Abstract
The antioxidant, antibacterial and antifungal properties of essential oils (EOs) of Juniperus thurifera L., a plant utilized in traditional, herbal medicine, were investigated. The EOs were extracted by use of a Clevenger apparatus and phytochemicals identified by gas chromatography coupled with mass spectrometry (GC/MS/MS). The antioxidant capacity of EOs of J. thurifera was determined by 2,2-diphenyl-1-picrylhydrazil (DPPH), total antioxidant capacity (TAC), and ferric reducing antioxidant power (FRAP). Antimicrobial activity of EOs of J. thurifera was determined against four fungal strains, Candida albicans; ATCC 10231, Aspergillus niger; MTCC 282, Aspergillus flavus; MTCC 9606 and Fusarium oxysporum; MTCC 9913 and four bacterial strains, Staphylococcus aureus; ATCC 6633, Escherichia coli; K12, Bacillus subtilis; DSM 6333, and Pseudomonas aeruginosa; CIP A22, by use of the disk diffusion method, and microdilution method used to determine the minimum inhibitory concentration (MIC). EOs of J. thurifera consisted of 31 compounds and were dominated by α-thujene (25%), elemol (12%) and muurolol (12%). Antioxidant activity recorded an IC50 of 24 ± 0.71 µg/mL (DPPF), EC50 of 0.19 ± 0.01 mg/mL (FRAP), and 9.3 × 102 ± 38 mg EAA/g (TAC). The EOs of J. thurifera exhibited significant antibacterial activity against all bacterial strains under investigation, especially P. aeruginosa; CIP A22 with an inhibition diameter of 28 ± 1.5 mm and MIC of 4.8 × 10−2 ± 0. 001 µg/mL. EOs of J. thurifera also exhibited significant antifungal activity against C. albicans; ATCC 10231 and F. oxysporum; MTCC 9913 with an activity of 21 ± 2.1 mm, 32 ± 2.3%, and MIC of 9.5 × 10−2 ± 0.001 Bioactive molecules found in EOs of J. thurifera could be used as an alternative solution to antibiotics available on the market to combat microbial resistance.
Keywords:
juniper; natural products; tree; leaves; disease; bioactive compounds; microbial resistance 1. Introduction
Thuriferous juniper (Juniperus thurifera L.) is a monoecious, dioecious, conifer tree or shrub with scaly leaves of the cypress family (Cupressaceae) [1]. It plays an important role in the western Mediterranean basin and is regarded as a keystone species of low-temperature-adapted open woodlands, with steppe-like undercover [2]. In Morocco, the area of this species, which constitutes the upper limit of the forest in the Atlas Mountains, has been considerably restricted and, the vast majority of the stands have been degraded by over-exploitation and over-grazing, aggravated by an almost total absence of regeneration. Its current surface area, in the Atlas Mountains of Morocco, is estimated to be 20,000 ha [3,4]. Thuriferous juniper, with its extraordinary resistance, remarkable ability to withstand very severe climatic conditions, and indifference to the physical nature of soils, longevity of as much as 500 years, is unquestionably the predominant tree of the high Moroccan mountains [5]. Development of these natural plant resources is mainly based on extraction of essential oils (EOs), which are high value-added products, employed in the pharmaceutical, cosmetics and food industries [6,7,8]. The genus Juniperus contains essential medicinal plants with a long history of usage in traditional medicine. Its leaves are used to treat diabetes, diarrhoea, and rheumatism as a decoction [9]. Leaves and berries of Juniperus are utilized as an oral hypoglycemic medication [10], while leaves are used to treat bronchitis and as a diuretic [9]. Studies of the biological and biotechnological activities of the phytochemical compounds of plants is of interest and the antimicrobial activities of EOs have been reported [6,11,12,13]. These activities are attributed to oxygenated mono-terpenes [6]. Use of natural molecules to inhibit oxidation of fat, its consequences on health and its economic repercussions have been the subject of several studies [6,14]. Results of several studies of the antioxidant activities of EOs from a variety of aromatic plants have demonstrated that these properties are mainly ascribed to the presence of compounds containing hydroxyl group(s) [15,16,17]. Recently, the EOs and aqueous extracts of plants have attracted interest because of their richness in natural biologically active constituents including antioxidant, antimicrobial and insecticidal properties.
Under the existing restricted and inadequate arsenal of new therapies, the list of microorganisms that are becoming resistant to all commonly used antibiotics is growing, prompting the discovery of alternative classes of medications to prevent significant public health concerns, unconventional therapeutic interventions derived from natural resource exploitation have been intensively investigated [18,19]. Objectives of this study were to describe the chemical composition of the EOs of leaves of J. thurifera collected from the Jbel lakraa Massif in the Eastern, Middle Atlas of Morocco and to compare the results to those of previous studies and investigate the antioxidant, antibacterial and antifungal activities of the EOs, so that an evaluation of the economic value of the EOs of J. thurifera and their potential as replacements for antibiotics available on the market to combat microbial resistance could be conducted.
