Abstract
According to Chinese Pharmacopoeia (2020 edition), Abelmoschi Corolla (AC) is the dried corolla of Flos Abelmoschus manihot (FAM). Market research has found that AC is often mixed with the non-medicinal parts in FAM, including calyx, stamen, and pistil. However, previous studies have not clarified the relationship between the medicinal and non-medicinal parts of FAM. In this study, in order to investigate whether there is any distinction between the medicinal and non-medicinal parts of FAM, the characterization of the constituents in calyx, corolla, stamen, and pistil was analyzed by UFLC-Triple TOF-MS/MS. Multivariate statistical analysis was used to classify and screen differential constituents between medicinal and non-medicinal parts of FAM, and the relative contents of differential constituents were compared based on the peak intensities. Results showed that 51 constituents in medicinal and non-medicinal parts of FAM were identified, and the fragmentation pathways to different types of constituents were preliminarily deduced by the fragmentation behavior of the identified constituents. Furthermore, multivariate statistical analysis revealed that the medicinal and non-medicinal parts of FAM differed significantly; 20 differential constituents were screened out to reveal the characteristics of metabolic differences. Among them, the relative contents of 19 differential constituents in the medicinal part were significantly higher than those in non-medicinal parts. This study could be helpful in the quality evaluation of AC as well as provide basic information for the improvement of the market standard of AC.
1. Introduction
Abelmoschi Corolla (AC) is derived from the dried corolla of Flos Abelmoschus manihot (FAM), which has the functions of eliminating dampness and heat, subduing swelling, and detoxicating [1]. It is a traditional Chinese medicine (TCM) with a long medicinal history in China [2]. The research of pharmacology showed that AC has multiple pharmacological activities, such as anti-inflammatory, antioxidant [3,4], antitumor [5], anticonvulsant, antidepressant, and neuroprotective activities [6,7], as well as therapeutic actions on renal tubular injury and diabetic nephropathy [8,9,10].
As the main raw material of the Chinese patent medicine Huangkui capsule, AC has significant medicinal value and a huge market demand. Market research found that commercial medicinal material of AC was often mixed with non-medicinal parts of FAM, including calyx, stamen, and pistil. The reason for this phenomenon is that the non-medicinal parts of FAM are not removed during the collection and processing of corolla. However, the chemical constituents of non-medicinal parts of FAM were not characterized in previous studies, so the comparative study between medicinal and non-medicinal parts of FAM was not clear-cut. The mixing of non-medicinal parts may alter the composition of AC, reduce its quality stability, and then affect its efficacy. Therefore, it is of practical value to study the chemical constituents of medicinal and non-medicinal parts of FAM and reveal the characteristics of metabolic differences.
Because it combines the separation powers of liquid chromatography with the very sensitive detection qualities of mass spectrometry, the LC-MS equipment has been frequently employed in TCM research in recent years [11]. Among them, ultra-fast liquid chromatography coupled with triple quadrupole-time of flight tandem mass spectrometry (UFLC-Triple TOF-MS/MS) is efficient and rapid in the determination of the molecular weight and characteristic fragment ions, by which the structure of multiple constituents in TCM can be identified quickly [12]. Hence, the characterization of the constituents in calyx, corolla, stamen, and pistil was analyzed by UFLC-Triple TOF-MS/MS. We integrated metabolic profiling and multivariate statistical analysis to separate the medicinal and non-medicinal parts of FAM and to define their chemical markers. The strategy for comparative analysis on chemical constituents of medicinal and non-medicinal parts of FAM was shown in Figure 1. The study could determine the differential constituents of medicinal and non-medicinal parts of FAM, so as to provide basic data for standardizing the harvest and market standards of AC. Our investigation will not only contribute to the quality evaluation of AC, but also has great significance in the quality stability improvement of AC.
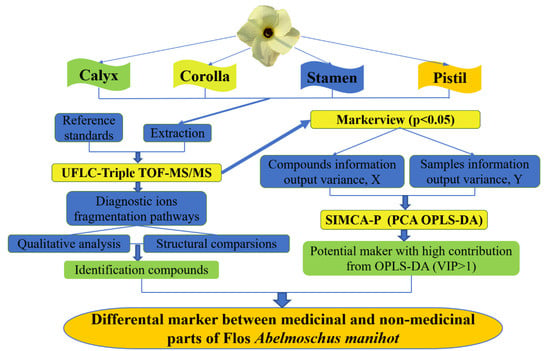
Figure 1.
The strategy for comparative analysis on chemical constituents of medicinal and non-medicinal parts of Flos Abelmoschus manihot (FAM).
2. Materials and Methods
2.1. Chemicals and Reagents
The standard substances of 3,4,5-trihydroxybenzoic acid, rutin, hyperin, and quercetin were purchased from the Chinese National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). 3,4-Dihydroxybenzoic acid was purchased from Shanghai Ronghe Pharmaceutical Technology Co., Ltd. (Shanghai, China). Chlorogenic acid, caffeic acid, and myricetin 3′-O-β-d-glucopyranoside were purchased from Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Dihydromyricetin and myricetin were purchased from Chengdu Aifa Bio-technology Co., Ltd. (Chengdu, China). Myricetin 3-O-β-d-glucopyranoside and quercetin 3-O-β-d-robinobioside were purchased from Liangwei Bio-technology Co., Ltd. (Nanjing, China). Quercetin 7-O-β-d-glucopyranoside, gossypetin 8-O-β-d-glucuronide, and quercetin 3-O-(6-O-acetyl-β-d-glucopyranoside) were purchased from Nanjing Casses Pharmaceutical Technology Co., Ltd. (Nanjing, China). Isoquercetin, quercetin 3′-O-β-d-glucoside, and tiliroside were purchased from Chengdu Chroma-Biotechnology Co., Ltd. (Chengdu, China). The purities of myricetin 3′-O-β-d-glucopyranoside was above 97% and other standards were greater than 98%, tested by HPLC analysis. Formic acid, acetonitrile, and methanol of HPLC grade were purchased from Merck (Darmstadt, Germany). A Milli-Q water purification system (Millipore, Bedford, MA, USA) was applied to make deionized water.
