Exogenous Melatonin Enhances Cold Resistance by Improving Antioxidant Defense and Cold-Responsive Genes’ Expression in Banana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cold Treatment
2.2. Measurement of Chlorophyll Fluorescence Parameters
2.3. Measurement of O2− and H2O2 Contents, Total Antioxidant Capacity and Antioxidant Enzyme Activities
2.4. Measurement of MDA, Proline and Soluble-Sugar Contents
2.5. Expression Analysis of Cold-Responsive Genes Using Quantitative Real-Time PCR (qRT-PCR)
2.6. Statistical Analysis
3. Results
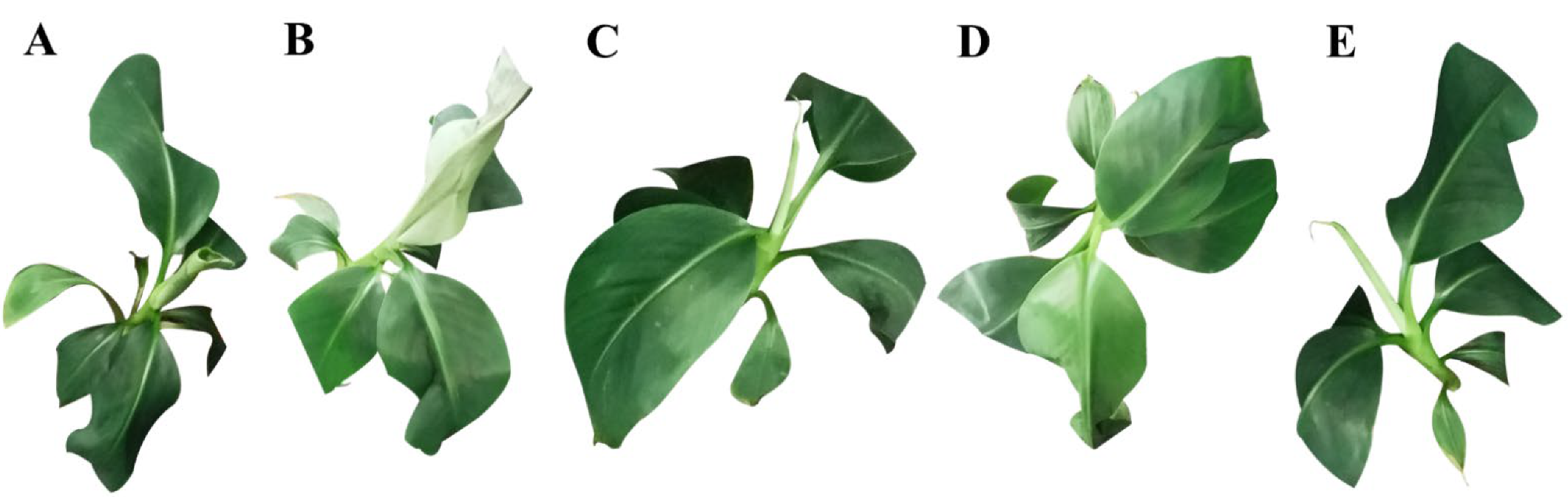
3.1. Effects of Different Concentrations of Melatonin on the Cold Resistance of Banana Seedlings
3.2. Melatonin Treatment Alleviates the Symptoms Caused by Cold Stress in Banana
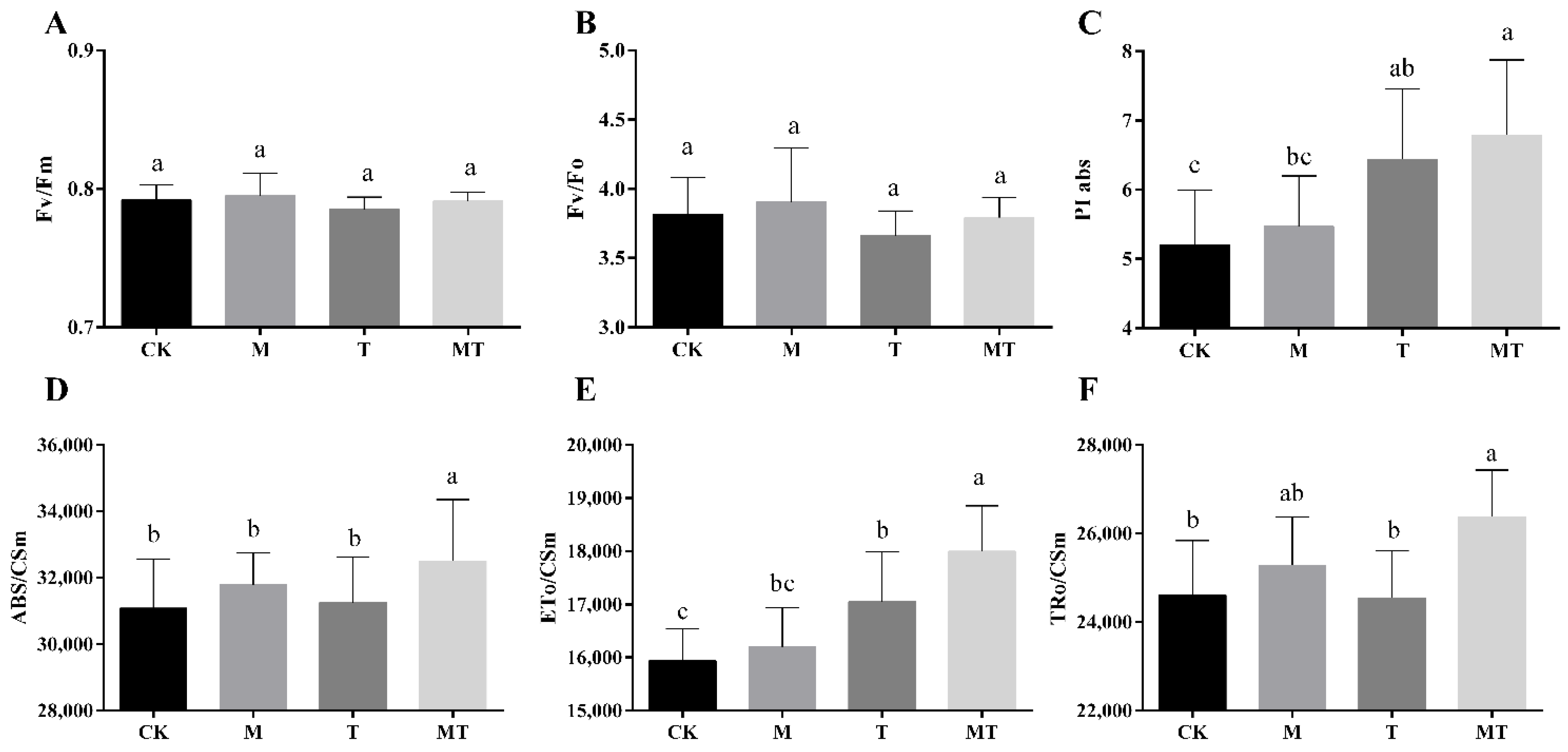
3.3. Effects of Melatonin on the Chlorophyll Fluorescence Parameters of Banana
