Salt Spray and Surfactants Induced Morphological, Physiological, and Biochemical Responses in Callistemon citrinus (Curtis) Plants
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatments
2.2. Plant Materials and Treatments
2.3. Chlorophyll Content, Gas Exchanges, Chlorophyll a Fluorescence and RWC Measurements
2.4. Estimation of Proline Content
2.5. Extraction and Assay of Antioxidant Enzymes
2.6. Climate Conditions
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farieri, E.; Toscano, S.; Ferrante, A.; Romano, D. Identification of ornamental shrubs tolerant to saline aerosol for coastal urban and peri-urban greening. Urban For. Urban Green. 2016, 18, 9–18. [Google Scholar] [CrossRef]
- Ferrante, A.; Trivellini, A.; Malorgio, F.; Carmassi, G.; Vernieri, P.; Serra, G. Effect of seawater on leaves of six plant species potentially useful for ornamental purposes in coastal areas. Sci. Hortic. 2011, 128, 332–341. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Rodríguez, P.; Morales, M.A.; Torrecillas, A. Contrasting physiological responses of dwarf sea-lavender and marguerite to simulated sea aerosol deposition. J. Environ. Qual. 2003, 32, 2238–2244. [Google Scholar] [CrossRef] [PubMed]
- Greaver, T.L.; Sternberg, L.L.D.S. Linking marine resources to ecotonal shifts of water uptake by terrestrial dune vegetation. Ecology 2006, 87, 2389–2396. [Google Scholar] [CrossRef]
- Franco, J.A.; Martínez-Sánchez, J.J.; Fernández, J.A.; Bañón, S. Selection and nursery production of ornamental plants for landscaping and xerogardening in semi-arid environments. J. Hortic. Sci. Biotechnol. 2006, 81, 3–17. [Google Scholar] [CrossRef]
- Niu, G.; Rodriguez, D.S. Relative salt tolerance of selected herbaceous perennials and groundcovers. Sci. Hortic. 2006, 110, 352–358. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D. Response of Mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Zollinger, N.; Koenig, R.; Cerny-Koenig, T.; Kjelgren, R. Relative salinity tolerance of intermountain western United States native herbaceous perennials. HortScience 2007, 42, 529–534. [Google Scholar] [CrossRef]
- Toscano, S.; Branca, F.; Romano, D.; Ferrante, A. An evaluation of different parameters to screen ornamental shrubs for salt spray tolerance. Biology 2020, 9, 250. [Google Scholar] [CrossRef]
- Wu, S.; Sun, Y.; Niu, G.; Pantoja, G.L.G.; Rocha, A.C. Responses of six Lamiaceae landscape species to saline water irrigation. J. Environ. Hortic. 2016, 34, 30–35. [Google Scholar] [CrossRef]
- Marcum, K.B.; Pessarakli, M.; Kopec, D. Relative salinity tolerance of 21 turf-type desert salt grasses compared to bermudagrass. HortScience 2005, 40, 827–829. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Romano, D.; Tribulato, A. Interactive effects of drought and saline aerosol stress on morphological and physiological characteristics of two ornamental shrub species. Horticulturae 2021, 7, 517. [Google Scholar] [CrossRef]
- Cheplick, G.P.; Demetri, H. Impact of saltwater spray and sand deposition on the coastal annual Triplasis purpurea (Poaceae). Am. J. Bot. 1999, 86, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Elhaak, M.A.; Migahid, M.M.; Wegmann, K. Ecophysiological studies on Euphorbia paralias under soil salinity and sea water spray treatments. J. Arid Environ. 1997, 35, 459–471. [Google Scholar] [CrossRef]
