Abstract
Sugar, an osmoregulatory substance used by plants to adapt to abiotic stresses such as drought and salinity, is one of the most important indexes of fruit quality. In this study, 0–150 mM saline–alkali solutions (NaCl:NaHCO3 = 3:1) were used to irrigate the roots of 10-year-old “Junzao” fruit trees during the growth period to explore the regulation mechanism of different concentrations of saline–alkali stress on sugar and reactive oxygen metabolism in jujube fruit at maturity. The results showed that under low stress (0~90 mM), the contents of sucrose, glucose, and fructose in the jujube fruit and the activities of sucrose phosphate synthase (SPS), sucrose synthase decomposition direction (SS-I), and sucrose synthase synthesis direction (SS-II) increased with increases in stress concentration, results that were consistent with the relative expression trends of the SPS and SS genes; however, the results were reversed under high concentrations (120 and 150 mM). The soluble acid invertase (S-AI) activity decreased with increases in stress concentration under low stress, and the results were reversed with high stress, which was consistent with the relative expression trends of the ZjcINV3, ZjnINV1, and ZjnINV3. Research regarding the response of antioxidant enzymes in fruits under saline–alkali stress showed that only the differences in peroxidase (POD) activity under saline–alkali stress were consistent with sugar accumulation; the proline (PRO), catalase (CAT) decreased and the malondialdehyde (MDA) superoxide dismutase (SOD) increased with increases in saline–alkali stress. These results indicate that the sugar metabolism and antioxidase jointly promote and regulate sugar accumulation in jujube fruits in a low saline–alkali environment.
1. Introduction
Xinjiang is a national high-quality fruit production area and the most advantageous production area of the country for jujube cultivation. In 2020, the cultivation area comprised about one-third of cultivation areas nationwide, and the annual yield comprised more than 50% of national production [1]; however, jujube is mostly grown in non-cultivated areas in which soil salinization is a serious problem that affects the growth of jujube trees and jujube quality.
Sugar is an important factor of fruit quality that is often affected by external environments such as light, water, salinity, and temperature [2]. Some natural phenomena in production and previous studies have shown that [3,4,5,6] mild salt stress can improve sugar accumulation in fruits and severe salt stress can decrease the sugar content. In a previous study, after sprayings with different concentrations of an NaCl solution, the fructose, glucose, and sucrose in grapefruit increased under low salinity (20~60 mM) and decreased under high salinity (100~150 mM) [6,7]. Differences in the sugar contents of fruits caused by different concentrations of salt stress have also been reported for tomatoes [4,8,9], Lycium chinensis [5], and figs [10]. These differences are often related to the activities of sucrose-metabolizing enzymes and gene expression levels [11]. Tomatoes [9] were treated with 25~75 mM NaCl, and the salt-stress-induced AI and SS-I enzyme activities promoted the accumulation of hexose. The up-regulation of SS, AI, and neutral invertase (NI) genes under 75 mM NaCl stress was shown to lead to increases in the contents of glucose, fructose, and sucrose in figs [10]. NaCl stress was shown to inhibit NI and AI and promoted SS, which reduced the hexose and soluble sugar contents in wolfberry fruit; low salt (0.3% and 0.6%) stress was shown to promote SPS activity and increase sucrose accumulation in fruit, and high salt (0.9%) stress led to the opposite result. Salt stress has a concentration-dependent effect on sugar metabolism [12,13] and leads to changes in oxidative stress in plants [14,15,16]. Research has shown that decreases in the total soluble sugar of kiwi fruit leaves [17] under NaCl stress are related to significantly increased proline levels. Begonia [18,19] was shown to adapt to saline–alkali stress by increasing the activities of SOD and POD and accumulating sugar to participate in osmotic regulation. The regulation and adaptation of sugar to stress in plants is an integrated response that involves gene expression regulation, post-transcriptional regulation, and the metabolite feedback of sugar metabolism at multiple biological levels [20,21].
At present, there have been few reports on the effect of saline–alkali stress on the sugar metabolism in jujube fruit. One report showed that the soluble sugar content in jujube fruit first increased and then decreased with increases in saline–alkali stress (0~0.6%), specifically demonstrating that 0.4% saline–alkali stress could significantly increase the soluble sugar in jujube fruit by 13% [22]; however, there is still no extensive research on the response of jujube fruit sugar accumulation in enzymatic and physiological of regulation under saline–alkali stress that uses a combination of physiology, molecular and oxidase [23]. Taking into account all these facts, the objectives of this study were to understand how jujube adjusts osmolytes and antioxidase activity to increase carbohydrate metabolic under the conditions of saline–alkali stress and to identify which genes are involved in the regulation of sugar metabolism in jujube.
2. Materials and Methods
2.1. Experimental Materials
The experiment was conducted in a jujube orchard of Alar City 10 Regiment, Aksu Prefecture, Xinjiang Region (40°59′ N 81°28′ E), located on the southern edge of the Taklamakan desert; it has a continental arid desert climate with an annual average temperature of 10.4 °C, an annual average precipitation of 40.1~82.5 mm, and an annual average evaporation of 1876.6~2558.9 mm. The materials used in the experiment were 10-year-old “Junzao” jujube trees, with wild jujube as rootstock. The row spacing was 4 m, and the plant spacing was 2 m (1250 trees/hm2). A rain shelter was arranged above the test site to prevent the impact of rainwater on treatment (Figure 1A). Connections between treatments were intercepted by digging a 100 cm deep ditch (Figure 1B) filled with a double-layer plastic film to prevent leakage and isolate plots (Figure 1C) so that the treatments of each plot did not affect each other. Four drip lines (two emitters with 0.3 m intervals; 3.2 L/h for each emitter) located 0.4 and 0.8 m away from the tree row provided the salt–alkali solution on both sides of the trees (Figure 1D). The soil was sandy loam, and the jujube trees were distributed in a 0–60 cm soil layer.