2.2. Plant Materials
Five batches of FAM samples were collected from Xinghua City (Jiangsu Province, China 32°98′17″ N, 119°90′44″ E) in October 2019. Each batch was carefully divided into four parts, calyx, corolla, stamen, and pistil, which were separately dried in the oven. The drying temperature was set at 50 °C. The samples were authenticated by Professor Xunhong Liu as the flower of Abelmoschus manihot (L.) Medic. of Malvaceae family and the voucher specimens were deposited in the laboratory of Chinese medicine identification, Nanjing University of Chinese Medicine. The voucher numbers of the samples were as follows: 190923CA1−190923CA5 (calyx), 190923CO1−190923CO5 (corolla), 190923ST1−190923ST5 (stamen), 190923PI1−190923PI5 (pistil).
2.3. UFLC-Triple TOF-MS/MS Analysis
2.3.1. Preparation of Standard and Sample Solutions
A mixed standard stock solution of 18 standard substances was prepared with 70% (v/v) methanol. The diluted solutions were stored at 4 °C for further UFLC-Triple TOF-MS/MS analysis.
The 0.5 g of calyx, corolla, stamen, and pistil powder were properly weighed and ultrasonically extracted with 20 mL 70% (v/v) methanol for 30 min, respectively. To compensate for the weight lost during extraction, the same solvent was added after cooling to room temperature. The extract was then filtered, and the filtrate was centrifuged at 12,000 rpm/min for 10 min. Afterwards, the supernatant was filtered via a 0.22 µm membrane before UFLC-Triple TOF-MS/MS analysis.
2.3.2. UFLC-Triple TOF-MS/MS Conditions
The chromatographic analysis was performed on an UFLC-20AD XR system (Shimadzu, Kyoto, Japan). The separation was conducted by an Agilent Zorbax SB-C18 column (250 mm × 4.6 mm, 5 µm) at 35 °C. The mobile phase contained 0.1% (v/v) aqueous formic acid water solution (A)–methanol:acetonitrile (1:1) (B) with the gradient elution: 0−3 min, 2% B; 3−10 min, 2−15% B; 10−14 min, 15−18% B; 14−20 min, 18−21% B; 20−30 min, 21−23% B; 30−45 min, 23−27% B; 45−50 min, 27−40% B; 50−52 min, 40−80% B; 52−54 min, 80−95% B. The injection volume was 10 µL and the flow rate was 1 mL/min.
A Triple TOFTM 5600 System MS/MS High Resolution Quadrupole Time-of-Flight Mass Spectrometer (AB SCIEX, Framingham, MA, USA) equipped with an electrospray ionization source was used for MS analysis in both positive and negative ion modes. The MS conditions were optimized as follows: the ion source temperature, 550 °C; the flow rate of curtain gas, 40 L/min; the flow rate of nebulization gas, 55 L/min; the flow rate of auxiliary gas, 55 L/min; the spray voltage, 4500 V in positive ion mode and -4500 V in negative ion mode; the declustering voltage, 100 V in positive ion mode and -100 V in negative ion mode. TOF MS and TOF MS/MS were scanned with the mass range of m/z 100−2000 and 50−1500, respectively.
2.3.3. Identification of the Constituents
A database of the chemical constituents of AC was formed based on previous research and the data were imported into the PeakView Software V.1.2 (AB SCIEX, Framingham, MA, USA). The chemical constituents of different parts of FAM were comprehensively characterized by comparing the retention time (tR), accurately measuring mass and multistage MS/MS fragmentation information with standard substances, databases, and related literatures.
2.4. Multivariate Statistical Analysis
The data of UFLC-Triple TOF-MS/MS were processed by PeakView Software V.1.2 (AB SCIEX, Framingham, MA, USA) and MarkerView 1.2.1 software (AB Sciex). Principal components analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS–DA) were performed using SIMCA-P 13.0 software (Umetrics AB, Umea, Sweden). PCA was used to categorize and identify different parts of FAM. OPLS-DA was performed to differentiate medicinal part and non-medicinal parts of FAM, as well as to identify the common differential constituents that cause the differences in each group of comparison by variable importance in the projection (VIP).
2.5. Relative Content Comparison of Differential Constituents
The relative contents of differential constituents in medicinal and non-medicinal parts of FAM were compared according to the peak intensities. To visualize and validate the distribution regularity of differential constituents among medicinal and non-medicinal parts of FAM, one-way analysis of variance (one-way ANOVA) was applied. Diagram of relative content comparison was charted by GraphPad Prism 8.0 software (GraphPad Software, San Diego, CA, USA).
3. Results
3.1. Identification of the Constituents in Medicinal and Non-Medicinal Parts of FAM
It was found that the best analytical selectivity and sensitivity was obtained in the negative ionization mode by comparing the data acquired in the two ion modes. As a result, we decided to collect data in the negative ion mode. The base peak chromatograms (BPCs) of the calyx (Figure 2A), corolla (Figure 2B), stamen (Figure 2C), and pistil (Figure 2D) extract in the negative ion mode are shown in Figure 2. Eventually, 51 constituents were identified, including 43 flavonoids, 6 organic acids, 1 ester, and 1 alkaloid. A total of 18 constituents were clearly identified by comparison with reference standards. Detailed information of the characteristic constituents is summarized in Table 1, with their structures presented in Figure S1.