- Scheiber, S.M.; Sandrock, D.; Alvarez, E.; Brennan, M.M. Effect of salt spray concentration on growth and appearance of ‘Gracillimus’ maiden grass and ‘Hamelin’ fountain grass. HortTechnology 2008, 18, 34–38. [Google Scholar] [CrossRef]
- Rizzo, V.; Toscano, S.; Farieri, E.; Romano, D. Antioxidative defense mechanism in Callistemon citrinus (Curtis) Skeels and Viburnum tinus L. ‘Lucidum’ in response to seawater aerosol and surfactants. J. Agric. Sci. Technol. 2019, 21, 911–925. [Google Scholar]
- Palmer, M.; Hatley, H. The role of surfactants in wastewater treatment: Impact, removal and future techniques: A critical review. Water Res. 2018, 147, 60–72. [Google Scholar] [CrossRef]
- Savé, R. What is stress and how to deal with it in ornamental plants? Acta Hortic. 2009, 813, 241–254. [Google Scholar] [CrossRef]
- Nicolotti, G.; Rettori, A.; Paoletti, E.; Gullino, M.L. Morphological and physiological damage by surfactant-polluted seaspray on Pinus pinea and Pinus halepensis. Environ. Monit. Assess. 2005, 105, 175–191. [Google Scholar] [CrossRef]
- Bussotti, F.; Bottacci, A.; Grossoni, P.; Mori, B.; Tani, C. Cytological and structural changes in Pinus pinea L. needles following the application of anionic surfactant. Plant Cell Environ. 1997, 20, 513–520. [Google Scholar] [CrossRef]
- Schreiber, L.; Bach, S.; Kirsch, T.; Knoll, D.; Schalz, K.; Riederer, M. A simple photometric device analysing cuticular transport physiology: Surfactant effect on permeability of isolated cuticular membranes of Prunus laurocerasus L. J. Exp. Bot. 1995, 46, 1915–1921. [Google Scholar] [CrossRef]
- Raddi, S.; Cherubini, P.; Lauteri, M.; Magnani, F. The impact of sea erosion on coastal Pinus pinea stands: A diachronic analysis combining tree-rings and ecological markers. For. Ecol. Manag. 2009, 257, 773–781. [Google Scholar] [CrossRef]
- Rettori, A.; Paoletti, E.; Nicolotti, G.; Gullino, M.L. Ecophysiological responses of Mediterranean pines to simulated sea aerosol polluted with an anionic surfactant: Prospects for biomonitoring. Ann. For. Sci. 2005, 62, 351–360. [Google Scholar] [CrossRef][Green Version]
- Bussotti, F.; Bottacci, A.; Grossoni, P.; Mori, B.; Tani, C. Anatomical and ultrastructural alterations in Pinus pinea L. needles treated with simulated sea aerosol. Agric. Med. 1995, 148–155. [Google Scholar]
- Tezara, W.; Martínez, D.; Rengifo, E.; Herrera, A. Photosynthetic responses of the tropical spiny shrubs Lycium nodosum (Solanaceae) to drought, soil salinity and saline spray. Ann. Bot. 2003, 92, 757–765. [Google Scholar] [CrossRef]
- Sudhir, P.; Murthy, S.D.S. Effects of salt stress on basic processes of photosynthesis. Photosynthetica 2004, 42, 481–486. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Sharma, N.; Gupta, N.K.; Gupta, S.; Hasegawa, H. Effect of NaCl salinity on photosynthetic rate, transpiration rate, and oxidative stress tolerance in contrasting wheat genotypes. Photosynthetica 2005, 43, 609–613. [Google Scholar] [CrossRef]
- Toscano, S.; Farieri, E.; Ferrante, A.; Romano, D. Physiological and biochemical responses in two ornamental shrubs to drought stress. Front. Plant Sci. 2016, 7, 645. [Google Scholar] [CrossRef] [PubMed]