2. Materials and Methods
2.1. Extraction of EOs from J. thurifera
J. thurifera was harvested from the mountains (lat: 33.68093368; long: 4.30823143) during October 2021, which was autumn. Specimens were identified by a botanist in the department of biology, Faculty of Sciences-FSDM-USMBA-Fez, and the plant is deposited in the Herbarium under number (FJT/02D20). Leaves were cleaned and subsequently dried at 35 °C for 72 h in a ventilated oven. Dried leaves were crushed with an electric blender, then EOs extracted by hydro-distillation on a Clevenger-type extractor [15]. Briefly, 200 g of the ground leaf material was mixed with 750 mL distilled water (dH2O) and extracted for about 120 min. Samples were partitioned into hexane (10%). At least three replicates were performed in this study.
2.2. GC/MS/MS Analysis of EOs
Constituents of EOs were identified and quantified by use of gas chromatography (TQ8040 NX; Shimadzu, Tokyo, Japan) attached to a triple quadrupole, tandem mass spectrometer (GC-MS). Chromatography was conducted on an apolar, capillary column RTxi-5 Sil MS column (30 m × 0.25 mm ID × 0.25 µm). Helium was used as carrier gas and the injection volume was 1 µL. Temperatures of the source and the interface were 200 °C and 280 °C, respectively. The chromatographic system was programmed with splitless injection (split opening at 4 min), injection temperature of 250 °C and pressure of 37.1 kPa. Temperature was programmed with an initial temperature of 50 °C for 2 min, ramp 1 was 5 °C/min to 160 °C for 2 min and ramp 2 was 5 °C/min to 280 °C for 2 min. Identification of phytochemicals in EOs was conducted by comparing the obtained retention indices with those of chemical compounds in the literature database [20].
2.3. Antioxidant Activity
2.3.1. DPPH Test
Antioxidant activity was determined by use of the DPPH assay according to previously published method [21]. Briefly, 800 µL of a methanolic solution of DPPH (0.2 mM) was mixed with 200 µL of different dilutions of EOs of J. thurifera (0–1 mg/mL), and subsequently incubated in the dark at RT for 30 min. Absorbances of samples were recorded at 517 nm and compared to those of a control consisting of 800 µL of DPPH solution. Samples, positive controls, quercetin or BHT were prepared under the same operating conditions. Decay of absorbance was measured with a spectrophotometer and percent inhibition (I%) calculated (Equation (1)).
I (%)= [(T0 − Tx)/T0] ∗ 100
By performing kinetics of this activity, concentrations corresponding to 50% inhibition (IC50), expressed as µg/mL, were determined, where the least IC50 corresponds to the greatest efficiency of EOs.
2.3.2. TAC Test
Antioxidant activity was determined by placing 100 µL of EOs at various concentrations after adding 1000 µL of a reagent composed of 0.6 M H2SO4, 28 mM Na2PO4 and 4 mM (NH4)2MoS4. Then, the tubes were tightly closed and incubated at 95 °C for 90 min. After cooling, absorbances were measured at 695 nm. The negative control consisted of 100 µL of methanol after the addition of 1000 µL of the above reagent [22]. The samples and controls were incubated under the same conditions. The obtained results were represented in mg ascorbic acid equivalents per gram (mg EAA/g).
2.3.3. FRAP Test
Reducing power was recorded by placing 200 µL of sample at several concentrations, into 500 µL of 0.2 M phosphate buffer (pH = 6.6), followed by 500 µL of 1% K3Fe (CN)6 in dH2O. Mixtures were subsequently placed into a water bath and incubated at 50 °C for 20 min. Next, about 500 µL trichloroacetic acid (TCA, 10%) was added followed by centrifugation. A 500 µL aliquot of the supernatant was transferred to another tube followed by the addition of 500 µL of dH2O and 100 µL of freshly-prepared FeCl3 (1%) in dH2O. Similarly, a blank without sample was included by replacing EOs of J. thurifera with methanol. Absorbances of reaction media were recorded at 700 nm and compared to the methanol blank, which allowed calibration of the apparatus (UV-VIS spectrophotometer). Positive controls were a solution of the standard antioxidants BHT and quercetin [23].
2.4. Antimicrobial Activity of EOs of J. thurifera
2.4.1. Microbial Strains Tested
Antimicrobial activity of J. thurifera EOs against four fungal strains, Candida albicans, ATCC 10231; Aspergillus niger, MTCC 282; Aspergillus flavus, MTCC 9606 and Fusarium oxysporum, MTCC 9913 and four strains of bacteria, Staphylococcus aureus, ATCC 6633; Escherichia coli, K12; Bacillus subtilis, DSM 6333 and Pseudomonas aeruginosa, CIP A22. The fungal and bacteria strains were provided by Sidi Mohammed Ben Abdellah University (Fez, Morocco) and Hassan II University Hospital (Fez, Morocco), respectively.