Figure 1.
(A) Rain shelter to prevent the impact of rainwater; (B) connections between treatments were intercepted by digging a 100 cm deep ditch; (C) the ditch was insulated with double-layer plastic; (D) the drip lines used to provide the salt–alkali solution.
2.2. Experimental Design
The experiment had a single-factor randomized block design. According to measurements of the saline–alkali soil in the Alar jujube orchard, the ratio of NaCl to NaHCO3 was 3:1. The concentrations of saline–alkali used in the study were 0 (CK), 30, 60, 90, 120, and 150 μM. Each sample group comprised three plots of four plants, with a total of 72 jujube trees; each group was irrigated with the saline solution every 14 days after the young fruiting stage for 3 irrigations in total.
2.3. Sample Collection
2.3.1. Plant Sample Collection
Samples were collected at fruit maturation (120 days after flowering), and 30 fruits were selected from a different part of each tree. The fruit samples were immediately put into an incubator and brought back to the laboratory before the fruit pulp was extracted and chopped. The pulp was quickly divided into three parts; one part was dried in a vacuum-freeze lyophilizer (GOLD-SIM, FD5-3) for 72 h, pulverized, extracted as powder, and stored at −20 °C in a cryogenic refrigerator for sugar content determination, while the other two parts were quickly frozen with liquid nitrogen and stored at −80 °C in a cryogenic refrigerator for enzymatic activity and enzyme gene determination.
2.3.2. Soil Sample Collection
Soil samples for each treatment were collected from three points under the trees: 0–20, 20–40, and 40–60 cm soil layers. Next, they were subjected to natural air drying, pushed through a 2 mm soil sieve, and stored in sealed bags.
2.4. Experimental Method
2.4.1. Measurement of the Soil Salt Contents
For the preparation of leachate, we first weighed 10 g of drying soil sample and placed it into a bottle, stirred in 50 mL of deionized water, and shook the mixture for 5 min. We then filtered the mixture with filter paper to obtain a clear 5:1 water–soil immersion filter solution.
For the measurement of soil salt contents, we poured 20 mL of the immersion solution into a dry evaporation dish and then placed it into a steam oven until the temperature rose from 105 to 110 °C and the solution reached a constant weight.
2.4.2. Extraction and Determination of Sugars
Sugars were extracted and measured according to the protocol of Pu [24]. We weighed 1 g of freeze-dried jujube powder and performed ultrasonic extraction with distilled water. After centrifugation, the supernatant was passed through a 0.45 μm microporous membrane. Sugars were detected with high-performance liquid chromatography (HPLC) using an Agilent 1206 HPLC system (Agilent Technologies, Waldbronn, Germany) with an evaporative light-scattering detector cell maintained at 60 °C. The column was a Waters XBridgeTM BEH Amide column (4.6 × 250 mm, 5 μm), the column temperature was 30 °C, the atomization tube temperature was 60 °C, and the drift tube temperature was 60 °C. The gas flow was 1.6 L/min, the gain value was 1.0, the mobile phase was acetonitrile/water (volume ratio of 76:24) to which 0.2% triethylamine was added, the injection volume was 10 μL, the running time was 18 min, and each sample was measured 3 times. We performed linear regression analysis on peak area (Y) with mass concentration (X); developed standard curves of sucrose (100 mg/mL), fructose (100 mg/mL), and glucose (100 mg/mL); and established a regression equation (Table 1). Then, we calculated the contents of the sugar components according to the peak area of the sample and the standard curve.

Table 1.
Linear equation and correlation coefficients of constituent sugars in mixed standard solution determined with HPLC–ELSD.
2.4.3. Enzyme Extraction and Activity Assays
Jujube and liquid nitrogen were placed in a mortar and ground into powder; then, 0.1 g of the powder was weighed and placed in a 2 mL centrifuge tube. The enzyme activity was determined with a sucrose phosphate synthase kit (SPS), a soluble acid convertase kit (S-AI), a neutral translate kit (NI), a sucrose synthetase kit (SS-I), a sucrose synthetase kit (SS-II), a proline kit (PRO), a malondialdehyde kit (MDA), a superoxide dismutase kit (SOD), a catalase kit (CAT), and a peroxidase kit (POD) produced by Suzhou Keming Biotechnology Co., Ltd., Suzhou, China. We used a microassay determination method.
2.4.4. RNA Extraction
We used the TransGen Biotech TransZol Plant kit to extract RNA. The mortar, medicine spoon, centrifuge tube, and gun head used for RNA extraction were treated with DEPC water and operated in a low-temperature, enzyme-free environment.
2.4.5. cDNA
cDNA synthesis was performed using the TransGen Biotech EasyScript® One-Step gDNA Removal and cDNA Synthesis SuperMix kits.
2.4.6. RT-qPCR
Fluorescence quantification was performed using the TransGen Biotech TransStart® Green qPCR SuperMix kit. The PCR reaction was conducted with the synthesized cDNA as the template. The reaction system comprised 10.5 L of the cDNA template, 0.2 L of the left and right primers, two sets of 5 μL of PerfectStartTM Green qPCR SuperMix, 0.2 L of Passive Reference Dye, and 3.9 L of ddH2O. The fluorescence quantification PCR (Applied BiosystemsT M QuantStudio TM 5, Thermo Fisher Scientific, Waltham, MA, USA) response procedure was set to 42 cycles of 94 °C degeneration for 30 s, 94 °C denaturation for 5 s, 55 °C annealing for 15 s, 72 °C extension for 10 s; finally, the system was heated at 1.6 °C/s to 95 °C s and then maintained for 15 s, allowed to cool down for 60 s at 1.6 °C/s and then held at 0.075 °C/s. The amplification of the selected and reference genes was conducted using three biological replicates each. The gene expression of the control group was used as the reference gene for analysis. The relative abundance of transcripts was calculated by using the 2−ΔΔCt method. Enzyme gene from 11 gene by Zhang Chunmei [25], and primer sequence information is shown in Table 2.