- Bowler, C.; Montagu, M.V.; Inze, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Malan, C.; Greyling, M.M.; Gressel, J. Correlation between CuZn superoxide dismutase and glutathione reductase, and environmental and xenobiotic stress tolerance in maize inbreds. Plant Sci. 1990, 69, 157–166. [Google Scholar] [CrossRef]
- Perl, A.; Perl-Treves, R.; Galili, S.; Aviv, D.; Shalgi, E.; Malkin, S.; Galun, E. Enhanced oxidative-stress defense in transgenic potato expressing tomato Cu, Zn superoxide dismutases. Theor. Appl. Genet. 1993, 85, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef] [PubMed]
- Ruley, A.T.; Sharma, N.C.; Sahi, S.V. Antioxidant defense in a lead accumulating plant, Sesbania drummondii. Plant Physiol. Biochem. 2004, 42, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Deeba, F.; Pandey, A.K.; Ranjan, S.; Mishra, A.; Singh, R.; Sharma, Y.K.; Shirke, P.A.; Pandey, V. Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem. 2012, 53, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chen, S.; Chen, F.; Cheng, X.; Zhang, F. The embryo rescue derived intergeneric hybrid between chrysanthemum and Ajania przewalskii shows enhanced cold tolerance. Plant Cell Rep. 2011, 30, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Jiang, J.; Chen, S.; Huang, C.; Fang, W.; Chen, F. Drought tolerance of intergeneric hybrids between Chrysanthemum morifolium and Ajania przewalskii. Sci. Hortic. 2012, 148, 17–22. [Google Scholar] [CrossRef]
- Mereu, S.; Gerosa, G.; Marzuoli, R.; Fusaro, L.; Salvatori, E.; Finco, A.; Spano, D.; Manes, F. Gas exchange and JIP-test parameters of two Mediterranean maquis species are affected by sea spray and ozone interaction. Environ. Exp. Bot. 2011, 73, 80–88. [Google Scholar] [CrossRef]
- Niinemets, Ü. Responses of forest trees to single and multiple environmental stresses from seedlings to mature plants: Past stress history, stress interactions, tolerance and acclimation. For. Ecol. Manag. 2010, 260, 1623–1639. [Google Scholar] [CrossRef]
- Gonthier, P.; Nicolotti, G.; Rettori, A.; Paoletti, E.; Gullino, M.L. Testing Nerium oleander as a biomonitor for surfactant polluted marine aerosol. Int. J. Environ. Health Res. 2010, 4, 1–10. [Google Scholar]
- Bussotti, F.; Grossoni, P.; Pantani, F. The role of marine salt and surfactants in the decline of Tyrrhenian coastal vegetation in Italy. Ann. For. Sci. 1995, 52, 251–261. [Google Scholar] [CrossRef]
- Lippi, G.; Serra, G.; Vernieri, P.; Tognoni, F. Response of potted Callistemon species to high salinity. Acta Hortic. 2003, 609, 247–250. [Google Scholar] [CrossRef]
- Mugnai, S.; Ferrante, A.; Petrognani, L.; Serra, G.; Vernieri, P. Stress-induced variation in leaf gas exchange and chlorophyll a fluorescence in Callistemon plants. Res. J. Biol. Sci. 2009, 4, 913–921. [Google Scholar]
- Vernieri, P.; Mugnai, S.; Borghesi, E.; Petrognani, L.; Serra, G. Non-chemical growth control of potted Callistemon laevis Anon. Agr. Med. 2006, 136, 85. [Google Scholar]
- Álvarez, S.; Sánchez-Blanco, M.J. Comparison of individual and combined effects of salinity and deficit irrigation on physiological, nutritional and ornamental aspects of tolerance in Callistemon laevis plants. J. Plant Physiol. 2015, 185, 65–74. [Google Scholar] [CrossRef]
