Abstract
Citrus trees are a very important crops that are cultivated worldwide, but not much knowledge is known about the ecophysiological responses to climatic changes in trees under natural conditions. The aim of this study was to investigate their adaptive capacity in response to seasonal phenological and environmental changes. The trial included Citrus trees (sweet orange, bitter orange, lemon, mandarin) growing under non-regular cropping conditions in a Monumental Charterhouse in Tuscany, in a subtropical Mediterranean climate with hot summer conditions. During a 1-year field trial, we determined the variations in chlorophyll fluorescence parameters and leaf biochemical traits (content of chlorophylls and carotenoids, total phenolic content (TPC), total antioxidant capacity (TAC), and total non-structural carbohydrates). In all Citrus spp., interspecific mean values of photochemical efficiency peaked during the summer, while a marked photoinhibition occurred in the winter in concomitance with higher interspecific mean values of leaf TPC, TAC, and non-structural carbohydrates. The trees showed the pivotal role played by photosynthetic acclimation as a survival strategy to tolerate abiotic stress in the climate change hotspot of Mediterranean environment. This study is included in a wider project aimed at a new valorization of Citrus trees as genetic resource and its by-products with added-value applications for innovative functional foods.
1. Introduction
The Citrus genus belongs to the order of Sapindales, the Rutaceae family, and includes 140 genera and 1300 species [1]. This genus originated in a wide area of the Eastern world and the domestication of the edible types began several thousand years ago [2]. Recent studies identified citron (C. medica L.), pummelo (C. maxima (Burm.) Merrill), and mandarin (C. reticulata Blanco) as the three biological Citrus taxa from which sweet orange (C. sinensis L. Osb.), bitter orange (C. aurantium L.), lemon (C. limon (L.) Burm.), and other species originated by hybridization [2,3]. Citrus spp. are very important fruit crops worldwide for providing essential sources of vitamin C and nutraceutical compounds as juice or fresh fruits: the global production of orange in 2019 exceeded 47 million metric tons [4], and sweet orange is one of the most produced and processed fruits globally [5]. Alongside essential vitamins C, E, B, and provitamin A, Citrus fruits (peel, pulp, oil, juice, and whole fruit) present high contents of bioactive secondary metabolites, i.e., flavonoids, carotenoids, limonoids, polyphenols, and essential oils, largely contributing to the dietary supply of antioxidative and anticarcinogenic substances, preventing cardiovascular and degenerative diseases [6,7,8,9].
Climatic conditions (temperature and water supply) determined the distribution of Citrus. Temperature is the main limiting abiotic environmental factor, and areas with high temperatures and low freezing frequency allow the cultivation of both early and late varieties [10]. Irrigation is always necessary in arid, semi-arid, and even humid regions, and water supply must be regularly distributed from late winter to autumn to enhance flowering, fruit yield, and quality [11]. Citrus plants are sensitive to low temperatures and wind, which damage the species with large fruits, e.g., sweet orange and lemon. Sweet orange, which prefers sunny locations and mild winters, must be sheltered from cold winds and late frosts to protect flowers and buds [10]. Low temperatures in sweet orange induce imbalances in metabolic processes and impair the photosynthetic apparatus, decreasing leaf stomatal conductance, transpiration, chlorophyll content, and net CO2 exchange rate [11]. Soil is another abiotic factor that affects fruit quality in Citrus: the optimal is deep, clay-calcareous, drained, and fertilized; the optimization of nitrogen use efficiency plays a major role in Citrus management [12]. Sandy soils, if fertilized and irrigated, are used for early varieties; clay-loam soils are mainly used for late varieties [10].
Despite their global importance as fruit crops, few ecophysiological studies on Citrus spp. are available in literature for plants grown in natural conditions [13,14,15] and specific aspects, such as the variability of photosynthetic efficiency during the year of cultivation and the influence of environmental conditions on photosynthesis, which need to be further explored. In the Monumental Charterhouse of Calci (Pisa, Italy), various Citrus species are present. The building is a wide historical complex founded in 1366 by Carthusian monks. For centuries (until 1972), they lived in single monastic cells, each one with its walled garden called “hortus conclusus”, where vegetables, herbs, and fruit trees (Citrus above all) were cultivated for food and medicinal purposes. Currently, the Charterhouse is part of the Natural History Museum of the University of Pisa, and the old Citrus trees are still productive even though unmanaged, untreated, and seldom pruned.
The present study was part of an EU-project aimed to valorize the traditional Citrus species widely present in the entire region of Tuscany, as cultivated or ornamental plants typical of the Mediterranean territory. Attention was focused on the characterization of physiological properties of Citrus trees, which offers the opportunity to develop and valorize sustainable by-products, acquiring a great potential as sources of valuable bio-compounds and raw materials due to their wide biomass, as already reported [8,16,17]. The specific aim was to investigate the physiological and biochemical adaptations in four Citrus spp. (sweet orange, bitter orange, lemon, mandarin) grown under non-regular cropping conditions. During the seasonal climatic changes, the variations of chlorophyll fluorescence parameters (potential efficiency of PSII photochemistry, Fv/Fm; actual photon yield of PSII photochemistry, ΦPSII; non-photochemical quenching, NPQ) and leaf biochemical traits (pigments, non-structural carbohydrates, total polyphenols content, and total antioxidant capacity) were determined in concomitance with the main phenological stages to investigate the adaptive capacity of these Citrus species to typical environmental restraints of the Mediterranean climate in the absence of chemical inputs (fertilizers, pesticides) and other regular crop management practices.
2. Materials and Methods
2.1. Site Description and Plant Materials
The study was conducted at the Monumental Charterhouse of Calci (Pisa, Italy) (43°43′19″ N, 10°31′22″ E, 50 m a.s.l.). The climatic conditions corresponded to a Mediterranean climate with a hot, dry summer and a relatively mild, wet winter, classified as Csa subtype according to Köppen-Geiger [18,19,20]. Edaphic properties of soil were determined (77.8% sand; 16.8% silt; 5.4% clay; pH = 8.2; EC = 1034.5 μS cm−1; C = 2.27%; N = 0.28%). The trial was carried out in 2018 and 2019 on four different species of Citrus grown in the monastic gardens: Citrus sinensis L. Osbeck (sweet orange), Citrus aurantium L. (bitter orange), Citrus reticulata Blanco (mandarin orange), and Citrus limon L. Osbeck (lemon). Plants were grown under unmanaged conditions: not fertilized, treated with pesticides, irrigated, or pruned. Environmental conditions of macroclimate (monthly mean air temperature, Tair; monthly minimum air temperature, Tmin; monthly maximum air temperature, Tmax; monthly mean relative humidity, RH; mean daily air temperature on sampling dates, Tair d) were collected from web databases of meteorological data recorded by local weather stations (S. Giusto, Pisa) throughout the whole trial (https://www.ilmeteo.it/portale/archivio-meteo/Calci; accessed on 14 March 2022); all meteorological measurements were recorded at 15-min intervals on a data logger Davis Vantage Pro 2 (Table 1 and Table 2). Mean annual temperatures and annual precipitations were 15.6 °C and 1032.9 mm in 2018, 15.2 °C and 857 mm in 2019, respectively. Mean winter temperatures (December–March) were 7.9 °C and 8 °C in 2018 and 2019, respectively, while the mean summer temperature was 22.8 °C in both years. January 2019 was the coldest month (5.3 °C), while the hottest was July 2018 (24.2 °C).

