Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District
Abstract
1. Introduction
2. Materials and Methods
2.1. Extent and Locations of Areas
2.2. Sampling of Leaves and Soil
2.3. Soil and Plant Tissue Preparation
2.4. Soil Chemical and Nutrients Analysis
2.5. Leaves Nutrients Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Soil Particles
3.2. Soil pH and Electrical Conductivity (EC)
3.3. Soil Organic Matter (SOM)
3.4. Soil Calcium Carbonates
3.5. Total Nitrogen
3.6. Extractable Phosphorus
3.7. Extractable Potassium
3.8. Extractable Boron (B)
3.9. Extractable Copper (Cu)
3.10. Extractable Iron (Fe)
3.11. Extractable Manganese (Mn)
3.12. Extractable Zinc (Zn)
3.13. Leaves Nitrogen, Phosphorus, and Potassium
3.14. Leaves Zinc, Iron, Copper, Manganese, and Boron
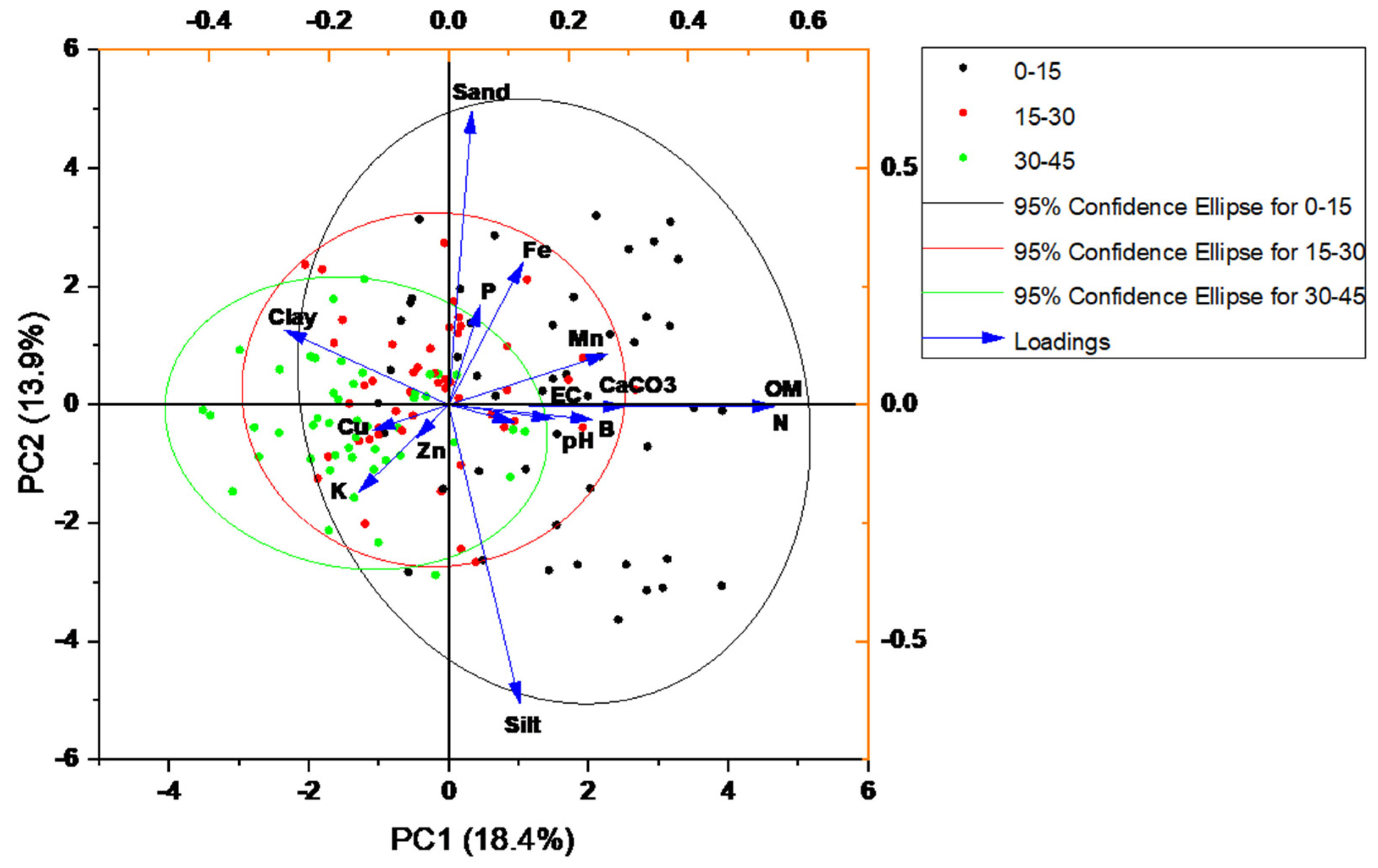
3.15. Pearson Correlation of Soil and Plant Attributes
4. Conclusions
5. Future Recommendation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, M.; Ahmad, N.; Anwar, S.A.; Majeed, T. Effect of micronutrients on citrus fruit yield growing on calcareous soils. In Advances in Plant and Animal Boron Nutrition; Fangsen, X.U., Goldbach, H.E., Brown, P.H., Bell, R.W., Fujiwara, T., Hunt, C.D., Goldberg, S., Shi, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 179–182. [Google Scholar]
- Razzaq, K.; Khan, A.S.; Malik, A.U.; Shahid, M.; Ullah, S. Foliar application of zinc influences the leaf mineral status, vegetative and reproductive growth, yield and fruit quality of “kinnow” mandarin. J. Plant Nutr. 2013, 36, 1479–1495. [Google Scholar] [CrossRef]
- Quiñones, A.; Martínez-Alcántara, B.; Legaz, F. Influence of irrigation system and fertilization management on seasonal distribution of N in the soil profile and on N-uptake by citrus trees. Agric. Ecosyst. Environ. 2007, 122, 399–409. [Google Scholar] [CrossRef]
- Fageria, N.K.; Baligar, C.; Clark, R.B. Micronutrients in crop production. Adv. Agron. 2002, 77, 185–268. [Google Scholar] [CrossRef]
- Usanmaz, S.; Atti, M.K. Animal manures and some chemicals as soil supplement to improve growth, yield and fruit quality of tomato. Pak. J. Bot. 2020, 52, 1289–1298. [Google Scholar] [CrossRef]
- Ahmed, N.; Khalid, S.; Grewal, A.G.; Ali, M.A.; Anjum, M.A.; Rahi, A.A.; Danish, S. Performance of mango scion cultivars under various levels of artificially induced salinity stress. Pak. J. Bot. 2020, 52, 1143–1158. [Google Scholar] [CrossRef]
- Leon-Calvario, I.; De La Fuente, M.C.; Benavides-Mendoza, A.; Juárez-Maldonado, A.; Sandoval-Rangel, A. Tomato grafted and cultivated in saline medium and its relation on nutraceutical compounds of the fruits. Pak. J. Bot. 2020, 52, 811–819. [Google Scholar] [CrossRef]
- Yasin Ashraf, M.; Yaqub, M.; Akhtar, J.; Athar Khan, M.; Ali Khan, M.; Ebert, G. Control of excessive fruit drop and improvement in yield and juice quality of kinnow (Citrus Deliciosa X Citrus Nobilis) through nutrient management. Pak. J. Bot. 2012, 44, 259–265. [Google Scholar]
