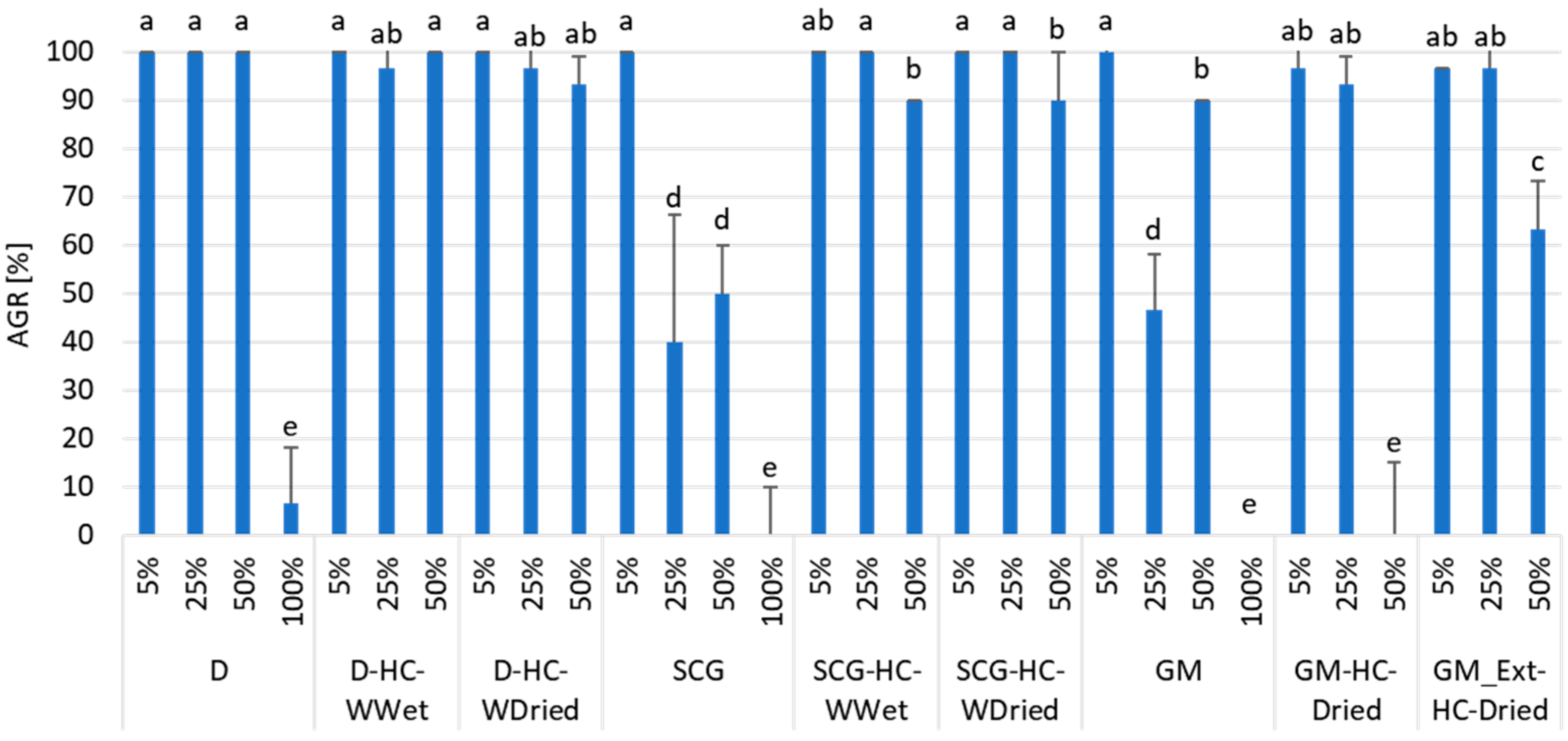
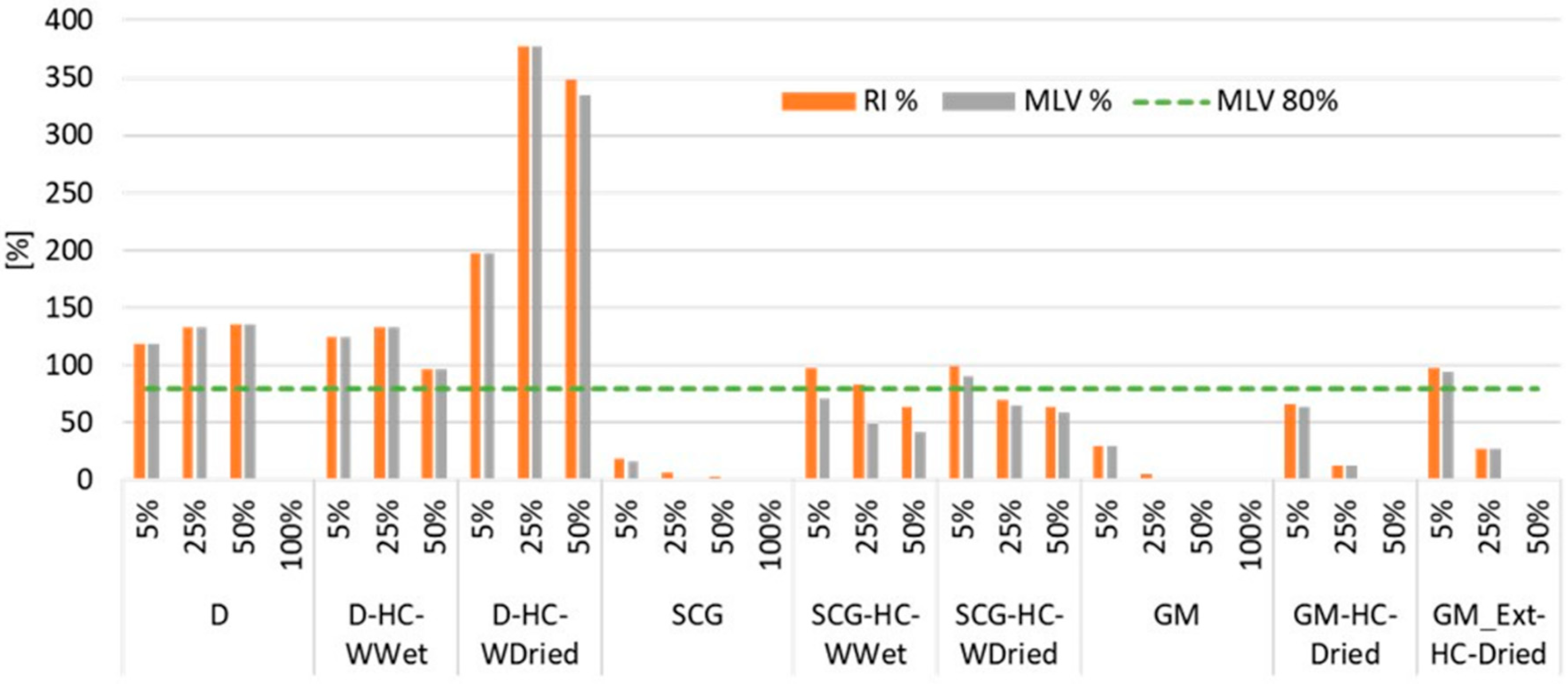
3.2. Effect of Feedstocks and Hydrochars on Seed Germination and Root Length
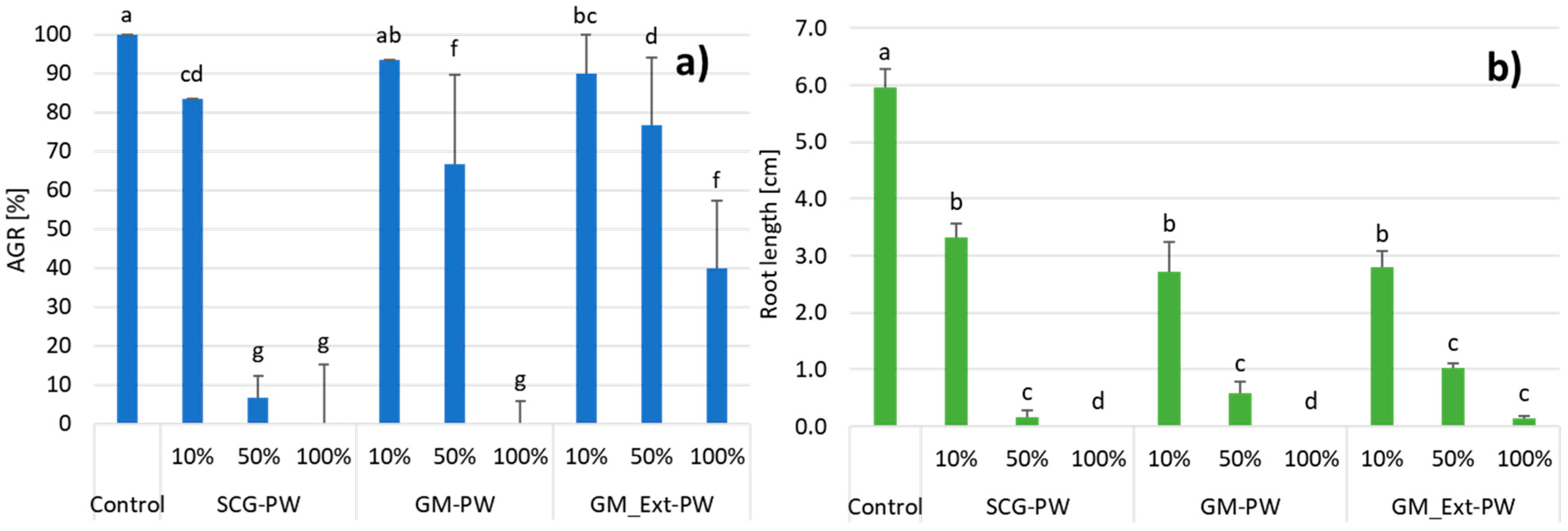
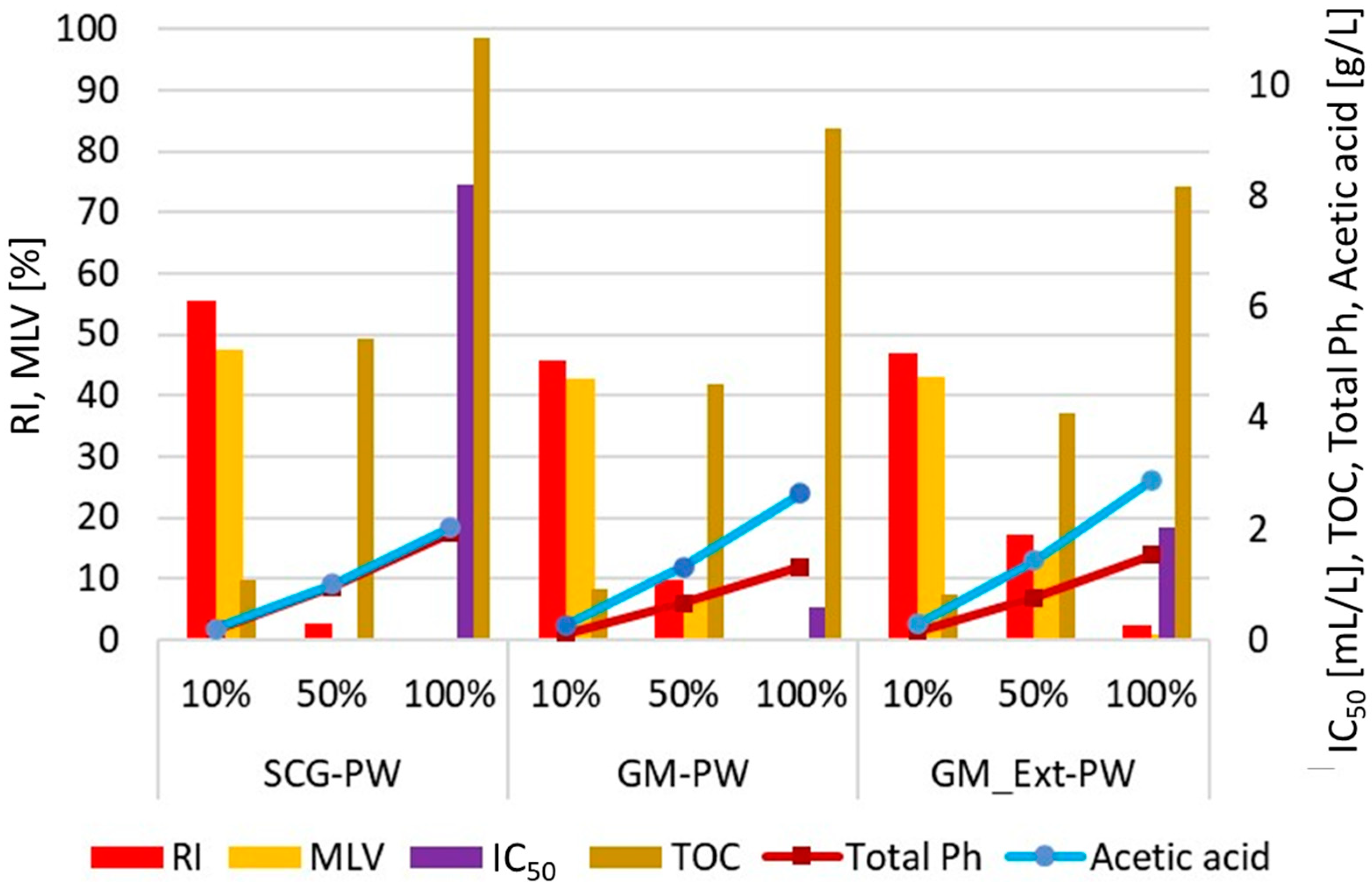
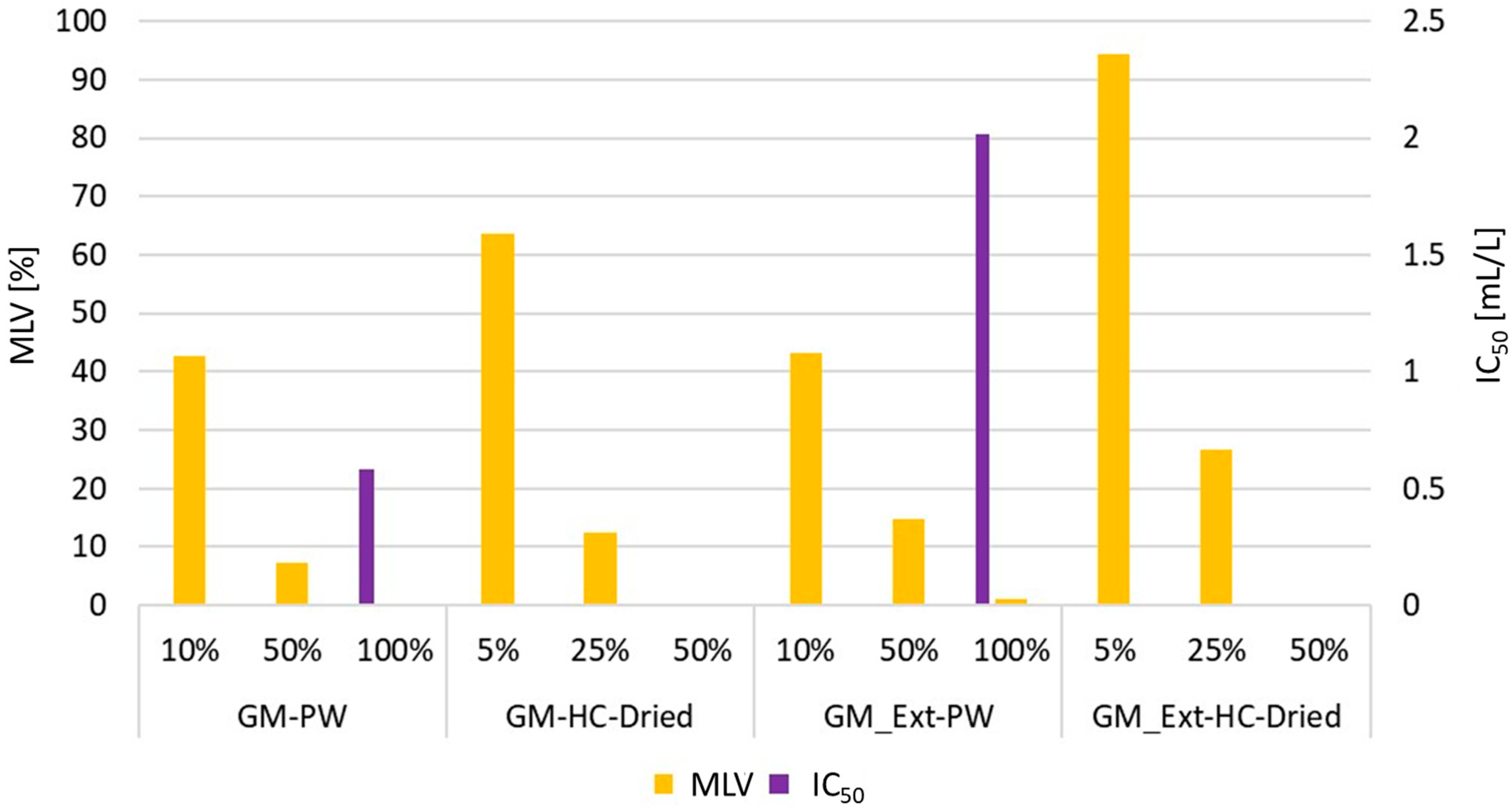
3.3. Effect of Process Water on Seed Germination
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Méndez, A.; Cárdenas-Aguiar, E.; Paz-Ferreiro, J.; Plaza, C.; Gascó, G. The Effect of Sewage Sludge Biochar on Peat-Based Growing Media. Biol. Agric. Hortic. 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Glenk, K.; Martin-Ortega, J. The Economics of Peatland Restoration. J. Environ. Econ. Policy 2018, 7, 345–362. [Google Scholar] [CrossRef] [Green Version]
- Barbier, E.B.; Burgess, J.C. Economics of Peatlands Conservation, Restoration and Sustainable Management; United Nations Environment Programme: Nairobi, Kenya, 2021; p. 53. [Google Scholar]
- Prasad, M.; Chrysargyris, A.; McDaniel, N.; Kavanagh, A.; Gruda, N.S.; Tzortzakis, N. Plant Nutrient Availability and PH of Biochars and Their Fractions, with the Possible Use as a Component in a Growing Media. Agronomy 2019, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Vasander, H.; Tuittila, E.-S.; Lode, E.; Lundin, L.; Ilomets, M.; Sallantaus, T.; Heikkilä, R.; Pitkänen, M.-L.; Laine, J. Status and restoration of peatlands in northern Europe. Wetl. Ecol. Manag. 2003, 11, 51–63. [Google Scholar] [CrossRef]
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.-M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic Use of Peat and Charred Material in Growing Media—An Option to Reduce the Pressure on Peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Nocentini, M.; Panettieri, M.; García de Castro Barragán, J.M.; Mastrolonardo, G.; Knicker, H. Recycling Pyrolyzed Organic Waste from Plant Nurseries, Rice Production and Shrimp Industry as Peat Substitute in Potting Substrates. J. Environ. Manag. 2021, 277, 111436. [Google Scholar] [CrossRef]