Table 1.
Environmental conditions during the experimental period between June 2018 and May 2019. Meteorological data recorded with an automatic weather station: monthly mean air temperature (Tair); maximum (Tmax) and minimum (Tmin) monthly air temperatures; monthly mean relative humidity (RH); wind speed, number of rain days and monthly mean precipitations (mm).

Table 2.
Daily temperatures on leaf sampling dates during the experimental period (27 June 2018–15 May 2019). Meteorological data recorded with automatic weather station: mean daily air temperature (Tair); maximum (Tmax) and minimum (Tmin) daily air temperatures; mean daily relative humidity (RH), wind speed and daily precipitations (mm).
2.2. Experimental Design
Leaves were sampled between June 2018 and May 2019, during the main phenological stages of Citrus fruit development, namely in May (flowering), June (early fruitlets), July (rapid growth period), October (fruit maturity), and January (ripening) [21]. Leaf samplings were carried out collecting 3–5 fully expanded and sun exposed mature leaves from five plants of each species, during the morning in full sun and used for fluorescence measurements and biochemical analyses. Fresh leaf samples were directly analyzed for relative water content (RWC) without washing; for biochemical analysis, fresh leaf samples were gently washed with deionized water, wiped with filter paper, and refrigerated until further analysis (pigments and RWC). For carbohydrates and total phenolic content, leaf samples were oven dried in an electric dryer (T 80 VN, Tecnovetro, Monza, Italy) at 60 °C until constant dry weight and stored in closed vials at 20 °C until processed.
2.3. Chlorophyll Fluorescence Measurements
Chlorophyll fluorescence measurements were performed on five fully expanded and exposed leaves of five plants per species by means of a miniaturized pulse amplitude-modulated fluorometer (Mini-PAM, Heinz Walz GmbH, Effeltrich, Germany). Actual photon yield of PSII photochemistry (ΦPSII), non-photochemical quenching (NPQ), and potential efficiency of PSII photochemistry (Fv/Fm) (ratio of variable fluorescence to maximum chlorophyll fluorescence of PSII in dark adapted leaves) were measured in the morning between 10 a.m. and 12 a.m. local time. Measurements of Fv/Fm were performed after 30 min of acclimation in the dark. The actual photon yield of PSII photochemistry was determined as ΦPSII = (Fm′ − Fs)/Fm′ at steady state [22], where Fm′ is the maximum fluorescence yield (with PSII reaction centers in reduced state imposing a saturating flash during exposition to actinic light) and Fs is the fluorescence at the actual state of PSII reaction centers during actinic light. Non-photochemical quenching was determined according to the Stern-Volmer equation as
where Fm is the maximum fluorescence yield in the dark, as reported in [23]. Data reported are means (±SE) of three analytical replications of five independent samples for each species (n = 15).
NPQ = (Fm/Fm′) − 1,
2.4. Leaf Structural and Biochemical Traits
Relative water content (RWC) was determined in three fresh leaf discs (14 mm-diameter) from five plants of each species (two duplicates, with triplicate analysis). After fresh weighting, leaf discs were immersed in deionized water for 24 h, excess water wiped with tissue paper, then weighted at full turgor. Dry weight was measured after oven drying leaf discs at 60 °C for 48 h. RWC was calculated as
RWC (%) = [(fresh weight − dry weight)/(turgor weight − dry weight)] × 100.
For the determination of chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl tot), and total carotenoids (Car), three fresh leaf discs of 14 mm-diameter (from five plants of each species, two duplicates) were homogenously sampled from the mid-lamina area of internerval regions, added with 5 mL of 100% MeOH and stored at 4–5 °C in the dark for 24 h. The absorbance of the extracts was measured at 665, 652, and 470 nm with a UV-VIS spectrophotometer (Shimadzu UV-1800, Shimadzu Corporation, Kyoto, Japan), as described in [24]. Leaf contents of chlorophylls and carotenoids were expressed as μg cm−2. Total soluble sugars and starch were determined as reported in [25] with minor modifications. Dried leaves (1 g DW each sample, from five different plants of each species, triplicate analysis) were added with 80% EtOH, incubated at 60 °C for 15 min, and centrifuged, and supernatants were used to determine soluble sugars. Pellets were extracted with 52% perchloric acid and centrifuged, and supernatants used for starch analysis. Aliquots of 0.2 mL were added to 0.8 mL of 0.2% (w/v) anthrone solution, incubated at 90 °C for 8 min and cooled. The Abs at 630 nm was read with a UV-1800 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). Contents of total soluble sugars and starch were obtained by comparing Abs with a standard curve of d-glucose and expressed as mg glucose g DW−1. For the detection of total phenolic content (TPC) and antioxidant activity, dried leaves (0.5 g FW of each sample, from five different plants of each species, triplicate analysis) were ground in a mortar, extracted with 2 mL of 70% MeOH, mixed at 4 °C for 30 min in the dark, thus centrifuged at 14.000× g for 20 min, and pellets were discarded. Aliquots of the soluble extract (0.05 mL) were used for the determination of TPC with Folin-Ciocalteau’s phenol reagent, as described in [26]. The Abs of each sample was measured at 725 nm with a UV-VIS spectrophotometer, and TPC was expressed as mg of gallic acid equivalents per g DW (mg GAE g DW−1). Total antioxidant capacity (TAC) of leaf extracts was evaluated by the DPPH method [27] with minor modifications [26]. Leaf extracts (0.05 mL) were added to 0.25 mL of methanolic DPPH solution (0.1 mM) and volume adjusted to 1 mL. After incubation in the dark (30 min), Abs was measured at 517 nm with a UV-VIS spectrophotometer. TAC was expressed as μmol of Trolox equivalents per g DW (μmol Trolox g DW−1). Data reported for leaf biochemical traits were means (±SE) of three replications of five independent samples for each species (n = 15).
2.5. Statistical Analysis
Statistical analysis was performed by ANOVA using the STATISTICA software package (StatSoft for Windows, 1998, Tulsa, OK, USA). The seasonal variation of structural (RWC), physiological (Fv/Fm, ΦPSII, NPQ), and biochemical (pigments, TPC, TAC) parameters were analyzed by two-way ANOVA to test main and interactive effects of month and species. When F test was significant, separation of means was performed by Fisher’s least significance difference (LSD) test with p ≤ 0.05.
3. Results
3.1. Chlorophyll Fluorescence Measurements
All the photosynthetic parameters (Fv/Fm, ΦPSII, NPQ) measured in Citrus leaves were characterized by a similar sinusoidal pattern with a minimum in January (Figure 1).
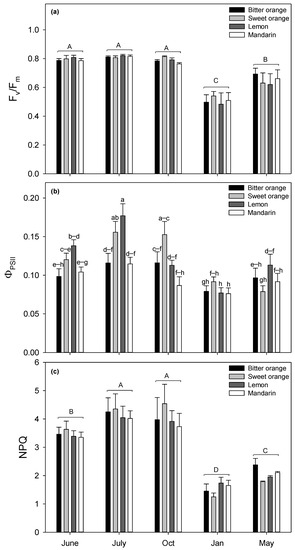
Figure 1.