- Ashraf, M.Y.; Hussain, F.; Ashraf, M.; Akhter, J.; Ebert, G. Modulation in yield and juice quality characteristics of citrus fruit from trees supplied with zinc and potassium foliarly. J. Plant Nutr. 2013, 36, 1996–2012. [Google Scholar] [CrossRef]
- Muhammad, I.; Abbasi, N.A.; Azhar, H.; Hafiz, I.A. Phenological behaviour and effect of different chemicals on pre-harvest fruit drop of sweet orange CV. “Salustiana”. Pak. J. Bot. 2011, 43, 453–457. [Google Scholar]
- Meena, D.; Bhatnagar, P.; Kumar, N.; Meena, L. Effect of foliar spray of nutrients on yield and quality of Nagpur mandarin (Citrus reticulata Blanco.). Pharma Innov. J. 2021, 10, 1122–1127. [Google Scholar]
- Tariq, M.; Sharif, M.; Shah, Z.; Khan, R. Effect of foliar application of micronutrients on the yield and quality of sweet orange (Citrus sinensis L.). Pak. J. Biol. Sci. 2007, 10, 1823–1828. [Google Scholar] [CrossRef]
- Ilyas, A.; Ashraf, M.Y.; Hussain, M.; Ashraf, M.; Ahmed, R.; Kamal, A. Effect of micronutrients (Zn, Cu and B) on photosynthetic and fruit yield attributes of citrus reticulata Blanco var. kinnow. Pak. J. Bot. 2015, 47, 1241–1247. [Google Scholar]
- Tahir, R. Impact of Foliar Application of Zn on Growth Yield and Quality Production of Citrus: A Review. Indian J. Pure Appl. Biosci. 2020, 8, 529–534. [Google Scholar] [CrossRef]
- Zaman, L.; Shafqat, W.; Qureshi, A.; Sharif, N.; Raza, K.; Din, S.; Ikram, S.; Jaskani, M.J.; Kamran, M. Effect of foliar spray of zinc sulphate and calcium carbonate on fruit quality of Kinnow mandarin (Citrus reticulata Blanco). J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 157–161. [Google Scholar] [CrossRef]
- Qiao, X.; He, Y.; Wang, Z.; Li, X.; Zhang, K.; Zeng, H. Effect of foliar spray of zinc on chloroplast β-carbonic anhydrase expression and enzyme activity in rice (Oryza sativa L.) leaves. Acta Physiol. Plant. 2014, 36, 263–272. [Google Scholar] [CrossRef]
- Vashisth, T.; Kadyampakeni, D. Diagnosis and management of nutrient constraints in citrus. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 723–737. ISBN 9780128187326. [Google Scholar]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Jin, L.F.; Yarra, R.; Yin, X.X.; Liu, Y.Z.; Cao, H.X. Identification and function prediction of iron-deficiency-responsive microRNAs in citrus leaves. 3 Biotech 2021, 11, 121. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.A.; Morgan, K.T.; Kadyampakeni, D.M.; Mahmoud, K.A. The effect of foliar and ground-applied essential nutrients on huanglongbing-affected mature citrus trees. Plants 2021, 10, 925. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmed, W.; Shahbaz, M. Role of foliar feeding of micronutrients in yield maximization of cotton in Punjab. Turk. J. Agric. For. 2013, 37, 420–426. [Google Scholar] [CrossRef]
- Kumar, D.; Patel, K.P.; Ramani, V.P.; Shukla, A.K.; Meena, R.S. Management of Micronutrients in Soil for the Nutritional Security. In Nutrient Dynamics for Sustainable Crop Production; Springer: Singapore, 2020; pp. 103–134. ISBN 9789811386602. [Google Scholar]