- Raja, A.M.; Khalaf, N.H.; Alkubaisy, S.A. Utilization of Date Palm Waste Compost as Substitute For Peat Moss. IOP Conf. Ser. Earth Environ. Sci. 2021, 904, 012041. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a Growing Media Additive and Peat Substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar Made from Green Waste as Peat Substitute in Growth Media for Calathea Rotundifola Cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Dalias, P.; Prasad, M.; Mumme, J.; Kern, J.; Stylianou, M.; Christou, A. Low-Cost Post-Treatments Improve the Efficacy of Hydrochar as Peat Replacement in Growing Media. J. Environ. Chem. Eng. 2018, 6, 6647–6652. [Google Scholar] [CrossRef] [Green Version]
- Roehrdanz, M.; Greve, T.; de Jager, M.; Buchwald, R.; Wark, M. Co-Composted Hydrochar Substrates as Growing Media for Horticultural Crops. Sci. Hortic. 2019, 252, 96–103. [Google Scholar] [CrossRef]
- Allen, S.G.; Kam, L.C.; Zemann, A.J.; Antal, M.J. Fractionation of Sugar Cane with Hot, Compressed, Liquid Water. Ind. Eng. Chem. Res. 1996, 35, 2709–2715. [Google Scholar] [CrossRef]
- Elliott, D.C.; Beckman, D.; Bridgwater, A.V.; Diebold, J.P.; Gevert, S.B.; Solantausta, Y. Developments in Direct Thermochemical Liquefaction of Biomass: 1983–1990. Energy Fuels 1991, 5, 399–410. [Google Scholar] [CrossRef]
- Álvarez, M.L.; Gascó, G.; Plaza, C.; Paz-Ferreiro, J.; Méndez, A. Hydrochars from Biosolids and Urban Wastes as Substitute Materials for Peat. Land Degrad. Develop. 2017, 28, 2268–2276. [Google Scholar] [CrossRef]
- Puccini, M.; Ceccarini, L.; Antichi, D.; Seggiani, M.; Tavarini, S.; Hernandez Latorre, M.; Vitolo, S. Hydrothermal Carbonization of Municipal Woody and Herbaceous Prunings: Hydrochar Valorisation as Soil Amendment and Growth Medium for Horticulture. Sustainability 2018, 10, 846. [Google Scholar] [CrossRef] [Green Version]
- Bates, A.K. The Biochar Solution: Carbon Farming and Climate Change; New Society Publishers: Gabriola Island, BC, Canada, 2010; ISBN 978-0-86571-677-3. [Google Scholar]