- Elshatshat, S.A. The effect of simulated seawater on water permeability of isolated leaf cuticular layers. Int. J. Agric. Biol. 2010, 12, 150–152. [Google Scholar]
- Moran, R.; Porath, D. Chlorophyll determination in intact tissues using N,NDimethylformamide. Plant Physiol. 1980, 65, 78–79. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Bian, S.; Jiang, Y. Reactive oxygen species, antioxidant enzyme activities and gene expression patterns in leaves and roots of Kentucky bluegrass in response to drought stress and recovery. Sci. Hortic. 2009, 120, 264–270. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Meth. Enzymol. 1984, 105, 121–130. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Setter, T.L.; DiTommaso, A.; Weston, L.A. Differential growth response to salt stress among selected ornamentals. J. Plant Nutr. 2007, 30, 1109–1126. [Google Scholar] [CrossRef]
- Marosz, A. Effect of soil salinity on nutrient uptake, growth, and decorative value of four ground cover shrubs. J. Plant Nutr. 2004, 27, 977–989. [Google Scholar] [CrossRef]
- Wahome, P.K. Mechanisms of salt (NaCl) stress tolerance in horticultural crops—A mini review. Acta Hortic. 2003, 609, 127–131. [Google Scholar] [CrossRef]
- Carter, C.T.; Grieve, C.M. Salt tolerance of floriculture crops. In Ecophysiology of High Salinity Tolerant Plants; Ḵẖān, M.A., Khan, M.A., Weber, D.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 279–287. [Google Scholar]
- Grattan, S.R.; Grieve, C.M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Touchette, B.W.; Rhodes, K.L.; Smith, G.A.; Poole, M. Salt spray induces osmotic adjustment and tissue rigidity in smooth cordgrass, Spartina alterniflora (Loisel.). Estuaries Coasts 2009, 32, 917–925. [Google Scholar] [CrossRef]
- Alarcón, J.J.; Morales, M.A.; Torrecillas, A.; Sánchez-Blanco, M.J. Growth, water relations and accumulation of organic and inorganic solutes in the halophyte Limonium latifolium cv. Avignon and its interspecific hybrid Limonium caspia × Limonium latifolium cv. Beltlaard during salt stress. J. Plant Physiol. 1999, 154, 795–801. [Google Scholar] [CrossRef]
- Morant-Manceau, A.; Pradier, E.; Tremblin, G. Osmotic adjustment, gas exchanges and chlorophyll fluorescence of a hexaploid triticale and its parental species under salt stress. J. Plant Physiol. 2004, 161, 25–33. [Google Scholar] [CrossRef]
- Goldstein, G.; Drake, D.R.; Alpha, C.; Melcher, P.; Heraux, J.; Azocar, A. Growth and photosynthetic responses of Scaevola sericea, a Hawaiian coastal shrub, to substrate salinity and salt spray. Int. J. Plant Sci. 1996, 157, 171–179. [Google Scholar] [CrossRef]
- De Vos, A.C.; Broekman, R.; Groot, M.P.; Rozema, J. Ecophysiological response of Crambe maritima to airborne and soil-borne salinity. Ann. Bot. 2010, 105, 925–937. [Google Scholar] [CrossRef]
- Bernstein, L.; Francois, L.E.; Clark, R.A. Salt tolerance of ornamental shrubs and ground covers. Am. Soc. Hortic. Sci. J. 1972, 97, 550–556. [Google Scholar]
- Niu, G.; Cabrera, R.I. Growth and physiological responses of landscape plants to saline water irrigation: A review. HortScience 2010, 45, 1605–1609. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Parvaiz, A.; Satyawati, S. Salt stress and phyto-biochemical responses of plants-a review. Plant Soil Environ. 2008, 54, 89. [Google Scholar] [CrossRef]