- Rafiullah, R.; Tariq, M.; Khan, F.; Shah, A.H.; Fahad, S.; Wahid, F.; Ali, J.; Adnan, M.; Ahmad, M.; Irfan, M.; et al. Effect of micronutrients foliar supplementation on the production and eminence of plum. Qual. Assur. Saf. Crop. Foods 2020, 12, 32–40. [Google Scholar] [CrossRef]
- Rahi, A.A.; Anjum, M.A.; Iqbal Mirza, J.; Ahmad Ali, S.; Marfo, T.D.; Fahad, S.; Danish, S.; Datta, R. Yield Enhancement and Better Micronutrients Uptake in Tomato Fruit through Potassium Humate Combined with Micronutrients Mixture. Agriculture 2021, 11, 357. [Google Scholar] [CrossRef]
- Bibi, F.; Ahmad, I.; Bakhsh, A.; Kiran, S.; Danish, S.; Ullah, H.; Rehman, A.-U. Effect of foliar application of boron with calcium and potassium on quality and yield of mango cv. summer bahisht (SB) Chaunsa. Open Agric. 2019, 4. [Google Scholar] [CrossRef]
- Havlin, J.L. Soil: Fertility and Nutrient Management. In Landscape and Land Capacity; Wang, Y., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 2020. [Google Scholar]
- Weil, R.R.; Brady, N.C. The Nature and Properties of Soils, 15th ed.; Brady, N., Weil, R.R., Eds.; Pearson Education Limited: London, UK, 2017; ISBN 9780133254488. [Google Scholar]
- Taalab, A.S.; Ageeb, G.W.; Siam, H.S.; Mahmoud, S.A. Some Characteristics of Calcareous soils. A review. Middle East J. Agric. Res. 2019, 8, 96–105. [Google Scholar]
- Chaudhari, P.R.; Ahire, D.V.; Ahire, V.D.; Chkravarty, M.; Maity, S. Soil Bulk Density as related to Soil Texture, Organic Matter Content and available total Nutrients of Coimbatore Soil. Int. J. Sci. Res. Publ. 2013, 3, 2250–3153. [Google Scholar]
- Srivastava, A.K.; Singh, S. Soil and plant nutritional constraints contributing to citrus decline in Marathwada region, India. Commun. Soil Sci. Plant Anal. 2005, 35, 2537–2550. [Google Scholar] [CrossRef]
- Hayat, N.; Ahmad, W.; Khan, F.A.; Ali, M.; Afzal, M.; Jahangeer, A. Soil Fertility Atlas of Pakistan-Punjab, 1st ed.; Ahmad, W., Niino, Y., Zia, M.H., Mahmood, K., Amad, N., Salim, M., Asraf, A., Shakir, M.A., Eds.; Food and Agricultural Organization, NARC: Islamabad, Pakistan, 2017. [Google Scholar]
- Campbell, C.R.; Plank, C.O. Preparation of plant tissue for laboratory analysis. In Handbook of Reference Methods for Plant Analysis; Kalra, Y., Ed.; CRC Press: Washington, DC, USA, 1998; pp. 37–49. [Google Scholar]
- Rhoades, J.D. Salinity: Electrical Conductivity and Total Dissolved Solids. In Methods of Soil Analysis, Part 3, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 5, pp. 417–435. [Google Scholar]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1982; Volume 9, pp. 199–224. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Crop Science Society of America, and Soil Science Society of America: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Loeppert, R.H.; Suarez, D.L. Carbonate and Gypsum. In Methods of Soil Analysis, Part 3, Chemical Methods; Soil Science Society of America: Madison, WI, USA, 2018; Volume 9, pp. 181–197. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen–total. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Soil Science Society of America, Madison: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Method of Soil Analysis, Agron. No. 9, Part 2: Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Pratt, P.F. Potassium. In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, 9.2; Norman, A.G., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1965; pp. 1022–1030. [Google Scholar]