- Schofield, H.K.; Pettitt, T.R.; Tappin, A.D.; Rollinson, G.K.; Fitzsimons, M.F. Biochar Incorporation Increased Nitrogen and Carbon Retention in a Waste-Derived Soil. Sci. Total Environ. 2019, 690, 1228–1236. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal Carbonization of Biomass Residuals: A Comparative Review of the Chemistry, Processes and Applications of Wet and Dry Pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef] [Green Version]
- Reza, M.T.; Freitas, A.; Yang, X.; Coronella, C.J. Wet Air Oxidation of Hydrothermal Carbonization (HTC) Process Liquid. ACS Sustain. Chem. Eng. 2016, 4, 3250–3254. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal Carbon from Biomass: A Comparison of the Local Structure from Poly- to Monosaccharides and Pentoses/Hexoses. Green Chem. 2008, 10, 1204. [Google Scholar] [CrossRef] [Green Version]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal Carbonization of Municipal Waste Streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Bargmann, I.; Rillig, M.C.; Buss, W.; Kruse, A.; Kuecke, M. Hydrochar and Biochar Effects on Germination of Spring Barley. J. Agro Crop. Sci 2013, 199, 360–373. [Google Scholar] [CrossRef]
- Thomas, S.C. Post-Processing of Biochars to Enhance Plant Growth Responses: A Review and Meta-Analysis. Biochar 2021, 3, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Fregolente, L.G.; Miguel, T.B.A.R.; de Castro Miguel, E.; de Almeida Melo, C.; Moreira, A.B.; Ferreira, O.P.; Bisinoti, M.C. Toxicity Evaluation of Process Water from Hydrothermal Carbonization of Sugarcane Industry By-Products. Environ. Sci. Pollut. Res. 2019, 26, 27579–27589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirth, B.; Mumme, J. Anaerobic Digestion of Waste Water from Hydrothermal Carbonization of Corn Silage. Appl. Bioenergy 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Ferrentino, R.; Merzari, F.; Grigolini, E.; Fiori, L.; Andreottola, G. Hydrothermal Carbonization Liquor as External Carbon Supplement to Improve Biological Denitrification in Wastewater Treatment. J. Water Process. Eng. 2021, 44, 102360. [Google Scholar] [CrossRef]