Seasonal variation of (a) potential efficiency of PSII photochemistry (Fv/Fm), (b) actual photon yield of PSII photochemistry (ΦPSII), and (c) non-photochemical quenching (NPQ) in Citrus spp. leaves. Data are means ± standard error (n = 5). A two-way ANOVA was applied; the significance levels (p) for plant species and month factors and their interactions are shown in Table 3. When the interaction between factors was significant (b), the Fisher LSD-test (p ≤ 0.05) was applied, and the separation of means was evidenced by different lowercase letters. When the interaction species × month was not significant (a,c), one-way ANOVA was applied and significant differences among months were reported with different uppercase letters (Fisher’s LSD test, p ≤ 0.05).
The highest interspecific mean Fv/Fm occurred in July (0.814) and the lowest in January (0.507), before raising in May (0.651). During the summer-autumn period, from June to October, Fv/Fm showed a stable mean value of 0.798. The statistically significant variable resulting from ANOVA analysis for Fv/Fm was the month, although June, July, and October did not differ from each other. No significant variability among species was found (Table 3).

Table 3.
Summary two-way ANOVA table for differences in relative water content (RWC), chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophylls (Chl tot), chlorophyll a/b ratio (Chl a/b), total carotenoids (Car), total carotenoids/total chlorophylls ratio (Car/Chl tot), potential efficiency of PSII photochemistry (Fv/Fm), actual photon yield of PSII photochemistry (ΦPSII), non-photochemical quenching (NPQ), total phenolic content (TPC), total antioxidant capacity (TAC), soluble sugars and starch due to species, month, and interaction of species and month.
All the variables in two-way ANOVA analysis of variance for ΦPSII resulted as significant, as reported in Table 3. ΦPSII was significantly affected by species, months, and their interaction. Interspecific mean ΦPSII was the maximum in July (0.141) and minimum in January (0.080). Intraspecific values of ΦPSII were the highest in lemon and sweet orange in July (0.177 and 0.156, respectively), while in October, sweet orange showed the highest ΦPSII (0.153). In January, quantum yield of PSII was the lowest in all species. During the whole trial, ΦPSII ranged less in mandarin and bitter orange, with respect to the other Citrus species. Regarding NPQ, two-way ANOVA results (Table 3) evidenced that species were not significantly different, and the significant variable was the month (albeit July and October did not statistically differ). Starting with an interspecific mean of 3.45 in June, NPQ peaked in July and October (4.16 and 4.03, respectively) and was minimum in January (1.52), before a slight increase in May (2.05).
3.2. Leaf Structural and Biochemical Traits
In two-way ANOVA analysis, mean RWC values were characterized by a significant interaction species × month (p ≤ 0.001), as well as a significant interspecific variability (Table 3). The interspecific mean value of RWC (Figure 2) was minimum in July in all the species (46.70%), especially in bitter orange (36.10%).
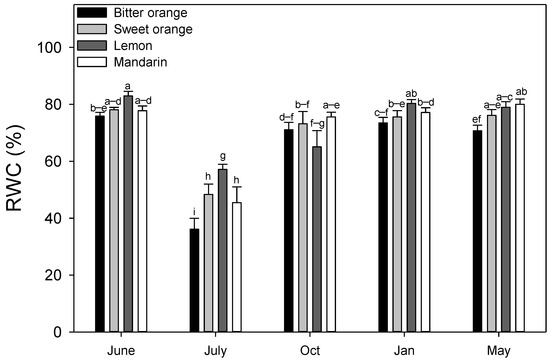
Figure 2.
Seasonal variation of the relative water content (RWC %) in Citrus spp. leaves. Data are means ± standard error (n = 5). Statistics as in Figure 1. Different lowercase letters indicate significant differences among means (p ≤ 0.05, Fisher’s LSD).
From October to May, mean RWC was high (75.60%), although in lemon the intraspecific mean was slightly lower in October (65.07%). Among the species, RWC peaked in lemon (82.90% in June). Moreover, bitter orange had the lowest RWC during the whole trial (except in October) and tended to show more stable values.
Data on leaf chlorophylls (Chl a, Chl b, Chl tot) were subjected to two-way ANOVA analysis of variance to determine the interaction between months and species; the significant variable was the month, and differences among species were not statistically significant (Table 3). During the whole trial, leaf contents of Chl a, Chl b, and Chl tot in all the Citrus spp. showed the same pattern (peaking in July, gradually decreasing in October, and reaching lower values in the other months), although no statistical difference was observed among species (Figure 3).
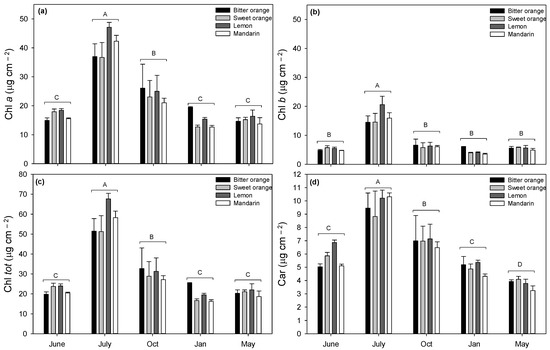
Figure 3.
Seasonal variation of leaf content (μg cm−2) of (a) chlorophyll a (Chl a), (b) chlorophyll b (Chl b), (c) total chlorophylls (Chl tot), and (d) total carotenoids (Car) in Citrus spp. leaves. Data are means ± standard error (n = 5). Statistics as in Figure 1. Different uppercase letters indicate significant differences among months (p ≤ 0.05, Fisher’s LSD).
The interspecific mean contents of Chl a, Chl b, and Chl tot were the highest in July, followed by October, while in other months, markedly lower values were found. Compared to July, interspecific means of Chl tot averagely decreased about one-half in October, reaching the minimum in January (57.16, 29.98 and 19.45 μg cm−2, respectively). The highest intraspecific content of Chl tot was found in lemon in July (67.70 μg cm−2).
For leaf carotenoids, the significant variable in two-way ANOVA analysis of variance was the month, and species were not statistically different for their carotenoid content (Table 3). Total carotenoids (Figure 3d) presented an accumulation pattern like chlorophylls: interspecific mean values were maximum in July (9.70 μg cm−2) and minimum in January and May (4.93 and 3.76 μg cm−2, respectively). Mandarin in July had the highest carotenoid content among species (10.30 μg cm−2). The interspecific mean value of the total carotenoids/total chlorophyll ratio (total Car/Chl; data not shown) was minimum in July (0.187) and higher in June, October, and January (0.25, on average).
Regarding non-structural carbohydrates, leaf contents of soluble sugars and starch (Figure 4) were determined in July and January in all Citrus spp.
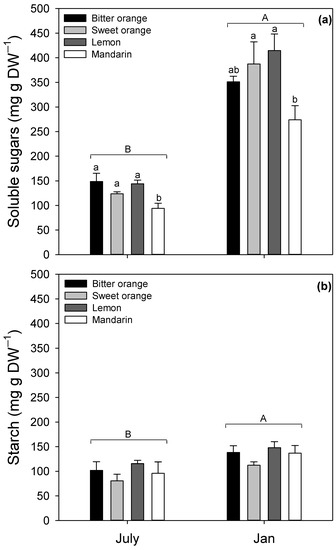
Figure 4.