- Soltanpour, P.N.; Schwab, A.P. A new soil test for simultaneous extraction of macroand micro-nutrients in alkaline soils. Commun. Soil Sci. Plant Anal. 1977, 8, 195–207. [Google Scholar] [CrossRef]
- Ryan, J.; Estefan, G.; Rashid, A. Soil and Plant Analysis Laboratory Manual, 2nd ed.; International Center for Agriculture in Dry Areas (ICARDA) Syria, The National Agricultural Research Center (NARC): Islamabad, Pakistan, 2001. [Google Scholar]
- Benton, J.J.; Wolf, B.; Mills, H.A. (Eds.) Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide—AbeBooks: 1878148001, 1st ed.; Micro-Macro Publishing Inc.: Athens, GA, USA, 1991. [Google Scholar]
- Bremner, M. Nitrogen-Total. In Methods of Soil Analysis Part 3. Chemical Methods-SSSA Book Series 5; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Johnston, C.T., Sumner, M.E., Eds.; John Wiley & Sons, Inc.: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Van Schouwenberg, J.C.; Walinge, I. Methods of Analysis for Plant Material; Agricultural University Wageningen: Wageningen, The Netherlands, 1973. [Google Scholar]
- Rashid, A. Mapping Zinc Fertility of Soils Using Indicator Plants and Soil Analyses; University of Hawaii at Manoa: Honolulu, HI, USA, 1986. [Google Scholar]
- Chapman, H.D.; Pratt, P.F. Methods of Analysis for Soils, Plants and Water; University of California, Division of Agricultural Sciences: Berkeley, CA, USA, 1961. [Google Scholar]
- Bingham, F.T. Boron. In Methods of Soil Analysis, Part 2; Agron. Monogr. No. 9; ASA and SSSA: Madison, WI, USA, 1982; pp. 437–447. [Google Scholar]
- Gaines, T.P.; Mitchell, G.A. Boron determination in plant tissue by the azomethine H method. Commun. Soil Sci. Plant Anal. 1979, 10, 1099–1108. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book International Co.: Singapore, 1997. [Google Scholar]
- OriginLab Corporation. OriginPro; OriginLab: Northampton, MA, USA, 2021. [Google Scholar]
- Muhammad, S.; Müller, T.; Joergensen, R.G. Relationships between soil biological and other soil properties in saline and alkaline arable soils from the Pakistani Punjab. J. Arid Environ. 2008, 72, 448–457. [Google Scholar] [CrossRef]
- Ahmad, M.; Ryan, J.; Paeth, R.C. Soil development as a function of time in the Punjab river plains of Pakistan. Soil Sci. Soc. Am. J. 1977, 41, 1162–1166. [Google Scholar] [CrossRef]
- Ahmad, I.; Bibi, F.; Ullah, H.; Munir, T.M. Mango fruit yield and critical quality parameters respond to foliar and soil applications of zinc and boron. Plants 2018, 7, 97. [Google Scholar] [CrossRef]