Table 2.
The primer sequence of qRT-PCR.
2.5. Statistical Analysis
DPS7.05 statistical analysis was performed. Values are presented as the mean (n = 3) ± standard error (SE). Duncan’s multiple range tests were conducted to determine whether there were significant differences between individual treatments at p < 0.05.
3. Results
3.1. Differences in Soil Salt Content under Different Saline–Alkali Treatments
As shown in Figure 2, the salt content in different soil layers gradually increased with increases in the saline–alkali stress. With increases in the saline–alkali stress, the salinity in the 0–20, 20–40, and 40–60 cm soil layers under saline–alkali treatment increased by 35.31~54.70%, 31.36~50.11%, and 19.66~39.62%, respectively, compared to the control. The fitting curve of salt content in the 0–60 soil layer under saline–alkali treatment was y = 0.0275X + 5.5005, R2 = 0.9860, and it increased by 2.02, 2.37, 3.31, 4.13, and 4.98 g/kg compared to the control total salt of 4.65 g/kg. The fitted growth curve was y = 0.0256X + 1.0611, R2 = 0.9842, and the total salt content of the treatment gradually increased.
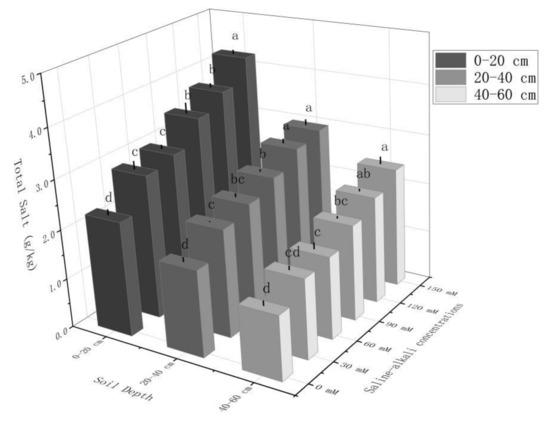
Figure 2.
Total salinity in 0–20, 20–40, and 40–60 cm soil layers under different saline–alkali treatments. Note: Small letters indicate significant differences at a 5% level (p ≤ 0.05).
3.2. Effects of Different Saline–Alkali Stress Treatments on the Contents of Sugar Components in Jujube Fruit
The differences in the sugar components and total sugar content of the jujube fruit under saline–alkali treatment are shown in Table 3. Under low stress (0~90 mM), the fructose and glucose contents in the fruit increased with increases in the saline–alkali stress, and under high stress (120~150 mM), it decreased with increases in stress concentration; when the stress was 90 mM, the fructose and glucose contents were the highest (99.03 and 86.34 mg/g, respectively), and significantly higher than those of the CK treatment by 23.4% and 24.8%, respectively, and significantly higher than those of 120 mM by 11.0% and 15.1%, respectively. The sucrose content increased with increases in stress concentration under 0~60 mM and was the highest under 60 mM (206.11 mg/g), which was significantly higher than the values of the control and 30 mM by 22.6 and 10.1%, respectively. Under 90~150 mM of stress, the sucrose content decreased with increases in stress concentration, but there were no significant differences among treatments. The content of soluble sugar was considered to be the sum of the three sugar components, and its content changed with the stress of saline–alkali in a trend consistent with those of fructose and glucose. At 90 mM, the soluble sugar content was the highest (387.52 mg/g), which was significantly higher than that of the CK treatment by 22.1%. At 30 mM of stress, the total soluble sugar content was higher than that at 60, 120, and 150 mM by 7.8%, but the difference was not significant. The sugar content changes in jujube fruit first increased and then decreased with increases in saline–alkali stress.

Table 3.
Differences in sugar component contents in jujube fruit under different saline stress conditions.
3.3. Comparison of Key Enzyme Activities of Sucrose Metabolism under Different Saline–Alkali Stress Treatments
The differences in the activities of five sucrose-metabolizing enzymes in jujube fruits under saline–alkali treatment are shown in Figure 3. Under low stress (0~90 mM), the activities of SPS, SS-I, and SS-II significantly increased with increases in stress. The activities of SPS and SS-I showed significant decreasing trends and SS-II showed a downward trend under 120 to 150 mM of saline–alkali stress, but the differences were not significant. The activity of SS-I was higher than that of SS-II. The NI activity increased with the increased stress, and there were no significant differences between treatments except for the CK treatment. The activity S-AI first decreased and then increased with increasing stress. When the stress was 90 mM, the activity of S-AI was the lowest (10.21 μg/min/g).
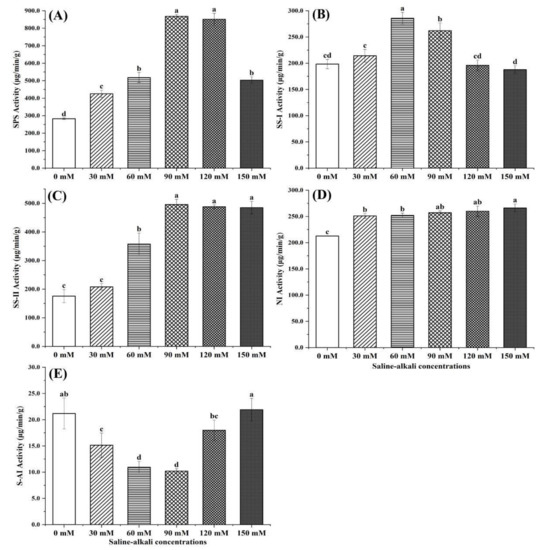
Figure 3.