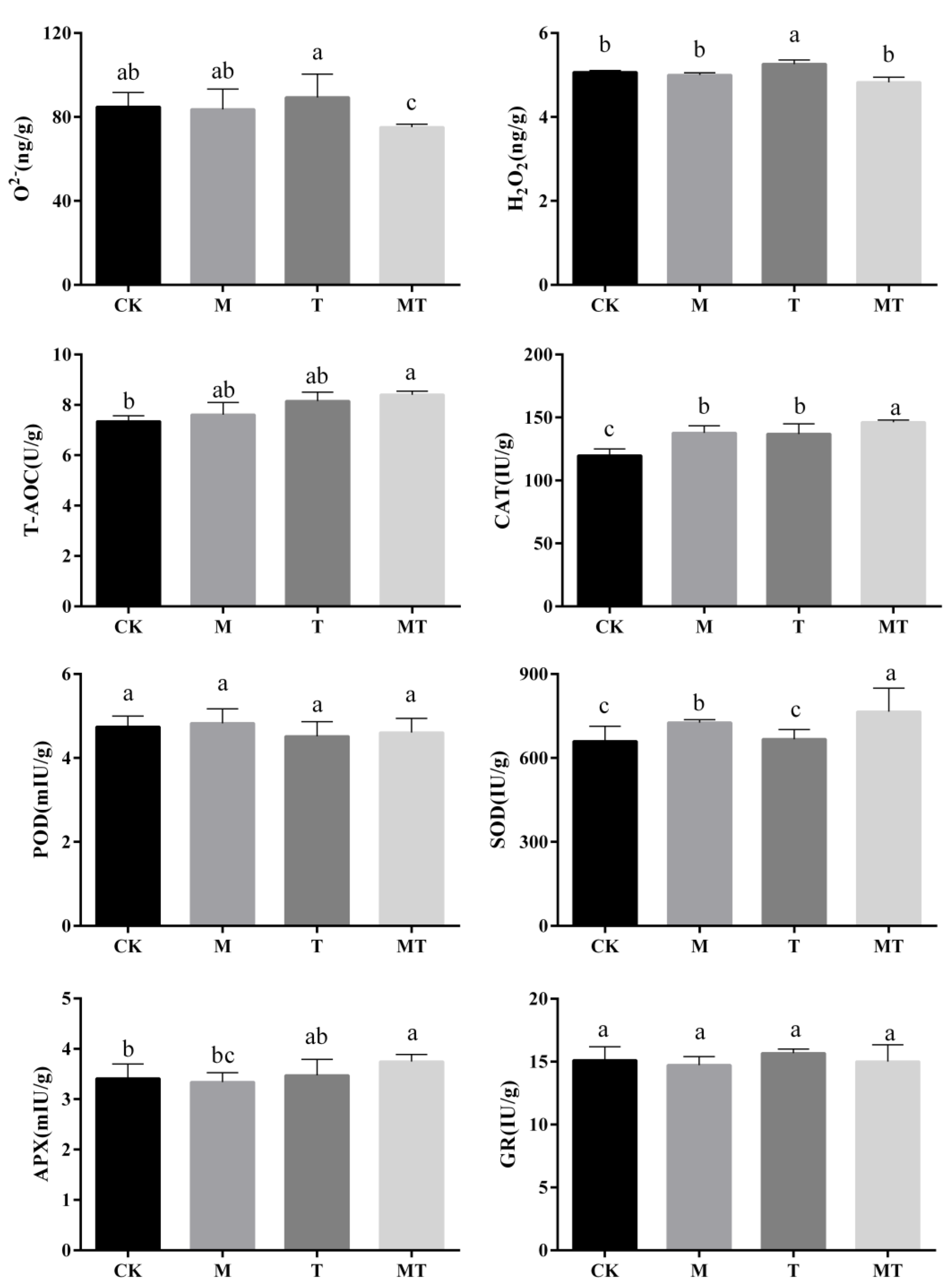
3.4. Influences of Melatonin on the O2− and H2O2 Contents and Total Antioxidant Capacity (T-AOC) in Banana Leaves
3.5. Influences of Melatonin on the Antioxidant Enzyme Activities in Banana Leaves
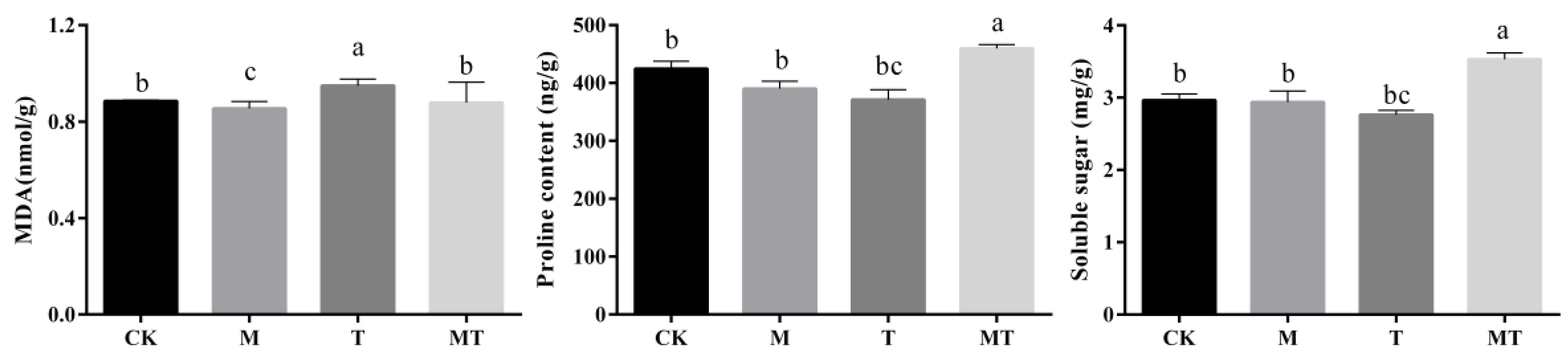
3.6. Effects of Melatonin on Osmoregulatory Substance Accumulations
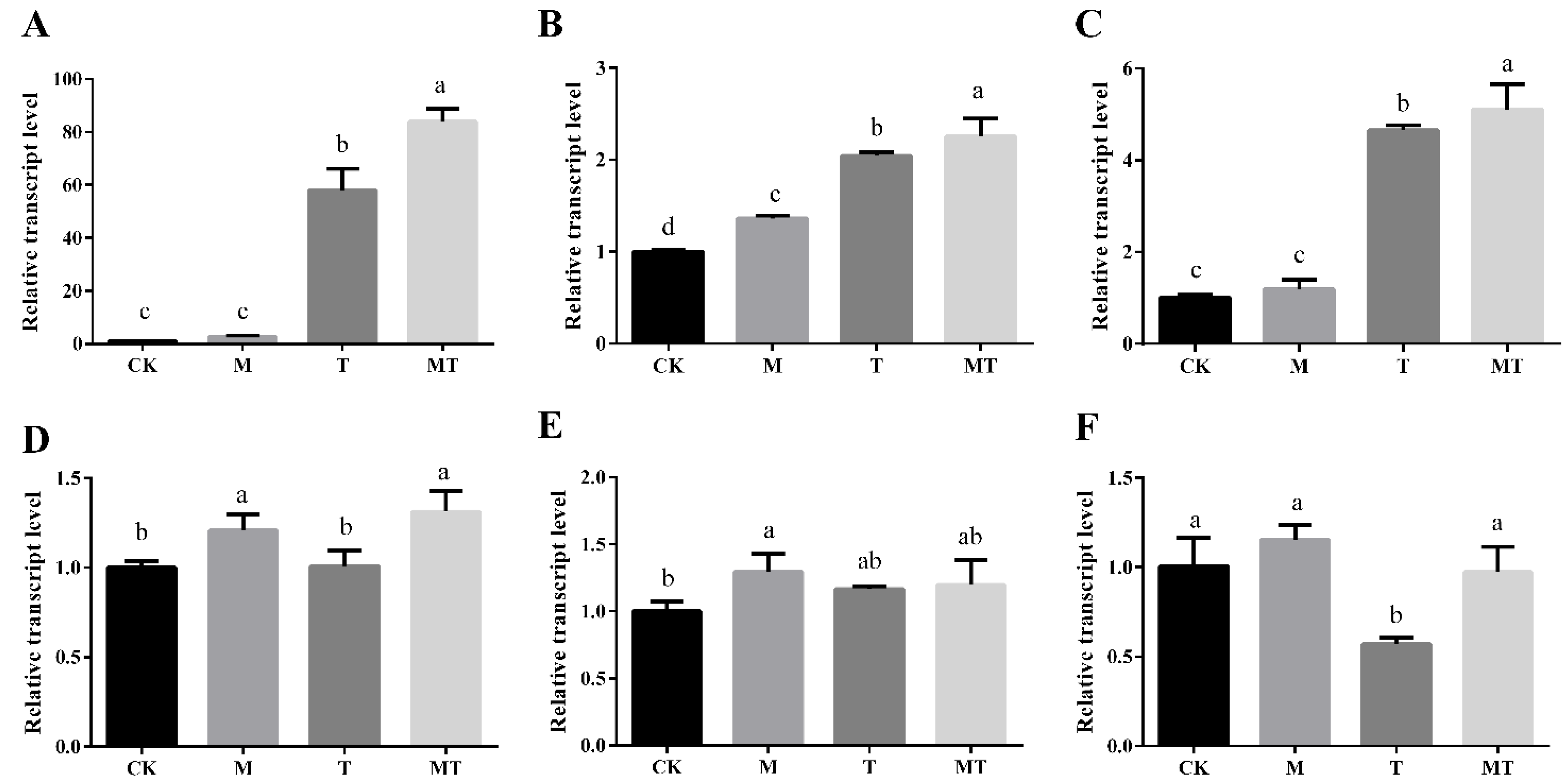
3.7. Influences of Melatonin on the Expression of Cold-Responsive Genes in Banana
4. Discussion
4.1. Application of Exogenous Melatonin Enhanced the Photosynthesis Ability of Banana under Cold Stress
4.2. Exogenous Melatonin Improves Banana Cold Resistance by Enhancing the Antioxidant Defense of Banana
4.3. Exogenous Melatonin Induces the Expression of Cold-Responsive Genes to Reprogram Banana Cold Responses
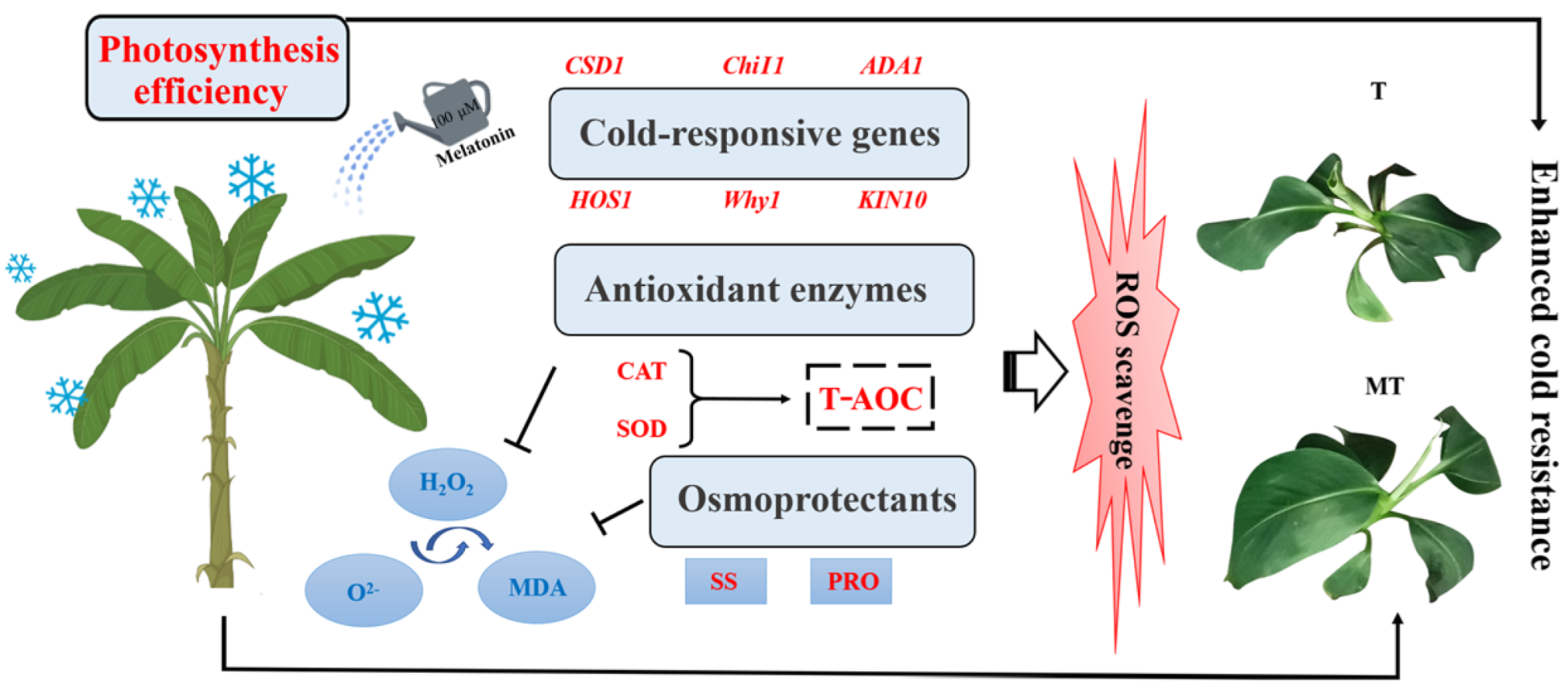