- Khalid Chaudhry, U.; Shahzad, S.; Nadir Naqqash, M.; Saboor, A.; Yaqoob, S.; Salim, M.; Khalid, M. Integration of biochar and chemical fertilizer to enhance quality of soil and wheat crop (Triticum aestivum L.). J. Biodivers. Environ. Sci. 2016, 9, 348–358. [Google Scholar] [CrossRef]
- Lindsay, W.L. Chemical Equilibria in Soils; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Ashraf, M. Distribution and Indexation of Plant Available Nutrients of District Layyah, Punjab Pakistan. Am. J. Agric. For. 2015, 3, 16. [Google Scholar] [CrossRef][Green Version]
- Malik, D.M.; Khan, M.A.; Chaudhry, T.A. Analysis Manual for Soils, Plants and Waters; Soil Fertility Survey and Soil Testing Institute, Department of Agriculture: Lahore, Pakistan, 1984. [Google Scholar]
- Liu, Z.; He, T.; Cao, T.; Yang, T.; Meng, J.; Chen, W. Effects of biochar application on nitrogen leaching, ammonia volatilization and nitrogen use efficiency in two distinct soils. J. Soil Sci. Plant Nutr. 2017, 17, 515–528. [Google Scholar] [CrossRef]
- Smith, W.; Grant, B.; Qi, Z.; He, W.; VanderZaag, A.; Drury, C.F.; Vergè, X.; Balde, H.; Gordon, R.; Helmers, M.J. Assessing the Impacts of Climate Variability on Fertilizer Management Decisions for Reducing Nitrogen Losses from Corn Silage Production. J. Environ. Qual. 2019, 48, 1006–1015. [Google Scholar] [CrossRef]
- Fontana, M.; Bragazza, L.; Guillaume, T.; Santonja, M.; Buttler, A.; Elfouki, S.; Sinaj, S. Valorization of calcium phosphite waste as phosphorus fertilizer: Effects on green manure productivity and soil properties. J. Environ. Manag. 2021, 285, 112061. [Google Scholar] [CrossRef]
- Rashid, A. Nutrient Indexing Surveys and Micronutrient Requirement of Crops; Pakistan Agricultural Research Council: Islamabad, Pakistan, 1994. [Google Scholar]
- Martens, D.C.; Lindsay, W.L. Testing soils for copper, iron, manganese, and zinc. In Soil Testing and Plant Analysis; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 229–264. [Google Scholar]
- Rahman, N.; Hangs, R.; Peak, D.; Schoenau, J. Chemical and molecular scale speciation of copper, zinc, and boron in agricultural soils of the canadian prairies. Can. J. Soil Sci. 2021, 101, 581–595. [Google Scholar] [CrossRef]
- Rashid, A. Secondary and micronutrient. In Soil Science; Rashid, A., Memon, K.S., Eds.; National Book Foundation: Islamabad, Pakistan, 1996; pp. 341–385. [Google Scholar]
- Rashid, A. Establishment and management of micronutrient deficiencies in Pakistan: A review. Soil Environ. 2005, 24, 1–22. [Google Scholar]
- Reuter, D.; Robinson, J.B. Plant Analysis: An Interpretation Manual, 2nd ed.; Reuter, D., Robinson, J.B., Eds.; CSIRO Publishing: Clayton, Australia, 1997; ISBN 0643101268. [Google Scholar]
- Tu, A.; Xie, S.; Zheng, H.; Li, H.; Li, Y.; Mo, M. Long-term effects of living grass mulching on soil and water conservation and fruit yield of citrus orchard in south China. Agric. Water Manag. 2021, 252, 106897. [Google Scholar] [CrossRef]
- Rehman, M.; Saleem, M.H.; Fahad, S.; Maqbool, Z.; Peng, D.; Deng, G.; Liu, L. Medium nitrogen optimized Boehmeria nivea L. growth in copper contaminated soil. Chemosphere 2021, 266, 128972. [Google Scholar] [CrossRef]