- EBC. European Biochar Certificate—Guidelines for a Sustainable Production of Biochar; Version 9.5E; European Biochar Foundation (EBC): Arbaz, Switzerland, 2021. [Google Scholar]
- IBI. Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. 2015. Available online: http://www.biochar-international.org/characterizationstandard (accessed on 28 December 2021).
- Cross, A.; Sohi, S.P. A Method for Screening the Relative Long-Term Stability of Biochar. GCB Bioenergy 2013, 5, 215–220. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Kuśmierz, M. Biochar Properties Regarding to Contaminants Content and Ecotoxicological Assessment. J. Hazard. Mater. 2013, 260, 375–382. [Google Scholar] [CrossRef]
- Domene, X.; Hanley, K.; Enders, A.; Lehmann, J. Short-Term Mesofauna Responses to Soil Additions of Corn Stover Biochar and the Role of Microbial Biomass. Appl. Soil Ecol. 2015, 89, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Norton, J.; Ouyang, Y. Controls and Adaptive Management of Nitrification in Agricultural Soils. Front. Microbiol. 2019, 10, 1931. [Google Scholar] [CrossRef] [Green Version]
- Olszyk, D.M.; Shiroyama, T.; Novak, J.M.; Johnson, M.G. A Rapid-Test for Screening Biochar Effects on Seed Germination. Commun. Soil Sci. Plant Anal. 2018, 49, 2025–2041. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.M.; Trabue, S.; Heaton, E. Germination Tests for Assessing Biochar Quality. J. Environ. Qual. 2012, 41, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowski, T.T. Seed Germination and Seedling Development. In Seed Germination, Ontogeny, and Shoot Growth; Elsevier: Amsterdam, The Netherlands, 1971; pp. 41–93. ISBN 978-0-12-424201-2. [Google Scholar]
- Makhaye, G.; Mofokeng, M.M.; Tesfay, S.; Aremu, A.O.; van Staden, J.; Amoo, S.O. Influence of Plant Biostimulant Application on Seed Germination. In Biostimulants for Crops from Seed Germination to Plant Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 109–135. ISBN 978-0-12-823048-0. [Google Scholar]
