Fungal and Oomycete Diseases of Minor Tropical Fruit Crops
Abstract
1. Introduction
| Dragon Fruit (Hylocereus spp.) | |||
| Disease | Causal Pathogen/ Hylocereus spp. | Country | References |
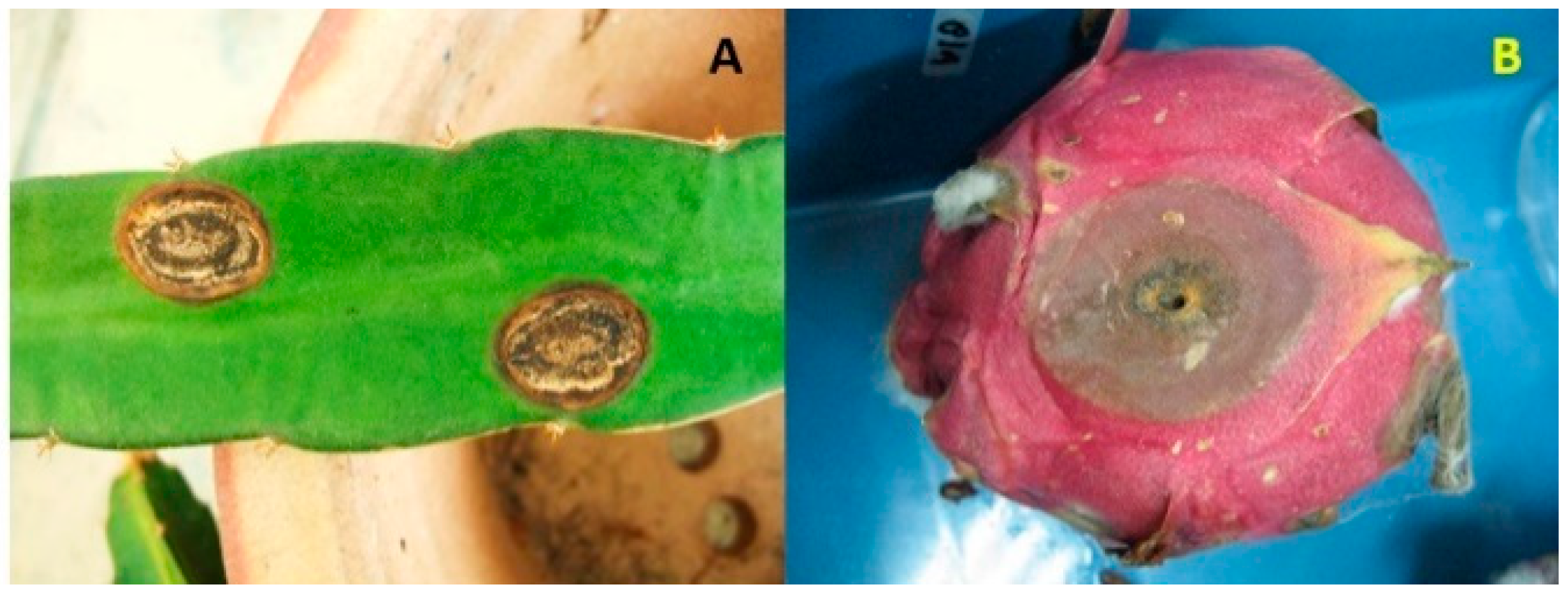
| Anthracnose (fruit and stem) | Colletotrichum gloeosporioides sensu lato (stem of H. undatus; stem of H. megalanthus; stem and fruit of Hylocereus spp.) | Miami-Dade County, Florida, USA; Brazil, Malaysia | Palmateer et al. [4], Takahashi et al. [5], Masyahit et al. [6] |
| Colletotrichum gloeosporioides (young stem and fruit of H. undatus) | China, Taiwan | Ma et al. [7], Lin et al. [8] | |
| Colletotrichum siamense (stem and fruit of H. undatus; stem of H. polyrhizus) | Thailand, China | Meetum et al. [9], Zhao et al. [10] | |
| Colletotrichum karstii (stem of H. undatus) | Brazil | Nascimento et al. [11] | |
| Colletotrichum fructicola (stem of H. undatus and H. monacanthus) | the Philippine | Evallo et al. [12] | |
| Colletotrichum truncatum (fruit of H. undatus, stem of H. polyrhizus) | Malaysia, China | Guo et al. [13], Iskandar Vijaya et al. [14] | |
| Colletotrichum aenigma (stem and fruit of H. undatus) | Thailand | Meetum et al. [9] | |
| Colletotrichumsiamense (stem of H. undatus) | Andaman Islands, India | Abirami et al. [15] | |
| Stem lesion/spot | Curvularia lunata (stem of H. polyrhizus) | Malaysia | Masratul Hawa et al. [16] |
| Storage fruit rot | Gilbertella persicaria (H. costaricensis) | China | Guo et al. [17] |
| Fruit blotch and stem rot | Bipolaris cactivora (H. undatus) | South Florida, Israel, Thailand, Vietnam | Tarnowski et al. [18], Ben-Ze’ev et al. [19], He et al. [20], Oeurn et al. [21] |
| Stem blight | Alternaria sp. (H. undatus) | South Florida, USA | Patel and Zhang [22] |
| Post-harvest disease | Alternaria alternata (H. undatus) | Brazil | Castro et al. [23] |
| Stem and Fruit Spot | Aureobasidium pullulans (Hylocereus spp.) | China | Wu et al. [24] |
| Stem blight | Sclerotium rolfsii (H. undatus) | China | Zheng et al. [25] |
| Stem reddish brown spot | Nigrospora sphaerica (H. undatus) | China | Liu et al. [26] |
| Stem reddish brown spot | Nigrospora lacticolonia and N. sphaerica (H. polyrhizus) | Malaysia | Kee et al. [27] |
| Stem canker, black rot, brown spot, fruit internal browning, fruit canker | Neoscytalidium dimidiatum (H. undatus and H. monacanthus) | Israel, Taiwan, Malaysia, China; Florida, USA; Puerto Rico | Chuang et al. [28], Lan et al. [29], Ezra et al. [30], Masratul Hawa et al. [31], Yi et al. [32], Sanahuja et al. [33], Serrato-Diaz and Goenaga [34] |
| Stem gray blight | Diaporthe arecae, Diaporthe eugeniae, Diaporthe hongkongensis, Diaporthe phaseolorum, and Diaporthe tectonendophytica (H. polyrhizus) | Malaysia | Huda-Shakirah et al. [35] |
| Stem rot | Diaporthe ueckerae (H. polyrhizus and H. undatus) | Taiwan | Wang et al. [36] |
| Fusarium proliferatum, Fusarium fujikuroi (H. polyrhizus) | Malaysia | Masratul Hawa et al. [37], [38] | |
| Fusarium solani (Hylocereus sp.) | Bali, Indonesia Banyuwangi Regency, Indonesia | Rita et al. [39], Sholihah et al. [40] | |
| Fusarium sp. (Hylocereus sp.) | Lombok Utara and Central Bangka Regency, Indonesia | Isnaini et al. [41], Kurniasari et al. [42] | |
| Neocosmospora rubicola/F. solani species complex (H. costaricensis) | Dongfang, Hainan Province, China | Zheng et al. [43] | |
| Basal rot | Fusarium oxysporum (H. undatus, Selenicereus megalanthus, H. polyrhizus) | Gran Buenos Aires, Argentina; Colombia, Bangladesh | Wright et al. [44], Salazar-González et al. [45], Mahmud et al. [46] |
| Stem blight | Fusarium oxysporum (H. polyrhizus) | Malaysia | Mohd Hafifi et al. [47] |
| Fruit rot | Fusarium lateritium, Fusarium semitectum | Vietnam | Le et al. [48] |
| Fusarium oxysporum, Fusarium dimerum (H. undatus) | Shanghai, China | Zhi-Jing et al. [49] | |
| Fusarium dimerum, Fusarium equiseti (H. undatus) | Mekong, Delta, Vietnam | Ngoc et al. [50] | |
| Guava (Psidium guajava L.) | |||
| Disease | Causal pathogen | Country | References |
| Fusarium wilt | Fusarium oxysporum f. sp. psidii | India | Prasad et al. [51], Misra and Gupta [52] |
| Fusarium oxysporum, Fusarium solani | India | Misra and Pandey [53] | |
| Fusarium proliferatum, Fusarium chlamydosporum | India | Misra and Gupta [52], Gupta and Misra [54] | |
| Fusarium oxysporum f. sp. psidii, Fusarium solani | India | Dwivedi and Dwivedi [55], Misra et al. [56,57], Misra [58] | |
| Fusariumoxysporum f. sp. psidii, Fusarium falciforme | India | Gangaraj et al. [59] | |
| Decline | Fusarium oxysporum f. sp. psidii, Fusarium solani f. sp. psidii (no information on the nematode) | District of Punjab, Pakistan | Aftab [60] |
| Fusarium solani (Meladogyne mayaguensis) | Brazil | Gomes et al. [61] | |
| Fusarium oxysporum (Meloidogyne incognita) | Haryana, India | Madhu et al. [62] | |
| Fusarium oxysporum f.sp. psidii(Meloidogyne enterolobii) | Ratlam district, India | Singh [63] | |
| Fusarium solani (Meloidogyne enterolobii) | Brazil | Veloso et al. [64] | |
| Fusarium solani, Fusarium oxysporum (no information on the nematode) | Pakistan | Khizar et al. [65] | |
| Anthracnose | Colletotrichum gloeosporioides complex | Italy | Weir et al. [66] |
| Colletotrichum siamense complex | India and Mexico | Sharma et al. [67], Rodríguez-Palafox et al. [68] | |
| Colletotrichum abscissum, Colletotrichum simmondsii | Brazil | Bragança et al. [69], Cruz et al. [70] | |
| Colletotrichum guajavae | India | Damm et al. [71] | |
| Crown rot | Fusarium verticillioides | India | Sanjeev and Brijpal [72], Baloch et al. [73] |
| Aspergillus fumigatus, Aspergillus niger, Aspergillus tamarii, Aspergillus japonicus, Aspergillus flavus | Phillipine | Valentino et al. [74] | |
| Fruit rot | Fusarium oxysporum | Egypt, Nigeria | Mathew [75], Amadi et al. [76], Embaby and Korkar, [77], Mairami et al. [78] |
| Aspergillus awamori | Pakistan | Akhtar et al. [79] | |
| Phytophthora nicotianae | Bangladesh | Pervez et al. [80] | |
| Neoscytalidium dimidiatum | Malaysia | Ismail et al. [81] | |
| Lasiodioplodia theobromae | Malaysia | Zee et al. [82] | |
| Canker | Diplodia natalensis, Pestalotia psidii | India | Misra 2012 [83] |
| Passion Fruit (Passiflora edulis Sim.) | |||
| Disease | Causal Pathogen | Country | References |
| Wilt | Fusarium oxysporum f. sp. passiflorae | Brazil, North America, Portugal, New Zealand, | Rooney-Latham et al. [84], Garcia et al. [85], Melo et al. [86], Thangavel et al. [87] |
| Fusrium oxysporum | Iksan and Jeju, Korea | Joa et al. [88] | |
| Fusrium solani | Zimbabwe | Cole et al. [89] | |
| Fusarium incarnatum, Fusarium solani, Fusarium proliferatum | Colombia | Henao-Henao et al. [90] | |
| Fusarium nirenbergiae | Italy | Aiello et al. [91] | |
| Collar rot | Fusarium solani f.sp. passiflorae (Fusarium solani) | Brazil, USA, China, Uganda | Emechebe et al. [92], Ploetz [93], Li et al. [94], Ssekyewa et al. [95], Bueno et al. [96], Marostega et al. [97], Zhou et al. [98] |
| Canker | Fusarium solani Fusarium oxysporum f.sp. pasiflorae | Florida, USA | Manicom et al. [99], Ploetz [100], Anderson and Chambers [101] |
| Fusarium solani | Taiwan and Uganda (reported as Nectria canker) | Emecahebe et al. [92], Lin and Chang [102] | |
| Dieback | Fusarium solani | Kenya | Wangungu et al. [103], Power and Verhoeff [104] |
| Fusarium oxysporum. subglutinans, Fusarium pseudoanthophilum, Fusarium solani, Fusarium. semitectum | Kenya | Amata et al. [105] | |
| Stem bulging | Gibberella fujukuroi, Fusarium sp. | Sri Lanka | Wanniarachchi et al. [106], Rajapaksha et al. [107] |
| Anthracnose | Colletotrichum boninense, Colletotrichum boninense, Colletotrichum truncatum, Colletotrichum gloeosporioides, Glomerella sp. | Florida | Tarnowski and Ploetz [108] |
| Colletotrichum boninense | Brazil | Tozze Jr. et al. [109] | |
| Colletotrichum queenslandicum | Northern Territory, Australia | James et al. [110] | |
| Colletotrichum brevisporum | Fujian Province, China | Du et al. [111] | |
| Colletotrichum truncatum | China and Taiwan | Zhuang et al. [112] Chen and Huang [113] | |
| Colletotrichum brasiliense | China | Shi et al. [114] | |
| Colletotrichum constrictum | Yunnan, China | Wang et al. [115] | |
| Lychee (Litchi chinense Sonn.) | |||
| Disease | Causal Pathogen/Plant Parts | Country | References |
| Anthracnose | Colletotrichum gloeosporioides sensu lato | Fitzell and Coates [116], Coates et al. [117] | |