- Kadyampakeni, D.M.; Chinyukwi, T. Are macronutrients and micronutrients therapeutic for restoring performance of trees affected by citrus greening? A discussion of current practices and future research opportunities. J. Plant Nutr. 2021, 44, 2949–2969. [Google Scholar] [CrossRef]
- Mihoub, A.; Amin, A.E.E.A.Z.; Motaghian, H.R.; Saeed, M.F.; Naeem, A. Citric Acid (CA)—Modified Biochar Improved Available Phosphorus Concentration and Its Half-Life in a P-Fertilized Calcareous Sandy Soil. J. Soil Sci. Plant Nutr. 2021, 1–10. [Google Scholar] [CrossRef]
- Jones, W.W.; Embleton, T.W. Development and current status of citrus leaf analysis as aguide to fertilization in California. In Proceedings of the Ist International Citrus Symposium, University of, University of California Riverside, CA, USA, 16–26 March 1969; pp. 1669–1671. [Google Scholar]
- Mulder, D. Les elements mineurs en culture fruitiere. In Proceedings of the Convegno Nazionale Frutticoltura, Montana de Saint Vincent, Italy, 26–29 September 1953; pp. 98–118. (In French). [Google Scholar]
- Villa, Y.B.; Khalsa, S.D.S.; Ryals, R.; Duncan, R.A.; Brown, P.H.; Hart, S.C. Organic matter amendments improve soil fertility in almond orchards of contrasting soil texture. Nutr. Cycl. Agroecosyst. 2021, 120, 343–361. [Google Scholar] [CrossRef]
- Saboor, A.; Ali, M.A.; Danish, S.; Ahmed, N.; Fahad, S.; Datta, R.; Ansari, M.J.; Nasif, O.; ur Rahman, M.H.; Glick, B.R. Effect of arbuscular mycorrhizal fungi on the physiological functioning of maize under zinc-deficient soils. Sci. Rep. 2021, 11, 18468. [Google Scholar] [CrossRef]
- Chernov, T.I.; Zhelezova, A.D.; Tkhakakhova, A.K.; Ksenofontova, N.A.; Zverev, A.O.; Tiunov, A.V. Soil microbiome, organic matter content and microbial abundance in forest and forest-derived land cover in Cat Tien National Park (Vietnam). Appl. Soil Ecol. 2021, 165, 103957. [Google Scholar] [CrossRef]
- Waqeel, J.; Khan, S.T. Microbial Biofertilizers and Micronutrients Bioavailability: Approaches to Deal with Zinc Deficiencies. In Microbial Biofertilizers and Micronutrient Availability; Khan, S.T., Malik, A., Eds.; Springer: Cham, Switzerland, 2022; pp. 239–297. [Google Scholar]
| Attributes | Units | Soil Depth (cm) | Range | Mean |
|---|---|---|---|---|
| Sand | % | 0–15 | 53–77 | 65.29 |
| 15–30 | 56–76 | 66.52 | ||
| 30–45 | 55–72 | 62.15 | ||
| Silt | 0–15 | 6–36 | 18.90 | |
| 15–30 | 7–33 | 16.75 | ||
| 30–45 | 7–30 | 16.96 | ||
| Clay | 0–15 | 8–21 | 15.81 | |
| 15–30 | 9–22 | 16.73 | ||
| 30–45 | 10–33 | 20.87 |
| pH | - | Soil Depth (cm) | Range | Mean | Acidic | Slightly Alkaline | Highly Alkaline |
| 0–15 | 7.76–8.58 | 8.24 | |||||
| 15–30 | 7.62–8.93 | 8.27 | |||||
| 30–45 | 7.69–8.70 | 8.25 | |||||
| EC | dSm−1 | Soil Depth (cm) | Range | Mean | Normal | Slightly Saline | Highly Saline |
| 0–15 | 0.20–1.88 | 1.14 | |||||
| 15–30 | 0.21–1.96 | 1.18 | |||||
| 30–45 | 0.20–1.96 | 1.12 | |||||