- Mofokeng, M.M.; Araya, H.T.; Araya, N.A.; Makgato, M.J.; Mokgehle, S.N.; Masemola, M.C.; Mudau, F.N.; du Plooy, C.P.; Amoo, S.O. Integrating Biostimulants in Agrosystem to Promote Soil Health and Plant Growth. In Biostimulants for Crops from Seed Germination to Plant Development; Elsevier: Amsterdam, The Netherlands, 2021; pp. 87–108. ISBN 978-0-12-823048-0. [Google Scholar]
- Bierhuizen, J.F.; Wagenvoort, W.A. Some Aspects of Seed Germination in Vegetables. 1. The Determination and Application of Heat Sums and Minimum Temperature for Germination. Sci. Hortic. 1974, 2, 213–219. [Google Scholar] [CrossRef]
- Marmiroli, M.; Bonas, U.; Imperiale, D.; Lencioni, G.; Mussi, F.; Marmiroli, N.; Maestri, E. Structural and Functional Features of Chars From Different Biomasses as Potential Plant Amendments. Front. Plant Sci. 2018, 9, 1119. [Google Scholar] [CrossRef] [Green Version]
- Celletti, S.; Bergamo, A.; Benedetti, V.; Pecchi, M.; Patuzzi, F.; Basso, D.; Baratieri, M.; Cesco, S.; Mimmo, T. Phytotoxicity of Hydrochars Obtained by Hydrothermal Carbonization of Manure-Based Digestate. J. Environ. Manag. 2021, 280, 111635. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L.; Müller, C. Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J. Environ. Qual. 2012, 41, 1023–1032. [Google Scholar] [CrossRef]
- Kaur-Bhambra, J.; Wardak, D.L.R.; Prosser, J.I.; Gubry-Rangin, C. Revisiting Plant Biological Nitrification Inhibition Efficiency Using Multiple Archaeal and Bacterial Ammonia-Oxidising Cultures. Biol Fertil. Soils 2021, 58, 241–249. [Google Scholar] [CrossRef]
- Norton, J.M.; Stark, J.M. Chapter Fifteen—Regulation and Measurement of Nitrification in Terrestrial Systems. In Methods in Enzymology; Klotz, M.G., Ed.; Research on Nitrification and Related Processes, Part A; Academic Press: Cambridge, MA, USA, 2011; Volume 486, pp. 343–368. [Google Scholar]
- Ficara, E.; Rozzi, A. PH-Stat Titration to Assess Nitrification Inhibition. J. Environ. Eng. 2001, 127, 698–704. [Google Scholar] [CrossRef]
- Pagga, U.; Bachner, J.; Strotmann, U. Inhibition of Nitrification in Laboratory Tests and Model Wastewater Treatment Plants. Chemosphere 2006, 65, 1–8. [Google Scholar] [CrossRef]
- Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 Laying down Rules on the Making Available on the Market of EU Fertilising Products and Amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and Repealing Regulation (EC) No 2003/2003. Off. J. Eur. Union 2019, L170, 1–114.
- Regulation (EU) 2021/1165 of 15 July 2021 Authorising Certain Products and Substances for Use in Organic Production and Establishing Their Lists. Off. J. Eur. Union 2021, L253, 13–48.
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent Trends in Biochar Production Methods and Its Application as a Soil Health Conditioner: A Review. SN Appl. Sci. 2020, 2, 1307. [Google Scholar] [CrossRef]
- Perra, M.; Lozano-Sánchez, J.; Leyva-Jiménez, F.-J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; de Gioannis, G.; Manca, M.L.; Manconi, M. Extraction of the Antioxidant Phytocomplex from Wine-Making by-Products and Sustainable Loading in Phospholipid Vesicles Specifically Tailored for Skin Protection. Biomed. Pharmacother. 2021, 142, 111959. [Google Scholar] [CrossRef] [PubMed]
- Harper, S.H.T.; Lynch, J.M. Microbial Effects on the Germination and Seedling Growth of Barley. New Phytol. 1980, 84, 473–481. [Google Scholar] [CrossRef]
- Bengtsson, G.; Bengtson, P.; Månsson, K.F. Gross Nitrogen Mineralization-, Immobilization-, and Nitrification Rates as a Function of Soil C/N Ratio and Microbial Activity. Soil Biol. Biochem. 2003, 35, 143–154. [Google Scholar] [CrossRef]
- Brust, G.E. Management Strategies for Organic Vegetable Fertility. In Safety and Practice for Organic Food; Elsevier: Amsterdam, The Netherlands, 2019; pp. 193–212. ISBN 978-0-12-812060-6. [Google Scholar]
- Watson, C.A.; Atkinson, D.; Gosling, P.; Jackson, L.R.; Rayns, F.W. Managing Soil Fertility in Organic Farming Systems. Soil Use Manag. 2006, 18, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Cervera-Mata, A.; Fernández-Arteaga, A.; Navarro-Alarcón, M.; Hinojosa, D.; Pastoriza, S.; Delgado, G.; Rufián-Henares, J.Á. Spent Coffee Grounds as a Source of Smart Biochelates to Increase Fe and Zn Levels in Lettuces. J. Clean. Prod. 2021, 328, 129548. [Google Scholar] [CrossRef]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and Phytotoxic Risk Assessment of Fresh and Treated Hydrochar from Hydrothermal Carbonization Compared to Biochar from Pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef]