2.4.2. Assessment of Antimicrobial Activity
The antimicrobial activity of J. thurifera EOs was determined by use of the disc diffusion method [24]. Petri dishes containing Mueller–Hinton (MH) and Malt Extract (ME) culture media were inoculated with the four bacterial strains and C. albicans, respectively, by the double-layer method, from cultures freshly grown in MH and ME medium, decimal dilutions were made in sterile saline (0.9%) until turbidity of 0.5 McFarland (108 CFU/mL) was reached, 100 µL were added to tubes containing 5 mL of soft agar (0.5% agar), then the inoculated tubes were spread in Petri dishes containing MH and ME medium. For A. niger, A. flavus, and F. oxysporum the antifungal activity was determined by the direct confrontation method in the ME medium. Sterile 6 mm Whatman paper discs were positioned into the centre of the petri dish and then impregnated with 20 μL of J. thurifera EOs, and also with conventional antimicrobial drugs; streptomycin and erythromycin for bacterial strains and fluconazole for fungal strains according to the methodology of the European Committee for Antimicrobial Susceptibility Testing (EUCAST). Then, bacteria- and fungi-inoculated dishes were incubated at temperatures of 30 °C and 37 °C optimal for the bacterial and fungal strains and C. albicans, respectively. Inhibition diameters and percentages of inhibition were calculated 18–24 h post inoculation (hpi) for the bacterial strains and after 24–48 hpi for C. albicans, and 7 days post inoculation for F. oxysporum, A. niger and A. flavus [24,25].
2.4.3. Minimum Inhibitory Concentration (MIC) Determination
Minimum inhibitory concentrations (MIC) of J. thurifera EOs against the four bacterial and four fungal strains were determined by use of the microdilution as previously described [25]. Briefly, a sterile 96-well microplate was used and 50 µL of sterile MH or ME medium was added for bacterial and fungal strains, respectively. Serially diluted EOs of J. thurifera at a volume of 100 µL prepared in 10% (v/v) DMSO was pipetted into the first row. This was followed by the addition of 30 µL of microbial strains. Plates were incubated for 24 h, 48 h or 7 d for bacteria, C. albicans and fungi (Fusarium oxysporum, A. niger, A. flavus), respectively; at 37 °C or 30 °C [25,26]. Each well received 20 µL of water 2,3,5-triphenyl tetrazolium chloride solution (0.2%) to visualize bacterial growth. MIC was defined as the least concentration that did not create a red colour [26].
2.5. Statistical Analyses
Results were expressed as means of triplicates ± SD (standard deviation). Shapiro–Wilks test was employed to determine the normality of distribution, while the t-test was used to check for homogeneity of variances. Analysis of variance (ANOVA) was performed, with Tukey’s HSD test, as a post hoc test for multiple comparisons. Differences were considered significant at probability level (p) < 0.05.
3. Results and Discussion
3.1. Identification of Chemicals Comprising EOs of J. thurifera by GC/MS
The yield of EOs of 0.96%, from leaves of J. thurifera provided was greater than that reported previously [27]. Essentially, all of the mass of the EOs of J. thurifera (99.99%) was accounted for by 31 phytochemical compounds (Table 1 and Figure 1). Previously, 99.46% of the mass of EOs of J. thurifera was reported to be accounted for by 24 compounds [28]. The phytochemical composition of the EOs of J. thurifera is dominated by α-thujene (25%), elemol (12%) and muurolol (12%) (Figure 2). The chemical composition of EOs of J. thurifera was quantitatively and qualitatively different from that reported previously [27]. In another recent study β-pinene (36%) were determined to be the predominant compounds in EOs of J. thurifera, whereas in this study β-pinene accounted for only 1.9% of the mass of EOs.

Table 1.
Phytochemical compounds identified by GC/MS/MS in EOs of J. thurifera.
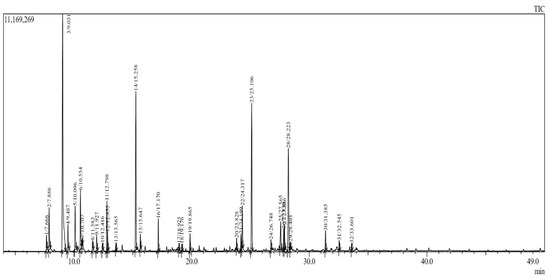
Figure 1.
Chromatograph of compounds identified by GC/MS in EOs of J. thurifera. Peaks represent absolute abundances, whereas numbers on the x-axis represent retention times in min.