| Colletotrichum gloeosporioides (immature fruit and asymptomatic flowers) | Mexico | Martinez-Bolanos et al. [118] | |
| Colletotrichum fioriniae (fruit) | China | Ling et al. [119] | |
| Colletotrichum karstii (leaves) | Guangxi, China | Zhao et al. [120] | |
| Pepper spot | Colletotrichum gloeosporioides sensu lato (fruit) | Australia | Cooke and Coates [121], Anderson et al. [122] |
| Colletotrichum siamense (fruit) | Taiwan, China | Ni et al. [123] Ling et al. [124] | |
| Blight of leaf, panicle and fruit | Alternaria alternata | Bihar, India | Kumar et al. [125] |
| Fruit rot (Brown rot) | Fusarium incarnatum | Hainan, China | Guo et al. [126] |
| Downy blight | Phytophthora litchi | Taiwan, Southern China | Kao and Leu [127], Wang et al. [128] |
| Longan (Dimocarpus longan Lour.) | |||
| Disease | Causal Pathogen | Country | References |
| Downy blight (young leaves, panicles, flowers and fruits) | Phytophthora litchi | Taiwan | Ann et al. [129] |
| Inflorescence wilt, vascular and flower necrosis | Fusarium decemcellulare | Puerto Rico | Serrato-Diaz et al. [130] |
| Fruit rot (Brown rot) | Phytophthora palmivora | Thailand | Kooariyakul and Bhavakul [131] |
| Lasiodiplodia theobromae | Puerto Rico | Serrato-Diaz et al. [132] | |
| Lasiodiplodia pseudotherobromae | Thailand | Pipattanapuckdee et al. [133] | |
| Pericarp browning | Phomopsis longanae, L. theobromae | China | Chen et al. [134] Sun et al. [135] |
| Dieback | Lasiodiplodia hormozganensis, Lasiodiplodia iraniensis, Lasiodiplodia pseudotheobromae, and Lasiodiplodia theobromae | Puerto Rico | Serrato-Diaz et al. [136] |
| Inflorescence blight | Lasiodiplodia theobromae | Puerto Rico | Serrato-Diaz et al. [132] |
| Durian (Durio zibethinus L.) | |||
| Disease | Causal Pathogen | Country | References |
| Patch canker or stem canker, fruit rot, seedling dieback, foliar blight and root rot | Phytophthora palmivora | Malaysia, Indonesia, Thailand, Brunei, Vietnam | Pongpisutta and Sangchote [137], Lim [138], Lim [139], Sivapalan et al. [140], Tho et al. [141] |
| Stem rot | Fusarium solani and L. pseudotheobromae | Thailand | Chantarasiri and Boontanom [142] |
| Leaf blight | Rhizoctonia solani | Vietnam and Peninsular Malaysia | Thuan et al. [143], Lim et al. [144] |
| Foliar blight and Dieback | Rhizoctonia solani | Malaysia | Lim et al. [144] |
| Leaf spot | Phomopsis durionis | Thailand | Tongsri et al. [145] |
| Fruit rot | Sclerotium rolfsii | Malaysia | Lim and Kamaruzaman [146] |
| Colletotrichum gloeosporioides, Lasiodiplodia theobromae | Thailand | Sangchote et al. [147] | |
| Aspergillus spp., Penicillium sp., Fusarium equiseti (secondary invaders or weak pathogens) | Brunei | Sivapalan et al. [148] | |
| Durian decline | Pythium vexans, Phytophthora palmivora | Queensland, Australia and Indonesia | O’Gara et al. [149] |
| Phytophthora palmivora, Pythium cucurbitacearum, Pythium vexans | Indonesia | Santoso et al. [150] | |
| Root rot and canker lesion | Phytophthora nicotianae | Sabah, Malaysia | Bong [151] |
| Root and stem rot | Pythium cucurbitacearum, Pythium vexans (syn. Phytopytium vexans), Pythium deliense | Queensland, Australia; Malaysia, Thailand, Indonesia, Vietnam | Lim and Sangchote [152], Vawdrey et al. [153], Thao et al. [154] |
| Rambutan (Nephelium lappaceum L.) | |||
| Disease | Causal Pathogen | Country | References |
| Fruit rot | Botryodiplodiatheobromae, Colletotrichum gloeosporioides, Gliocephalotrichum bulbilium, Pestalotiopsis sp., Phomopsis sp., Glomerella sp. | Hawaii, Puerto Rico, Malaysia, Thailand and Sri Lanka, China | Farungsang et al. [155], Sivakumar et al. [156], Sangchote et al. [157], He et al. [158] |
| Lasmenia sp., Gliocephalotrichum spp., Pestalatiopsis virgatula | Hawaii | Nishijima et al. [159], Keith [160] | |
| Gliocephalotrichum bulbilium, Gliocephalotrichum simplex, Colletotrichum fructicola, Colletotrichum queenslandicum | Puerto Rico | Serrato-Diaz et al. [161], Serrato-Diaz, et al. [162], Serrato-Diaz et al. [163] | |
| Gliocephalotrichum bacillisporum | Malaysia | Intan Sakinah and Latiffah [164] | |
| Corky bark | Dolabra nepheliae | Malaysia, Hawaii, Puerto Rico and Honduras | Booth and Ting [165], Combs et al. [166], Rossman et al. [167] |
| Stem canker | Dolabra nepheliae | Hawaii, Puerto Rico and Honduras | Rossman et al. [168,169] |
| Corky bark and dieback | Lasiodiplodia brasiliensis, L. hormozganensis, Lasiodiplodia iraniensis, Lasiodiplodia pseudotheobromae, Lasiodiplodia theobromae, Neofusicoccum batangarum, Neofusicoccum parvum | Puerto Rico | Serrato-Diaz et al. [130] |
| Powdery mildew | Oidium nephelii | Sri Lanka, the Philippine, Thailand and Malaysia | Garcia [170], Coates et al. [171], Rajapakse et al. [172] |
| Inflorescence wilt, flower and vascular necrosis | Fusarium decemcellulare | Puerto Rico | Serrato-Diaz et al. [130] |
| Leaves necrosis of rambutan seedlings | Pseudocercospora nephelii | Brunei, Malaysia (Sabah and Selangor) | Peregrine et al. [173] |
| Mangosteen (Garcinia mangostana L.) | |||
| Disease | Causal Pathogen | Country | References |
| Leaf blight | Pestalotiopsis flagisettula | Thailand, Malaysia, North Queensland, Australia, Hawaii | Lim and Sangchote [174], Keith and Matsumoto [175] |
| Brown leaf spots and blotches | Pestalotiopsis sp. | Hawaii | Keith and Matsumoto [175] |
| Diplodia fruit rot | Diplodia theobromae | Thailand | Lim and Sangchote [174] |
| Fruit rot | Gliocephalotrichum bulbilium | Guangzhou, China | Li et al. [176] |
| Gliocephalotrichum bulbilium, Graphium sp. | Thailand | Sangchote and Pongpisutta [177] | |
| Mucor irregularis | Wujing Town, Shanghai | Wang et al. [178] | |
| Black aril rot | Lasiodiplodia theobromae | Hawaii | Ketsa and Paull [179] |
| White aril rot | Phomopsis sp. | Hawaii | Ketsa and Paull [179] |
| Soft aril rot | Pestalotiopsis sp. | Hawaii | Ketsa and Paull [179] |
| Decline | Lasidiplodia theobromae, Lasiodiplodia parva | Bahia, Brazil | Paim et al. [180] |
| Brown root rot | Phellinus noxius | - | Lim and Sangchote [174] |
| Stem canker and dieback | Pestalotiopsis sp. | - | Lim and Sangchote [174] |
| Thread blight | Marasmiellus scandens | - | Lim and Sangchote [174] |
2. Fungal Diseases of Dragon Fruit
2.1. Anthracnose
2.2. Stem Rot
2.3. Fruit Rot
3. Fungal Diseases of Guava
3.1. Wilt Disease
3.2. Guava Decline
3.3. Anthracnose
3.4. Crown Rot
3.5. Fruit Rot
3.6. Canker
4. Fungal Diseases of Passion Fruit
4.1. Vascular Wilt
4.2. Collar Rot
4.3. Canker
4.4. Anthracnose
4.5. Brown Spot and Septoria Spot
5. Fungal and Oomycete Diseases of Lychee
6. Fungal and Oomycete Diseases of Longan
7. Fungal and Oomycete Diseases of Durian
8. Fungal Diseases of Rambutan
8.1. Fruit Rot
8.2. Corky Bark, Stem Canker, and Dieback
8.3. Powdery Mildew
8.4. Other Fungal Diseases
9. Fungal Diseases of Mangosteen
10. Management of Fungal and Oomycetes Diseases
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Galán Saúco, V. Potential of minor tropical fruits to become important fruit crops. Acta Hortic. 2013, 975, 581–591. [Google Scholar] [CrossRef]
- Altendorf, S. Minor tropical Fruits (Mainstreaming a niche market). Food Outlook 2018, 8, 67–75. [Google Scholar]
- Ploetz, R.C. Tropical fruit crops and the diseases that affect their production. In Tropical Biology and Conservation Management; Del Claro, K., Oliveira, P.S., Rico-Gray, V., Eds.; EOLSS Publications: Oxford, UK, 2004; Volume 3, pp. 1–22. [Google Scholar]
- Palmateer, A.J.; Ploetz, R.C.; van Santen, E.; Correll, J.C. First occurrence of anthracnose caused by Colletotrichum gloeosporioides on pitahaya. Plant Dis. 2007, 91, 631. [Google Scholar] [CrossRef]
- Takahashi, L.M.; Rosa, D.D.; Basseto, M.A.; de Souza, H.G.; Furtado, E.L. First report of Colletotrichum gloeosporioides on Hylocereus megalanthus in Brazil. Australas. Plant Dis. Notes 2008, 3, 96–97. [Google Scholar] [CrossRef][Green Version]
- Masyahit, M.; Sijam, K.; Awang, Y.; Mohd Satar, M.G. The first report of the occurrence of anthracnose disease caused by Colletotrichum gloeosporioides (Penz.) Penz. & Sacc. on dragon fruit (Hylocereus spp.) in Peninsular Malaysia. Am. J. Appl. Sci. 2009, 6, 902–912. [Google Scholar]
- Ma, W.J.; Yang, X.; Wang, X.R.; Zeng, Y.S.; Liao, M.D.; Chen, C.J.; Sun, S.; Jia, D.M. First report of anthracnose disease on young stems of bawanghua (Hylocereus undatus) caused by Colletotrichum gloeosporioides in China. Plant Dis. 2014, 98, 991–992. [Google Scholar] [CrossRef]