Leaf content of non-structural carbohydrates (mg g DW−1) in Citrus spp. in January and July: (a) soluble sugars; (b) starch. Data are means ± standard error (n = 5). Statistics as in Figure 1. Different lowercase (a) and uppercase (a,b) letters indicate significant differences among species and months, respectively (p ≤ 0.05, Fisher’s LSD).
Non-structural carbohydrates showed a significant variability among species and months, as evidenced by two-way ANOVA analysis of variance (Table 3). The content of leaf carbohydrates was higher in January compared to July (soluble sugars +179.5%; starch +36%, on average). The interspecific mean content of soluble sugars (Figure 4a) was 127.61 mg g DW−1 in July and 356.76 mg g DW−1 in January, while starch was 98.40 and 133.85 mg g DW−1 in July and January, respectively. Soluble sugars (Figure 4a) ranged from 94.17 mg g DW−1 in mandarin (July) to 414.48 mg g DW−1 in lemon (January) during the whole trial. Intraspecific ranges of soluble sugars (mg g DW−1) were 148.61–351.14 in bitter orange, 144.01–414.48 in lemon, 123.66–387.47 in sweet orange, and 94.17–273.95 in mandarin. Among the species, lemon had the highest content of soluble sugars during the trial (414.48 mg g DW−1 in January). Starch content (Figure 4b) varied from 80.63 mg g DW−1 in sweet orange (July) to 148.02 mg g DW−1 in lemon (January). Intraspecific ranges of starch (mg g DW−1) resulted in 101.81–138.29 in bitter orange, 115.50–148.02 in lemon, 80.63–112.32 in sweet orange, and 95.68–136.77 in mandarin. Among the species, lemon showed the highest starch content during the whole trial (148.02 mg g DW−1 in January). Starch resulted in lower than soluble sugars both in January and July (–62.48% and –22.80%, respectively).
For TPC and TAC, all the variables resulted in statistically significant two-way ANOVA analysis of variance, as reported in Table 3. Interspecific mean values of leaf TPC (Figure 5a) showed a minimum in July and a peak of accumulation in January (8.03 and 13.02 mg GAE g DW−1, respectively), with an average increase by 62%.
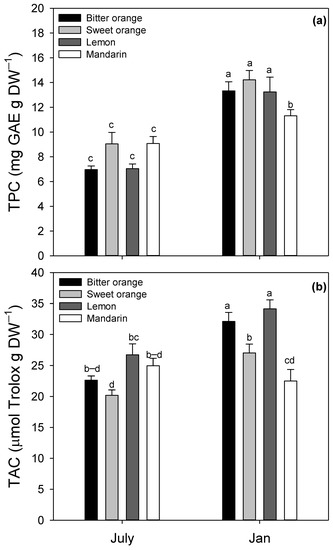
Figure 5.
Leaf content of (a) total phenolic compounds (TPC, mg GAE g DW−1) and (b) total antioxidant capacity (TAC, μmol Trolox g DW−1) in Citrus spp. in January and July. Data are means ± standard error (n = 5). Statistics as in Figure 1. Different lowercase letters indicate significant differences among means (p ≤ 0.05, Fisher’s LSD).
Leaf TPC exhibited a significant variability among species and months, as evidenced by two-way ANOVA. TPC ranged from 6.96 mg GAE g DW−1 in bitter orange (July) to 14.22 mg GAE g DW−1 in sweet orange (January). Ranges of TPC (mg GAE g DW−1) were 6.96–13.33 in bitter orange, 7.04–13.24 in lemon, 9.04–14.22 in sweet orange, and 9.07–11.31 in mandarin. Among the species, sweet orange showed the highest TPC during the whole trial (January). Leaf TAC displayed a pattern similar to TPC (Figure 5b). The interspecific mean value of TAC was 23.63 μmol Trolox g DW−1 in July, averagely increasing by 22.40% up to 28.94 μmol Trolox g DW−1 in January. Among the species, TAC was the highest in lemon in January and lowest in sweet orange in July (34.14 and 20.19 μmol Trolox g DW−1, respectively).
4. Discussion
4.1. Seasonal Variation of Chlorophyll Fluorescence Parameters
Citrus spp. are perennial evergreen plants of tropical origin with C3 metabolism, thus subjected to large seasonal variation of climatic parameters throughout the annual cycle and establishing a complex interaction with the environment [13]. Nevertheless, the information about their physiological responses to climatic changes under natural conditions, especially in the northern hemisphere, is still lacking up to now. The Italian peninsula falls within the Mediterranean region, characterized by a typical climatic seasonality with hot-dry summers and cold-rainy winters [28]. In our test site, classified as a hot-summer Mediterranean climate (Csa), according to Köppen-Geiger [18,20], plants grow without regular crop management practices, thus offering a unique opportunity to study the adaptation of Citrus species to seasonal climatic changes under non-regular cropping conditions in the Mediterranean environment.
Climactic factors play a pivotal role in regulating photosynthesis, although the variability of photosynthetic efficiency in response to seasonal climatic changes in Citrus spp. still need to be fully investigated. Most studies on Citrus photosynthesis were carried out in controlled or semi-controlled trials in subtropical areas [29,30,31,32,33,34], and only a few of them were carried out in the Mediterranean area on field-grown plants throughout the year [15,35]. These studies pointed out the importance of temperature dynamics and daily climatic changes when considering the inter-seasonal variability of photosynthetic rates in Citrus [14,15]. In particular, photosynthetic rates are limited by daily Tmax in summer and Tmin in winter [13,36]. Since the plants in our trial were Citrus species of monumental value grown under unmanaged conditions, the direct comparison of photosynthetic and biochemical results with available data on Citrus orchards under regular cropping conditions needs to take into account the differences in agronomic management and plant material. However, the interspecific mean values of Fv/Fm in our study were comparable to those reported in [15] during the summer-autumn period (from June to October) and were very close to the optimal value of around 0.8 measured for most plant species [37]. Conversely, contrary to what observed in [15], the potential photochemical efficiency of PSII drastically decreased in winter, reaching values of 0.507 in January. Since a Fv/Fm < 0.725 denotes the occurrence of photoinhibition in Citrus spp. [13], our data clearly indicate a marked photoinhibition due to low winter temperatures, also observed by [35]. Hence, the higher winter Fv/Fm values observed in Valencia (Spain) [15], could be explained considering that this site is located in a climatic area classified as semi-arid Mediterranean (BsK), with warmer winters compared to our site (Csa) [18]. As reported in Table 2, winter 2019 was rather rigid in our trial site (monthly mean T = 5.3 °C; Tmin = 0.8 °C in January). In January, all Citrus spp. showed an average reduction of Fv/Fm by 37% compared to summer-autumn, thus greater than the decrease by 23.5% from September to January found for Citrus spp. grown in a climatically equivalent Mediterranean area (San Giuliano, Corsica, France; Csa according to Köppen-Geiger) [35].