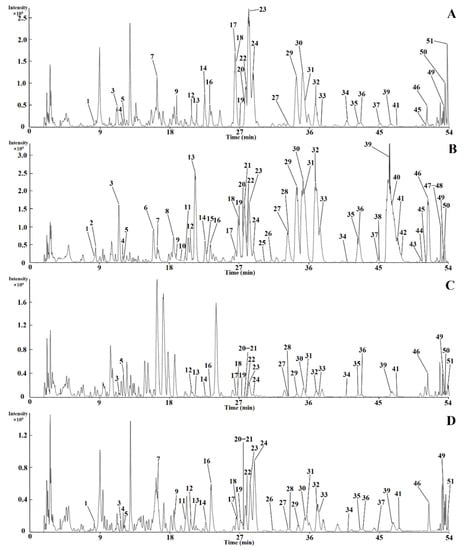
Figure 2.
The base peak chromatograms (BPCs) of the calyx (A), corolla (B), stamen (C), and pistil (D) extract in negative ion mode. Note: the peak numbers denoted were the same as those in Table 1.

Table 1.
Identification of 51 constituents in calyx, corolla, stamen, and pistil by UFLC-Triple TOF-MS/MS.
3.1.1. Identification of Flavonoids
As the main active substance of AC, flavonoids have always been the research hotspot. In this study, a total of 43 flavonoids were identified from the extract of calyx, corolla, stamen, and pistil, including flavone, flavonols, and dihydroflavonol. At the same time, flavonols can be carefully divided into hibiscus parent flavonols, gossypetin parent flavonols, myricetin parent flavonols, quercetin parent flavonols, and kaempferol parent flavonols, respectively. The common substituents group on the A and B rings in flavonoids were hydroxyl, methoxy, and acetyl, and it was also extremely common for saccharides or glucuronic acids to interact with hemiacetal hydroxyl groups to form flavonoid glycosides. The basic fracture paths of flavonoids were the loss of these neutral pieces and Retro-Diels-Alder (RDA) cleavage of the C ring [13]. Figure 3 depicts several RDA cleavage mechanisms of related flavonoids. The molecular ion peak intensity of flavonoid glycosides was often modest, and the base peak was frequently the fragment peak of aglycon.
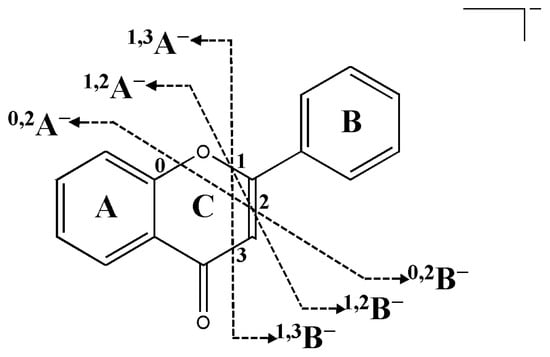
Figure 3.
Schematic diagram of the fracture site of related flavonoids in negative ion mode.
Dihydroflavonol and flavone Compounds 11 and 34 were identified as dihydroflavonol and flavone, respectively. Compound 11 produced a [M−H]− ion at m/z 319.05 and abundance fragment ions, such as ions at m/z 301.06, 193.01, 165.02, and 151.00. The product ion at m/z 301.06 was generated from the elimination of H2O from the molecular ion. The m/z 193.01 was the basic peak due to the removal of the B ring. The m/z 165.02 and 151.00 were 0,2A− (A fragment with A ring after the 0,2 bonds of C ring were broken) and 1,3A− (A fragment with A ring after the 1,3 bonds of C ring were broken). Therefore, compound 11 was identified as dihydromyricetin and further confirmed by the reference substance. The molecular ion peak of compound 34 was generated at m/z 593.15, suggesting that the molecular formula of the compound was C27H30O15 and abundant fragment ions were generated at 285.04, 255.03, and 227.04. After removing the glycosyl and CH2 from m/z 593.15 ion, the product ion of m/z 285.04 was generated. The fragment ions of m/z 255.03 and 227.04 were formed by dropping CH2O and CO. Hence, compound 34 was proposed as 4′-methoxyl-5,7-dihydroxyl flavone-[-O-β-D-xylopyranosyl-(1→3)]-O-β-d-glucopyranoside, consistent with previous studies [14].
Hibiscus parent flavonols Compounds 6, 8, and 28 were identified as hibiscus parent flavonols with the adducted ion as [M−H]− or [M+HCOO]−. The primary distinctive fragment ions of hibiscus parent flavonols were 1,2A− (A fragment with A ring after the 1,2 bonds of C ring were broken) and 1,3A− obtained by RDA fragmentation. Compounds 6 and 8 produced [M+HCOO]− ion at m/z 541.08 and abundance fragment ions, such as ions at m/z 333.03, 315.01, 287.02, 195.00, and 167.00. The m/z 333.03 was the product ion [M−H−glc]− due to cleavage of the glycosidic bond. The product ions at m/z 315.01 and 287.02 were generated from the elimination of H2O and CO from m/z 333.03 ion. The m/z 195.00 and 167.00 were 1,2A− and 1,3A− obtained by RDA fragmentation. The adducted ion of compound 28 was observed as [M−H]− and the fragment ions basic peak [M−H−glu]− was observed at m/z 333.03 by the loss of 176 Da. Meanwhile, the m/z 195.00 and 167.00 were 1,2A− and 1,3A− obtained by RDA fragmentation. Therefore, compounds 6, 8, and 28 were identified as hibiscetin-3-O-glucoside, floramanoside B, and floramanoside C, respectively, which is consistent with previous studies [15]. See Table 1 for details.