- Lin, C.P.; Ann, P.J.; Huang, H.C.; Chang, T.T.; Tsai, J.N. Anthracnose of pitaya (Hylocereus spp.) caused by Colletotrichum spp., a new postharvest disease in Taiwan. J. Taiwan Agric. Res. 2017, 66, 171–183. [Google Scholar]
- Meetum, P.; Leksomboon, C.; Kanjanamaneesathian, M. First report of Colletotrihum aenigma and C. siamense, the causal agents of anthracnose disease of dragon fruit in Thailand. J. Plant Pathol. 2015, 97, 402. [Google Scholar]
- Zhao, H.J.; Chen, S.C.; Chen, Y.F.; Zou, C.C.; Wang, X.L.; Wang, Z.H.; Liu, A.R.; Ahammed, G.J. First report of red dragon fruit (Hylocereus polyrhizus) anthracnose caused by Colletotrichum siamense in China. Plant Dis. 2018, 102, 1175. [Google Scholar] [CrossRef]
- Nascimento, M.B.; Belle, C.; Azambuja, R.H.; Maich, S.L.; Neves, C.G.; Souza Junior, I.T.; Farias, C.R.; de Barros, D.R. First report of Colletotrichum karstii causing anthracnose spot on pitaya (Hylocereus undatus) in Brazil. Plant Dis. 2019, 103, 2137. [Google Scholar] [CrossRef]
- Evallo, E.; Taguiam, J.D.; Bengoa, J.; Maghirang, R.; Balendres, M. First report of Colletotrichum fructicola, causing anthracnose of Hylocereus plants, in the Philippines. Czech Mycol. 2021, 73, 79–90. [Google Scholar] [CrossRef]
- Guo, L.W.; Wu, Y.X.; Ho, H.H.; Su, Y.Y.; Mao, Z.C.; He, P.F.; He, Y.Q. First report of dragon fruit (Hylocereus undatus) anthracnose caused by Colletotrichum truncatum in China. J. Phytopathol. 2014, 162, 272. [Google Scholar] [CrossRef]
- Iskandar Vijaya, S.; Anuar, I.; Latiffah, Z. Characterization and pathogenicity of Colletotrichum truncatum causing stem anthracnose of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J. Phytopathol. 2014, 163, 67–71. [Google Scholar] [CrossRef]
- Abirami, K.; Sakthivel, K.; Neelam, S.; Bhaskaran, V.; Gautam, R.K.; Jerard, B.A.; Kumar, A. Occurrence of anthracnose disease caused by Colletotrichum siamense on dragon fruit (Hylocereus undatus) in Andaman Islands, India. Plant Dis. 2018, 103, 768. [Google Scholar] [CrossRef]
- Masratul Hawa, M.; Salleh, B.; Latiffah, Z. First report of Curvularia lunata on red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. Plant Dis. 2009, 93, 971. [Google Scholar] [CrossRef]
- Guo, L.W.; Wu, Y.X.; Mao, Z.C.; Ho, H.H.; He, Y.Q. Storage rot of dragon fruit caused by Gilbertella persicaria. Plant Dis. 2012, 96, 1826. [Google Scholar] [CrossRef]
- Tarnowski, T.; Palmateer, A.; Crane, J. First report of fruit rot on Hylocereus undatus caused by Bipolaris cactivora in South Florida. Plant Dis. 2010, 94, 1506. [Google Scholar] [CrossRef]
- Ben-Ze’ev, I.; Assoiline, I.; Levy, E.; Elkind, G. First report of Bipolaris cactivora causing fruit blotch and stem rot of dragon fruit (pitaya) in Israel. Phytoparasitica 2011, 39, 195–197. [Google Scholar] [CrossRef]
- He, P.F.; Ho, H.; Wu, X.X.; Hou, M.S.; He, Y.Q. Bipolaris cactivora causing fruit rot of dragon fruit imported from Vietnam. Plant Pathol. Quar. 2012, 2, 31–35. [Google Scholar] [CrossRef]
- Oeurn, S.; Jitjak, W.; Sanoamuang, N. Fungi on dragon fruit in Loei Province, Thailand and the ability of Bipolaris cactivora to cause post-harvest fruit rot. KKU Res. J. 2015, 20, 405–418. [Google Scholar]
- Patel, J.S.; Zhang, S. First report of Alternaria blight of pitahaya (Hylocereus undatus) caused by Alternaria sp. in South Florida of the United States. Plant Dis. 2017, 101, 1046. [Google Scholar] [CrossRef][Green Version]
- Castro, J.C.; Endo, E.H.; de Souza, M.R.; Zanqueta, E.B.; Polonio, J.C.; Pamphile, J.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P.; de Abreu Filho, B.A. Bioactivity of essential oils in the control of Alternaria alternata in dragon fruit (Hylocereus undatus Haw.). Ind. Crops Prod. 2017, 97, 101–109. [Google Scholar] [CrossRef]
- Wu, J.B.; Zhan, R.L.; Liu, F.; Cang, J.M. First report of a stem and fruit spot of pitaya caused by Aureobasidium pullulans in China. Plant Dis. 2017, 101, 249. [Google Scholar] [CrossRef]
- Zheng, F.; Chen, L.W.; Ma, R.; Xu, G.; Ding, X.F.; Zheng, F.Q.; Xie, C.P. First report of southern blight in pitaya (Hylocereus undatus) caused by Sclerotium rolfsii in China. Plant Dis. 2018, 102, 441. [Google Scholar] [CrossRef]
- Liu, F.; Wu, J.B.; Zhan, R.L.; Ou, X.C. First report of reddish-brown spot disease on pitaya caused by Nigrospora sphaerica in China. Plant Dis. 2016, 100, 1792. [Google Scholar] [CrossRef]
- Kee, Y.J.; Hafifi, A.B.; Huda-Shakirah, A.R.; Wong, K.; Jin, X.; Siti Nordahliawate, M.; Zakaria, L.; Masratul Hawa, M. First report of reddish brown spot disease of red-fleshed dragon fruit (Hylocereus polyrhizus) caused by Nigrospora lacticolonia and Nigrospora sphaerica in Malaysia. Crop Prot. 2019, 122, 165–170. [Google Scholar] [CrossRef]
- Chuang, M.F.; Ni, H.F.; Yang, H.R.; Shu, S.L.; Lai, S.Y.; Jiang, Y.L. First report of stem canker disease of pitaya (Hylocereus undatus and H. polyrhizus) caused by Neoscytalidium dimidiatum in Taiwan. Plant Dis. 2012, 96, 906. [Google Scholar] [CrossRef]
- Lan, G.B.; He, Z.F.; Xi, P.G.; Jiang, Z.D. First report of brown spot disease caused by Neoscytalidium dimidiatum on Hylocereus undatus in Guangdong, Chinese Mainland. Plant Dis. 2012, 96, 1702. [Google Scholar] [CrossRef]
- Ezra, D.; Liarzi, O.; Gat, T.; Hershcovich, M.; Dudai, M. First report of internal black rot caused by Neoscytalidium dimidiatum on Hylocereus undatus (pitahaya) fruit in Israel. Plant Dis. 2013, 97, 1513. [Google Scholar] [CrossRef]
- Masratul Hawa, M.; Salleh, B.; Latiffah, Z. Identification and molecular characterizations of Neoscytalidium dimidiatum causing stem canker of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. J. Phytopath. 2013, 161, 841–849. [Google Scholar]
- Yi, R.H.; Mo, J.J.; Wu, F.F.; Chen, J. Fruit internal brown rot caused by Neoscytalidium dimidiatum on pitahaya in Guangdong province, China. Austral. Plant Dis. Notes 2015, 10, 13. [Google Scholar] [CrossRef]
- Sanahuja, G.; Lopez, P.; Palmateer, A.J. First report of Neoscytalidium dimidiatum causing stem and fruit canker of Hylocereus undatus in Florida. Plant Dis. 2016, 100, 1499. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Goenaga, R. First report of Neoscytalidium dimidiatum causing stem canker on dragon fruit (Hylocereus spp.) in Puerto Rico. Plant Dis. 2021, 105, 2728. [Google Scholar] [CrossRef] [PubMed]
- Huda-Shakirah, A.R.; Kee, Y.J.; Wong, K.L.; Latiffah, Z.; Masratul Hawa, M. Diaporthe species causing stem gray blight of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. Sci. Rep. 2021, 11, 3907–3918. [Google Scholar] [CrossRef]
- Wang, Y.C.; Liu, J.H.; Huang, C.C.; Hong, C.F. First report of dragon fruit (Hylocereus undatus) stem rot caused by Diaporthe ueckerae in Taiwan. Plant Dis. 2021. [Google Scholar] [CrossRef]
- Masratul Hawa, M.; Salleh, B.; Latiffah, Z. Characterization and pathogenicity of Fusarium proliferatum causing stem rot of Hylocereus polyrhizus in Malaysia. Ann. Appl. Biol. 2013, 163, 269–280. [Google Scholar] [CrossRef]
- Masratul Hawa, M.; Faziha, I.N.; Nik Izham, M.N.; Latiffah, Z. Fusarium fujikuroi associated with stem rot of red-fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. Ann. Appl. Biol. 2017, 170, 434–446. [Google Scholar] [CrossRef]
- Rita, W.S.; Suprapta, D.N.; Sudana, I.; Swantara, I.M. First report on Fusarium solani, a pathogenic fungus causing stem rot disease on dragon fruits (Hylocereus sp.) in Bali. J. Biol. Agric. Healthc. 2013, 3, 93–99. [Google Scholar]
- Sholihah, R.I.; Sritamin, M.; Wijaya, I.N. Identification of Fusarium solani fungi associated with stem rot disease in dragon fruit plant (Hylocereus sp.) at Bangorejo District, Banyuwangi Regency. J. Trop. Agric. 2019, 8, 91–102. [Google Scholar]
- Isnaini, M.; Muthahanas, I.; Jaya, I.K.D. Preliminary Study on Stem Rot Diseases in Pitaya Grown in North Lombok; 2009; pp. 109–114. Available online: https://d1wqtxts1xzle7.cloudfront.net/35477156/Mulat_Kdamar_ok-with-cover-page-v2.pdf?Expires=1649668891&Signature=WEflxJd8wnYb80lzyH8A5FAza~-McaDNb3fxzxrc0Xm1Li333hmvz570ptb39GYNHb5YNYfwvgvucgM9iP8dQlwwUm6Oom1bWBvHGNvGzjesP7Yvii5ujO5wckbfNoRvk6P7tC0Bbz4ueu29Wi4iZa2JMceo6meMppVceAPrNhRIOFD2E4CFpiruAX~B3cbu0gDggx6~1h9Dob47nuN1lkFefxDOZ1C4-jaSlIpnJ0cLzVMZf2GwBPNny-CsbDtYkmI17E6L3Qbkd-smeoSYgYgCPf~COEVx24lbFuRAHWWnpwLVckGy6KT8Y2axzeUo9rYqHrfeK~DdZ1fCwuZlbQ__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 2 March 2022).