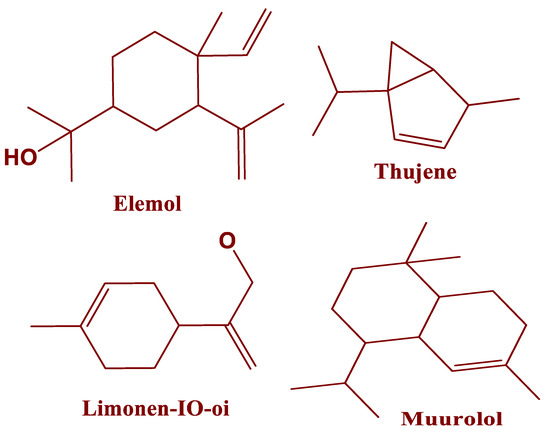
Figure 2.
Molecular structures of phytochemical compounds in EOs of J. thurifera.
3.2. Antioxidant Activity
When antioxidant activities of EOs of J. thurifera were evaluated by three methods (Figure 3a), the percentage of inhibition of free radical (DPPH) was directly proportional to the concentrations of the EOs of J. thurifera. For a concentration of 13 µg/mL of EOs of J. thurifera, the percentage of DPPH inhibition was 85 ± 0.24% and for a concentration of 27 µg/mL the percentage of inhibition was approximately 91 ± 0.17% (Figure 3a). Antioxidant capacity was determined from the IC50, which is the concentration necessary to reduce 50% of the DPPH radical. The smaller the IC50 value, the greater the antioxidant activity of a compound [29]. Free radical activities of EOs of J. thurifera, BHT and quercetin revealed that IC50 of EOs of J. thurifera is of the order of 23.6 ± 0.71 µg/mL, 14.2 ± 0.14 µg/mL and 15.9 ± 0.56 µg/mL (Figure 3b), respectively. Evaluation of antioxidant capacity by use of the FRAP method revealed that the effective concentration (EC-50) is in the range of 0.19 ± 0.01 mg/mL (EOs of J. thurifera), 3.6 × 10−2 ± 0.003 mg/mL (BHT) and 2.8 × 10−2 ± 0.002 mg/mL (quercetin) (Figure 4a). Total antioxidant capacity (Figure 4b) of EOs of J. thurifera was 9.3 × 102 ± 38 mg EAA/g versus 5.3 × 102 ± 22 mg EAA/g (BHT) and 6.6 × 102 ± 46.67 mg EAA/g (quercetin).
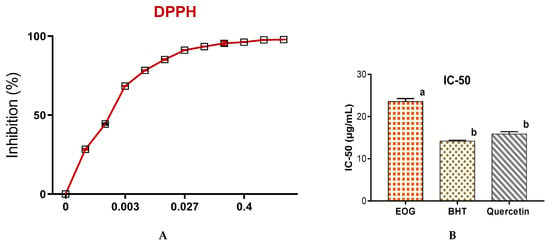
Figure 3.
Antioxidant activities of EOs of J. thurifera by the DPPH method (A) and the concentration of IC-50 (B). Bars with the same letters do not differ significantly (p < 0.05).
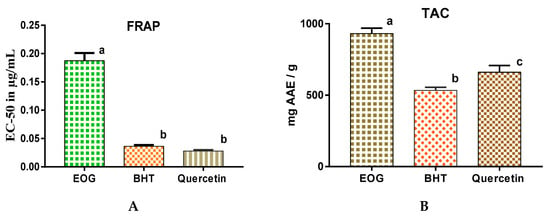
Figure 4.
Antioxidant activities of EOs of J. thurifera by the FRAP method (A) and antioxidant capacity of EOs of J. thurifera (B). Bars with the same letters do not differ significantly (p < 0.05).
Assessment of oxidative stress (OS, oxidation in vivo) has become important since this type of oxidation can be involved in several health effects including rheumatoid arthritis, atherosclerosis, diabetes, aging and cancer [30,31,32]. Natural antioxidants present in plant extracts and EOs can provide protection against OS by two main mechanisms, namely scavenging reactive oxygen species (ROS) and blocking lipid peroxidation [33,34]. There is a correlation between the antioxidant power of EOs and its phytochemical composition. In this context, it has been previously documented that the antioxidant capacities of EOs are associated with their phytochemical composition via the hydroxyl function present in their constituents, and the richer an oil is in phenolic compounds and terpenes, the more effective its antioxidant capacity [35,36,37,38,39,40].
The phytochemical profile of EOs of J. thurifera (Table 1) revealed that EOs of J. thurifera are rich in terpenic compounds, such as thujene, γ-terpinene, cymene and linalool which are known for their antioxidant potentials [41]. Recent studies have shown that sabinene is an antioxidant compound [42]. Similarly, cymene possesses a potent anti-nociceptive behavior although it exhibited lesser antioxidant potential [43].