Citrus spp. have a low temperature threshold of 13 °C, under which the photosynthetic metabolism is seriously disturbed [32,35]. Even if Citrus spp. can tolerate temperatures from 0 °C to −7 °C for brief periods, they are considered cold tender plants [38]. Plants of tropical origin, such as Citrus spp., show a limited ability to acclimate to low temperatures in cold periods of the northern hemisphere, when photosynthesis, stomatal conductance, and chlorophyll content decrease [11]. Ecophysiological studies referred to low air temperatures as a relevant climatic parameter inhibiting photosynthetic rates and enzymatic activities in the Calvin cycle; in particular, low night Tair is the main factor responsible for winter photoinhibition in Citrus, stomatal closure, and reduced Rubisco carboxylation [13,35,39,40], while low Tsoil disrupts root functionality and decreases shoot hydration due to a higher hydraulic resistance [32]. Moreover, in a recent study, Fv/Fm declined of 35% in Citrus plants exposed to freezing temperatures (−7 °C, 36 h) clearly indicated a marked reduction of the photochemical activity of PSII in cold-stressed leaves [41]. Photoinhibition is a photoprotective mechanism based on a decrease of energy at PSII level to avoid photodamage or photooxidation [42]. Dynamic photoinhibition is frequent in Citrus leaves: Fv/Fm rapidly changes in response to daily climatic conditions, recovering within hours as Citrus do not show photodamage [13,15,31,32,33,43]. Hence, the reduced Fv/Fm during winter could represent a photoprotective mechanism to avoid irreversible photodamage in the peculiar microenvironmental conditions of our site.
The optimal range for photosynthesis in Citrus is 25–30 °C, thus the lowest photosynthetic rates occur in summer and winter with daily temperatures outside this range [15]. As perennial plants exposed to large environmental variations, Citrus spp. dispose of a photosynthetic apparatus that can acclimate to high temperatures, and in subtropical climates, their photosynthesis is slightly limited up to 40 °C, given that plants enhance the thermal stability of chloroplast membranes and photosynthetic enzymes. A lethal temperature of 55 °C makes severe heat injuries in Citrus unlikely [13,34]. In our test site, monthly maximum temperatures fell within the optimal photosynthetic range of 25–30 °C, indicated in [15], only in June and July (26.2 °C and 29.2 °C, respectively). The maximum seasonal values of ΦPSII and NPQ were recorded in July, highlighting a high energy dissipation capacity of Citrus spp. in summer through both photochemical and non-photochemical processes, especially in lemon and sweet orange. This high energy dissipation capacity in summer was associated with the highest seasonal values of both chlorophyll and carotenoid concentrations, suggesting that these Citrus species were able to maintain an optimal photosynthetic activity and safely dissipate the excess light energy in summer despite the strong reduction in precipitation and lowering of leaf water status. These data agree with previous findings, showing that high irradiance in summer did not impair the photosynthesis in orange trees due to the concomitant increases of heat dissipation of excessive light energy at PSII level and other metabolic electron sink processes, which avoid photoinhibition under high irradiance conditions [32,33]. Moreover, other authors reported that Citrus species exhibited the maximum seasonal photosynthetic rates during the spring-summer period [13,44,45]. Among the Citrus species studied, lemon showed the highest photochemistry activity of PSII in July, associated with relatively higher chlorophyll and RWC values, highlighting how the photosynthetic apparatus of this species was particularly suitable for withstanding the hot and dry Mediterranean summer conditions of the site.
4.2. Leaf Structural and Biochemical Traits
Leaf RWC plays a role as an indicator of the degree of drought stress by providing a measurement of the leaf water deficit [46]. The climatic conditions (e.g., monthly precipitations and temperatures; Table 1) affected leaf RWC of all Citrus spp. in our trial (Figure 2). The minimum interspecific average RWC occurred in July (46.7%), during the summer drought, which typically occurs in this Mediterranean area. Despite the minimum seasonal value of RWC, the contents of Chl tot and Car were maximum in July independently by species (Figure 3), suggesting that leaf pigment content mainly depends on seasonal changes of intensity and composition of the solar radiation but only marginally on precipitations and plant water status. This observation is also supported by previous results in Citrus not being significantly affected by water stress [47]. In fact, leaf chlorophyll content in Citrus is influenced by thermal environment, since sweet orange plants displayed a higher total chlorophyll content with air temperature (Tair) of 30 °C compared to 25 °C [34]. Thus, the higher content of Chl tot in July is probably because Tair in our trial site peaked during the period from July to August, with frequent occurrence of the daily maximum Tair > 30 °C (Table 2). On the other hand, the minimum chlorophyll leaf contents detected in January in all Citrus spp. (Figure 3) can be deduced as a consequence of the aforementioned winter photoinhibition due to low air and soil temperatures, as observed by other authors [11,32,33]. Multiple factors can affect Chl tot content in Citrus mature leaves (i.e., light intensity, temperatures, nutrition, pathogens, genetic factors); nevertheless, our data were in agreement with a previous work [48]. Moreover, our values of Chl tot and Car during the year (expressed as mg m−2) were consistent with those (388 and 94 mg m−2, respectively) reported in orange leaves [49] and also supported by a similar Car/Chl ratio (our interspecific mean Chl a/b ratio was 2.72 in July and 3.85 in October). Furthermore, mean Chl a/b ratio during the whole trial (3.18) was concordant with [47] and [50] in mature, turgid Citrus leaves (3.33 and 3.37, respectively). It is worth noting that the highest seasonal content of carotenoids was recorded in concomitance with the lowest leaf starch concentration, according with [51], in which the carotenoid accumulation is associated with starch degradation in Citrus.
Although the environmental conditions account for most of the variation of photosynthetic rates in Citrus [15], leaf carbohydrates can modulate photosynthesis through a mechanism of source-sink imbalance [13,14,15,52,53]. The seasonal pattern of carbohydrates in all tissues of Citrus spp. has been previously investigated [54], showing a gradual decrease of sugars and starch contents from anthesis (late spring) until the end of fruit set (summer) due to a strong sink activity, followed by an accumulation in autumn and a peak in mid-winter (December-January). This winter accumulation of leaf carbohydrates can play a key role for cold tolerance in Citrus [54]. In our work, the leaf content of non-structural carbohydrates (soluble sugars and starch) increased by 217% in January compared to July, and this seasonal pattern is consistent according to [54]. Evidence presented here confirmed the negative correlation between starch accumulation and photosynthesis observed in Citrus leaves [52]; furthermore, the lower content of leaf carbohydrates in July, due to the strong sink demand of flowers and fruits, suggests an effect of stimulation on photosynthesis [53], since the highest photosynthetic activity, evaluated as photochemical efficiency, occurred in July throughout the whole trial.
Generally, Citrus spp. are considered vulnerable to the cold temperatures of winter [38,41]. Chilling (0–12 °C) increased electrolyte leakage, due to the loss of cell membrane integrity, while decreasing photosynthetic and respiration rates; moreover, the lower CO2 availability and CO2/O2 ratio in chloroplasts directs the electron flow to O2 with consequent oxidative stress and overproduction of ROS [35,55].