- Kurniasari, N.; Hidayati, N.A.; Wahyuni, T. Identifikasi cendawan yang berpotensi menyebabkan penyakit busuk kuning pada batang tanaman buah naga. EKOTONIA J. Penel. Biol. Botani Zool. Mikrob. 2019, 4, 1–6. [Google Scholar] [CrossRef]
- Zheng, F.; Xu, G.; Zheng, F.Q.; Ding, X.F.; Xi, C.P. Neocosmospora rubicola causing stem rot of pitaya (Hylocereus costaricensis) in China. Plant Dis. 2018, 102, 2653. [Google Scholar] [CrossRef]
- Wright, E.R.; Rivera, M.C.; Ghirlanda, A.; Lori, G.A. Basal rot of Hylocereus undatus caused by Fusarium oxysporum in Buenos Aires, Argentina. Plant Dis. 2007, 91, 323. [Google Scholar] [CrossRef] [PubMed]
- Salazar-González, C.; Serna, L.; Gómez-López, E. Caracterización molecular de Fusarium asociado a pudrición basal del fruto en pitahaya (Selenicereus megalanthus). Agron. Mesoam. 2016, 27, 277–286. [Google Scholar] [CrossRef]
- Mahmud, N.U.; Chakraborty, M.; Paul, S.K.; Gupta, D.R.; Surovy, M.Z.; Rahman, M.; Islam, M.T. Report of basal rot of dragon fruit caused by Fusarium oxysporum in Bangladesh. Plant Dis. 2021, 105, 218. [Google Scholar] [CrossRef] [PubMed]
- Mohd Hafifi, A.B.; Kee, Y.J.; Masratu Hawa, M. First report of Fusarium oxysporum as a causal agent of stem blight of red-Fleshed dragon fruit (Hylocereus polyrhizus) in Malaysia. Plant Dis. 2019, 103, 1040. [Google Scholar] [CrossRef]
- Le, V.T.; Nguyen, N.; Nguyen, D.D.; Dang, K.T.; Nguyen, T.N.C.; Dang, M.V.H.; Chau, N.H.; Trink, N.L. Quality Assurance System for Dragon Fruit; ACIAR Proceedings: Canberra, Auatralia, 2000; Volume 100, pp. 101–114.
- Zhi-Jing, C.; Yi-Wen, W.; Yue, Y.; Ling, X. Pathogens analysis of soft rot disease of imported pitaya in Shanghai. Microb. China 2011, 38, 1499–1506. [Google Scholar]
- Ngoc, N.K.; Phong Nguyen, N.V.; An, P.T.M.; Woolf, A.B.; Fullerton, R.A. Effect of storage temperatures on postharvest diseases of dragon fruit (Hylocereus undatus Haw.) in the Mekong Delta Region, Vietnam. Acta Hortic. 2018, 1213, 453–460. [Google Scholar] [CrossRef]
- Prasad, N.; Mehta, P.R.; Lal, S.B. Fusarium wilt of guava (Psidium guajava L.) in Uttar Pradesh, India. Nature 1952, 169, 753. [Google Scholar] [CrossRef]
- Misra, A.K.; Gupta, V.K. Pathogenicity of Fusarium spp. isolates of guava wilt. J. Mycol. Plant Pathol. 2010, 40, 72–77. [Google Scholar]
- Misra, A.K.; Pandey, B.K. Guava wilt disease—A challenge for the coming millennium. In Proceedings of the National Symposium on Challenges & Prospects of Plant Pathology in the Coming Millennium, Uttar Pradesh, India, 9–11 December 1999. [Google Scholar]
- Gupta, V.K.; Misra, A.K. Fusarium chlamydosporum, causing wilt disease of guava (Psidium guajava L.) in India. Arch. Phytopath. Pflanzenschutz. 2012, 45, 2425–2428. [Google Scholar] [CrossRef]
- Dwivedi, N.; Dwivedi, S.K. Soil solarization: An ecofriendly technique to eradicate soil Fusaria causing wilt disease in guava (Psidium guajava). Int. J. Fruit Sci. 2020, 20, S1765–S1772. [Google Scholar] [CrossRef]
- Mishra, R.K.; Pandey, B.K.; Singh, V.; Mathew, A.J.; Pathak, N.; Zeeshan, M. Molecular detection and genotyping of Fusarium oxysporum f. sp. psidii isolates from different agro-ecological regions of India. J. Microb. 2013, 51, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Pandey, B.K.; Pathak, N.; Zeeshan, M. BOX-PCR-and ERIC-PCR-based genotyping and phylogenetic correlation among Fusarium oxysporum isolates associated with wilt disease in Psidium guajava L. Biocatal. Agric. Biotechnol. 2015, 4, 25–32. [Google Scholar] [CrossRef]
- Misra, A.K. Progressive steps in understanding and solving guava wilt-A national problem. Indian Phytopath. 2017, 70, 1–11. [Google Scholar] [CrossRef]
- Gangaraj, R.; Nagaraja, A.; Gaba, S.; Das, A.; Prameeladevi, T.; Debbarma, R.; Choudhary, S.P.; Kumari, A.; Kamil, D. Occurrence, identification and pathogenicity of Fusarium species associated with guava wilt disease in India. Arch. Phytopathol. Pflanzenschutz 2021, 11, 1–23. [Google Scholar] [CrossRef]
- Aftab, A.B. Studies on Guava Decline and Disease Management. Master’s Thesis, University of Agriculture, Faisalabad, Pakistan, 2009. [Google Scholar]
- Gomes, V.M.; Souza, R.M.; Mussi-Dias, V.; Silveira, S.F.; Dolinski, C. Guava decline: A complex disease involving Meloidogyne mayaguensis and Fusarium solani. J. Phytopath. 2011, 159, 45–50. [Google Scholar] [CrossRef]
- Madhu, M.R.; Verma, K.K.; Vinod, K. Distribution, prevalence and intensity of guava decline in western Haryana. J. Entomol. Zool. Stud. 2019, 7, 521–524. [Google Scholar]
- Singh, N. Emerging problem of guava decline caused by Meloidogyne enterolobii and Fusarium oxysporum f.sp. psidii. Indian Phytopath. 2020, 73, 373–374. [Google Scholar] [CrossRef]
- Veloso, J.S.; Câmara, M.P.S.; Souza, R.M. Guava decline: Updating its etiology from ‘Fusarium solani’ to Neocosmospora falciformis. Eur. J. Plant Pathol. 2021, 159, 455–460. [Google Scholar] [CrossRef]
- Khizar, R.; Sundas, H.; Tanzeel, A.; Kashif, N. Guava Decline a Serious Threat in Pakistan. Available online: https://www.technologytimes.pk/2017/12/23/guava-serious-threat-pakistan/2017 (accessed on 31 May 2021).
- Weir, B.S.; Johnston, P.R.; Damm, U. The Colletotrichum gloeosporioides species complex. Stud. Mycol. 2012, 73, 115–180. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar-Pinnaka, A.; Shenoy, B.D. Resolving the Colletotrichum siamense species complex using ApMat marker. Fungal Divers. 2015, 71, 247–264. [Google Scholar] [CrossRef]
- Rodríguez-Palafox, E.E.; Vásquez-López, A.; Márquez-Licona, G.; Lima, N.B.; Lagunes-Fortiz, E.; Tovar-Pedraza, J.M. First report of Colletotrichum siamense causing Anthracnose of guava (Psidium guajava) in Mexico. Plant Dis. 2021, 105, 3290. [Google Scholar] [CrossRef] [PubMed]
- Bragança, C.A.D.; Damm, U.; Baroncelli, R.; Massola Júnior, N.S.; Crous, P.W. Species of the Colletotrichum acutatum complex associated with anthracnose diseases of fruit in Brazil. Fungal Biol. 2016, 120, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.F.; Medeiros, N.L.; Benedet, G.L.; Araújo, M.B.; Uesugi, C.H.; da Ferreira, M.A.S.V.; Peixoto, J.R.; Blum, L.E.B. Control of post-harvest anthracnose infection in guava (Psidium guajava) fruits with phosphites, calcium chloride, acetyl salicylic acid, hot water, and 1-MCP. Hortic. Environ. Biotechnol. 2015, 56, 330–340. [Google Scholar] [CrossRef]
- Damm, U.; Cannon, P.F.; Woudenberg, J.H.C.; Crous, P.W. The Colletotrichum acutatum species complex. Stud. Mycol. 2012, 73, 37–113. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, K.; Brijpal, B. First report of crown rot disease of guava caused by Fusarium verticilloides in India. Indian J. Hortic. 2017, 74, 132–134. [Google Scholar]
- Baloch, A.; Dad, S.; Baloch, R.A.; Jatoi, G.H.; Muhammad, A.; Bhatti, Z.U.B. Effect of medium, temperature and pH, on in-vitro growth of Botryodiplodia theobromae isolated from guava. Pak. J. Biotechnol. 2018, 15, 123–127. [Google Scholar]
- Valentino, M.J.; Pineda, F.G.; Fandialan, M.F. Phytopathogenicity of fungi associated with crown rot of guava (Psidium guajava). Plant Pathol. Quar. 2015, 50, 7–13. [Google Scholar] [CrossRef]
- Mathew, S. The prevalence of fungi on the post harvested guava (Psidium guajava L.) in Aksum. Int. J Pharm. Sci. Res. 2010, 1, 145–149. [Google Scholar]
- Amadi, J.; Nwaokike, P.; Olahan, G. Isolation and identification of fungi involved in the post-harvest spoilage of guava (Psidium guajava) in Awka Metropolis. Int. J. Eng. Appl. Sci. 2014, 4, 7–12. [Google Scholar]
- Embaby, E.S.M.; Korkar, H.M. Decay of guava fruit (Psidium guajava Linn.) quality caused by some mold fungi. Int. J. Agric. Technol. 2015, 11, 713–730. [Google Scholar]
- Mairami, F.M.; Ndana, R.W.; Umar, I.D. Isolation and identification of fungal species associated with fruits spoilage in Bwari Market Abuja, Nigeria. J. Adv. Appl. Microbiol. 2018, 12, 401–407. [Google Scholar] [CrossRef][Green Version]
- Akhtar, N.; Hanif, K.; Shafiq, M.; Anwar, W. New report of Aspergillus awamori fruit rot of guava in Pakistan. J. Anim. Plant Sci. 2018, 28, 1537–1541. [Google Scholar]
- Pervez, Z.; Alam, M.S.; Islam, M.S.; Ahmed, N.U.; Mahmud, M.R. First report of Phytophthora guava fruit rot in Bangladesh. J. Plant Pathol. Microb. 2018, 9, 1. [Google Scholar] [CrossRef]
- Ismail, S.I.; Ahmad Dahlan, K.; Abdullah, S.; Zulperi, D. First report of Neoscytalidium dimidiatum causing fruit rot on guava (Psidium guajava) in Malaysia. Plant Dis. 2021, 105, 220. [Google Scholar] [CrossRef] [PubMed]
- Zee, K.Y.; Asib, N.; Ismail, S.I. First report of Lasiodiplodia theobromae causing postharvest fruit rot on guava (Psidium guajava) in Malaysia. Plant Dis. 2021, 105, 2716. [Google Scholar] [CrossRef]
- Misra, P.K. Effect of planting distance on yield performance of turmeric varieties intercropped with guava plantation. J Pharmacogn. Phytochem. 2012, 1, 137. [Google Scholar]
- Rooney-Latham, S.; Blomquist, C.L.; Scheck, H.J. First report of Fusarium wilt caused by Fusarium oxysporum f. sp. passiflorae on passion fruit in North America. Plant Dis. 2011, 95, 1478. [Google Scholar] [CrossRef]
- Garcia, E.; Paiva, D.; Costa, J.; Portugal, A.; Ares, A. First report of Fusarium Wilt caused by Fusarium oxysporum f. sp. Passiflorae on passion fruit in Portugal. Plant Dis. 2019, 103, 2680. [Google Scholar] [CrossRef]