Gossypetin and myricetin parent flavonols 7 and 11 constituents were identified as flavonoids with gossypetin and myricetin parent flavonols, respectively, including 12, 13, 25, 39, 40, 43, 44 and 14, 15, 16, 18, 19, 20, 21, 26, 33, 38, 41. We found that gossypetin and myricetin parent flavonols were more likely to lose H2O and CO fragments. In addition, the main characteristic fragments of these two flavonols were ions obtained from the cleavage of RDA. Taking compounds 39 and 20 as examples, the molecular ions were located at m/z 493.06 and 479.08, suggesting that the molecular formulas of the two compounds were C21H18O14 and C21H20O13, respectively. They all have base peaks at m/z 317.03, indicating that compounds 39 and 20 had a glycosidic acid and a glucose group, respectively. The main difference between the two was that the former fragment ions were [1,2A]− (m/z 195.00), [1,3A]− (m/z 167.00), and [1,3A−CO]− (m/z 139.00), while the latter fragment ions were [1,2A]− (m/z 179.00), [1,3A]− (m/z 151.00), and [1,2B]− (m/z 137.02). The difference is due to the different positions of hydroxyl groups in aglycon. Therefore, compounds 39 and 20 were identified as gossypetin 8-O-β-d-glucuronide and myricetin 3-O-β-d-glucopyranoside and further confirmed by the reference substance.
Quercetin parent flavonols 17 constituents including 17, 22, 23, 24, 27, 29, 30, 31, 32, 35, 37, 42, 45, 46, 47, 48, and 49 were identified as flavonoids with quercetin parent flavonols. They were more likely to lose H2O and CO fragments and the ions obtained by RDA fragmentation were the main characteristic fragments. Taking compound 27 as example, the molecular ions was located at m/z 463.09, suggesting that the molecular formulas was C21H20O12. The m/z 301.03 was the product ion [M−H−glc]− due to cleavage of the glycosidic bond. The fragments ions of m/z 253.05 and 237.54 were formed by dropping CO and H2O. The m/z 179.00 and 151.00 were 1,2A− and 1,3A− obtained by RDA fragmentation. Therefore, compound 27 was identified as isoquercitrin and confirmed by the reference substance. The specific fragment information was shown in Table 1.
Kaempferol parent flavonols Compounds 36, 50, and 51 were identified as kaempferol parent flavonols. Taking compound 50 as an example, the molecular ion was located at m/z 593.13, suggesting that the molecular formula was C30H26O13. The m/z 447.09 was the fragments ion [M−H−C9H6O2]− by dropping hydroxycinnamoyl group, m/z 285.04 was the product ion [M−H−C9H6O2−glc]− due to the cleavage of the glycosidic bond. The fragments ions of m/z 257.05 and 239.03 were ions [M−H−C9H6O2−glc−CO]− and [M−H−C9H6O2−glc−CO−H2O]− formed by dropping CO and H2O. Therefore, compound 50 was identified as tiliroside which confirmed by the reference substance.
3.1.2. Identification of Organic Acids
The adducted ion of organic acids was observed as [M−H]− in the negative mode. The MS/MS spectra of compounds 1, 4, 9 usually had a basic peak at [M−H−CO2]−, and then produce [M−H−CO2−H2O]− by the loss of H2O. The basic peak of compound 7 was the removal of caffeic acid group [M−H−C9H6O3]−, followed by the loss of 2H2O and CO produce [M−H−C9H6O3−2H2O−CO]−. Compounds 1, 4, 7, 9 were identified as 3,4,5-trihydroxybenzoic acid, 3,4-dihydroxybenzoic acid, chlorogenic acid, and caffeic acid, respectively, which were further confirmed by the reference substance. The basic peak of compounds 2, 3 were [M−H−glc]−. At the same time, the other fragments were consistent with the identified components 1, 4. Therefore, compounds 2, 3 were identified as gallic acid 3-O-β-glucoside and protocatecheuic acid 3-O-β-D-glucoside. See Table 1 for details.
3.1.3. Identification of Ester
Compound 5 gave precursor ion [M−H]− at m/z 299.08, suggesting that its molecular formula was C13H16O8. The fragment ion was observed at m/z 137.02 by the loss of 162 Da, attributed to the loss of a glucose group. The loss of H2O from [M−H−glc]− resulted in the fragment at m/z 119.03, showing the existence of hydroxyl group. Hence, it was identified as 4-hydroxybenzoic acid β-D-glucosyl ester by referring to the previous studies [16].
3.1.4. Identification of Alkaloid
Compound 10 gave precursor ion [M−H]− at m/z 252.09, suggesting that its molecular formula was C12H15NO5. The fragment ions basic peak [M−H−H2O]− was observed at m/z 234.08 by the loss of 18 Da, indicating the presence of hydroxyl group. Afterwards, the loss of CO2 from [M−H−H2O]− produced the fragment at m/z 190.09. Thus, it was identified as acortatarine A, consistent with previous studies [17].
3.2. Multivariate Statistical Analysis
PCA was conducted to classify the different parts of FAM. The first two principal components accounted for more than 75% of the total variance, could be used to represent overall information of samples (R2X [1] = 0.554, R2X [2] = 0.235). The PCA scores plot indicated that the medicinal part and non-medicinal parts of FAM were divided into two clusters (Figure 4). Corolla were gathered in the positive axis, non-medicinal parts of FAM were distributed in the negative axis, indicating that there was a significant difference between the medicinal and non-medicinal parts of FAM.
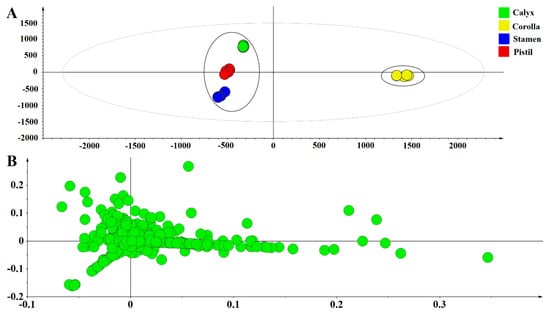
Figure 4.