- Maunuksela, L.; Herranen, M.; Torniainen, M. Quality Assessment of Biogas Plant End Products by Plant Bioassays. Int. J. Environ. Sci. Dev. 2012, 305–310. [Google Scholar] [CrossRef]
- Wang, G. Key Factors Affecting Seed Germination in Phytotoxicity Tests during Sheep Manure Composting with Carbon Additives. J. Hazard. Mater. 2022, 421, 126809. [Google Scholar] [CrossRef]
- Schmidt, E.L.; Belser, L.W. Nitrifying Bacteria. In Agronomy Monographs; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 2015; pp. 1027–1042. ISBN 978-0-89118-977-0. [Google Scholar]
- Papadopoulou, E.S.; Bachtsevani, E.; Lampronikou, E.; Adamou, E.; Katsaouni, A.; Vasileiadis, S.; Thion, C.; Menkissoglu-Spiroudi, U.; Nicol, G.W.; Karpouzas, D.G. Comparison of Novel and Established Nitrification Inhibitors Relevant to Agriculture on Soil Ammonia- and Nitrite-Oxidizing Isolates. Front. Microbiol. 2020, 11, 581283. [Google Scholar] [CrossRef]


| Material/Code | Pre-/Post-Treatment | Water Content [wt%] | Mixing Ratio between Material and Peat [wt%, FM] | ||||
|---|---|---|---|---|---|---|---|
| Peat | none (used as it is) | 74.42 | - | - | - | 100 (used as control) | |
| Feedstocks | D | separated at biogas plant | 70.87 | 5 | 25 | 50 | 100 |
| SCG | none (used as it is) | 57.85 | 5 | 25 | 50 | 100 | |
| GM | none (used as it is) | 6.17 | 5 | 25 | 50 | 100 | |
| GM_Ext | extraction in ethanol solution | 7.40 | n.t | n.t | n.t | n.t | |
| Hydrochars | D-HC | washed after HTC | 83.59 | 5 | 25 | 50 | n.t |
| washed and dried | 0 * | 5 | 25 | 50 | n.t | ||
| SCG-HC | washed after HTC | 70.54 | 5 | 25 | 50 | n.t | |
| washed and dried | 0 * | 5 | 25 | 50 | n.t | ||
| GM-HC | after HTC | 66.40 | n.t | n.t | n.t | n.t | |
| dried | 0 * | 5 | 25 | 50 | n.t | ||