- Melo, N.J.A.; Negreiros, A.M.; Medeiros, H.L.; Júnior, R.S. Evaluation of Fusarium wilt disease in passion fruit species inoculated with Fusarium oxysporum f.sp. passiflorae. J. Phytopath. 2020, 168, 81–87. [Google Scholar] [CrossRef]
- Thangavel, R.; Pattemore, J.A.; Rebijith, K.B.; Grbavac, N.; Ganev, S.; Chan, N.; Pearson, H.G.; Alexander, B.J.R. Fusarium oxysporum f. sp. Passiflorae infecting passionfruit in New Zealand in a changing taxonomic landscape. Australas. Plant Pathol. 2021, 50, 365–377. [Google Scholar] [CrossRef]
- Joa, J.-H.; Choi, I.-Y.; Choi, M.-K.; Heo, B.-S.; Jang, J.-O.; Shin, H.-D. Fusarium wilt caused by Fusarium oxysporum on passionfruit in Korea. Res. Plant Dis. 2018, 24, 75–80. [Google Scholar] [CrossRef]
- Cole, D.L.; Hedges, T.R.; Ndowora, T. A wilt of passion fruit (Passiflora edulis f. edulis Sims) caused by Fusarium solani and Phytophthora nicotianae var. parasitica. Trop. Pest Manag. 1992, 38, 362–366. [Google Scholar] [CrossRef]
- Henao-Henao, E.D.; Hernández-Medina, C.A.; Salazar-González, C.; Velasco-Belalcazar, M.L.; Gómez-López, E.D. Identificación molecular de aislamientos de Fusarium asociados a maracuyá en el Valle del Cauca, Colombia. Agron. Mesoam. 2018, 29, 56–65. [Google Scholar] [CrossRef]
- Aiello, D.; Fiorenza, A.; Leonardi, G.R.; Vitale, A.; Polizzi, G. Fusarium nirenbergiae (Fusarium oxysporum species complex) causing the wilting of passion fruit in Italy. Plants 2021, 10, 2011. [Google Scholar] [CrossRef] [PubMed]
- Emechebe, A.M.; Mukiibi, J. Nectria collar and root rot of passion fruit in Uganda. Plant Dis. Rep. 1976, 60, 227–231. [Google Scholar]
- Ploetz, R.C. Sudden wilt of passionfruit in Southern Florida caused by Nectria haematococca. Plant Dis. 1991, 75, 1071–1073. [Google Scholar] [CrossRef]
- Li, D.F.; Yang, J.Q.; Zhang, X.Y.; Sun, L.F. Identification of the pathogen causing collar rot of passion fruit in Fujian. Acta Phytopath. Sin. 1993, 23, 372. [Google Scholar]
- Ssekyewa, C.; Opio, A.F.; Swinburne, T.R.; Van Damme, P.L.; Abubakar, Z.M. Sustainable management of collar rot disease of passion fruits in Uganda. Int. J. Pest Manag. 1999, 45, 173–177. [Google Scholar] [CrossRef]
- Bueno, C.J.; Fischer, I.H.; Rosac, D.D.; Firminod, A.C.; Harakavae, R.; Oliveira, C.M.G.; Furtado, E.L. Fusarium solani f. sp. passiflorae: A new forma specialis causing collar rot in yellow passion fruit. Plant Pathol. 2014, 63, 382–389. [Google Scholar] [CrossRef]
- Marostega, T.N.; Lara, L.P.; Oliveira, D.D.; Chimello, A.M.; Gilio, T.A.; Preisigke, S.D.; Araujo, K.L.; Serafim, M.E.; Neves, L.G. Molecular and aggressiveness characterization of isolates of Fusarium solani and Fusarium oxysporum f. sp. passiflorae associated to passion fruit wilting. J. Agric. Sci. 2019, 11, 407–420. [Google Scholar]
- Zhou, Y.; Liu, Y.; Tang, J.; Xu, X. Stem collar rot of passion fruit caused by Fusarium solani in Zhanjiang, China. J. Plant Pathol. 2021, 103, 739. [Google Scholar] [CrossRef]
- Manicom, B.; Ruggiero, C.; Ploetz, R.C.; de Goes, A. Diseases of Passion Fruit. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Cambridge, MA, USA, 2003; pp. 413–441. [Google Scholar]
- Ploetz, R.C. Fusarium-induced diseases of tropical, perennial crops. Phytopathology 2006, 96, 648–652. [Google Scholar] [CrossRef]
- Anderson, J.D.; Chambers, A.H. University of Florida Passion Fruit in Florida: Investigation of Fungal Pathogens Causing Wilt and Canker. In Proceedings of the ASHS Virtual Conference, Gainesville, FL, USA, 10–13 August 2020. [Google Scholar]
- Lin, Y.S.; Chang, H.J. Collar rot of passion fruit possibly caused by Nectria haematococca in Taiwan. In Ecology and Management of Soilborne Plant Pathogens; Parker, C.A., Rovira, A.D., Moore, K.J., Wong, P.T.W., Kollmorgen, J.F., Eds.; APS: St Paul, MN, USA, 1985; pp. 41–45. [Google Scholar]
- Wangungu, C.W.; Mwangi, M.; Gathu, R.; Muasya, R.; Mbaka, J.; Kori, N. Reducing dieback disease incidence of passion fruit in Kenya through management practices. In Proceedings of the African Crop Science Conference, Maputo, Mozambique, 10–13 October 2011. [Google Scholar]
- Power, R.H.; Verhoeff, K. Dieback of passion fruit in Surinam. J. Phytopathol. 1984, 110, 336–345. [Google Scholar] [CrossRef]
- Amata, R.; Otipa, M.; Waiganjo, M.; Wasilwa, L.; Erbaugh, M.; Miller, S. Management of dieback disease of passion fruits. Acta Hortic. 2012, 1007, 363–368. [Google Scholar] [CrossRef]
- Wanniarachchi, S.D.R.; Karunatileke, M.S.; Wickramasekara, P.G.R.K.; Peiris, V.K.; Wickramasinghe, C.D. Stem bulging disorder in passionfruit (Passiflora edulis f. flavicarpa). Acta Hortic. 2017, 1178, 175–178. [Google Scholar] [CrossRef]
- Rajapaksha, R.G.; Wahundeniya, I.; Premarathna, M.P.; Marasinghe, J.; Silva, N.R.; Edirimanna, E.R.; Kohombange, S. Identification and controlling of stem bulging of passion fruit (Passiflora edulis) in Sri Lanka. Int. J. Environ. Agric. Biotechnol. 2019, 4, 785–788. [Google Scholar] [CrossRef][Green Version]
- Tarnowski, T.L.B.; Ploetz, R.C. First report of Colletotrichum boninense, C. capsici, and a Glomerella sp. as causes of postharvest anthracnose of passion fruit in Florida. Plant Dis. 2010, 94, 786. [Google Scholar] [CrossRef]
- Tozze, H.J., Jr.; Fischer, I.H.; Camara, M.P.S.; Massola, N.S., Jr. First report of Colletotrichum boninense infecting yellow passion fruit (Passiflora edulis f. flavicarpa) in Brazil. Austral. Plant Dis. Notes 2010, 5, 70–72. [Google Scholar]
- James, R.S.; Ray, J.; Tan, Y.P.; Shivas, R.G.; James, R. Colletotrichum siamense, C. theobromicola and C. queenslandicum from several plant species and the identification of C. asianum in the Northern Territory, Australia. Austral. Plant Dis. Notes 2014, 9, 138. [Google Scholar] [CrossRef]
- Du, Y.-X.; Shi, N.-N.; Chen, W.-L.; Ruan, H.C.; Yang, X.-J.; Gan, L.; Dai, Y.-L.; Chen, F.-R. Identification of Colletotrichum brevisporum causing anthracnose on passion fruit. Canad. J. Plant Pathol. 2017, 39, 527–532. [Google Scholar] [CrossRef]
- Zhuang, W.-Y.; Zhuang, W.Y.; Guo, L.; Guo, S.Y.; Guo, Y.L.; Mao, X.L.; Sun, S.X.; Wei, S.X.; Wen, H.A.; Yu, Z.H.; et al. Higher Fungi of Tropical China; Mycotaxon Ltd.: Ithaca, NY, USA, 2001. [Google Scholar]
- Chen, Y.-H.; Huang, T.-P. First report of anthracnose caused by Colletotrichum capsici on passion fruit in Taiwan. Plant Dis. 2018, 102, 2648. [Google Scholar] [CrossRef]
- Shi, G.Y.; Zeng, Q.; Wei, Y.W.; Hu, C.J.; Ye, X.L.; Jiao, C. First report of anthracnose caused by Colletotrichum brasiliense on violet passion fruit in China. Plant Dis. 2022. [Google Scholar] [CrossRef]
- Wang, N.; Chi, F.; Ji, Z.; Zhou, Z.; Zhang, J. First report of passion fruit anthracnose caused by Colletotrichum constrictum. Plant Dis. 2021, 105, 4158. [Google Scholar] [CrossRef]
- Fitzell, R.D.; Coates, L.M. Lychee-diseases. In Postharvest Diseases of Horticultural Crops; Tropical fruit; Coates, L., Cook, T., Persley, D., Beatties, B., Wade, N., Ridgeway, R., Eds.; Department of Primary Industries: Brisbane, Australia, 1995; Volume 2, pp. 41–42. [Google Scholar]
- Coates, L.; Zhou, E.; Sittigul, C. Diseases. In Litchi and Longan: Botany, Production and Uses; Menzel, C., Waite, F.K., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 261–272. [Google Scholar]
- Martínez-Bolaños, M.; Téliz-Ortíz, D.; Mora-Aguilera, A.; Valdovinos-Ponce, G.; Nieto-Ángel, D.; García-Pérez, E.; Sánchez-López, V. Anthracnose (Colletotrichum gloeosporioides Penz.) of litchi fruit (Litchi chinensis Soon.) in Oaxaca, México. Rev. Mex. Fitopatol. 2015, 32, 141–155. [Google Scholar]
- Ling, J.F.; Peng, A.; Jiang, Z.; Xi, P.; Song, X.; Cheng, B.; Cui, Y.; Chen, X. First report of anthracnose fruit rot caused by Colletotrichum fioriniae on litchi in China. Plant Dis. 2020, 105, 1225. [Google Scholar] [CrossRef]
- Zhao, J.; Yu, Z.; Wang, Y.; Li, Q.; Tang, L.; Guo, T.; Huang, S.; Mo, J.; Hsiang, T. Litchi anthracnose caused by Colletotrichum karstii in Guangxi, China. Plant Dis. 2021, 105, 3295. [Google Scholar] [CrossRef]
- Cooke, A.W.; Coates, L.M. Pepper spot: A preharvest disease of lychee caused by Colletotrichum gloeosporioides. Austral. Plant Pathol. 2002, 31, 303–304. [Google Scholar] [CrossRef]
- Anderson, J.; Aitken, E.; Dann, E.; Coates, L. Morphological and molecular diversity of Colletotrichum spp. causing pepper spot and anthracnose of lychee (Litchi chinensis) in Australia. Plant Pathol. 2013, 62, 279–288. [Google Scholar] [CrossRef]
- Ni, H.F.; Huang, C.W.; Wu, C.J.; Yang, H.R.; Lin, C.Y.; Chang, J.Y.; Chang, J.W. First report of pepper spot disease of lychee caused by Colletotrichum siamense in Taiwan. J. Plant Pathol. 2017, 99, 799–818. [Google Scholar]
- Ling, J.F.; Song, X.B.; Xi, P.G.; Cheng, B.P.; Cui, Y.P.; Chen, X.; Peng, A.T.; Jiang, Z.D.; Zhang, L.H. Identification of Colletotrichum siamense causing litchi pepper spot disease in mainland China. Plant Pathol. 2019, 68, 1533–1542. [Google Scholar] [CrossRef]
- Kumar, V.; Anal, A.K.; Rai, S.; Nath, V. Leaf, panicle and fruit blight of litchi (Litchi chinensis) caused by Alternaria alternata in Bihar state, India. Can. J. Plant Pathol. 2018, 40, 84–89. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, J.; Zhang, Z.; Li, M.; Gong, D.; Hong, X.; Li, H.; Wang, Y.; Zhao, C.; Hu, M. First report of Fusarium incarnatum causing fruit rot of litchi in China. Plant Dis. 2021, 105, 2018. [Google Scholar] [CrossRef]