Differences of the sucrose metabolic enzyme activities in jujube fruits under different saline–alkali stress conditions. (A) SPS activity; (B) SS-I activity; (C) SS-II activity; (D) NI activity; (E) S-AI activity. Note: values indicate the means ± SE, n = 3. Small letters indicate significant differences at a 5% level (p ≤ 0.05).
3.4. Effects of Different Saline–Alkali Stress Treatments on Gene Expression of Key Enzymes of Sucrose Metabolism in Jujube Fruit
11 genes and a reference gene (UBQ2) selected by Zhang [25] were used for study sugar accumulation in the jujube fruits under the saline–alkali treatment. As shown in Figure 4, the relative expression levels of 10 genes were all lower than those of the CK treatment, and only the expression level of the ZjSPS4 gene was higher than that of the CK treatment, which first increased and then decreased with increases in stress. The expression levels of the sucrose synthase genes ZjSS1, ZjSS2, and ZjSS3 and the invertase genes first increased and then decreased with increases in concentration (except for in the CK treatment), and when the stress was 90 mM, the expression levels were significantly higher than in other treatments. The expression of the ZjvINV2, ZjcINV3, ZjnINV1, and ZjnINV3 genes first decreased and then increased, and when the stress was 60 mM, the expression levels were significantly higher than in other saline treatments.
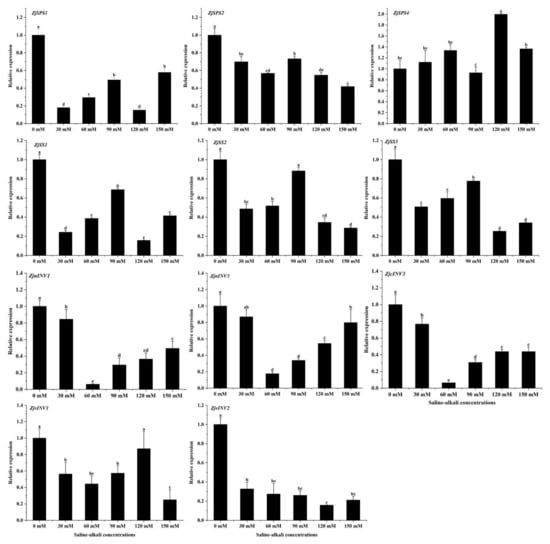
Figure 4.
Differences in key enzyme genes of sucrose metabolism in jujube fruits under saline–alkali stress. Note: values indicate the means ± SE, n = 3. Small letters indicate significant differences at a 5% level (p ≤ 0.05).
3.5. Effects of Different Saline–Alkali Treatments on Proline, Malondialdehyde, and Antioxidant Enzymes in Jujube Fruit
The effect of saline–alkali treatment on the antioxidant enzyme activity of jujube fruits is shown in Figure 5. The PRO content decreased with increases in saline–alkali stress and was significantly lower than that of the CK treatment (3.84 mg/g). The content at 30 mM (2.56 mg/g) was 38.7% higher than that of the CK treatment, but there were no significant differences among treatments. The SOD and MDA trends were similar, and there were no significant differences between the 30 mM (480.01 U/g) and CK treatments; the SOD content under 60~150 mM of stress was significantly higher than that under the 30 mM and CK treatments. The CAT content had the same trend as the PRO content, and its activity (223.57 umol/min/g) under the 30 mM treatment was 35.3% higher than that under the 150 mM treatment. The POD content first increased and then decreased with increases in salt stress. The POD content under the 60 mM treatment (46.59 U/g) was significantly higher than that under the CK and 150 mM treatments by 30.2% and 133.2%, respectively.
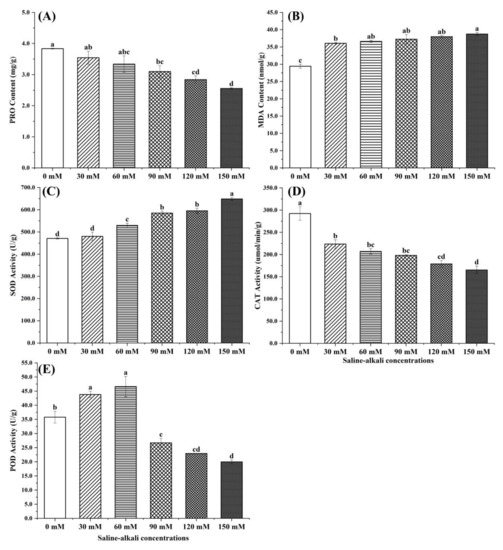
Figure 5.
Effects of different saline–alkali treatments on the contents of PRO (A), MDA (B), SOD (C), CAT (D), and POD (E) in jujube fruit. Note: values indicate the means ± SE, n = 3. Small letters indicate significant differences at a 5% level (p ≤ 0.05).
4. Discussion
As an osmotic regulator, sugar plays an important role in maintaining osmotic balance in response to saline–alkali stress. Sugar is an important indicator of fruit quality and an important osmotic regulator used by plants to resist stress, and their accumulation in plants experiencing salinity stress is an adaptive mechanism [26,27]. Sucrose, glucose, and fructose are the main soluble sugar components in fruit; they act as signaling molecules in plant metabolic processes and defense responses, and respond differently to stress. According to previous studies [28,29], in order to resist stress, plants can accumulate sugar to increase intracellular solute concentration, maintain osmotic balance and consequently normal cell turgor, prevent the excessive dehydration of protoplasts, and reduce the harm caused by stress, this is consistent with the response of this study under low salt stress (0~90 mM); however, excessive stress will lead to an imbalance of permeability and the inhibition of sugar accumulation [6,7,21], this is consistent with the inhibition of sugar accumulation under high salinity stress (120~150 mM) in this study.