- Griffiths, M.E.; Orians, C.M. Salt spray effects on forest succession in rare coastal sandplain heathlands: Evidence from field surveys and Pinus rigida transplant experiments. J. Torrey Bot. Soc. 2004, 131, 23–31. [Google Scholar] [CrossRef]
- Sánchez-Blanco, M.J.; Ferrández, T.; Navarro, A.; Bañon, S.; Alarcón, J.J. Effects of irrigation and air humidity preconditioning on water relations, growth and survival of Rosmarinus officinalis plants during and after transplanting. J. Plant Physiol. 2004, 161, 1133–1142. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Waldhoff, D.; Furch, B.; Junk, W.J. Fluorescence parameters, chlorophyll concentration, and anatomical features as indicators for flood adaptation of an abundant tree species in Central Amazonia: Symmeria paniculata. Environ. Exp. Bot. 2002, 48, 225–235. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Lao, M.T. The effects of salt stress on ornamental plants and integrative cultivation practices. Sci. Hortic. 2018, 240, 430–439. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C. Proline accumulation in plants: A review. Amino Acids 2008, 35, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.; Gill, R.; Ahlawat, S.; Anjum, N.A.; Sharma, K.K.; Ansari, A.A.; Hasanuzzaman, M.; Ramakrishna, A.; Chauhan, N.; Tuteja, N.; et al. Targeting the redox regulatory mechanisms for abiotic stress tolerance in crops. In Biochemical, Physiological and Molecular Avenues for Combating Abiotic Stress Tolerance in Plants; Wani, S.H., Ed.; Academic Press Elsevier: London, UK, 2018; pp. 151–220. [Google Scholar] [CrossRef]
- Bernstein, L. Osmotic adjustment of plants to saline media. II. Dynamic phase. Am. J. Bot. 1963, 50, 360–370. [Google Scholar] [CrossRef]
- De Gara, L.; Foyer, C.H. Ying and Yang interplay between reactive oxygen and reactive nitrogen species controls cell functions. Plant Cell Environ. 2017, 40, 459–461. [Google Scholar] [CrossRef]
- Skłodowska, M.; Gapińska, M.; Gajewska, E.; Gabara, B. Tocopherol content and enzymatic antioxidant activities in chloroplasts from NaCl-stressed tomato plants. Acta Physiol. Plant. 2009, 31, 393–400. [Google Scholar] [CrossRef]
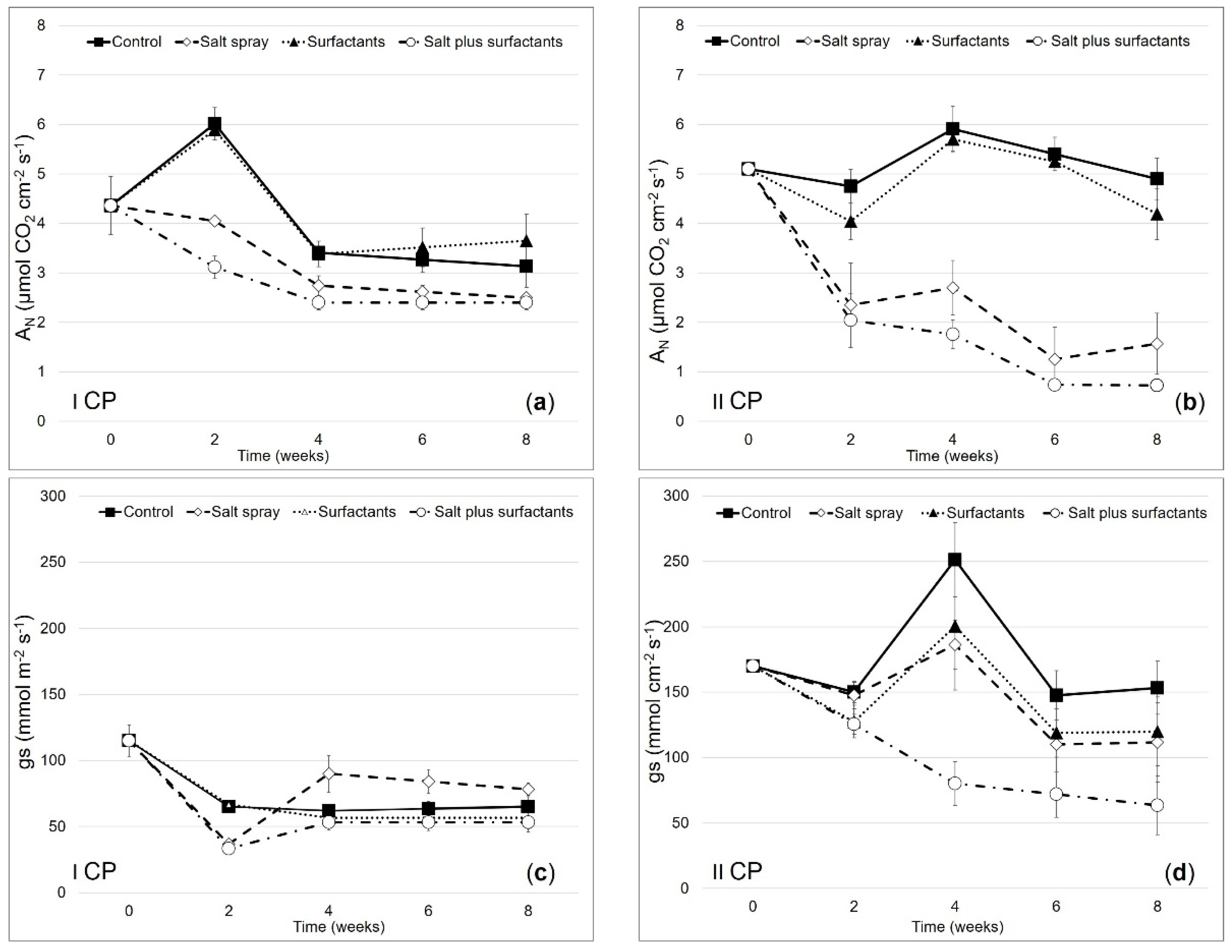
 ) and II CP (
) and II CP ( ) treated with distilled water (control,
) treated with distilled water (control,  and
and  ), salt spray (
), salt spray ( and
and  ), surfactants (
), surfactants ( and
and  ), and salt plus surfactants (
), and salt plus surfactants ( and
and  ). Values are means ± SE (n = 3). Values with the same letter are not significantly different as determined by Tukey’s test (p < 0.05).
). Values are means ± SE (n = 3). Values with the same letter are not significantly different as determined by Tukey’s test (p < 0.05).
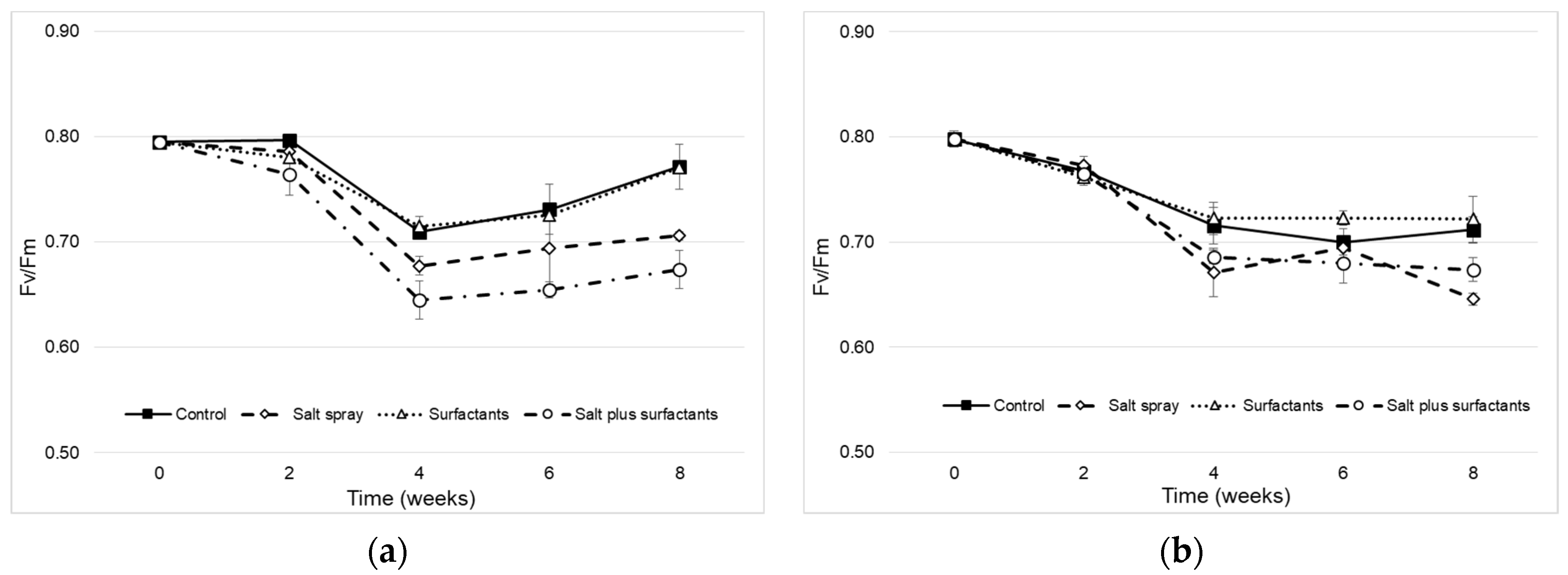
 ) and II CP (
) and II CP ( ) treated with distilled water (control,
) treated with distilled water (control,  and
and  ), salt spray (
), salt spray ( and
and  ), surfactants (
), surfactants ( and
and  ), and salt plus surfactants (
), and salt plus surfactants ( and
and  ). Values are means ± SE (n = 3). Values with the same letter are not significantly different as determined by Tukey’s test (p < 0.05).