- Kao, C.W.; Leu, L.S. Sporangium germination of Peronophythora litchii, the causal of litchi downy blight. Mycologia 1980, 72, 737–748. [Google Scholar] [CrossRef]
- Wang, H.C.; Sun, H.Y.; Stammler, G.; Ma, J.X.; Zhou, M.G. Baseline and differential sensitivity of Peronophythora litchii (lychee downy blight) to three carboxylic acid amide fungicides. Plant Pathol. 2009, 58, 571–576. [Google Scholar] [CrossRef]
- Ann, P.J.; Tsai, J.N.; Yang, H.R. First report of leaf and stem downy blight of longan seedlings caused by Peronophythora litchii in Taiwan. Plant Dis. 2012, 96, 1224. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Perez-Cuevas, M.; Rivera-Vargas, L.I.; Goenaga, R.; French-Monar, R.D. First report of Fusarium decemcellulare causing inflorescence wilt and vascular and flower necrosis of rambutan (Nephelium lappaceum), longan (Dimocarpus longan), and mango (Mangifera indica). Plant Dis. 2015, 99, 1187. [Google Scholar] [CrossRef]
- Kooariyakul, S.S.; Bhavakul, K. Brown rot of longan fruits caused by Phytophthora palmivora in Thailand. Acta Hortic. 2005, 665, 395–404. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Rivera-Vargas, L.I.; Goenaga, R.; French-Monar, R.D. First report of Lasiodiplodia theobromae causing inflorescence blight and fruit rot of longan (Dimocarpus longan L.) in Puerto Rico. Plant Dis. 2014, 98, 279. [Google Scholar] [CrossRef]
- Pipattanapuckdee, A.; Boonyakait, D.; Tiyayon, C.; Seehanam, P.; Ruangwong, O. Lasiodiplodia pseudotheobromae causes postharvest fruit rot of longan in Thailand. Australas. Plant Dis. Notes 2019, 14, 21. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, H.; Zhang, S.; Sun, J.; Lin, Y.; Wang, H.; Lin, M.; Shi, J. Phomopsis longanae Chi-induced disease development and pericarp browning of harvested longan fruit in association with energy metabolism. Front. Microbiol. 2018, 9, 1454. [Google Scholar] [CrossRef]
- Sun, J.; Lin, H.; Zhang, S.; Lin, Y.; Wang, H.; Lin, M.; Hung, Y.C.; Chen, Y. The roles of ROS production-scavenging system in Lasiodiplodia theobromae (Pat.) Griff. & Maubl. induced pericarp browning and disease development of harvested longan fruit. Food Chem. 2018, 247, 16–22. [Google Scholar] [PubMed]
- Serrato-Diaz, L.M.; Aviles-Noriega, A.; Soto-Bauzó, A.; Rivera-Vargas, L.I.; Goenaga, R.; Bayman, P. Botryosphaeriaceae fungi as causal agents of dieback and corky bark in rambutan and longan. Plant Dis. 2020, 104, 105–115. [Google Scholar] [CrossRef]
- Pongpisutta, R.; Sangchote, S. Phytophthora fruit rot of durian (Durio zibethinus L.). In Proceedings of the Postharvest Handling of Tropical Fruits, Chiang Mai, Thailand, 19–23 July 1993. [Google Scholar]
- Lim, T.K. Durian Diseases and Disorders; Tropical Press Sdn. Bhd.: Kuala Lumpur, Malaysia, 1990. [Google Scholar]
- Lim, T.K. Durian production in the world and status of Phytophthora palmivora. In Management of Phytophthora Diseases in Durian; Guest, D.I., Ed.; Australian Centre for International Agricultural Research (ACIAR), University of Melbourne: Melbourne, Australia, 1998. [Google Scholar]
- Sivapalan, A.; Hj Hamdan, F.; Junaidy, M. Patch canker of Durio zibethinus caused by Phytophthora palmivora in Brunei Darussalam. Plant Dis. 1997, 81, 113. [Google Scholar] [CrossRef] [PubMed]
- Tho, K.E.; Baconguis, R.; Raymundo, A.; Dalisay, T. Surveys for stem canker and stem borer of durian in the coastal areas of Cambodia. ACPP APPS 2011 New Frontiers in Plant Pathology for Asia and Oceania Inaugural joint. In Proceedings of the 4th Asian Conference on Plant Pathology and the 18th Biennial Australasian Plant Pathology Society Conference, Darwin Convention Centre, Darwin, Australia, 26–29 April 2011. [Google Scholar]
- Chantarasiri, A.; Boontanom, P. Fusarium solani and Lasiodiplodia pseudotheobromae, fungal pathogens causing stem rot disease on durian trees (Durio zibethinus) in Eastern Thailand. New Dis. Rep. 2021, 44, e12026. [Google Scholar] [CrossRef]
- Thuan, T.; Tho, N.; Tuyen, B.C. First report of Rhizoctonia solani subgroup AG 1-ID causing leaf blight on durian in Vietnam. Plant Dis. 2008, 92, 648. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K.; Ng, C.C.; Chin, C.L. Etiology and control of durian foliar blight and dieback caused by Rhizoctonia solani. Ann. Appl. Biol. 2008, 111, 301–307. [Google Scholar]
- Tongsri, V.; Songkumarn, P.; Sangchote, S. Leaf spot characteristics of Phomopsis durionis on durian (Durio zibethinus Murray) and latent infection of the pathogen. Acta Univ. Agric. Silvic. Mendel. Brun. 2016, 64, 185–193. [Google Scholar] [CrossRef]
- Lim, T.K.; Kamaruzaman, S. A rot of detached durian fruits caused by Sclerotium rolfsii. Pertanika 1989, 12, 11–14. [Google Scholar]
- Sangchote, S.; Jaisong, S.; Sangsiri, T. Fruit rot disease on durian, pathogen resistance to fungicide and control. In Proceedings of the 10th National Plant Protection Conference, Kum Phukam Resident, Chiang Mai, Thailand, 22–24 February 2012. [Google Scholar]
- Sivapalan, A.; Metussin, R.; Harndan, F.; Zain, R.M. Fungi associated with postharvest fruit rots of Durio graveolens and D. kutejensis in Brunei Darussalam. Australas. Plant Pathol. 1998, 27, 274–277. [Google Scholar] [CrossRef]
- O’Gara, E.; Sangchote, S.; Fitzgerald, L.; Wood, D.; Seng’, A.; Guest, D.I. Infection biology of Phytophthora palmivora Butl. in Durio zibethinus L. (durian) and responses induced by phosphonate. In Diversity and Management of Phytophthora in Southeast Asia; BPA Print Group Pty Ltd.: Melbourne, Australia, 2004; pp. 42–52. [Google Scholar]
- Santoso, P.J.I.; Nyoman, I.; Aryantha, P.; Adi, P.; Sony, S.S. Identification of Pythium and Phytophthora sssociated with durian (Durio sp.) in Indonesia: Their molecular and Morphological characteristics and distribution. Asian J. Plant Pathol. 2015, 9, 59–71. [Google Scholar] [CrossRef]
- Bong, C.L. Destructive diseases of selected fruit trees and species. In Fruits, Nuts and Spices, Proceedings of an in-House Seminar and Workshop; Lagud Sebrang: Tenom, Malaysia, 1990; pp. 24–26. [Google Scholar]
- Lim, T.K.; Sangchote, S. Diseases of Durian. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 241–252. [Google Scholar]
- Vawdrey, L.; Langdon, P.; Martin, T. Incidence and pathogenicity of Phytophthora palmivora and Pythium vexans associated with durian decline in far northern Queensland. Australas. Plant Pathol. 2005, 34, 127–128. [Google Scholar] [CrossRef][Green Version]
- Thao, L.; Hien, L.; Liem, N.; Thanh, H.; Khanh, T.; Binh, V.; Trang, T.; Anh, P.; Tu, T. First report of Phytopythium vexans causing root rot disease on durian in Vietnam. New Dis. Rep. 2020, 41, 2044-0588. [Google Scholar] [CrossRef]
- Farungsang, U.; Sangchote, S.; Farungsang, N. Appearance of quiescent fruit rot fungi on rambutan stored at 13 °C and 25 °C. Acta Hortic. 1992, 321, 903–907. [Google Scholar] [CrossRef]
- Sivakumar, D.; Wijeratnam, R.S.W.; Wijesundera, R.L.C.; Abeysekera, M. Post-harvest diseases of rambutan (Nephelium lappaceum) in the Western Province. J. Natl. Counc. Sci. Lanka 1997, 25, 225–229. [Google Scholar] [CrossRef]
- Sangchote, S.; Farungsang, U.; Farungsang, N. Pre- and postharvest infection of rambutan by pathogens and effects of postharvest treatments. In Disease Control and Storage Life Extension in Fruit, Proceedings of an International Workshop, Chiang Mai, Thailand, 22–23 May 1997; Australian Centre for International Agricultural Research: Canberra, Australia, 22–23 May 1998. [Google Scholar]
- He, C.P.; Zheng, X.-L.; Li, R.; Wu, W.-H.; Yu, X.-M.; Ke, X.-Y.; Zheng, F.-C. Identification and biological characters of rambutan gray spot disease. J. Fruit Sci. 2010, 27, 270–274. [Google Scholar]
- Nishijima, K.A.; Follett, P.A.; Bushe, B.C.; Nagao, M.A. First report of Lasmenia sp. and two species of Gliocephalotrichum on rambutan in Hawaii. Plant Dis. 2002, 86, 71. [Google Scholar] [CrossRef]
- Keith, L.M. First report of Pestalotiopsis virgatula causing Pestalotiopsis fruit rot on rambutan in Hawaii. Plant Dis. 2008, 92, 835. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Latoni-Brailowsky, E.I.; Rivera-Vargas, L.I.; Goenaga, R.; French-Monar, R.D. First report of Gliocephalotrichum bulbilium and G. simplex causing fruit rot of rambutan in Puerto Rico. Plant Dis. 2012, 96, 1225. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Latoni-Brailowsky, E.I.; Rivera-Vargas, L.I.; Goenaga, R.; Crous, P.W.; French-Monar, R.D. First report of Calonectria hongkongensis causing fruit rot of rambutan (Nephelium lappaceum). Plant Dis. 2013, 97, 1117. [Google Scholar] [CrossRef]
- Serrato-Diaz, L.M.; Rivera-Vargas, L.I.; Goenaga, R.J.; Navarro, E.D.; French-Monar, R.D. First report of Colletotrichum fructicola and C. queenslandicum causing fruit rot of rambutan (Nephelium lappaceum). Plant Dis. 2017, 101, 1043. [Google Scholar] [CrossRef]
- Intan Sakinah, M.A.; Latiffah, Z. First report of Gliocephalotrichum bacillisporum causing fruit rot of rambutan (Nephelium lappaceum) in Malaysia. Plant Dis. 2013, 97, 1110. [Google Scholar] [CrossRef] [PubMed]
- Booth, C.; Ting, W.P. Dolabra nepheliae gen. nov., sp. nov., associated with canker of Nephelium lappaceum. Trans. Br. Mycol. Soc. 1964, 47, 235–237. [Google Scholar] [CrossRef]
- Combs, B.; Nickum, M.; Nelson, S. Corky bark disease of rambutan. Plant Dis. 2012, 1–5. Available online: https://www.ctahr.hawaii.edu/oc/freepubs/pdf/pd-86.pdf (accessed on 2 March 2022).