The principal component analysis scores scatter plot (A) and loading scatter plot (B) of medicinal and non-medicinal parts of FAM.
OPLS-DA was used to further distinguish the medicinal and non-medicinal parts of FAM, and to find out the important constituents that cause the differences with VIP values. The OPLS-DA score scatter plot, VIP plot, and S-Plot for comparison of the medicinal and non-medicinal parts of FAM were shown in Figure 5A–C. The OPLS-DA model demonstrated good adaptability (R2X = 0.938, 0.949, and 0.937, respectively, R2Y = 0.999, 0.999, and 0.999, respectively) and predictability (Q2 = 0.999, 0.998, and 0.998, respectively). Calyx and corolla, stamen and corolla, pistil and corolla were all separated into two clusters along PC1 axis. The result revealed that the difference between the medicinal and non-medicinal parts of FAM was significant, which was completely consistent with the result of PCA.
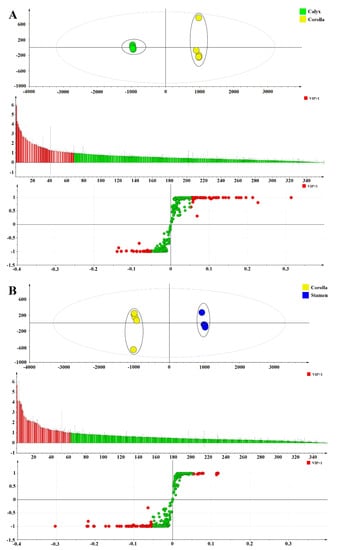
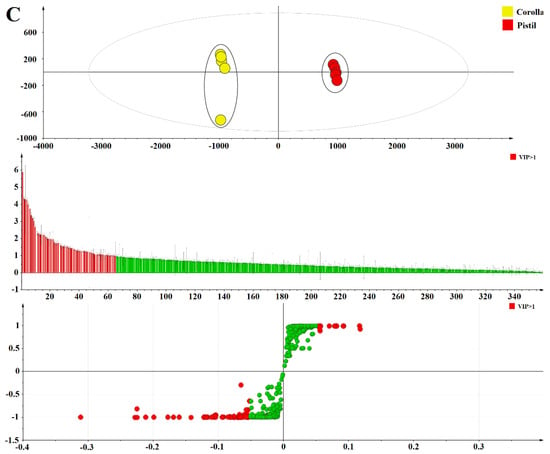
Figure 5.
The orthogonal partial least squares discriminant analysis score scatter plot, VIP plot, and S-Plot of calyx and corolla (A), stamen and corolla (B), pistil and corolla (C).
The identification of potential chemical markers to distinguish the medicinal and non-medicinal parts of FAM was the focus of this study. Based on their VIP values (i.e., larger than 1.0), 20 constituents were screened out to discriminate the medicinal and non-medicinal parts of FAM, including protocatecheuic acid 3-O-β-d-glucoside (3), hibiscetin-3-O-glucoside (6), floramanoside B (8), gossypetin 3-O-β-glucopyranoside-8-O-β-glucuronopyranoside (12), gossypetin 3-O-β-glucuronopyranoside-8-O-β-glucopyranoside (13), myricetin 3-O-β-d-galactopyranoside (18), myricetin 3-O-β-d-glucopyranoside (20), myricetin 3-O-rutinose (21), quercetin 3-O-β-d-xylopyranosyl-(1→2)-O-β-d-galactopyranoside (23), floramanoside C (28), quercetin 3-O-β-d-robinobioside (29), hyperin (30), rutin (31), isoquercitrin (32), myricetin 3′-O-β-d glucopyranoside (33), 6″-acetylhyperin (35), gossypetin 8-O-β-d-glucuronide (39), myricetin (41), quercetin 3′-O-β-d-glucoside (46), and floramaroside E (48). Therefore, these constituents could be selected as differential constituents to distinguish the medicinal and non-medicinal parts of FAM.
3.3. Relative Content Comparison of Differential Constituents
The relative contents of differential constituents in medicinal and non-medicinal parts of FAM were compared based on peak intensity. Furthermore, the one-way ANOVA followed by least significant difference test (variance homogeneity) or Tamhane’s test (variance heterogeneity) was carried out to illustrate the abundance variation of 20 differential constituents. As shown in Figure 6. The relative contents of 19 differential constituents (protocatecheuic acid 3-O-β-d-glucoside, hibiscetin-3-O-glucoside, floramanoside B, gossypetin 3-O-β-glucopyranoside-8-O-β-glucuronopyranoside, gossypetin 3-O-β-glucuronopyranoside-8-O-β-glucopyranoside, myricetin 3-O-β-d-galactopyranoside, myricetin 3-O-β-d-glucopyranoside, myricetin 3-O-rutinose, floramanoside C, quercetin 3-O-β-d-robinobioside, hyperin, rutin, isoquercitrin, myricetin 3′-O-β-d glucopyranoside, 6″-acetylhyperin, gossypetin 8-O-β-d-glucuronide, myricetin, quercetin 3′-O-β-d-glucoside, floramaroside E) in corolla were significantly higher than those in non-medicinal parts, only quercetin 3-O-β-d-xylopyranosyl-(1→2)-O-β-d-galactopyranoside in calyx of non-medicinal part was higher than that of medicinal part.