). Values are means ± SE (n = 3). Values with the same letter are not significantly different as determined by Tukey’s test (p < 0.05).
 ) and II CP (
) and II CP ( ) treated with distilled water (control,
) treated with distilled water (control,  and
and  ), salt spray (
), salt spray ( and
and  ), surfactants (
), surfactants ( and
and  ), and salt plus surfactants (
), and salt plus surfactants ( and
and  ). Values are means ± SE (n = 3). Values with the same letter are not significantly different by Tukey’s test (p < 0.05).
). Values are means ± SE (n = 3). Values with the same letter are not significantly different by Tukey’s test (p < 0.05).
 ) and II CP (
) and II CP ( ) treated with distilled water (control,
) treated with distilled water (control,  and
and  ), salt spray (
), salt spray ( and
and  ), surfactants (
), surfactants ( and
and  ), and salt plus surfactants (
), and salt plus surfactants ( and
and  ). Values are means ± SE (n = 3). Values with the same letter are not significantly different by Tukey’s test (p < 0.05).
). Values are means ± SE (n = 3). Values with the same letter are not significantly different by Tukey’s test (p < 0.05).
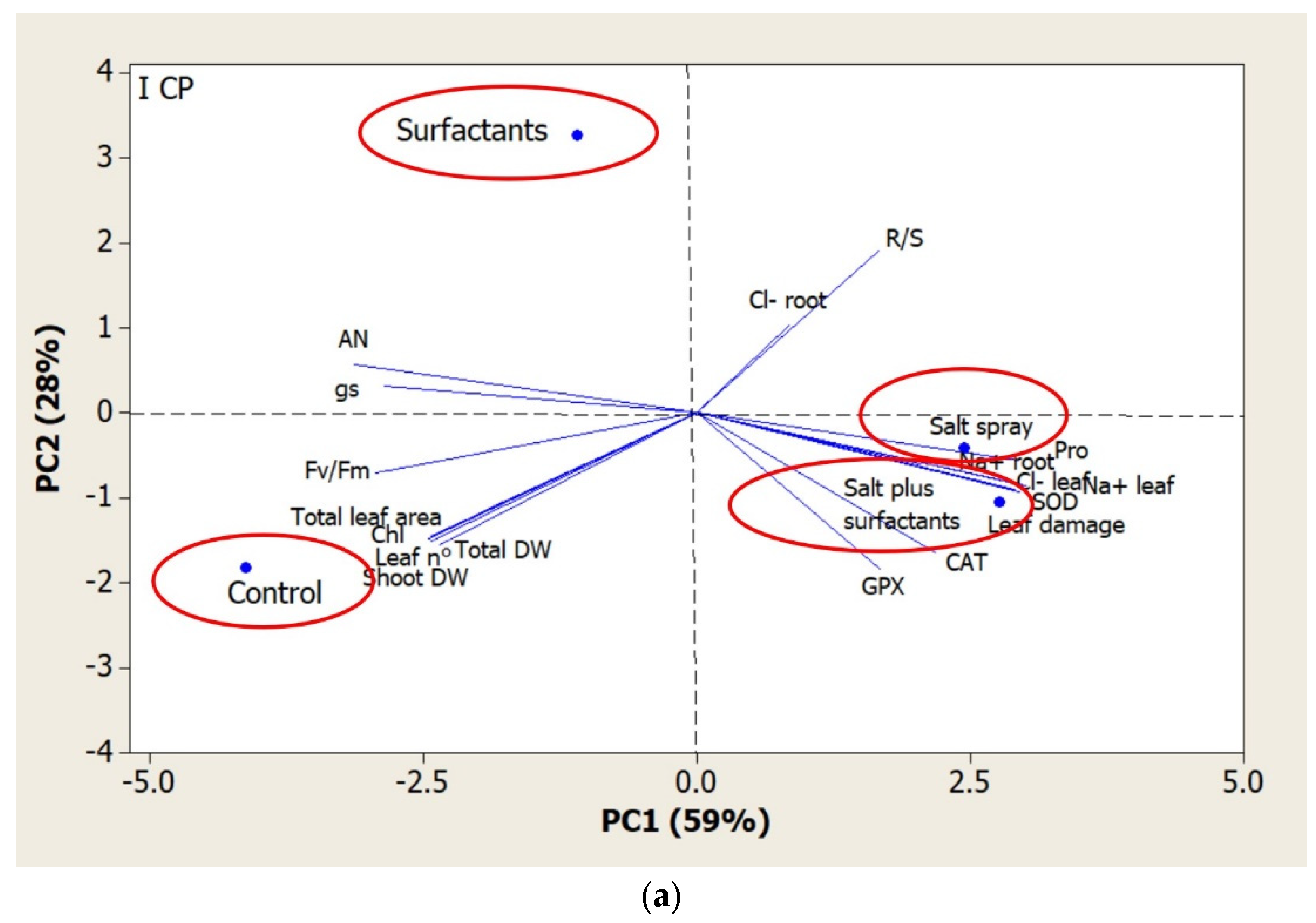
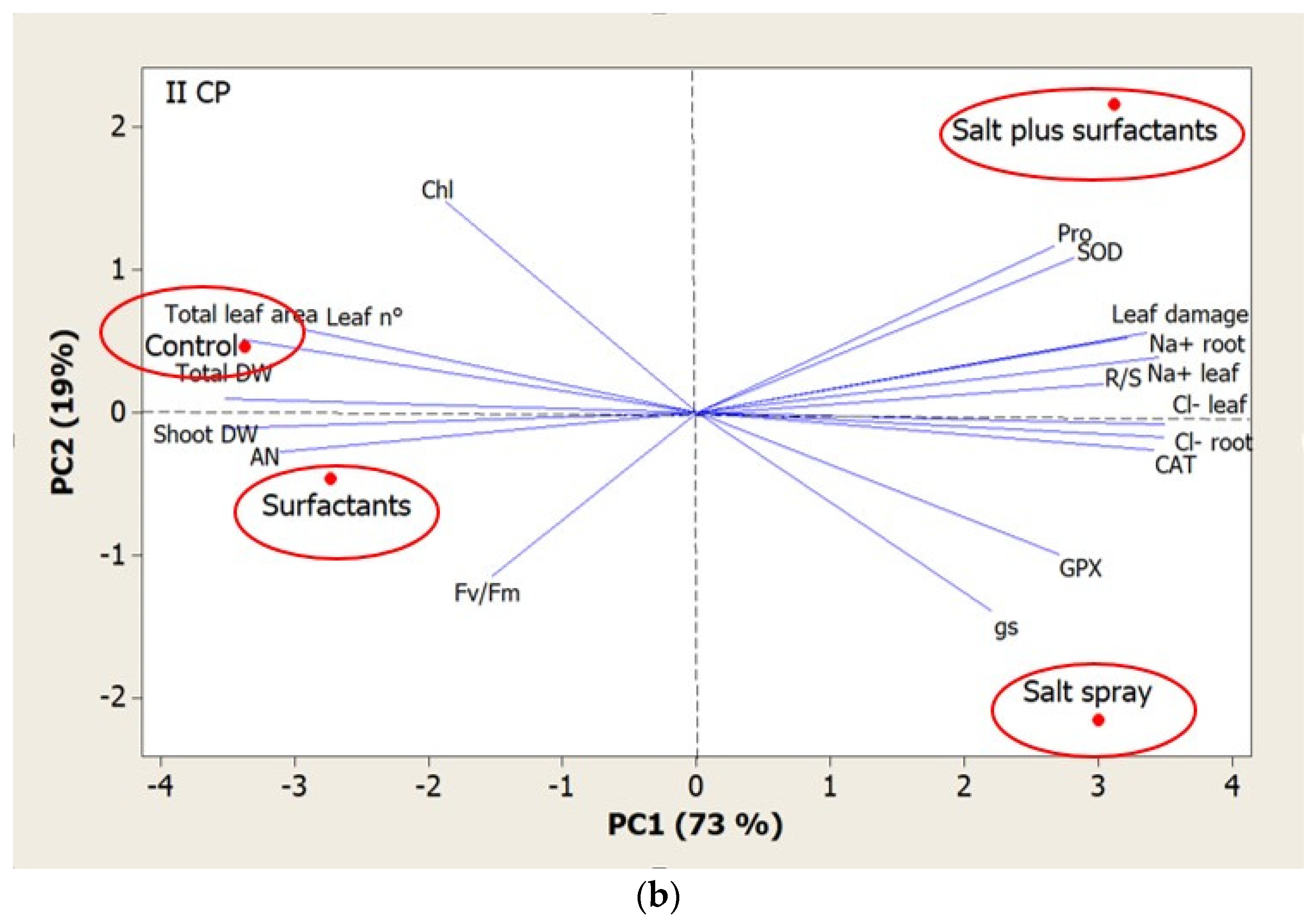
| Total Dry Biomass | Shoot Dry Biomass | R/S Ratio | Leaf Number | Total Leaf Area | Chlorophyll Content | Na+ Leaves | Cl− Leaves | Na+ Root | Cl− Root | Leaf Damage | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Main Effects | |||||||||||
| Cycle periods (CP) | F 12.21 ** | F 24.42 *** | F 178.26 *** | F 21.50 *** | F 25.59 *** | F 0.68 ns | F 19.74 *** | F 852.36 *** | F 674.85 *** | F 98.51 *** | F 0.84 ns |