In our trial (Table 1), the monthly mean temperature in January 2019 was 5.3 °C, therefore inducing chilling and higher leaf contents of soluble sugars and starch (Figure 4a,b). This situation has already been reported in Citrus spp. [38,56] and in Olea europaea L., another evergreen species traditionally widespread in Tuscany [57]. Furthermore, a recent proteomics study [41] has clearly demonstrated the increase of soluble sugars and starch in Citrus leaves during cold stress. Citrus plants with high sugar/starch ratios in leaves can survive freezing without injury [11]. An additional physiological ground is the relation between the winter increase in carbohydrate content and the synthesis of soluble carbohydrates acting as osmolytes during cold stress acclimatation [58]. In this regard, the soluble sucrose is accumulated as cryoprotectant and osmolyte, playing a pivotal role against the dehydration caused by freezing [59].
Citrus leaves are known to be a rich source of polyphenols with antioxidant properties, such as flavonoids, flavonols, anthocyanins, polymerized phenols, and hydrolyzable tannins [7,60]. Regarding the likely ROS inactivation, TPC and TAC were analyzed in January and July in the leaves of all Citrus spp. In our study, the interspecific mean values of leaf TPC and TAC in January increased by 62.1% and 22.4%, respectively, compared to July (Figure 5), and the values are consistent with previous data reported for Citrus leaves [61,62]. Phenylalanine ammonia lyase (PAL) activity in Citrus leaves is stimulated by cold stress, with consequent higher levels of phenylpropanoid secondary metabolites and accumulation of polyphenols with antioxidant activity, able to modulate ROS homeostasis [41,58]. In this regard, Tajvar et al. (2011) found a gradual increase of the antioxidant capacity in orange leaves during chilling (0–9 °C), reaching maximum values with freezing (from 0 °C to −6 °C) [38]. Considering that the Mediterranean climate is characterized by temperatures with wide seasonal excursions, the patterns of leaf TPC and TAC in our study (Figure 5), both higher in January, denote the key role of cold stress in the accumulation of polyphenols with antioxidant activity [38,41].
The occurrence of these environmental conditions in our trial site, inducing both chilling and freezing, explains the higher leaf content of carbohydrates in January and also the simultaneous increase of leaf TPC and TAC, consistent with previous data reported [38,41]. The highly positive correlation (data not shown) also found between the contents of leaf TPC and total soluble sugars is further evidence that phenols and non-structural carbohydrates paralleled in our trial. In our study, Citrus spp. showed the same seasonal changes, although mandarin exhibited winter values of TPC, TAC, and soluble sugars lower than other species, suggesting less activation of the antioxidant system and osmolyte protection to counteract the cold-induced photoinhibition. It is worth mentioning that, in a previous study [35], mandarin appeared to be most tolerant to low temperatures in comparison to pummelo and kumquat.
As a whole, chlorophyll fluorescence measurements indicate that Citrus spp. in the present study were well adapted to their microenvironment and climatic seasonal changes, maintaining the photosynthetic activity, even in the absence of proper nutrient supplies and regular cropping conditions. Our data are consistent with the previously observed occurrence of winter photoinhibition in Citrus, since both photochemical efficiency of photosystem II and leaf chlorophyll contents (a, b, total) displayed minimum values in January. On the other hand, all the Citrus species showed an optimal photosynthetic activity and leaf pigment concentration during the summer period, independent of the reduction in water availability. Among Citrus spp., lemon exhibited the best adaptability of the photosynthetic apparatus to Mediterranean summer conditions, associated with the maintenance of a better leaf water status. The source-sink imbalance regulates the leaf carbohydrate content through the seasons, in relation to the climatic conditions, the photosynthetic efficiency, and the phenological stage. The low temperatures observed in winter lead to an increase of leaf antioxidant compounds (polyphenols) to modulate ROS homeostasis. The winter increases in the patterns of accumulation of leaf carbohydrates and TPC, also observed in different species [63], must be considered an example of the complex interactions of primary and secondary metabolism in the responses of Citrus spp. to cold stress.
5. Conclusions
In the Charterhouse gardens, the ornamental Citrus trees have historical and landscape values, and this study represents a promising start point to determine their physiological status. In the present trial, these plants exhibited the ability to adapt and compensate to environmental limitations and showed the critical importance of the acclimation of photosynthesis and antioxidant system as a survival strategy for Citrus spp. in the Mediterranean environment within the current global climate change. These Citrus spp. could offer the opportunity to develop and valorize sustainable by-products, acquiring a great potential as sources of raw materials and valuable bio-compounds.
Author Contributions
Conceptualization, L.P. (Laura Pistelli), A.S. and L.P. (Luisa Pistelli); methodology, M.C., L.P. (Laura Pistelli), and A.S.; software, A.S. and I.M.; validation, M.C., A.S. and L.P. (Laura Pistelli); formal analysis, M.M., M.C. and A.S.; investigation, L.P. (Laura Pistelli), A.S., M.C. and L.P. (Luisa Pistelli); resources, L.P. (Luisa Pistelli); data curation, M.C.; writing—original draft preparation, M.C., A.S. and L.P. (Laura Pistelli); writing—review and editing, M.C., A.S. and L.P (Laura Pistelli); visualization, L.P. (Laura Pistelli), A.S., M.C. and L.P. (Luisa Pistelli); supervision, A.S. and L.P. (Laura Pistelli); project administration, L.P. (Luisa Pistelli); funding acquisition, L.P. (Luisa Pistelli). All authors have read and agreed to the published version of the manuscript.
Funding
This work was part of the research project “European Project INTERREG-MARITTIMO Mare di agrumi, tourist brand, and green biotechnologies for the development of companies on a common resource: Citrus fruits” (CUP C26D16007240) (Luisa Pistelli).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors thank the Natural History Museum of the University of Pisa for the opportunity to study the Citrus trees located in the monastic gardens of the Monumental Charterhouse of Calci (Pisa, Italy).
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- El Sawi, S.A.; Ibrahim, M.E.; El-Rokiek, K.G.; El-Din, S.A.S. Allelopathic potential of essential oils isolated from peels of three citrus species. Ann. Agric. Sci. 2019, 64, 89–94. [Google Scholar] [CrossRef]
- Wu, G.A.; Prochnik, S.; Jenkins, J.; Salse, J.; Hellsten, U.; Murat, F.; Perrier, X.; Ruiz, M.; Scalabrin, S.; Terol, J.; et al. Sequencing of diverse mandarin, pummelo and orange genomes reveals complex history of admixture during citrus domestication. Nat. Biotechnol. 2014, 32, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Curk, F.; Ancillo, G.; Ollitrault, F.; Perrier, X.; Jacquemoud-Collet, J.P.; Garcia-Lor, A.; Navarro, L.; Ollitrault, P. Nuclear species-diagnostic SNP markers mined from 454 amplicon sequencing reveal admixture genomic structure of modern Citrus varieties. PLoS ONE 2015, 10, e0125628. [Google Scholar] [CrossRef] [PubMed]
- USDA (United States Department of Agriculture-Foreign Agricultural Service). USDA Crop Statistics. 2020. Available online: https://apps.fas.usda.gov/psdonline/app/index.html#/app/advQuery (accessed on 6 July 2021).