3.3. Antimicrobial Activity of J. thurifera EOs
3.3.1. Antibacterial Activity of J. thurifera EOs
EOs extracted from leaves of J. thurifera exhibited antibacterial activity in comparison with the concentration used and which antibiotic was used, streptomycin sulphate or erythromycin especially against P. aeruginosa CIP A22 with an inhibition diameter of 27.67 ± 1.53 mm and a MIC of 0.0475 ± 0.00 µg/mL, against S. aureus, ATCC 6633 with an inhibition diameter of 20.33 ± 0.58 mm and a MIC of 0.095 ± 0.00 µg/mL, against E. coli K12 with an inhibition diameter of 15.67 ± 3.05 mm and a MIC of 0.095 ± 0.00 µg/mL and against B. subtilis DSM 6333 with an inhibition diameter of 14.33 ± 1.15 mm and a MIC of 0.095 ± 0.00 µg/mL (Table 2).

Table 2.
Antibacterial activity of J. thurifera EOs in comparison with the antibiotics streptomycin and erythromycin.
Antibacterial activity of J. thurifera EOs might be due to their chemical composition, J. thurifera EOs are rich in terpene compounds, especially thujene, γ-terpinene, cymene, and linalool which are well known for their antibacterial activity [41]. They are also rich with sabinene and cymene which are compounds with antibacterial activity [44]. Results of the study reported here were different from results of a previous study [44], which indicated that extracts of J. thurifera L. leaves growing in eastern Algeria were active only against S. aureus, ATCC and methicillin-resistant S. aureus bacteria and the greatest activity with an inhibition diameter of 14 mm for a concentration of 1 g/mL. However, no inhibition was detected for extracts against E. coli ATCC or P. aeruginosa ATCC. However, the antibacterial activity of extracts of leaves of Juniperus phoenicea L was observed against both Gram-positive and Gram-negative bacteria [45]. J. thurifera EOs exhibited significant antibacterial activity against Gram-positive and Gram-negative bacteria, especially against S. aureus, E. coli, and P. aeruginosa with inhibition diameters of 31.12 ± 3.11, 13.23 ± 2.59, and 18.27 ± 2.29 mm, respectively [46]. Those results were similar to those observed in the study, the results of which are presented here for E. coli, but are the opposite of the results for S. aureus and P. aeruginosa, for which the greater antibacterial activity might have been due to the different physicochemical composition of the EOs observed in the study reported here, which are consistent with results of several other studies [47], which found S. aureus was sensitive to the EOs of J. thurifera from Algeria. Furthermore, two strains of Pseudomonas proved to be resistant, [28]. In that study, the EOs from twigs of J. thurifera collected in the Eastern range of the Middle Atlas Mountains of Morocco exhibited significant antibacterial activity against E. coli, B. subtilus, M. luteus, and S. aureus. Similarly, EOs of J. thurifera had significant antibacterial activity against S. aureus, ATCC 33862 with an inhibition diameter of 27 mm and MIC of 450 µL/mL, against E. coli, ATCC 25922 with an inhibition diameter of 25.6 mm and MIC of 530 µL/mL and against P. mirabilis, ATCC 7002 with an inhibition diameter of 18.8 mm and MIC of 930 µL/mL [48].
3.3.2. Antifungal Activity of J. thurifera EOs
When compared with the fungicide fluconazole in the in vitro evaluation of antifungal activity of J. thurifera EOs against A. niger, A. flavus, F. oxysporum, and C. albicans in the disc diffusion test, these EOs exhibited significant activity against F. oxysporum, MTCC 9913 with percent inhibition of 32.47 ± 2.25 and MIC values of 0.095 µg/mL as well as with an inhibition diameter of 21.33 ± 2.08 mm and a MIC value of 0.095 µg/mL against C. albicans; ATCC 10231 (Table 3), In addition, J. thurifera EOs exhibited antifungal activities against F. oxysporum and C. albicans. However, J. thurifera EOs did not exhibit antifungal activity against A. niger or A. flavus. The antifungal activity of J. thurifera EOs may be mainly due to their chemical composition, J. thurifera EOs are particularly rich in thujene, pinene, and limonene which are well known for their antimicrobial activity, especially antifungal activity [16,17].

Table 3.
Antifungal activity of J. thurifera EOs in comparison with fluconazole.
Several studies have been devoted to the control of pathogenic and phytopathogenic fungi in general, and A. niger, A. flavus, F. oxysporum and C. albicans in particular, through the use of various bioactive substances, either natural or synthetic. The results of this study are opposite of those of another study [49], in which sesquiterpenes of J. thurifera EOs did not present any antifungal activity against C. albicans CECT;1394. Similarly, in another study [28] the EOs of J. thurifera twigs collected from the Eastern sector of the Middle Atlas Mountains of Morocco exhibited antifungal activities against A. niger, Penicillium expansum, and Penicillium digitatum. The results of the study presented here are consistent with those focused on substances of bacterial and fungal origin [24], which reported an isolate from Bacillus sp. Gn-A11-18 exhibiting antifungal activity of 31.33 ± 0.58 mm against C. albicans; ATCC 10231 and a percentage of inhibition of 29.66 ± 0.57% against A. niger. Similarly, results of another study [50] showed significant antifungal activity mainly against Alternaria alternata, F. oxysporum, F. solani, Rhizoctonia solani and Verticillium dahlia with percentage inhibitions ranging from 24 to 92.1%.