Effects of SPS and SS on sugar synthesis and decomposition by expression of related genes under saline–alkali stress. The regulation of metabolic enzyme gene expression and metabolic enzyme activity determines the amount of sugar accumulation in fruit [30,31,32]. Under saline–alkali stress, the changes in sucrose content were mainly affected by SPS and SS-I activities, and the changes in fructose and glucose were mainly affected by SS-II activities, which shows that sugar accumulation during fruit ripening is closely related to SPS and SS under saline-alkali stress. A similar change was also observed in tomatoes [4,8], wolfberries [5], and figs [10]; meanwhile, the relative expression levels of genes also changed. The relative expression level of ZjSS1, ZjSS2, and ZjSS3 was consistent with the change of SS-I enzyme activity under saline–alkali stress, but ZjSPS1, ZjSPS2 and ZjSPS4 did not show significant trends consistent with the SPS enzyme; it may be that the regulation of SPS activity by SPS genes may be controlled by both positive and negative regulation of multiple genes in saline-alkali stress [30,33,34,35].
Invertases and related genes’ response to saline-alkali stress regulates sugar content in jujube fruit. As invertases, S-AI and NI also regulate sugar accumulation in response to abiotic stress [36]. In this study, the activity of NI were no significant differences under different treatments, and all significantly higher than control, indicating that NI was significantly affected by saline–alkali stress, but there was no significant concentration-effect [12,37,38]. The changes of S-AI activity with the stress concentration, contrary to the accumulation of fructose and glucose. These results show that saline–alkali stress regulates the conversion level of sucrose to fructose and glucose by S-AI, as well as controlling intracellular sugar accumulation and osmotic concentration to maintain osmotic balance [5,39]. In addition, the responses of ZjcINV3, ZjnINV1, and ZjnINV2 to stress were consistent with the trends of S-AI activity, which suggests that they could be crucial in regulating S-AI activity in response to saline–alkali stress; this is consistent with the result that the change of Hf VIN1 gene expression and VIN enzyme activity with a decrease in temperature stress [40], which indicates that plants need more invertases to decompose sucrose into hexose under low temperature, salinity and other stresses, to provide more energy for cells to maintain increased respiratory consumption and enhance resistance to stress [36,37,39]. Hydrolyze sucrose by IN in vacuole for tissue utilization, and down-regulation of gene expression can respond to the needs of sucrose decomposition under stress [39,40].
Response of antioxidant enzymes to salinity stress and regulation of sugar content in jujube fruits. Sugar metabolism is often accompanied by biological oxidation in plants with stress, and the protective mechanism of active oxygen in cells plays an important role in responding to stress [14]. Salinity stress can cause an imbalance of reactive oxygen species; (ROS), PRO, MDA, SOD, POD, and CAT are important indexes of the plant stress response, and they act as potent salinity mitigators [15,41]. In the present study, PRO and CAT presented similar activity decreases with increases in stress concentration. It has been reported that proline is an osmoprotectant and scavenger of free radicals in plant cells, accumulating in cells to protect osmotic balance [42,43]. In this connection, PRO decreased indicating that osmotic balance gradually weakened in saline–alkali stress. It is possible that with the increase of saline–alkali stress concentration, the membrane permeability is destroyed, the proline anabolism in the jujube fruit is unbalanced, and the resistance to salinity gradually weakens [44,45]. The inhibition of CAT activity during saline stress is related to the destruction of cell metabolism and structure [46]. In this study, CAT activity was found to be restrained by the different saline–alkali stress concentrations in jujube fruit, which may be caused irreversible damage to the structure and function of CAT proteins [47].
The MDA level directly reflects the degree of cell membrane damage and the level of lipid peroxidation [47,48]; this elevation of MDA occurrence might be due to the impairment of the cell membrane, reduction of lipid peroxidation level insufficient activity of enzymatic antioxidant [49]. In the current experiment, the MDA content increased gradually in a concentration-dependent manner in the “Junzao” jujube fruit under saline–alkali stress, which indicates that membranes were increasingly severely damaged with increasing saline–alkali stress. A similar increase in MDA was also observed in Arabidopsis [50], tomato [51,52], wheat [53], maize [54], tobacco [55] with salt stress.
SOD content could reflect the antioxidant capacity of plants, and increases in activity are usually related to plant tolerance [41,47]. In order to resist saline–alkali stress, the jujube fruit can prevent cell damage by enhancing SOD activity, scavenging free radicals, and maintaining the metabolic balance of reactive oxygen [56,57]. The MDA and SOD presented similar activity increases with increasing saline–alkali stress, which indicates that the level of SOD under salt-alkali stress is closely related to the damage degree reflected by MDA to stress [47,56,58].
As a defensive enzyme, POD is mainly involved in ROS scavenging, and its activity is related to plant tolerance [41]. In this study, the change in POD activity was consistent with the response of sucrose in jujube fruits. Under low saline–alkali stress, the ability of POD to scavenge excessive ROS in cells may be closely related to sugar accumulation; however, the protective effect of the antioxidant enzyme system was limited [38,57]. Saline–alkali stress can lead to decreased enzyme activity when it reaches the minimum tolerance limit of plants (60 mM), at which point their defense mechanism is destroyed and POD enzyme activity decreases [38,41].
The concentration effect of stress on sugar accumulation is reflected in the enzyme activity and antioxidase. The responses of MDA, SOD, and NI to different saline–alkali stresses were consistent in this study, indicating that the hydrolysis of sucrose by NI to fructose and glucose was closely related to the degree of lipid peroxidation damage and the stress resistance of cell membrane [37,38]; this could be because that the responses of these antioxidase and soluble sugars to saline–alkali stress play roles in maintaining cellular redox homeostasis, promoting sugar accumulation, and maintaining osmotic material balance [44,56].