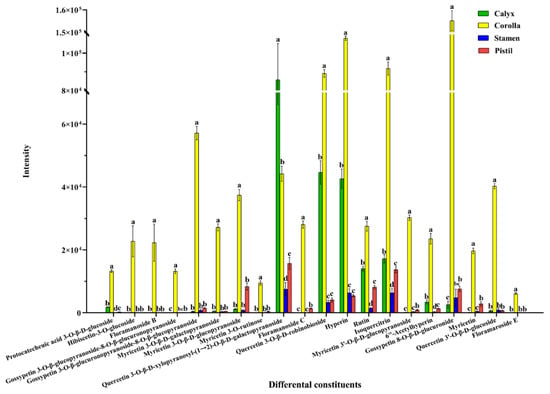
Figure 6.
The relative contents of 20 differential constituents in medicinal and non-medicinal parts of FAM. (Different letters indicate significant differences, p < 0.05).
4. Discussion
The clinical efficacy of TCM is determined by its chemical constitution [40]. The Chinese Pharmacopoeia officially recorded that the medicinal part of AC was only the corolla of FAM. However, the phenomenon of doping non-medicinal parts in AC was common. Therefore, the chemical constituents of medicinal and non-medicinal parts of FAM were analyzed. A total of 20 differential constituents, including 1 organic acid and 19 flavonoids, were screened out by multivariate statistical analysis and their relative contents were compared. Among them, the relative content of 19 differential constituents in the corolla of FAM was significantly higher than that in the non-medicinal parts. Many studies have shown that the flavonoids of AC such as hyperin and isoquercitrin possess anti-inflammatory and renal injury protective properties [41,42,43], which is consistent with the clinical efficacy of AC [44,45]. Due to the great difference in chemical constituents between the medicinal part and the non-medicinal parts, the latter may not have the same therapeutic effect as the medicinal part. The screened differential constituents might be the pharmacodynamic substances of AC, which provide ideas for the research of pharmacodynamic substance basis of AC.
In summary, 51 constituents from medicinal and non-medicinal parts of FAM were identified and their metabolic profiles were compared. 20 differential constituents were screened to distinguish the medicinal part and non-medicinal parts of FAM. The great difference in the relative content of them indicates that the non-medicinal parts of FAM are hardly a substitute for the corolla part. Our study could be conducive to the quality evaluation and quality stability improvement of AC and provide a scientific basis for strictly regulating the harvest and market standards of AC.
Supplementary Materials
The following is available online at https://www.mdpi.com/article/10.3390/horticulturae8040317/s1, Figure S1. Chemical structures of constituents identified in medicinal and non-medicinal parts of Flos Abelmoschus manihot.
Author Contributions
Conceptualization, X.L., S.Y. and Z.C.; data curation, S.Y., Y.M., L.W. and Z.C.; formal analysis, S.Y., N.W., J.Y., D.W. (Dianguang Wang) and D.W. (Dandan Wang); writing—original draft preparation, S.Y.; writing—review and editing, X.L., L.Z., Z.C., C.C., S.L. and H.G.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions of China (NO. ysxk-2014) and General Project of Natural Science Research in Universities of Jiangsu Province (20KJD360001).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article or in supplementary material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- The State Pharmacopoeia Commission of P. R. China. Pharmacopoeia of the People’s Repulic of China; Part I; China Medical Science and Technology Press: Beijing, China, 2020; p. 319. [Google Scholar]
- State Administration of Traditional Chinese Medicine. Chinese Materia Medica. Part 5; Shanghai Science and Technology Press: Shanghai, China, 1999; pp. 331–332. [Google Scholar]
- Qiu, Y.; Ai, P.F.; Song, J.J.; Liu, C.; Li, Z.W. Total flavonoid extract from Abelmoschus manihot (L.) Medic flowers attenuates d-galactose-induced oxidative stress in mouse liver through the Nrf2 pathway. J. Med. Food. 2017, 20, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.Y.; Ai, G.; Zhang, X.J.; Xu, H.J.; Huang, Z.M. Investigations of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic against α-naphthylisothiocyanate-induced cholestatic liver injury in rats. J. Ethnopharmacol. 2015, 172, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.H.; Qian, J.; Li, Z.; Gong, A.; Zhong, S.; Qiao, L.; Qian, S.; Zhang, Y.; Dou, R.; Li, R.; et al. Bioactive compounds from Abelmoschus manihot L. alleviate the progression of multiple myeloma in mouse model and improve bone marrow microenvironment. OncoTargets Ther. 2020, 13, 959–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Xue, C.; Duan, J.A.; Qian, D.; Tang, Y.; You, Y. Anticonvulsant, antidepressant-like activity of Abelmoschus manihot ethanol extract and its potential active components in vivo. Phytomedicine 2011, 18, 1250–1254. [Google Scholar] [CrossRef]