4. Conclusions
The results of this study indicated that the EOs extracted from J. thurifera had excellent antioxidant and antimicrobial potencies against clinically important drug-resistant microbes. These results are intriguing since they suggest that EOs extracted from J. thurifera could potentially be used as an alternative to traditional antioxidant antimicrobial treatments. However, prior to any prospective application of the studied EOs as natural medicines to control microorganisms, evaluation of the potential side effects on non-target organisms along with pre-clinical and clinical works on non-human primates and humans will be required.
Author Contributions
Conceptualization: S.L., A.E.B., A.E.M. and M.B.; formal analysis: S.L., A.M.S. and A.A.; investigation: S.L., A.A.A., A.C., M.A., A.M.S. and A.A.; methodology: A.M.S. and A.A.; supervision: A.M.S., A.A. and A.B.; writing original draft, and editing: M.B., S.L., A.M.S., A.A., M.A.M.A.-S. and J.P.G.; visualization: M.A.M.A.-S.; validation: J.P.G. and M.A.M.A.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Researchers Supporting Project number (RSP-2022R437), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data reported here is available from the authors upon request.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2022R437) King Saud University, Riyadh, Saudi Arabia. Giesy was supported by a Discovery Grant from the Natural Science and Engineering Research Council of Canada, the Canada Research Chair program and a Distinguished Visiting Professorship in the Department of Environmental Sciences, Baylor University, and Waco, TX, USA.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Godron, M. Le genévrier thurifère (Juniperus thurifera L.) dans le Parc National du Mercantour (Alpes-Maritimes). Lettres Bot. 1983, 130, 227–242. [Google Scholar]
- Gauquelin, T.; Bertaudière, V.; Cambecèdes, J.; Largier, G. Le genevrier thurifere (Juniperus thurifera l.) dans les Pyrenees: Etat de conservation et perspectives. Acta Bot. Barc. 2003, 49, 83–94. [Google Scholar]
- Gauquelin, T.; Bertaudiere, V.; Montes, N.; Badri, W.; Asmode, J.F. Endangered stands of thuriferous juniper in the western Mediterranean basin: Ecological status, conservation and management. Biodivers. Conserv. 1999, 8, 1479–1498. [Google Scholar] [CrossRef]
- Emberger, L. Sur une formule climatique applicable en géographie botanique. C. R. Acad. Sci. 1930, 191, 389–390. [Google Scholar]
- Gauquelin, T.; Lebreton, P. Le genévrier thurifère, Juniperus thurifera L. (cupressacées): Analyse biométrique et biochimique; propositions systématiques. Ecol. Mediterr. 1988, 14, 31–42. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Halima, M.B.; Chaabouni, M.M. Composition chimique et activités antioxydante, antimicrobienne et insecticide de l’huile essentielle de Juniperus phœnicea. J. Société Chim. Tunis. 2008, 10, 119–125. [Google Scholar]
- El Moussaoui, A.; Jawhari, F.Z.; El Ouahdani, K.; Bousta, D.; Bari, A. Valorization of the Pharmacological Potential of Phytochemical Compounds Contained in the Crude Extract of the Root of a Plant of Withania frutescens L. Phytothérapie 2021, 19, 77–82. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Jawhari, F.Z.; Bourhia, M.; Maliki, I.; Sounni, F.; Mothana, R.A.; Bousta, D.; Bari, A. Withania frutescens: Chemical characterization, analgesic, anti-inflammatory, and healing activities. Open Chem. 2020, 18, 927–935. [Google Scholar] [CrossRef]
- Bellakhdar, J. The Traditional Moroccan Pharmacopoeia, Medicine and Arabic Popular Knowledge; Ibis Press: Paris, France, 1997. [Google Scholar]
- Amer, M.M.A.; Wasif, M.M.; Abo-Aytta, A.M.; Gabr, F.A.A. Chemical and biological evaluation of Juniperus phoenicea as a hypoglycamic agent. J. Agric. Res. 1994, 21, 1077–1091. [Google Scholar]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.M. Antimicrobial activity of essential oils from Tunisian aromatic plants. Flavour Fragr. J. 2003, 18, 380–383. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.M.; Ben Aissa, R.; Zgoulli, S.; Thonart, P.; Carlier, A.; Marlier, M.; Lognay, G.C. Volatile Constituents and Antimicrobial Activity of Lavandula stoechas L. Oil from Tunisia. J. Essent. Oil Res. 2011, 17, 584–586. [Google Scholar] [CrossRef]
- Ben Hamida-Ben Ezzeddine, N.; Abdelkéfi, M.M.; Ben Aissa, R.; Chaabouni, M.M. Antibacterial Screening of Origanum majorana L. Oil from Tunisia. J. Essent. Oil Res. 2011, 13, 295–297. [Google Scholar] [CrossRef]