Stress has a concentration effect on sugar accumulation in jujube fruit. Previous studies have demonstrated that salt stress affects carbohydrate contents in the fruit, depending on the magnitude of stress [10,17]. Moderate salt stress can improve the soluble solids, or soluble sugar content in fruit [4,5,6,7,8,9], but the sugar content of grapes (100~150mM) [6,7], figs (100 mM) [10], tomatoes (0.3%) [8], and Lycium chinensis (0.9%) [5] were inhibited at the high saline stress. Similar results were obtained in this study, and under 90 mM saline–alkali stress treatment, the content of fructose, glucose, soluble total sugar, and the activity of SPS, SS-II, and POD in jujube fruit reaches the highest; meanwhile, there is still no extensive research on that activity of enzymes and expression of genes in response to saline–alkali stress and affect sugar accumulation in fruit [9,10,13], especially in jujube fruit. The change of S-AI and ZjcINV3, ZjnINV1, ZjnINV2 showed an opposite trend compared with the increase in sugar in this study. It has been evidenced that the transcript levels and regulation of invertases play an important role in regulating sugar accumulation in response to stress [10,11,13,37,38,39,40], which suggests a different transcriptional level of invertase-encoding genes resulting in sugar accumulation in the fruit.
5. Conclusions
In summary, this research explored the effects of enzyme activities, gene expression, and oxidase markers on sugar accumulation in jujube fruit under saline–alkali stress. In “Junzao” fruit, low saline–alkali stress promoted the increase in soluble sugar content, and high saline–alkali stress inhibited the accumulation of sugar. Increases in SPS and SS-II activities and the inhibition of AI activities are the main reasons for the accumulation of sucrose, glucose, and fructose, while down-regulation of ZjcINV3, ZjnINV1, and ZjnINV3 may be the key genes on sugar accumulation in fruit. The activity of POD reflects the ability of “Junzao” fruit to resist saline–alkali stress by promoting sugar accumulation and maintaining osmotic balance. Studying the relationship between environmental factors and sugar accumulation of jujube could provide a theoretical basis for the ecological regulation of fruit sugars’ metabolism, as well as provide relevant references for explaining the influence of salinized soil on jujube fruit quality in Xinjiang.
Author Contributions
Y.W.: data curation, methodology, formal analysis, writing—original draft, and writing—review and editing. Y.F.: methodology and writing—review and editing. M.Y.: investigation, and formal analysis. J.Y. and X.Z.: methodology, project administration, and supervision. J.B.: writing—review, and editing. Q.Z.: funding acquisition and supervision. C.W.: funding acquisition, conceptualization, project administration, supervision, writing—original draft, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Major scientific and technological projects of XPCC (2017DB006); Innovation and Entrepreneurship Platform and Base Construction Project of XPCC (2019CB001); and Scientific Research Innovation Project of postgraduate in Xinjiang Autonomous Region (XJ2020G270).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data generated or analyzed during this study are included in this published article.
Acknowledgments
The authors would like to thank the University of Tarim for supporting the project.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| SPS | sucrose phosphate synthase |
| SS-I | Sucrose synthase decomposition direction |
| SS-II | Sucrose synthase synthesis direction |
| NI | Neutral invertase |
| S-AI | Soluble acid invertase |
| PRO | Proline |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| POD | Peroxidase |
| ROS | Reactive oxygen species |
References
- Wei, S.; Hongtao, D.; Yanfeng, Z.; Cun, W.; Hairong, L.; Dengke, L. Problems and countermeasures of Chinese jujube industry in Aksu area. J. Fruit Resour. 2021, 2, 84–87. (In Chinese) [Google Scholar]
- Chen, T.; Zhang, Z.; Li, B.; Qin, G.; Tian, S. Molecular basis for optimizing sugar metabolism and transport during fruit development. aBIOTECH 2021, 2, 330–340. [Google Scholar] [CrossRef]
- Hao, Y. Developing and utilizing saline-alkali land to produce high quality fruit. Chin. Fruit Ind. Inf. 2013, 30, 30–31. (In Chinese) [Google Scholar]
- Ladewig, P.; Trejo-TéLlez, L.I.; Servin-Juarez, R.; Contreras-Oliva, A.; Gomez-Merino, F.C. Growth, yield and fruit quality of Mexican tomato landraces in response to salt stress. Not. Bot. Horti Agrobot. Cluj-Napoca. 2021, 49, 12005. [Google Scholar] [CrossRef]
- Juan, Y.; Xing, X.; Yuqing, W.; Rongxia, Z. Sugar sand sucrose-metabolizing enzymesin fruits of Lycium barbarum under salt stress. J. Ningxia Agric. Coll. 2004, 25, 28–31. (In Chinese) [Google Scholar]
- Li, X.L.; Wang, C.R.; Li, X.Y.; Yao, Y.X.; Hao, Y.J. Modifications of Kyoho grape berry quality under long-term NaCl treatment. Food Chem. 2013, 139, 931–937. [Google Scholar] [CrossRef]
- Sun, H.; Sun, T.-Y.; Xu, L.-L.; Du, Y.-P. Effects of the long-term treatment of low-concentrated salt on grape berry quality and transcriptome. Plant Physiol. J. 2017, 53, 2197–2205. (In Chinese) [Google Scholar] [CrossRef]
- Wang, W.; Cai, L.; Long, Z.; Zhang, X.; Zhao, F. Effects of non-uniform salt stress on growth, yield, and quality of tomato. Soil Sci. Plant Nutr. 2021, 67, 545–556. [Google Scholar] [CrossRef]