| GM_Ext-HC | after HTC | 69.20 | n.t | n.t | n.t | n.t | |
| dried | 0 * | 5 | 25 | 50 | n.t | ||
| Materials | Pre-/Post-Treatment | TOC [g L−1] | Dilution Ratio (Process Water:Distilled Water) [wt%] | ||||
| Process water | SCG-PW | diluted with distilled water | 10.85 | 0 | 10 | 50 | 100 |
| GM-PW | diluted with distilled water | 9.21 | 0 | 10 | 50 | 100 | |
| GM_Ext-PW | diluted with distilled water | 8.17 | 0 | 10 | 50 | 100 | |
| Material | D | D-HC | SCG | SCG-HC | GM | GM-HC | GM_Ext | GM_Ext-HC |
|---|---|---|---|---|---|---|---|---|
| C:N | 31.47 | 20.90 | 21.36 | 25.25 | 23.56 | 33.30 | 23.75 | 37.18 |
| PW Samples | pH | EC [mS cm−1] | TOC [g L−1] | COD [gO2 L−1] | Total Ph [g L−1] | Acetic acid [g L−1] | IC50 [mL L−1] |
|---|---|---|---|---|---|---|---|
| D | 5.34 | 8.79 | 11.72 | 35.00 | N.D. | 2.36 | N.D. |
| SCG | 4.03 | 3.74 | 10.02 (±1.16) | 29.58 (±0.48) | 1.92 (±0.03) | 2.01 (±0.04) | 8.21 |
| GM | 4.37 | 12.53 | 10.20 (±0.02) | 29.78 (±0.02) | 1.32 (±0.02) | 2.64 (±0.12) | 0.58 |
| GM_Ext | 4.26 | 11.71 | 8.64 (±0.03) | 26.47 (±0.05) | 1.52 (±0.02) | 2.87 (±0.15) | 2.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farru, G.; Dang, C.H.; Schultze, M.; Kern, J.; Cappai, G.; Libra, J.A. Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media. Horticulturae 2022, 8, 325. https://doi.org/10.3390/horticulturae8040325
Farru G, Dang CH, Schultze M, Kern J, Cappai G, Libra JA. Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media. Horticulturae. 2022; 8(4):325. https://doi.org/10.3390/horticulturae8040325
Chicago/Turabian StyleFarru, Gianluigi, Chau Huyen Dang, Maja Schultze, Jürgen Kern, Giovanna Cappai, and Judy A. Libra. 2022. "Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media" Horticulturae 8, no. 4: 325. https://doi.org/10.3390/horticulturae8040325
APA StyleFarru, G., Dang, C. H., Schultze, M., Kern, J., Cappai, G., & Libra, J. A. (2022). Benefits and Limitations of Using Hydrochars from Organic Residues as Replacement for Peat on Growing Media. Horticulturae, 8(4), 325. https://doi.org/10.3390/horticulturae8040325