- Moussaoui, A.E.L.; Bourhia, M.; Jawhari, F.Z.; Mechchate, H.; Slighoua, M.; Bari, A.; Ullah, R.; Mahmood, H.M.; Ali, S.S.; Ibenmoussa, S.; et al. Phytochemical Identification, Acute, and Sub-Acute Oral Toxicity Studies of the Foliar Extract of Withania frutescens. Molecules 2020, 25, 4528. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.D. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Kadiri, M.; Bourhia, M.; Agour, A.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Chedadi, M.; Sfaira, M.; et al. Promising Antioxidant and Anticorrosion Activities of Mild Steel in 1.0 M Hydrochloric Acid Solution by Withania frutescens L. Essential Oil. Front. Chem. 2021, 9, 760. [Google Scholar] [CrossRef]
- El Moussaoui, A.; Borgia, M.; Jawhari, F.Z.; Salamatullah, A.M.; Ullah, R.; Bari, A.; Mahmood, H.M.; Sohaib, M.; Serhii, B.; Rozhenko, A.; et al. Chemical profiling, antioxidant, and antimicrobial activity against drug-resistant microbes of essential oil from Withania frutescens L. Appl. Sci. 2021, 11, 5168. [Google Scholar] [CrossRef]
- Celiktas, O.Y.; Kocabas, E.E.H.; Bedir, E.; Sukan, F.V.; Ozek, T.; Baser, K.H.C. Antimicrobial activities of methanol extracts and essential oils of Rosmarinus officinalis, depending on location and seasonal variations. Food Chem. 2007, 100, 553–559. [Google Scholar] [CrossRef]
- Semantic Scholar. Chemical Compositions, Antibacterial and Antioxidant Activities of Essential Oil and Various Extracts of Geranium sanguineum L. Flowers. Available online: https://www.semanticscholar.org/paper/Chemical-compositions%2C-antibacterial-and-activities-Hammami-Triki/6020c187f613631977960bcb42c76c88ed9eded6 (accessed on 22 February 2022).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatograpy/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-11-4. [Google Scholar]
- El Atki, Y.; Aouam, I.; El Kamari, F.; Taroq, A.; Lyoussi, B.; Oumokhtar, B.; Abdellaoui, A. Phytochemistry, antioxidant and antibacterial activities of two Moroccan Teucrium polium L. subspecies: Preventive approach against nosocomial infections. Arab. J. Chem. 2020, 13, 3866–3874. [Google Scholar] [CrossRef]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef]
- Öztürk, M. Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem. 2012, 134, 48–54. [Google Scholar] [CrossRef]
- El Barnossi, A.; Moussaid, F.; Iraqi Housseini, A. Antifungal activity of Bacillussp. Gn-A11-18isolated from decomposing solid green household waste in water and soil against Candida albicans and Aspergillus niger. In Proceedings of the E3S Web of Conferences, Virtual, 7–8 September 2020; EDP Sciences: Les Ulis, France, 2020; Volume 150. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Chebbac, K.; Ghneim, H.K.; El Moussaoui, A.; Bourhia, M.; El Barnossi, A.; Ouaritini, Z.B.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant and Antimicrobial Activities of Chemically-Characterized Essential Oil from Artemisia aragonensis Lam. against Drug-Resistant Microbes. Molecules 2022, 27, 1136. [Google Scholar] [CrossRef]
- Akkad, S.; Akssira, M. Étude de la composition des huile es de Juniperus thurifera L. var. africana à l’aide du couplage GC-MS. In Proceedings of the Deuxième Colloque International: Le Genévrier Thurifère et Les Forêts d’Altitude dans les Montagnes du Pourtour Méditerranéen, Livre des résumés, Hautes-Alpes, France, 17–21 April 2001; Volume 8, pp. 166–170. [Google Scholar]
- Mansouri, N.; Satrani, B.; Ghanmi, M.; El Ghadraoui, L.; Aafi, A.; Farah, A. Valorization of the essential oils of Moroccan Juniperus thurifera and Juniperus oxycedrus. Phytotherapie 2010, 8, 166–170. [Google Scholar] [CrossRef]
- Heim, E.K.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoïds antioxydants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hybertson, B.M.; Gao, B.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Aspects Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhou, Q.; Gong, W.; Wang, Y.; Nie, Z.; He, H.; Li, J.; Wu, J.; Wu, C.; Zhang, J. Studies on the antioxidant and hepatoprotective activities of polysaccharides from Talinum triangulare. J. Ethnopharmacol. 2011, 136, 316–321. [Google Scholar] [CrossRef]