- Rossman, A.Y.; Schoch, C.L.; Farr, D.F.; Nishijima, K.; Keith, L.; Goenaga, R. Dolabra nepheliae on rambutan and lychee represents a novel lineage of phytopathogenic Eurotiomycetes. Mycoscience 2010, 51, 300–309. [Google Scholar] [CrossRef]
- Rossman, A.Y.; Goenaga, R.; Keith, L. First report of Dolabra nepheliae on rambutan and litchi in Hawaii and Puerto Rico. Plant Dis. 2007, 91, 1685. [Google Scholar] [CrossRef]
- Rossman, A.; Melgar, J.; Walker, D.; Gonzalez, A.; Ramirez, T.; Rivera, J. First report of Dolabra nepheliae associated with corky bark disease of rambutan and pulasan in Honduras. Plant Dis. 2012, 96, 765. [Google Scholar] [CrossRef]
- Garcia, A.S. The powdery mildew disease of rambutan. Philipp. PhytoPathol. 1983, 19, 15–16. [Google Scholar]
- Coates, L.M.; Sangchote, S.; Jononson, D.I.; Sittigul, C. Diseases of longan, lychee and rambutan. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 307–325. [Google Scholar]
- Rajapakse, R.; Edirimanna, E.; Kahawatta, J. Management of powdery mildew disease of rambutan (Nephelium lappaceum L.) in Sri Lanka. J. Agric. Sci. 2006, 2, 8–14. [Google Scholar] [CrossRef][Green Version]
- Peregrine, W.T.H.; Kassim, A.; Ahmad, A.; Sutton, B.C. A serious disease of seedling rambutan caused by Pseudocercospora nephelii sp. nov. Plant Pathol. 1990, 39, 197–201. [Google Scholar] [CrossRef]
- Lim, T.K.; Sangchote, S. Diseases of mangosteen. In Diseases of Tropical Fruit Crops; Ploetz, R.C., Ed.; CABI Publishing: Wallingford, UK, 2003; pp. 365–372. [Google Scholar]
- Keith, L.M.; Matsumoto, T.K. First report of Pestalotiopsis leaf blotch on mangosteen in Hawaii. Plant Dis. 2013, 97, 146. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Chen, W.X.; Liu, A.Y.; Chen, Q.L.; Feng, S.J. First report of Gliocephalotrichum bulbilium causing fruit rot of postharvest mangosteen in China. Plant Dis. 2014, 98, 994. [Google Scholar] [CrossRef] [PubMed]
- Sangchote, S.; Pongpisutta, R. Fruit rots of mangosteen and their control. In Proceedings of the 7th International Congress of Plant Pathology, Edinburgh, Scotland, UK, 9–16 August 1998. [Google Scholar]
- Wang, T.; Sun, C.; Zhu, P. First report of Mucor irregularis causing postharvest fruit rot on Garcinia mangostana in China. Plant Dis. 2021, 106. [Google Scholar] [CrossRef] [PubMed]
- Ketsa, S.; Paull, R.E. Mangosteen (Garcinia mangostana L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits: Mangosteen to White Sapote; Yahia, E.M., Ed.; Woodhead Publishing: Swaston, UK, 2011; pp. 1–32. [Google Scholar]
- Paim, E.; Silveira, A.; Bezerra, J.; Luz, E.; Sacramento, C. Etiology of the decline of mangosteen in the Southern Bahia. Rev. Bras. Frutic. 2012, 34, 1074–1083. [Google Scholar] [CrossRef][Green Version]
- Perween, T.; Mandal, K.; Hasan, M.A. Dragon fruit: An exotic super future fruit of India. J. Pharmacogn. Phytochem. 2018, 7, 1022–1026. [Google Scholar]
- Morton, J. Fruits of Warm Climates; Florida Flair Books: Miami, FL, USA, 1987; pp. 281–286. [Google Scholar]
- Tel Zur, N. R&D of pitahayas-dragon fruit-vine cacti: Limitations and challenges and the current global market. Acta Hortic. 2015, 1067, 365–370. [Google Scholar]
- Ortiz-Hernandez, Y.D.; Carillo-Salazar, J.A. Pitahaya (Hylocereus spp.). Commun. Sci. 2012, 3, 220–237. [Google Scholar]
- Balendres, M.A.; Bengoa, J. Diseases of dragon fruit (Hylocereus species): Etiology and current management options. Crop Prot. 2019, 126, 104920. [Google Scholar] [CrossRef]
- Masratul Hawa, M. Characterizations, Pathogenicity and Chemical Control of Fusarium Species from Stem Rot of Dragon Fruit (Hylocereus polyrhizus) in Malaysia. Ph.D. Thesis, Universiti Sains Malaysia, Penang, Malaysia, 2014. [Google Scholar]
- Wang, C.L.; Lin, C.C. Fruit rot of pitaya and stem rot of cacti in Taiwan. Plant Pathol. Bull. 2005, 14, 269–274. [Google Scholar]
- Taba, S.; Miyahira, N.; Nasu, K.; Takushi, T.; Moromizato, Z.-I. Fruit rot of strawberry pear (pitaya) caused by Bipolaris cactivora. J. Gen. Plant Pathol. 2007, 73, 374–376. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, Z.; Li, M.; Chen, L.; Hu, M. Identification and biological characteristics of dragon fruit (Hylocereus undatus Britt) Fusarium rot pathogen. Chi. J. Trop. Crops 2016, 37, 164–171. [Google Scholar]
- Rojas-Sandoval, J.; Acevedo-Rodríguez, P. Psidium guajava (guava). Available online: https://www.cabi.org/isc/datasheet/45141#todistribution (accessed on 1 July 2021).
- Shukla, P.K.; Fatima, T.; Rajan, S. Research on Fusarium wilt disease of guava. Indian Phytopathol. 2019, 72, 629–636. [Google Scholar] [CrossRef]
- Ansar, M.; Saleem, A.; Iqbal, A. Cause and control of guava decline in the Punjab (Pakistan). Pak. J. Phytopathol. 1994, 6, 41–44. [Google Scholar]
- Hamiduzzaman, M.M.; Meah, M.B.; Ahmad, M.U. Effect of Fusarium oxysporum and nematode interaction on guava wilt. Bangladesh J. Plant Pathol. 1997, 13, 9–11. [Google Scholar]
- Hussain, M.Z.; Rahman, M.A.; Islam, M.N.; Latif, M.A.; Bashar, M.A. Morphological and molecular identification of Fusarium oxysporum Sch. isolated from guava wilt in Bangladesh. Bangladesh J. Bot. 2012, 41, 49–54. [Google Scholar] [CrossRef]
- Schoeman, M.H.; Benade, E.; Wingfield, M.J. The symptoms and cause of guava wilt in South Africa. J. Phytopathol. 1997, 145, 37–41. [Google Scholar] [CrossRef]
- Lim, T.K.; Manicom, B.Q. Diseases of guava. In Disease of Tropical Fruit Crops; CABI Publications: Wallingford, UK, 2003; pp. 275–289. [Google Scholar]
- Negi, S.S.; Misra, A.K.; Rajan, S. Guava wilt. In Proceedings of the National Seminar on New Horizon in Production and Post Harvest Management of Tropical and Subtropical Fruits, IARI, New Delhi, India, 8–9 December 1998. [Google Scholar]
- Misra, A.K.; Shukla, S.K. Assessment of loss due to Guava wilt around Lucknow. In Proceedings of the National Seminar on Production and Post-Harvest Technology of Guava, Department of Horticulture, CSAUA&T, Kanpur, India, 9–10 January 2002. [Google Scholar]
- Gupta, V.K.; Misra, A.K.; Gaur, R.K.; Jain, P.K.; Gaur, D.; Saroj, S. Current status of Fusarium wilt disease of guava (Psidium guajava L.) in India. Biotechnology 2010, 9, 176–195. [Google Scholar] [CrossRef]
- Gomes, V.M.; Souza, R.M.; Midorikawa, G.; Miller, R.; Almeida, A.M. Guava decline: Evidence of nationwide incidence in Brazil. Nematropica 2012, 42, 153–162. [Google Scholar]
- Almeida, A.M.; Gomes, V.M.; Souza, R.M. Greenhouse and field assessment of rhizobacteria to control guava decline. Bragantia 2011, 70, 837–842. [Google Scholar] [CrossRef][Green Version]
- Back, M.A.; Haydock, P.P.J.; Jenkinson, P. Disease complexes involving plant parasitic nematodes and soilborne pathogens. Plant Pathol. 2002, 51, 683–697. [Google Scholar] [CrossRef]
- Gomes, V.M.; Souza, R.M.; Da Silveira, S.F.; Almeida, A.M. Guava decline: Effect of root exudates from Meloidogyne enterolobii-parasitized plants on Fusarium solani in vitro and on growth and development of guava seedlings under controlled conditions. Eur. J. Plant Pathol. 2013, 137, 393–401. [Google Scholar] [CrossRef]
- Omar, A.A.W. Occurrence of Colletotrichum anthracnose disease of guava fruit in Egypt. Int. J. Pest. Manag. 2001, 47, 147–152. [Google Scholar]
- Amusa, N.A.; Ashaye, O.A.; Amadi, J.; Dapo, O.O. Guava fruit anthracnose and the effects on its nutritional and market values in Ibadan. Niger. J. Appl. Sci. 2005, 6, 539–542. [Google Scholar] [CrossRef]
- Merida, M.; Palmateer, A.J. Florida Plant Disease Management Guide: Guava (Psidium guajava); Plant Pathology Department; UF/IFAS Extension; Department of Agriculture; Cooperative Extension Service; Universidad de Florida: Gainesville, FL, USA, 2013; p. 232. [Google Scholar]
- Intan Sakinah, M.A.; Suzianti, I.V.; Latiffah, Z. Molecular characterization and pathogenicity of Colletotrichum sp. from guava. Arch. Phytopathol. Plant Protect. 2014, 47, 1549–1556. [Google Scholar]
- Latiffah, Z.; Nurul Zaadah, J.; Suzianti, I.V.; Intan Sakinah, M.A. Molecular characterization of Colletotrichum isolates associated with anthracnose of mango fruit. Sains Malays. 2015, 44, 651–656. [Google Scholar]
- Bailey, M.; Sarkhosh, A.; Rezazadeh, A.; Anderson, J.; Chambers, A.; Crane, J. The Passion fruit of Florida. EDIS 2021, 1, 1–13. [Google Scholar] [CrossRef]
- Cazarin, C.B.B.; Rodriguez-Nogales, A.; Algieri, F.M.; Utrilla, P.; Rodríguez-Cabezas, M.; Garrido-Mesa, J. Intestinal anti-inflammatory effects of Passiflora edulis peel in the dextran sodium sulphate model of mouse colitis. J. Funct. Foods 2016, 26, 565–576. [Google Scholar] [CrossRef]
- Ortiz, E.; Hoyos-Carvajal, L. Standard methods for inoculations of F. oxysporum and F. solani in Passiflora. Afr. J. Agric. Res. 2016, 11, 1569–1575. [Google Scholar]
- Gardner, D. Pathogenicity of Fusarium oxysporum f. sp. passiflorae to banana poka and other Passiflora spp. in Hawaii. Plant Dis. 1989, 73, 476–478. [Google Scholar] [CrossRef]
- Liberato, J.R.; Laranjeira, F.F. Fusarium Wilt of Passionfruit (Fusarium oxysporum f. sp. passiflorae) 2005. Updated on 21 January 2007, 9:21:11 a.m. Available online: http://www.padil.gov.au (accessed on 20 May 2021).