| SOM | % | Soil Depth (cm) | Range | Mean | Low | Medium | High |
| 0–15 | 0.25–0.93 | 0.59 | |||||
| 15–30 | 0.20–0.85 | 0.41 | |||||
| 30–45 | 0.05–0.67 | 0.30 | |||||
| CaCO3 | Soil Depth (cm) | Range | Mean | Low | Medium | High | |
| 0–15 | 1.45–17.5 | 7.94 | |||||
| 15–30 | 1.30–12.6 | 5.78 | |||||
| 30–45 | 1.50–18.9 | 8.38 |
| Nutrients | Units | Soil Depth (cm) | Range | Mean | Low | Medium | High |
|---|---|---|---|---|---|---|---|
| N | % | 0–15 | 0.013–0.047 | 0.03 | |||
| 15–30 | 0.01–0.043 | 0.02 | |||||
| 30–45 | 0.0–0.03 | 0.02 | |||||
| P | mg kg−1 | 0–15 | 3.4–10.67 | 7.51 | |||
| 15–30 | 2.76–9.97 | 6.20 | |||||
| 30–45 | 1.14–8.97 | 4.59 | |||||
| K | 0–15 | 66–137 | 103.46 | ||||
| 15–30 | 60–132 | 99.47 | |||||
| 30–45 | 108–151 | 121.62 |
| Nutrients | Units | Soil Depth (cm) | Range | Mean | Low | Medium | High |
|---|---|---|---|---|---|---|---|
| B | mg kg−1 | 0–15 | 0.19–0.84 | 0.50 | |||
| 15–30 | 0.10–0.66 | 0.35 | |||||
| 30–45 | 0.06–0.55 | 0.26 | |||||
| Cu | 0–15 | 0.18–0.48 | 0.28 | - | |||
| 15–30 | 0.15–0.32 | 0.21 | |||||
| 30–45 | 0.06–0.24 | 0.14 | |||||
| Fe | 0–15 | 0.33–7.02 | 4.19 | ||||
| 15–30 | 0.25–5.78 | 2.61 | |||||
| 30–45 | 0.04–3.34 | 1.29 | |||||
| Mn | 0–15 | 0.15–5.03 | 1.29 | ||||
| 15–30 | 0.12–2.12 | 0.72 | |||||
| 30–45 | 0.06–1.33 | 0.42 | |||||
| Zn | 0–15 | 0.30–1.55 | 0.78 | ||||
| 15–30 | 0.24–1.25 | 0.48 | |||||
| 30–45 | 0.11–0.57 | 0.30 |
| Nutrients | Units | Range | Mean | Deficient | Low | Adequate | High |
|---|---|---|---|---|---|---|---|
| N | % | 0.50–2.30 | 1.2 | ||||
| P | 0.02–0.22 | 0.1 | |||||
| K | 0.03–1.36 | 0.6 | |||||
| Zn | mg kg−1 | 1.33–33.0 | 13.9 | ||||
| Fe | 1.33–57.9 | 28.5 | |||||
| Cu | 1.00–14.9 | 6.65 | |||||
| Mn | 1.75–41.5 | 23.1 | |||||
| B | 2.20–33.14 | 16.27 |
| Soil | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Attributes | Depth | Sand | Silt | Clay | pH | EC | OM | CaCO3 | Zn | Fe | Mn | Cu | B | N | P |
| Sand | −0.2015 | ||||||||||||||
| Silt | −0.1112 | −0.8327 | |||||||||||||
| Clay | 0.5189 | −0.0829 | −0.4827 | ||||||||||||
| pH | 0.0321 | −0.0089 | −0.0025 | 0.0185 | |||||||||||
| EC | −0.0138 | 0.0321 | −0.0073 | −0.0376 | 0.2787 | ||||||||||
| OM | −0.5566 | 0.0475 | 0.1031 | −0.2607 | 0.2386 | 0.0828 | |||||||||
| CaCO3 | −0.3611 | −0.0585 | 0.0765 | −0.0451 | −0.0024 | 0.1191 | 0.2862 | ||||||||
| Zn | 0.2072 | −0.0492 | −0.0001 | 0.0780 | −0.0306 | 0.0993 | −0.0131 | 0.0531 | |||||||
| Fe | −0.2756 | 0.2646 | −0.1615 | −0.1278 | −0.1371 | −0.0639 | 0.0948 | 0.1173 | −0.0313 | ||||||
| Mn | −0.3649 | 0.0856 | −0.0133 | −0.1115 | −0.0262 | 0.0593 | 0.2562 | 0.1523 | −0.1542 | 0.1107 | |||||
| Cu | 0.3103 | −0.0443 | 0.0425 | −0.0065 | −0.1075 | −0.0288 | −0.1571 | −0.0149 | 0.0963 | −0.0253 | −0.0493 | ||||
| B | −0.2853 | 0.0169 | 0.0824 | −0.1750 | 0.0975 | 0.0905 | 0.3065 | 0.0785 | 0.0320 | 0.0949 | 0.2165 | −0.0236 | |||
| N | −0.5526 | 0.0435 | 0.1043 | −0.2567 | 0.2410 | 0.0819 | 0.9997 | 0.2843 | −0.0161 | 0.0900 | 0.2561 | −0.1556 | 0.3073 | ||