- Shaowei, L.; Fei, Q.; Tianlai, L. Effects of NaCl stress on photosynthetic characteristics and sucrose metabolism of tomato leaves. North. Hortic. 2012, 3, 14–18. (In Chinese) [Google Scholar] [CrossRef]
- Mascellani, A.; Natali, L.; Cavallini, A.; Mascagni, F.; Caruso, G.; Gucci, R.; Havlik, J.; Bernardi, R. Moderate salinity stress affects expression of main sugar metabolism and transport genes and soluble carbohydrate content in ripe fig fruits (Ficus carica L. cv. Dottato). Plants 2021, 10, 1861. [Google Scholar] [CrossRef]
- Guanglian, L.; Min, Z.; Chunhui, H.; Dongfeng, J.; Xiaobiao, X. Progress in research on sugar metabolism and related enzyme genes in fruit. Acta Agric. Univ. Jiangxiensis 2020, 42, 187–195. [Google Scholar] [CrossRef]
- Qijun, M. Molecular Mechanism by which Apple Sucrose Transporter MdSUT2.2 Involves in Regulating Sugar Content in Response to Drought and Salt Stresses. Doctor’s Thesis, Shandong Agricultural University, Taian, China, 2018. (In Chinese). [Google Scholar]
- Gao, Z.; Sagi, M.; Lips, S.H. Carbohydrate metabolism in leaves and assimilate partitioning in fruits of tomato (Lycopersicon esculentum L.) as affected by salinity. Plant Sci. 1998, 135, 149–159. [Google Scholar] [CrossRef]
- Alhasnawi, A.N.; Kadhimi, A.A.; Isahak, A.; Mohamad, A.; Doni, F.; Mohtar, W.; Yusoff, W.; Zain, C.R.B.M. Salinity stress in plant and an important antioxidant enzyme. Life Sci. J. 2014, 11, 913–920. [Google Scholar] [CrossRef]
- Ali, A.; Yun, D. Salt stress tolerance; what do we learn from halophytes? J. Plant Biol. 2017, 60, 431–439. [Google Scholar] [CrossRef]
- Arif, Y.; Singh, P.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salinity induced physiological and biochemical changes in plants: An omic approach towards salt stress tolerance. Plant Physiol. Biochem. 2020, 156, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Zhang, Y.J.; Li, Z.; Bai, D.F.; Zhong, Y.P.; Fang, J.B. Effect of salt stress on growth, physiological and biochemical characters of four kiwifruit genotypes. Sci. Hortic. 2020, 271, 109473. [Google Scholar] [CrossRef]
- Jia, X.; Wang, H.; Svetla, S.; Zhu, Y.F.; Hu, Y.; Cheng, L.; Zhao, T.; Wang, Y.X. Comparative physiological responses and adaptive strategies of apple Malus halliana to salt, alkali and saline-alkali stress. Sci. Hortic. 2019, 245, 154–162. [Google Scholar] [CrossRef]
- Jia, X.; Zhu, Y.; Zhang, R.; Zhu, Z.; Zhao, T.; Cheng, L.; Gao, L.; Liu, B.; Zhang, X.; Wang, Y. Ionomic and metabolomic analyses reveal the resistance response mechanism to saline-alkali stress in Malus halliana seedlings. Plant Physiol. Biochem. 2020, 147, 77–90. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Liu, Y.; Yang, S.; Zhang, J.; Gong, X.; Ma, F. Overexpression of MdMIPS1 enhances salt tolerance by improving osmosis, ion balance, and antioxidant activity in transgenic apple. Plant Sci. 2020, 301, 110654. [Google Scholar] [CrossRef]
- Jie, W.; He-Li, W.; Cui-Yun, W.; Zhang, Q.; Jiang, Y.; Xiang-Yu, L. Effects of mixed salt-alkali stress on the internal quality of Zizyphus jujuba ‘Huizao’. Agric. Res. Arid Areas 2015, 33, 144–147. (In Chinese) [Google Scholar]
- Soltabayeva, A.; Ongaltay, A.; Omondi, J.O.O.; Srivastava, S. Morphological, Physiological and Molecular Markers for Salt-Stressed Plants. Plants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Ding, T.; Wang, W.; Xiang, Y.; Ye, X.; Li, M.; Liu, D. Effect of harvest, drying and storage on the bitterness, moisture, sugars, free amino acids and phenolic compounds of jujube fruit (Zizyphus jujuba cv. Junzao). J. Sci. Food Agric. 2018, 98, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Chunmei, Z. Molecular Mechanism Related to Themetabolism of Sugar, Acid and Domestication for Ziziphus Jujuba Mill. Doctor’s Thesis, Northwest Agriculture & Forestry University, Yangling, Shanxi, China, 2016. [Google Scholar]
- Tao, H.; Gexiang, Z.; Fuchao, Z.; Yu, C. Research progress in plant salt stress response. Mol. Plant Breed. 2018, 16, 3006–3015. (In Chinese) [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J. Abiotic stress signaling and responses in plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Lastdrager, J.; Hanson, J.; Smeekens, S. Sugar signals and the control of plant growth and development. J. Exp. Bot. 2014, 65, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Zhu, X.; Jia, H.; Wang, C. Research advances on physiological function of plant sucrose synthase. J. Nanjing Agric. Univ. 2017, 40, 759–768. (In Chinese) [Google Scholar]
- Li, J.; Wu, L.; Foster, R.; Ruan, Y.L. Molecular regulation of sucrose catabolism and sugar transport for development, defence and phloem function. J. Integr. Plant Biol. 2017, 59, 322–335. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Li, T.; Jiang, J. Effects of salinity on sucrose metabolism during tomato fruit development. Afr. J. Biotechnol. 2010, 9, 842–849. [Google Scholar] [CrossRef]
- Lu, S.; Li, T.; Jiang, J. Tomato key sucrose metabolizing enzyme activities and gene expression under NaCl and PEG iso-osmotic stresses. Agric. Sci. China. 2009, 8, 1046–1052. [Google Scholar] [CrossRef]
- Na, C.; DongDong, H.; Daoyuan, W.; Lijuan, P.; Xiaoyuan, C.; Ming, C.; Tong, W.; Mian, W.; Zhen, Y.; Shanlin, Y. Expression analysis of the sucrose synthase gene AhSuSy in different tissue and under abiotic stresses in peanut. J. Peanut Sci. 2013, 42, 25–32. (In Chinese) [Google Scholar]