- Hirooka, Y.; Kobayashi, T.; Natsuaki, K.T.; Uehara, K. Occurrence of Passiflora wilt in Japan caused by Haematonectria ipomoeae. Jpn. J. Phytopathol. 2003, 69, 1–8. [Google Scholar] [CrossRef][Green Version]
- Joy, P.P.; Sherin, C.G. Diseases of Passion Fruit (Passiflora edulis) and their Management. In Insect Pests Management of Fruit Crops; Pandey, A.J., Moll, P., Eds.; Nova Deli Biotech: Delhi, India, 2016; pp. 453–470. [Google Scholar]
- Fischer, I.H.; Rezende, J.A.M. Diseases of passion flower (Passiflora spp.). Pest Technol. 2008, 2, 1–19. [Google Scholar]
- Ploetz, R.C. Diseases of Tropical Fruit Crops; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Pegg, K.G.; Willingham, S.L.; O’Brien, R.G.; Cooke, A.W.; Coates, L.M. Base rot of golden passionfruit caused by a homothallic strain of Fusarium solani. Austral. Plant Pathol. 2002, 31, 305–306. [Google Scholar] [CrossRef]
- Francisco Neto, E.; Oliveira, J.C.; Centurion, M.A.P.C.; Nakamura, K. Influência da idade da folha, da luz e do método de inoculação na infecção de Passiflora por Colletotrichum gloeosporioides. Summa Phytopathol. 1995, 21, 25–30. [Google Scholar]
- Wolcan, S.; Larran, S. First report of anthracnose caused by Glomerella cingulata on passion fruit in Argentina. Plant Dis. 2000, 84, 706. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Wang, C.C.; Lin, H.L.; Wang, C.L. First report of Septoria blotch of passion fruit caused by Septoria passifloricola in Taiwan. Plant Dis. 2020, 105, 700. [Google Scholar] [CrossRef] [PubMed]
- Ochse, J.J.; Soule, M.J.; Dijkman, M.J.; Wehlburg, C. Other Fruit Crops. Tropical and Sub-Tropical Agriculture; The McMillan Company: New York, NY, USA, 1961; Volume 1, pp. 1–356. [Google Scholar]
- Menzel. The Lychee Crop in Asia and the Pacific. Major Diseases. 2002. Available online: http://www.fao.org/3/ac681e/ac681e09.htm#bm09.2 (accessed on 1 July 2021).
- Misra, A.K.; Pandey, B.K. Diseases of litchi and their management. In Diseases of Fruits and Vegetables and Their Management; Thind, T.S., Ed.; Kalyani Publishers: Ludhiana, India, 2001; pp. 150–155. [Google Scholar]
- Bagshaw, J.S.; Underhill, S.J.R.; Fitzell, R.D. Lychees-disorders and injuries. In Postharvest Diseases of Horticultural Crops; Coates, L., Cooke, A., Persley, D., Beattie, B., Wade, N., Ridgeway, R., Eds.; Department of Primary Industries: Queensland, Australia, 1995; Volume 2, pp. 43–44. [Google Scholar]
- Mitra, S.K.; Pan, J. Litchi and longan production and trade in the world. Acta Hortic. 2020, 1293, 1–6. [Google Scholar] [CrossRef]
- Ann, P.J.; Ko, W.H. Blossom blight of litchi in Taiwan caused by Peronohythora litchi. Plant Dis. 1984, 68, 826. [Google Scholar] [CrossRef]
- Tran, H.T.; Van, H.N.; Muniappan, R.; Amrine, J.W.; Naidu, R.A.; Gilbertson, R.L.; Sidhu, J.K. Integrated pest management of longan (Sapindales: Sapindaceae) in Vietnam. J. Int. Pest Manag. 2019, 10, 18. [Google Scholar] [CrossRef]
- Subhadrabandhu, S.; Schneemann, J.M.P.; Verheij, E.W.M. Durio zibethinus Murray. In PROSEA: Plant Resources of South-East Asia Edible Fruits and Nuts; Verheij, E.W.M., Coronel, R.E., Eds.; Pudoc Wageningen Netherlands: Wageningen, The Netherlands, 1991; Volume 2, pp. 157–161. [Google Scholar]
- Love, K.; Gasik, L.; Paull, R.E. Durian for Hawai’i. Fruit, Nut, and Beverage Crops; College of Tropical Agriculture and Human Resources, University of Hawaii: Oahu, HI, USA, 2019; pp. 1–22. [Google Scholar]
- Drenth, A.; Sendall, B. Economic Impact of Phytophthora Diseases in Southeast Asia. In Diversity and Management of Phytophthora in Southeast Asia; Drenth, A., Guest, D.I., Eds.; ACIAR Monograph, Australian Center for International Agricultural Research: Canberra, Australia, 2004; p. 114. [Google Scholar]
- Lee, B.S. Biological approaches for controlling Phytophthora root and trunk rot of durian. In Proceedings of the National Horticulture Conference, Kuala Lumpur, Malaysia, 16–17 November 1999. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society Press: St Paul, MN, USA, 1996. [Google Scholar]
- Lim, T.K.; Chan, L.G. Fruit rot of durian caused by Phytophthora palmivora. Pertanika 1986, 9, 269–276. [Google Scholar]
- Chan, L.G.; Lim, T.K. Control of Phytophthora palmivora on cocoa and durian seedlings. J. Plant Prot. Trop. 1987, 4, 9–13. [Google Scholar]
- Mohamed Azni, I.N.A.; Sundram, S.; Ramachandran, V. Pathogenicity of Malaysian Phytophthora palmivora on cocoa, durian, rubber and oil palm determines the threat of bud rot disease. For. Pathol. 2019, 49, e12557. [Google Scholar] [CrossRef]
- Solpot, T.C.; Cumagun, C.J.R. Phylogenetic analyses and cross-infection studies of Phytophthora species infecting cacao and durian in South-Central Mindanao, Philippines. J. Phytopathol. 2021, 170, 41–56. [Google Scholar] [CrossRef]
- Perrine-Walker, F. Phytophthora palmivora–cocoa interaction. J. Fungi 2020, 6, 167. [Google Scholar] [CrossRef] [PubMed]
- Herder, K. Rambutan, an Exotic Fruit with a Growing Presence in Europe. 2018. Available online: https://www.freshplaza.com/article/2188347/rambutan-an-exotic-fruit-with-a-growing-presence-in-europe/ (accessed on 13 November 2021).
- Alahakoon, P.; Jayawardana, N.; Madushani, K.; Nilmini, R. Introduction of bio-fungicides for controlling powdery mildew disease of rambutan. In Proceedings of the International Forestry and Environment Symposium, Sri Lanka, India, 26–27 November 2010. [Google Scholar]
- Cruz, F.S.D.J. Status Report on Genetic Resources of Mangosteen (Garcinia mangostana L.) in Southeast Asia; IPGRI Office for South Asia: Delhi, India, 2001. [Google Scholar]
- Kanwar, J.S.; Dhillon, W.S. Horticultural Practices and Their Impact on Plant Diseases. Training Course on Integrated Approaches in Plant Disease Management for Sustainable Agriculture; Department of Plant Pathology, Punjab Agricultural University: Ludhiana, India, 2002; pp. 202–208. [Google Scholar]
- Thind, S.K. Principles of disease management in fruit crops. Int. Clin. Pathol. J. 2017, 4, 123–137. [Google Scholar]
- Palmateer, A. Phytophthora Diseases Affecting Ornamentals & the Potential for Foliar Outbreaks; Bayer Cropscience LP: Saint Louis, MO, USA, 2017. [Google Scholar]
- De Waard, M.A.; Georgopoulos, S.; Hollomon, G.D.W.; Ishii, H.; Leroux, P.; Ragsdale, N.N.; Schwinn, F.J. Chemical control of plant diseases: Problems and prospects. Annu. Rev. Phytopathol. 1993, 31, 403–421. [Google Scholar] [CrossRef]
- Jackson-Ziems, T.A.; Giesler, J.G.; Harveson, R.M.; Wegulo, S.N.; Korus, K.; Adesemoye, T. Fungicide application timing and disease control. Plant Pathol. 2016, 486, 486. [Google Scholar]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.; Kannangara, S.D.; Promputtha, I. Fungi vs. Fungi in Biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell Infect Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- Yadav, S.M.; Patil, R.K.; Balai, L.P.; Niwas, R. Post-harvest diseases of horticultural crops and their management. Pop. Kheti. 2013, 1, 20–25. [Google Scholar]
- Mahajan, P.V.; Caleb, O.J.; Singh, Z.; Watkins, C.B.; Geyer, M. Postharvest treatments of fresh produce. Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2014, 372, 20130309. [Google Scholar] [CrossRef]
- Palou, L. Postharvest treatments with GRAS salts to control fresh fruit decay. Horticulturae 2018, 4, 46. [Google Scholar] [CrossRef]
- Dhall, R.K. Advances in edible coatings for fresh fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 435–450. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakaria, L. Fungal and Oomycete Diseases of Minor Tropical Fruit Crops. Horticulturae 2022, 8, 323. https://doi.org/10.3390/horticulturae8040323
Zakaria L. Fungal and Oomycete Diseases of Minor Tropical Fruit Crops. Horticulturae. 2022; 8(4):323. https://doi.org/10.3390/horticulturae8040323
Chicago/Turabian StyleZakaria, Latiffah. 2022. "Fungal and Oomycete Diseases of Minor Tropical Fruit Crops" Horticulturae 8, no. 4: 323. https://doi.org/10.3390/horticulturae8040323
APA StyleZakaria, L. (2022). Fungal and Oomycete Diseases of Minor Tropical Fruit Crops. Horticulturae, 8(4), 323. https://doi.org/10.3390/horticulturae8040323