- Panwar, D.; Saini, A.; Panesar, P.S.; Chopra, H.K. Unraveling the scientific perspectives of citrus by-products utilization: Progress towards circular economy. Trends Food Sci. Technol. 2021, 111, 549–562. [Google Scholar] [CrossRef]
- Menichini, F.; Loizzo, M.R.; Bonesi, M.; Conforti, F.; De Luca, D.; Statti, G.A.; de Cindio, B.; Menichini, F.; Tundis, R. Phytochemical profile, antioxidant, anti-inflammatory and hypoglycemic potential of hydroalcoholic extracts from Citrus medica L. cv Diamante flowers, leaves and fruits at two maturity stages. Food Chem. Toxicol. 2011, 49, 1549–1555. [Google Scholar] [CrossRef]
- Lagha-Benamrouche, S.; Madani, K. Phenolic contents and antioxidant activity of orange varieties (Citrus sinensis L. and Citrus aurantium L.) cultivated in Algeria: Peels and leaves. Ind. Crops Prod. 2013, 50, 723–730. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Taglieri, I.; Sgherri, C.; Flamini, G.; Macaluso, M.; Sanmartin, C.; Venturi, F.; Quartacci, M.F.; Pistelli, L.; Zinnai, A. Nutraceutical oils produced by olives and Citrus peel of Tuscany varieties as sources of functional ingredients. Molecules 2019, 24, 65. [Google Scholar] [CrossRef]
- Haraoui, N.; Allem, R.; Chaouche, T.M.; Belouazni, A. In-vitro antioxidant and antimicrobial activities of some varieties citrus grown in Algeria. Adv. Tradit. Med. 2020, 20, 23–34. [Google Scholar] [CrossRef]
- Inglese, P.; Bellavia, G.P. The Citrus in the Mediterranean Region. In Integrated Control of Citrus Pests in the Mediterranean Region; Vacante, V., Gerson, U., Eds.; Bentham Science Publisher: Sharjah, United Arab Emirates, 2012; pp. 3–18. [Google Scholar] [CrossRef]
- Vu, J.C.V. Photosynthetic Responses of Citrus to Environmental Changes. In Handbook of Plant and Crop Stress, 2nd ed.; Pessarakli, M., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1999; pp. 947–962. [Google Scholar]
- Martínez-Alcántara, B.; Quiñones, A.; Forner-Giner, M.A.; Iglesias, D.J.; Primo-Millo, E.; Legaz, F. Impact of fertilizer-water management on nitrogen use efficiency and potential nitrate leaching in citrus trees. Soil. Sci. Plant Nutr. 2012, 58, 659–669. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C. Some aspects of Citrus ecophysiology in subtropical climates: Revisiting photosynthesis under natural conditions. Braz. J. Plant Physiol. 2007, 19, 393–411. [Google Scholar] [CrossRef]
- Nebauer, S.G.; Renau-Morata, B.; Guardiola, J.L.; Molina, R.V. Photosynthesis down-regulation precedes carbohydrate accumulation under sink limitation in Citrus. Tree Physiol. 2011, 31, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Nebauer, S.G.; Arenas, C.; Rodríguez-Gamir, J.; Bordón, Y.; Fortunato-Almeida, A.; Monerri, C.; Guardiola, J.L.; Molina, R.V. Crop load does not increase the photosynthetic rate in Citrus leaves under regular cropping conditions. A study throughout the year. Sci. Hortic. 2013, 160, 358–365. [Google Scholar] [CrossRef]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of fruit industrial by-products—A case study on circular economy approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, I.; Sanmartin, C.; Venturi, F.; Macaluso, M.; Bianchi, A.; Sgherri, C.; Quartacci, M.F.; De Leo, M.; Pistelli, L.; Palla, F.; et al. Bread fortified with cooked purple potato flour and Citrus albedo: An evaluation of its compositional and sensorial properties. Foods 2021, 10, 942. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Seager, R.; Osborn, T.J.; Kushnir, Y.; Simpson, I.R.; Nakamura, J.; Liu, H. Climate variability and change of Mediterranean-type climates. J. Clim. 2019, 32, 2887–2915. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Cercós, M.; Colmenero-Flores, J.M.; Naranjo, M.A.; Ríos, G.; Carrera, E.; Ruiz-Rivero, O.; Lliso, I.; Morillon, R.; Tadeo, F.R.; et al. Physiology of Citrus fruiting. Braz. J. Plant Physiol. 2007, 19, 333–362. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta Gen. Subj. 1989, 900, 87–92. [Google Scholar] [CrossRef]
- Huarancca Reyes, T.; Scartazza, A.; Pompeiano, A.; Ciurli, A.; Lu, Y.; Guglielminetti, L.; Yamaguchi, J. Nitrate reductase modulation in response to changes in C/N balance and nitrogen source in Arabidopsis. Plant Cell Physiol. 2018, 59, 1248–1254. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Das, B.; Choudhury, B.; Kar, M. Quantitative estimation of changes in biochemical constituents of mahua (Madhuca indica syn. Bassia latifolia) flowers during postharvest storage. J. Food Process. Preserv. 2010, 34, 831–844. [Google Scholar] [CrossRef]
- Pistelli, L.; D’Angiolillo, F.; Morelli, E.; Basso, B.; Rosellini, I.; Posarelli, M.; Barbafieri, M. Response of spontaneous plants from an ex-mining site of Elba island (Tuscany, Italy) to metal(loid)s contamination. Environ. Sci. Pollut. R. 2017, 24, 7809–7820. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rana, G.; Katerji, N. Measurement and estimation of actual evapotranspiration in the field under Mediterranean climate: A review. Europ. J. Agron. 2000, 13, 125–153. [Google Scholar] [CrossRef]
- Vu, J.C.V.; Yelenosky, G. Water deficit and associated changes in some photosynthetic parameters in leaves of ‘Valencia’ orange (Citrus sinensis L. Osbeck). Plant Physiol. 1988, 88, 375–378. Available online: https://www.jstor.org/stable/4271580 (accessed on 14 March 2022). [CrossRef]
- Jifon, J.L.; Syvertsen, J.P. Moderate shade can increase net gas exchange and reduce photoinhibition in Citrus leaves. Tree Physiol. 2003, 23, 119–127. [Google Scholar] [CrossRef]
- Guo, Y.P.; Zhou, H.F.; Zhang, L.C. Photosynthetic characteristics and protective mechanisms against photooxidation during high temperature stress in two citrus species. Sci. Hortic. 2006, 108, 260–267. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Photosynthesis and water relations of well-watered orange plants as affected by winter and summer conditions. Photosynthetica 2009, 47, 215–222. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G.; Oliveira, R.F. Seasonal and diurnal changes in photosynthetic limitation of young sweet orange trees. Environ. Exper. Bot. 2009, 66, 203–211. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Espinoza-Núñez, E.; Ramos, R.A.; Machado, D.F.S.P. Moderate warm temperature improves shoot growth, affects carbohydrate status and stimulates photosynthesis of sweet orange plants. Braz. J. Plant Physiol. 2012, 24, 37–46. [Google Scholar] [CrossRef][Green Version]