| Treatments (T) | F 1.06 ns | F 1.21 ns | F 3.60 * | F 1.33 ns | F 1.60 ns | F 0.12 ns | F 123.46 *** | F 522.64 *** | F 346.21 *** | F 12.27 *** | F 440.64 *** |
| Interaction | |||||||||||
| CP × T | F 0.50 ns | F 0.66 ns | F 3.50 * | F 0.67 ns | F 0.57 ns | F 0.11 ns | F 7.37 ** | F 184.15 *** | F 164.62 *** | F 13.94 *** | F 9.23 ns |
| Treatments | Total Dry Weight (g Plant−1) | Shoot Dry Weight (g Plant−1) | R/S Ratio (g g−1) | Leaf (n·Plant−1) | Total Leaf Area (cm2 Plant−1) | Chlorophyll Content (µg cm−2) | Na+ Leaves (g kg−1 DW) | Cl− Leaves (g kg−1 DW) | Na+ Root (g kg−1 DW) | Cl− Root (g kg−1 DW) | Leaf Damage (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Winter (I CP) | Control | 11.3 ± 0.5a | 7.6 ± 0.4a | 0.5 ± 0.0b | 146.0 ± 6.3a | 478.3 ± 31.7a | 39.1 ± 2.8 | 2.6 ± 0.3b | 2.9 ± 0.4b | 3.4 ± 0.1 | 2.9 ± 0.6 | 0.0 ± 0.0b |
| Salt spray | 8.5 ± 0.8b | 4.8 ± 0.6b | 0.8 ± 0.1a | 56.5 ± 7.9b | 181.2 ± 29.7b | 27.8 ± 1.9 | 17.9 ± 1.1a | 14.8 ± 1.3a | 4.0 ± 0.5 | 3.3 ± 0.4 | 16.5 ± 1.2a | |
| Surfactant | 7.7 ± 0.5b | 4.1 ± 0.2b | 0.9 ± 0.1a | 41.2 ± 2.8b | 137.0 ± 11.2b | 31.5 ± 3.6 | 1.2 ± 0.1b | 2.0 ± 0.5b | 3.3 ± 0.2 | 2.7 ± 0.7 | 0.14 ± 0.0b | |
| Salt spray plus surfactant | 8.0 ± 0.3b | 4.7 ± 0.3b | 0.7 ± 0.1a | 56.2 ± 7.8b | 197.2 ± 34.5b | 35.8 ± 1.6 | 16.8 ± 1.2a | 14.8 ± 1.2a | 3.9 ± 0.5 | 3.4 ± 0.5 | 20.3 ± 1.2a | |
| Significance | ** | ** | * | *** | *** | ns | *** | *** | ns | ns | ** | |
| Summer (II CP) | Control | 21.6 ± 1.1a | 18.5 ± 1.0a | 0.2 ± 0.0 | 220.3 ± 12.5a | 860.0 ± 45.0a | 35.2 ± 2.0 | 3.2 ± 0.5c | 9.2 ± 0.5c | 4.6 ± 0.2c | 4.3 ± 0.2b | 0.0 ± 0.0c |