- Jietang, Z. Advances in research on invertase in plant development and response to abiotic and biotic stresses. J. Trop. Subtrop. Bot. 2016, 24, 352–358. (In Chinese) [Google Scholar]
- Dahro, B.; Wang, F.; Peng, T.; Liu, J.-H. PtrA/NINV, an alkaline/neutral invertase gene of Poncirus trifoliata, confers enhanced tolerance to multiple abiotic stresses by modulating ROS levels and maintaining photosynthetic efficiency. BMC Plant Biol. 2016, 16, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-W.; Nie, Y.-X.; Wan, Y.-Y.; Chen, S.-Y.; Sun, Y.; Wang, X.-J.; Bai, J.-G. Exogenous glucose regulates activities of antioxidant enzyme, soluble acid invertase and neutral invertase and alleviates dehydration stress of cucumber seedlings. Sci. Hortic. 2013, 162, 20–30. [Google Scholar] [CrossRef]
- Koch, K. Sucrose metabolism: Regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 2004, 7, 235–246. [Google Scholar] [CrossRef]
- Lu, B.; Zhiguo, Z.; Shijie, Z.; Dongmei, H.; Qiaoping, Q. Solation of three types of invertase genes from Hemerocallis fulva and their responses to low temperature and osmotic stress. Acta Hortic. Sin. 2021, 48, 300–312. (In Chinese) [Google Scholar] [CrossRef]
- Yang, S.Y.; Chen, X.Y.; Hui, W.K.; Ren, Y.; Ma, L. Progress in responses of antioxidant enzyme systems in plant to environmental stresses. J. Fujian Agric. For. Univ. 2016, 45, 481–489. (In Chinese) [Google Scholar] [CrossRef]
- Arteaga, S.; Yabor, L.; Díez, M.J.; Prohens, J.; Boscaiu, M.; Vicente, O. The use of proline in screening for tolerance to drought and salinity in common bean (Phaseolus vulgaris L.) genotypes. Agronomy 2020, 10, 817. [Google Scholar] [CrossRef]
- Yu, Z. Effects of Exogenous Proline on Growth and Proline Metabolism of Trifoliate Orange Rootstock under Boron Stress. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a multifaceted signalling molecule in plant responses to abiotic stress: Understanding the physiological mechanisms. Plant Biol. 2021, 24, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Naliwajski, M.; Skłodowska, M. The Relationship between the Antioxidant System and Proline Metabolism in the Leaves of Cucumber Plants Acclimated to Salt Stress. Cells 2021, 10, 609. [Google Scholar] [CrossRef]
- Sanoubar, R.; Cellini, A.; Gianfranco, G.; Spinelli, F. Osmoprotectants and antioxidative enzymes as screening tools for salinity tolerance in radish (Raphanus sativus). Hortic. Plant J. 2020, 6, 14–24. [Google Scholar] [CrossRef]
- Mansour, M.M.F. The plasma membrane transport systems and adaptation to salinity. J. Plant Physiol. 2014, 171, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.K.; Saha, D.; Skalicky, M.; Mishra, U.N.; Chauhan, J.; Behera, L.P.; Lenka, D.; Chand, S.; Kumar, V.; Dey, P.; et al. Crucial Cell Signaling Compounds Crosstalk and Integrative Multi-Omics Techniques for Salinity Stress Tolerance in Plants. Front. Plant Sci. 2021, 12, 670369. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Sohag, A.A.M.; Afrin, S.; Bashar, K.K.; Afrin, T.; Mahamud, A.G.M.S.U.; Polash, M.A.S.; Hossain, M.T.; Sohel, M.A.T.; Brestic, M.; et al. Differential Response of Sugar Beet to Long-Term Mild to Severe Salinity in a Soil–Pot Culture. Agriculture 2019, 9, 223. [Google Scholar] [CrossRef] [Green Version]
- Ellouzi, H.; Ben Hamed, K.; Cela, J.; Munné-Bosch, S.; Abdelly, C. Early effects of salt stress on the physiological and oxidative status of Cakile maritima (halophyte) and Arabidopsis thaliana (glycophyte). Physiol. Plant. 2011, 142, 128–143. [Google Scholar] [CrossRef]
- Gapińska, M.; Skłodowska, M.; Gabara, B. Effect of short- and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots. Acta Physiol. Plant. 2008, 30, 11–18. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Vanden Langenberg, K.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Esfandiari, E.; Gohari, G. Response of ROS-Scavenging systems to salinity stress in two different wheat (Triticum aestivum L.) cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca. 2017, 45, 287–291. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Xia, Z. Overexpression of a maize SUMO conjugating enzyme gene (ZmSCE1e) increases Sumoylation levels and enhances salt and drought tolerance in transgenic tobacco. Plant Sci. 2019, 281, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Aleem, M.; Aleem, S.; Sharif, I.; Wu, Z.; Aleem, M.; Tahir, A.; Atif, R.M.; Cheema, H.M.N.; Shakeel, A.; Lei, S.; et al. Characterization of SOD and GPX Gene Families in the Soybeans in Response to Drought and Salinity Stresses. Antioxidants 2022, 11, 460. [Google Scholar] [CrossRef]
- Wang, C.; Wen, D.; Sun, A.; Han, X.; Zhang, J.; Wang, Z.; Yin, Y. Differential activity and expression of antioxidant enzymes and alteration in osmolyte accumulation under high temperature stress in wheat seedlings. J. Cereal Sci. 2014, 60, 653–659. [Google Scholar] [CrossRef]
- Rasel, M.; Tahjib-Ul-Arif, M.; Hossain, M.A.; Hassan, L.; Farzana, S.; Brestic, M. Screening of Salt-Tolerant Rice Landraces by Seedling Stage Phenotyping and Dissecting Biochemical Determinants of Tolerance Mechanism multidimensional roles in salt-stressed plants. J. Plant Growth Regul. 2020, 40, 1853–1868. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).