| P | 0.0956 | 0.1504 | −0.1537 | 0.0387 | 0.0732 | −0.0505 | 0.0619 | 0.0605 | 0.0655 | 0.1750 | 0.0114 | 0.0991 | 0.0829 | 0.0620 | |
| K | 0.3218 | −0.1952 | 0.0568 | 0.2065 | −0.0051 | 0.0884 | −0.1096 | −0.0110 | 0.2074 | −0.1558 | −0.1785 | 0.0579 | −0.0408 | −0.1086 | 0.0365 |
| Plant | |||||||||||||||
| Attributes | N | P | K | Mn | Fe | Zn | Cu | - | |||||||
| P | −0.0515 | ||||||||||||||
| K | 0.1142 | 0.0137 | |||||||||||||
| Mn | −0.0224 | 0.1007 | −0.0266 | ||||||||||||
| Fe | 0.0912 | −0.0747 | 0.0670 | −0.0429 | |||||||||||
| Zn | −0.0208 | 0.0146 | 0.0096 | −0.0654 | 0.0198 | ||||||||||
| Cu | −0.0098 | 0.0426 | −0.0919 | 0.0368 | −0.0141 | 0.0134 | |||||||||
| B | 0.0353 | 0.0978 | −0.0683 | 0.0710 | −0.0831 | 0.0571 | 0.0170 | ||||||||
| Principal Component Name | PC1 | PC2 | Eigenvalue | Percentage of Variance (%) | Cumulative (%) |
|---|---|---|---|---|---|
| Loadings | |||||
| EC | 0.11039 | −0.03655 | 2.76299 | 18.41996 | 18.41996 |
| pH | 0.17578 | −0.02994 | 2.09231 | 13.94876 | 32.36872 |
| OM | 0.54404 | −0.00228 | 1.52309 | 10.15394 | 42.52266 |
| CaCO3 | 0.24058 | −0.03169 | 1.29373 | 8.62489 | 51.14754 |
| Zn | −0.05452 | −0.07174 | 1.05737 | 7.04912 | 58.19666 |
| Fe | 0.12402 | 0.30105 | 1.00438 | 6.69589 | 64.89255 |
| Mn | 0.26602 | 0.10773 | 0.94669 | 6.31126 | 71.20381 |
| Cu | −0.12618 | −0.05415 | 0.89797 | 5.98646 | 77.19028 |
| B | 0.29453 | −0.00369 | 0.8619 | 5.74597 | 82.93624 |
| K | −0.15047 | −0.18534 | 0.72676 | 4.84504 | 87.78128 |
| P | 0.05317 | 0.21096 | 0.6767 | 4.51137 | 92.29265 |
| N | 0.54338 | −0.00423 | 0.63379 | 4.22524 | 96.51788 |
| Sand | 0.03839 | 0.61922 | 0.52204 | 3.48025 | 99.99813 |
| Silt | 0.11845 | −0.63184 | 2.80154 × 10−4 | 0.00187 | 100 |
| Clay | −0.27392 | 0.15779 | 1.02939 × 10−31 | 6.86263 × 10−31 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, N.; Hussain, S.; Ali, M.A.; Minhas, A.; Waheed, W.; Danish, S.; Fahad, S.; Ghafoor, U.; Baig, K.S.; Sultan, H.; et al. Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District. Horticulturae 2022, 8, 61. https://doi.org/10.3390/horticulturae8010061
Ahmad N, Hussain S, Ali MA, Minhas A, Waheed W, Danish S, Fahad S, Ghafoor U, Baig KS, Sultan H, et al. Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District. Horticulturae. 2022; 8(1):61. https://doi.org/10.3390/horticulturae8010061
Chicago/Turabian StyleAhmad, Niaz, Sajjad Hussain, Muhammad Arif Ali, Asif Minhas, Waqar Waheed, Subhan Danish, Shah Fahad, Umber Ghafoor, Khurram Shehzad Baig, Haider Sultan, and et al. 2022. "Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District" Horticulturae 8, no. 1: 61. https://doi.org/10.3390/horticulturae8010061
APA StyleAhmad, N., Hussain, S., Ali, M. A., Minhas, A., Waheed, W., Danish, S., Fahad, S., Ghafoor, U., Baig, K. S., Sultan, H., Hussain, M. I., Ansari, M. J., Marfo, T. D., & Datta, R. (2022). Correlation of Soil Characteristics and Citrus Leaf Nutrients Contents in Current Scenario of Layyah District. Horticulturae, 8(1), 61. https://doi.org/10.3390/horticulturae8010061