| Salt spray | 14.1 ± 0.6b | 12.0 ± 0.7b | 0.2 ± 0.0 | 167.5 ± 12.8b | 624.5 ± 32.1b | 25.6 ± 2.5 | 21.2 ± 2.6b | 55.0 ± 0.6a | 7.4 ± 0.8b | 8.0 ± 0.4a | 17.1 ± 1.2b | |
| Surfactant | 20.0 ± 0.9a | 17.3 ± 0.8a | 0.2 ± 0.0 | 188.2 ± 8.4b | 814.0 ± 24.7a | 30.9 ± 3.5 | 1.5 ± 0.1c | 4.5 ± 0.7d | 3.2 ± 0.5d | 4.2 ± 0.1b | 0.0 ± 0.0c | |
| Salt spray plus surfactant | 14.3 ± 0.3b | 11.2 ± 0.8b | 0.2 ± 0.0 | 179.0 ± 8.5b | 691.9 ± 19.5b | 33.2 ± 1.4 | 28.9 ± 2.6a | 51.8 ± 0.8b | 9.9 ± 0.5a | 7.3 ± 0.6a | 27.7 ± 1.2a | |
| Significance | *** | *** | ns | * | *** | ns | *** | *** | *** | *** | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toscano, S.; La Fornara, G.; Romano, D. Salt Spray and Surfactants Induced Morphological, Physiological, and Biochemical Responses in Callistemon citrinus (Curtis) Plants. Horticulturae 2022, 8, 261. https://doi.org/10.3390/horticulturae8030261
Toscano S, La Fornara G, Romano D. Salt Spray and Surfactants Induced Morphological, Physiological, and Biochemical Responses in Callistemon citrinus (Curtis) Plants. Horticulturae. 2022; 8(3):261. https://doi.org/10.3390/horticulturae8030261
Chicago/Turabian StyleToscano, Stefania, Giovanni La Fornara, and Daniela Romano. 2022. "Salt Spray and Surfactants Induced Morphological, Physiological, and Biochemical Responses in Callistemon citrinus (Curtis) Plants" Horticulturae 8, no. 3: 261. https://doi.org/10.3390/horticulturae8030261
APA StyleToscano, S., La Fornara, G., & Romano, D. (2022). Salt Spray and Surfactants Induced Morphological, Physiological, and Biochemical Responses in Callistemon citrinus (Curtis) Plants. Horticulturae, 8(3), 261. https://doi.org/10.3390/horticulturae8030261