- Cheng, X.P.; Qin, S.; Dong, L.Y.; Zhou, J.N. Inhibitory effect of total flavone of Abelmoschus manihot L. Medic on NMDA receptor-mediated current in cultured rat hippocampal neurons. Neurosci. Res. 2006, 55, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; He, W.; Xia, P.; Sun, W.; Shi, M.; Zhou, Y.; Zhu, W.; Zhang, L.; Liu, B.; Zhu, J.; et al. Total extracts of Abelmoschus manihot L. attenuates adriamycin-induced renal tubule injury via suppression of ROS-ERK1/2-mediated NLRP3 inflammasome activation. Front. Pharmacol. 2019, 10, 567. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Dusabimana, T.; Kim, S.R.; Je, J.; Jeong, K.; Kang, M.C.; Cho, K.M.; Kim, H.J.; Park, S.W. Supplementation of Abelmoschus manihot ameliorates diabetic nephropathy and hepatic steatosis by activating autophagy in mice. Nutrients 2018, 10, 1703. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Ye, L.; Tao, J.; Ge, C.; Huang, L.; Yu, J. Total flavones of Abelmoschus manihot improve diabetic nephropathy by inhibiting the iRhom2/TACE signalling pathway activity in rats. Pharm. Biol. 2017, 56, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Beccaria, M.; Cabooter, D. Current developments in LC-MS for pharmaceutical analysis. Analyst 2020, 145, 1129–1157. [Google Scholar] [CrossRef]
- Cai, Z.; Liao, H.; Wang, C.; Chen, J.; Tan, M.; Mei, Y.; Wei, L.; Chen, H.; Yang, R.; Liu, X. A comprehensive study of the aerial parts of Lonicera japonica Thunb. based on metabolite profiling coupled with PLS-DA. Phytochem. Anal. 2020, 31, 786–800. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Chen, G. Studies on the Chemical Constituents and Anhyperglycemic Action of Abelmoschus manihot L. Medie; Academy of Military Medical Sciences: Beijing, China, 2006. [Google Scholar]
- Zhang, Y.; He, W.; Li, C.; Chen, Q.; Han, L.; Liu, E.; Wang, T. Antioxidative flavonol glycosides from the flowers of Abelmouschus manihot. J. Nat. Med. 2013, 67, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; An, Y.T.; Wang, T.; Shang, H.H.; Gao, X.M.; Zhang, Y. Isolation and identification of chemical constituents from the flowers of Abelmoschus manihot (L.) Medic (III). J. Shenyang Pharm. Univ. 2011, 28, 520–525. [Google Scholar]
- Xia, K.Y.; Zhang, C.L.; Cao, Z.Y.; Ge, H.T.; Tang, H.T. Chemical constituents from corolla abelmoschi. Strait Pharm. J. 2019, 31, 58–61. [Google Scholar]
- Liu, G.D.; Zhao, Y.W.; Li, Y.J.; Wang, X.J.; Si, H.H.; Huang, W.Z.; Wang, Z.Z.; Ma, S.P.; Xiao, W. Qualitative and quantitative analysis of major constituents from Dazhu Hongjingtian capsule by UPLC/Q-TOF-MS/MS combined with UPLC/QQQ-MS/MS. Biomed. Chromatogr. 2017, 31, 3887. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Y.; Liu, W.; Feng, F.; Xie, N. HPLC with quadrupole TOF-MS and chemometrics analysis for the characterization of Folium Turpiniae from different regions. J. Sep. Sci. 2013, 36, 2552–2561. [Google Scholar] [CrossRef]
- Huang, G.Q.; Liang, J.; Wei, J.Y.; Huang, D.F.; Lin, J.; Chen, X.S.; Liu, X.F. Analysis and identification of chemical constituents in hypoglycemic effective fractions of Longan Folium based on UPLC-Q-Orbitrap HRMS. Chin. J. Exp. Tradit. Med. Formulae 2021, 27, 127–138. [Google Scholar]
- Xiao, G.L.; Jiang, J.Y.; Xu, A.L.; Li, Y.X.; Bi, X.L. Analysis of chemical constituents in Bushao Tiaozhi capsules by UPLC-Q-TOF-MS. Chin. J. Exp. Tradit. Med. Formulae 2020, 26, 190–199. [Google Scholar]
- Liu, J.; Chen, L.; Fan, C.R.; Li, H.; Huang, M.Q.; Xiang, Q.; Xu, W.; Xu, W.; Chu, K.D.; Lin, Y. Qualitative and quantitative analysis of major constituents of Paeoniae Radix Alba and Paeoniae Radix Rubra by HPLC-DAD-Q-TOF-MS/MS. China J. Chin. Mater. Med. 2015, 40, 63–67. [Google Scholar]
- Yang, Y.X.; Liao, S.G.; Wang, Z.; Li, Y.J.; Liang, Y.; Hao, X.Y.; Wang, Y.L. Analysis of water-soluble chemical constituents of Indigoferae Stachyoidis Radix by UHPLC-DAD-Q-TOF-MS/MS. Chin. J. Exp. Tradit. Med. Formulae 2014, 20, 669–677. [Google Scholar]
- Chi, Y.M.; Zhu, H.Y.; Ju, L.; Zhang, Y.; Shen, X.N.; Hua, X.Y.; Nie, F. Analysis of flavonols compounds in flos Abelmoschus Manihot by high performance liquid chromatography-electrospray ionization/quadrupole-time of flight-mass/mass spectrometry. Anal. Chem. 2009, 37, 227–231. [Google Scholar]
- Guo, J.M.; Xue, C.F.; Duan, J.A.; Shang, E.X.; Qian, D.W.; Tang, Y.P.; Ouyang, Q.; Sha, M. Fast characterization of major constituents in huangkui capsule using UPLC/QTOF MSE and MassFragment. In Proceedings of the 9th National Symposium on Natural Medicine Resources Proceedings and Abstrcats, Guangzhou, China, 18 July 2010; pp. 691–699. [Google Scholar]
- Hui, T.T.; Xia, Z.T.; Zhang, L.L.; Wu, N.F.; Chen, X.P.; Zhou, S.P. Identification and characterization of constituents in Yushu granules by HPLC-ESI-MS. Chin. J. Pharm. Anal. 2013, 33, 586–594. [Google Scholar]