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Theocharis, A.; Clément, C.; Barka, E.A. Physiological and molecular changes in plants grown at low temperatures. Planta 2012, 235, 1091–1105. [Google Scholar] [CrossRef]
- Hu, W.H.; Wu, Y.; Zeng, J.Z.; He, L.; Zeng, Q.M. Chill-induced inhibition of photosynthesis was alleviated by 24-epibrassinolide pretreatment in cucumber during chilling and subsequent recovery. Photosynthetica 2010, 48, 537–544. [Google Scholar] [CrossRef]
- Møller, I.M. Plant mitochondria and oxidative stress: Electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu. Rev. Plant Biol. 2001, 52, 561–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halliwell, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, T.A.; Fariduddin, Q.; Yusuf, M. Low-temperature stress: Is phytohormones application a remedy? Environ. Sci. Pollut. Res. 2017, 24, 21574–21590. [Google Scholar] [CrossRef] [PubMed]
- Bauerfeind, M.A.; Winkelmann, T.; Franken, P.; Druege, U. Transcriptome, carbohydrate, and phytohormone analysis of Petunia hybrida reveals a complex disturbance of plant functional integrity under mild chilling stress. Front. Plant Sci. 2015, 6, 583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152. [Google Scholar] [CrossRef]
- Shi, H.T.; Ye, T.T.; Zhong, B.; Liu, X.; Chan, Z.L. Comparative proteomic and metabolomic analyses reveal mechanisms of improved cold stress tolerance in bermudagrass (Cynodon dactylon (L.) Pers.) by exogenous calcium. J. Integr. Plant Biol. 2014, 56, 1064–1079. [Google Scholar] [CrossRef]
- Hu, Z.R.; Fan, J.B.; Xie, Y.; Amombo, E.; Liu, A.; Gitau, M.M.; Khaldun, A.; Chen, L.; Fu, J.M. Comparative photosynthetic and metabolic analyses reveal mechanism of improved cold stress tolerance in bermudagrass by exogenous melatonin. Plant Physiol. Biochem. 