- Santini, J.; Giannettini, J.; Pailly, O.; Herbette, S.; Ollitrault, P.; Berti, L.; Luro, F. Comparison of photosynthesis and antioxidant performance of several Citrus and Fortunella species (Rutaceae) under natural chilling stress. Trees 2013, 27, 71–83. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Oliveira, R.F. Temperature response of photosynthesis and its interaction with light intensity in sweet orange leaf discs under nonphotorespiratory condition. Ciênc. Agrotecnol. 2006, 30, 670–678. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Tajvar, Y.; Fotouhi Ghazvini, R.; Hamidoghli, Y.; Sajedi, R. Antioxidant changes of Thomson navel orange (Citrus sinensis) on three rootstocks under low temperature stress. Hort. Environ. Biotechnol. 2011, 52, 576–580. [Google Scholar] [CrossRef]
- Allen, D.J.; Ort, D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001, 6, 36–42. [Google Scholar] [CrossRef]
- Santos, C.M.A.; Ribeiro, R.V.; Magalhães Filho, J.R.; Machado, D.F.S.P.; Machado, E.C. Low substrate temperature imposes higher limitation to photosynthesis of orange plants as compared to atmospheric chilling. Photosynthetica 2011, 49, 546–554. [Google Scholar] [CrossRef]
- Jiang, J.; Hou, R.; Yang, N.; Li, L.; Deng, J.; Qin, G.; Ding, D. Physiological and TMT-labeled proteomic analyses reveal important roles of sugar and secondary metabolism in Citrus junos under cold stress. J. Proteom. 2021, 237, 104145. [Google Scholar] [CrossRef]
- Keutgen, N.; Chen, K. Responses of citrus leaf photosynthesis, chlorophyll fluorescence, macronutrient and carbohydrate contents to elevated CO2. J. Plant Physiol. 2001, 158, 1307–1316. [Google Scholar] [CrossRef]
- Blanke, M.M. Photoinhibition in Citrus. In Proceedings of the International Society of Citriculture IX Congress, ISC2000, Orlando, FL, USA, 3–7 December 2000; pp. 619–622. [Google Scholar]
- Pimentel, C.; Bernacchi, C.; Long, S. Limitations to photosynthesis at different temperatures in the leaves of Citrus limon. Braz. J. Plant Physiol. 2007, 19, 141–147. [Google Scholar] [CrossRef]
- Insausti, P.; PLoSchuk, E.L.; Izaguirre, M.M.; Podworny, M. The effect of sunlight interception by sooty mold on chlorophyll content and photosynthesis in orange leaves (Citrus sinensis L.). Eur. J. Plant Pathol. 2015, 143, 559–565. [Google Scholar] [CrossRef]
- Mullan, D.; Pietragalla, J. Leaf Relative Water Content. In Physiological Breeding II: A Field Guide to Wheat Phenotyping; Pask, A.J.D., Pietragalla, J., Mullan, D.M., Reynolds, M.P., Eds.; CIMMYT: Mexico City, Mexico, 2012; pp. 25–27. [Google Scholar]
- Norman, S.M.; Maier, V.P.; Pon, D.L. Abscisic acid accumulation and carotenoid and chlorophyll content in relation to water stress and leaf age of different types of Citrus. J. Agric. Food Chem. 1990, 38, 1326–1334. [Google Scholar] [CrossRef]
- Turrell, F.M.; Weber, J.R.; Austin, S.W. Chlorophyll content and reflection spectra of Citrus leaves. Bot. Gaz. 1961, 123, 10–15. [Google Scholar] [CrossRef]
- Han, S.; Chen, L.; Jiang, H.; Smith, B.R.; Yang, L.; Xie, C. Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings. J. Plant Physiol. 2008, 165, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Melgar, J.C.; Syvertsen, J.P.; Martínez, V.; García-Sánchez, F. Leaf gas exchange, water relations, nutrient content and growth in citrus and olive seedlings under salinity. Biol. Plant. 2008, 52, 385–390. [Google Scholar] [CrossRef]
- Cao, H.; Wang, J.; Dong, X.; Han, Y.; Ma, Q.; Ding, Y.; Zhao, F.; Zhang, J.; Chen, H.; Xu, Q.; et al. Carotenoid accumulation affects redox status, starch metabolism, and flavonoid/anthocyanin accumulation in citrus. BMC Plant Biol. 2015, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, D.J.; Lliso, I.; Tadeo, F.R.; Talon, M. Regulation of photosynthesis through source: Sink imbalance in citrus is mediated by carbohydrate content in leaves. Physiol. Plant. 2002, 116, 563–572. [Google Scholar] [CrossRef]
- Ribeiro, R.V.; Machado, E.C.; Santos, M.G. Leaf temperature in sweet orange plants under field conditions: Influence of meteorological elements. Rev. Bras. Agrometeorol. 2005, 13, 353–368. [Google Scholar]
- Mataa, M.; Tominaga, S.; Kozaki, I. Seasonal changes of carbohydrate constituents in Ponkan (Citrus reticulata Blanco). J. Jpn. Soc. Hort. Sci. 1996, 65, 513–523. [Google Scholar] [CrossRef]
- Crifò, T.; Puglisi, I.; Petrone, G.; Reforgiato Recupero, G.; Lo Piero, A.R. Expression analysis in response to low temperature stress in blood oranges: Implication of the flavonoid biosynthetic pathway. Gene 2011, 476, 1–9. [Google Scholar] [CrossRef]
- Garcia-Luis, A.; Fornes, F.; Guardiola, J.L. Leaf carbohydrates and flower formation in Citrus. J. Am. Soc. Hortic. Sci. 1995, 120, 222–227. [Google Scholar] [CrossRef]
- Gulen, H.; Cansev, A.; Eris, A. Cold hardiness of olive (Olea europaea L.) cultivars in cold-acclimated and non-acclimated stages: Seasonal alteration of soluble sugars and phospholipids. J. Agric. Sci. 2009, 147, 459–467. [Google Scholar] [CrossRef]
- Chapman, J.M.; Muhlemann, J.K.; Gayomba, S.R.; Muday, G.K. RBOH-dependent ROS synthesis and ROS scavenging by plant specialized metabolites to modulate plant development and stress responses. Chem. Res. Toxicol. 2019, 32, 370–396. [Google Scholar] [CrossRef] [PubMed]
- Guy, C.L.; Huber, J.L.A.; Huber, S.C. Sucrose phosphate synthase and sucrose accumulation at low temperature. Plant Physiol. 1992, 100, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Muthiah, P.L.; Umamaheswari, M.; Asokkumar, K. In vitro antioxidant activities of leaves, fruits and peel extracts of Citrus. Int. J. Phytopharm. 2012, 2, 13–20. [Google Scholar] [CrossRef][Green Version]
- Ordoñez-Gómez, E.S.; Reátegui-Díaz, D.; Villanueva-Tiburcio, J.E. Polifenoles totales y capacidad antioxidante en cáscara y hojas de doce cítricos. Total polyphenols and antioxidant capacity of peel and leaves in twelve Citrus. Sci. Agropecu. 2018, 9, 113–121. [Google Scholar] [CrossRef]
- Doerfler, H.; Lyon, D.; Nägele, T.; Sun, X.; Fragner, L.; Hadacek, F.; Egelhofer, V.; Weckwerth, W. Granger causality in integrated GC–MS and LC–MS metabolomics data reveals the interface of primary and secondary metabolism. Metabolomics 2013, 9, 564–574. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).