- Ma, T.T.; Wang, Y.; Chen, X.Q.; Chen, X.P. LC/MS guided approach to discovering nephroprotective substances from Huangkui capsule. J. Zhejiang Univ. 2017, 46, 66–73. [Google Scholar]
- Fan, L. Quality Evaluation of Ampelopsis grossedentata Metabolic-Related Study of Its Bioactive Ingredient Dihydromyricetin; Huazhong University of Science and Technology: Wuhan, China, 2018. [Google Scholar]
- Mei, Y.; Wei, L.; Tan, M.; Wang, C.; Zou, L.; Chen, J.; Cai, Z.; Yin, S.; Zhang, F.; Shan, C.; et al. Qualitative and quantitative analysis of the major constituents in Spatholobi Caulis by UFLC-Triple TOF-MS/MS and UFLC-QTRAP-MS/MS. J. Pharm. Biomed. Anal. 2021, 194, 113803. [Google Scholar] [CrossRef]
- Keimu, A. Study on the Mass Pectrometric Analytical Method of Flavonoid Glycosides; Peking Union Medical College of China: Beijing, China, 2006. [Google Scholar]
- Rak, G.; Fodor, P.; Abrank, L. Three-step HPLC—ESI-MS/MS procedure for screening and identifying non-target flavonoid derivatives. Int. J. Mass Spectrom. 2010, 290, 32–38. [Google Scholar] [CrossRef]
- Shin, J.S.; Han, H.S.; Lee, S.B.; Myung, D.B.; Lee, K.; Lee, S.H.; Kim, H.J.; Lee, K.T. Chemical constituents from leaves of Hydrangea serrata and their anti−photoaging effects on UVB−irradiated human fibroblasts. Biol. Pharm. Bull. 2019, 42, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.M.; Guo, X.Y.; Quan, Q.H.; Ji, R.F.; Sun, Q.Q.; Tian, J.Y.; Tan, P.; Liu, Y.G. Analysis on chemical constituent from Cudrania tricus pidata Bur by LTQ-Orbitrap MS. J. Chin. Mass Spectrom. Soc. 2018, 39, 599–606. [Google Scholar]
- Wang, Y. Studies on Analysis of Flavonoids of Flowers, Stems and Leaves of Abelmoschus manihot (L.) Medic; Beijing University of Chinese Medicine: Beijing, China, 2015. [Google Scholar]
- Kang, Y.; Mao, Y.N.; Wang, F.F.; Wu, W.Q.; Liu, Y. Analysis of chemical components in leaves of Hippophae rhamnoides by UPLC-LTQ Orbitrap MS. Mod. Chin. Med. 2018, 20, 1340–1346. [Google Scholar]
- Xu, W.; Fu, Z.Q.; Lin, J.; Huang, X.C.; Chen, D.; Yu, H.M.; Huang, Z.H.; Fan, S.M. Qualitative and quantitative analysis of major constituents in Tetrastigma hemsleyanum by HPLC-Q-TOF-MS and UPLC-QqQ-MS. China J. Chin. Mater. Med. 2014, 39, 4365–4372. [Google Scholar]
- Zeng, M.L.; Shen, N.T.; Wu, S.W.; Li, Q. Analysis on chemical constituents in Tetrastigma hemsleyanum by UPLC-Triple-TOF/MS. Chin. Tradit. Herb. Drugs 2017, 48, 874–883. [Google Scholar]
- Zhang, L.; Wang, H.H.; Yang, S.H.; Tu, Z.C.; Li, J.; Chen, J.; Huang, Y.Z. Characterization of chemical constituents in ethyl acetate fraction of lotus leaves by ultra-high performance liquid chromatography-quadrupole time-of-flight tandem mass spectrometry. Food Sci. 2019, 40, 229–235. [Google Scholar]
- Gao, X.; Wan, Y.Y.; Li, C.Y.; Duan, X.B.; Ding, X.S.; Ju, W.Z. Systematic screening and assignment of flavones in total flavones of Abelmoschus manihot based on high-performance liquid chromatography time-of-flight mass spectrometry analysis and mass defect filter. Anal. Chem. 2020, 48, 262–274. [Google Scholar]
- Liu, C.X.; Chen, S.L.; Xiao, X.H.; Zhang, T.J.; Hou, W.B.; Liao, M.L. A new concept on quality marker of Chinese materia medica: Quality control for Chinese medicinal products. Chin. Tradit. Herb. Drugs. 2016, 47, 1443–1457. [Google Scholar]
- Wu, L.; Li, Q.; Liu, S.M.; An, X.F.; Huang, Z.M.; Zhang, B.; Yuan, Y.G.; Xing, C.Y. Protective effect of hyperoside against renal ischemia-reperfusion injury via modulating mitochondrial fission, oxidative stress, and apoptosis. Free Radic. Res. 2019, 53, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.D.; Tao, W.W.; Su, S.L.; Guo, S.; Zhu, Y.; Guo, J.M.; Qian, D.W.; Cong, X.D.; Tang, R.M.; Duan, J.A. Antidepressant activity of flavonoid ethanol extract of Abelmoschus manihot corolla with BDNF up-regulation in the hippocampus. Acta Pharm. Sin. 2017, 52, 222–228. [Google Scholar]
- An, X.F.; Zhang, L.; Yuan, Y.G.; Wang, B.; Yao, Q.M.; Li, L.; Zhang, J.S.; He, M.; Zhang, J.N. Hyperoside pre-treatment prevents glomerular basement membrane damage in diabetic nephropathy by inhibiting podocyte heparanase expression. Sci. Rep. 2017, 7, 6413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.; Wan, Y.G.; Wang, C.J.; Zhao, Q.; Wei, Q.X.; Tu, Y.; Yin, X.J. Mechanisms and effects of Abelmoschus manihot preparations in treating chronic kidney disease. China J. Chin. Mater. Med. 2012, 37, 2252–2256. [Google Scholar]
- Chen, Y.Z.; Gong, Z.X.; Cai, G.Y.; Gao, Q.; Chen, X.M.; Tang, L.; Wei, R.B.; Zhou, J.H. Efficacy and safety of Flos Abelmoschus manihot (Malvaceae) on type 2 diabetic nephropathy: A systematic review. Chin. J. Integr. Med. 2015, 21, 464–472. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).