- Rains, J.L.; Jain, S.K. Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 2011, 50, 567–575. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Mumper, R.J. Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Masella, R.; Di Benedetto, R.; Varì, R.; Filesi, C.; Giovannini, C. Novel mechanisms of natural antioxidant compounds in biological systems: Involvement of glutathione and glutathione-related enzymes. J. Nutr. Biochem. 2005, 16, 577–586. [Google Scholar] [CrossRef]
- Tiqwari, A.K. Imbalance in antioxidant defence and human diseases: Multiple approach of natural antioxidants therapytle. Curr. Sci. 2001, 81, 1179–1181. [Google Scholar]
- Bouhdid, S.; Skali, S.N.; Idaomar, M.; Zhiri, A.; Baudoux, D.; Amensour, M.; Abrini, J. Antibacterial and antioxidant activities of Origanum compactum essential oil. Afr. J. Biotechnol. 2008, 7, 1563–1570. [Google Scholar] [CrossRef]
- Gülçin, Ì.; Şat, I.G.; Beydemir, Ş.; Elmastaş, M.; Küfrevioǧlu, Ö.I. Comparison of antioxidant activity of clove (Eugenia caryophylata Thunb) buds and lavender (Lavandula stoechas L.). Food Chem. 2004, 87, 393–400. [Google Scholar] [CrossRef]
- Chun, S.S.; Vattem, D.A.; Lin, Y.T.; Shetty, K. Phenolic antioxidants from clonal oregano (Origanum vulgare) with antimicrobial activity against Helicobacter pylori. Process. Biochem. 2005, 40, 809–816. [Google Scholar] [CrossRef]
- Fayed, S.A. Antioxidant and Anticancer Activities of Citrus reticulate (Petitgrain Mandarin) and Pelargonium graveolens (Geranium) Essential Oils. Res. J. Agric. Biol. Sci. 2009, 5, 740–747. [Google Scholar]
- Zhuang, S.-R.; Chen, S.-L.; Tsai, J.-H.; Huang, C.-C.; Wu, T.-C.; Liu, W.-S.; Tseng, H.-C.; Lee, H.-S.; Huang, M.-C.; Shane, G.-T.; et al. Effect of citronellol and the Chinese medical herb complex on cellular immunity of cancer patients receiving chemotherapy/radiotherapy. Phytother. Res. 2009, 23, 785–790. [Google Scholar] [CrossRef]
- Momtaz, S.; Abdollahi, M. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. Int. J. Pharmacol. 2010, 6, 454–461. [Google Scholar] [CrossRef] [Green Version]
- Quiroga, P.R.; Asensio, C.M.; Nepote, V. Antioxidant effects of the monoterpenes carvacrol, thymol and sabinene hydrate on chemical and sensory stability of roasted sunflower seeds. J. Sci. Food Agric. 2015, 95, 471–479. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p-Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 2013, 459530. [Google Scholar] [CrossRef] [Green Version]
- Manel, M.; Nouzha, H.; Rim, M.; Imane, M.; Sana, A.; Yasmine, O. Antibacterial and antioxidant activity of Juniperus thurifera L. leaf extracts growing in East of Algeria. Vet. World 2018, 11, 373–378. [Google Scholar] [CrossRef]
- Ennajar, M.; Bouajila, J.; Lebrihi, A.; Mathieu, F.; Abderraba, M.; Raies, A.; Romdhane, M. Chemical composition and antimicrobial and antioxidant activities of essential oils and various extracts of Juniperus phoenicea L. (Cupressacees). J. Food Sci. 2009, 74, M364–M371. [Google Scholar] [CrossRef]
- Rahhal, R.; Hajjouji, H.E.L.; Gmouh, S.; Hsaine, M.; Fougrach, H. Chemical composition, antioxidant and antibacterial activities of the essential oils of Juniperus phoenicea, Juniperus thurifera and Juniperus oxycedrus. Mediterr. J. Chem. 2019, 9, 190–198. [Google Scholar]
- Zeraib, A.; Chalard, P. Characterization and chemosystematics of Algerian thuriferous juniper (Juniperus thurifera L.) Chemical composition and biological properties of essential oils from medicinal and aromatic species growing in the Aures region. View project Valorisation de la Biodiversité végétale dans une zone steppique d’El Haourane (M’sila, Algérie) View project. Artic. J. Appl. Bot. Food Qual. 2014, 87. [Google Scholar] [CrossRef]
- Bahri, F.; Harrak, R.; Achak, N.; Romane, A. Natural Product Research: Formerly Natural Product Letters Chemical composition and antibacterial activities of the essential oils isolated from Juniperus thurifera L. var. Nat. Prod. Res. 2012, 27, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, J.; Gilardoni, G.; Ramón, E.; Tosi, S.; Picco, A.M.; Bicchi, C.; Vidari, G. Phytochemical Study of the Ecuadorian Species Lepechinia Mutica (Benth.) Epling and High Antifungal Activity of Carnosol against Pyricularia Oryzae. Pharmaceuticals 2018, 11, 33. [Google Scholar] [CrossRef] [Green Version]
- Barrero, A.F.; Del Moral, J.F.Q.; Armando, L.; Herrador, M.M. Antimicrobial Activity of Sesquiterpenes from the Essential Oil of Juniperus thurifera Wood. Planta Med. 2005, 71, 67–71. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).