2016, 100, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Yang, Y.Q.; Sun, K.; Chen, Y.; Chen, X.; Li, X.H. Exogenous melatonin enhances cold, salt and drought stress tolerance by improving antioxidant defense in tea plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’hont, A.; Denoeud, F.; Aury, J.M.; Baurens, F.C.; Carreel, F.; Garsmeur, O.; Noel, B.; Bocs, S.; Droc, G.; Rouard, M.; et al. The banana (Musa acuminata) genome and the evolution of monocotyledonous plants. Nature 2012, 488, 213–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Bodjrenou, D.; Zhang, S.; Wang, B.; Pan, H.; Yeh, K.W.; Lai, Z.X.; Cheng, C.Z. The endophytic fungus Piriformospora indica reprograms banana to cold resistance. Int. J. Mol. Sci. 2021, 22, 4973. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Cheng, C.Z.; Chen, F.; Ni, S.S.; Lin, Y.L.; Lai, Z.X. High-throughput sequencing of small RNAs revealed the diversified cold-responsive pathways during cold stress in the wild banana (Musa itinerans). BMC Plant Biol. 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H. Development report and situation forecast of banana industry in 2015. World Trop. Agric. Inf. 2016, 8, 31–37. [Google Scholar]
- Wang, F.; Guo, J.; Ke, Y.; Li, C. China banana industry development report in 2016 and development trend in 2017. China Trop. Agric. 2017, 3, 25–29. (In Chinese) [Google Scholar]
- Liu, W.H.; Cheng, C.Z.; Lai, G.T.; Lin, Y.L.; Lai, Z.X. Molecular cloning and expression analysis of KIN10 and cold-acclimation related genes in wild banana ‘Huanxi’ (Musa itinerans). SpringerPlus 2015, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.S.; Wu, J.H.; Li, C.Y.; Wei, Y.R.; Sheng, O.; Hu, C.H.; Kuang, R.B.; Huang, Y.H.; Peng, X.X.; McCardle, J.A.; et al. Quantitative proteomic analysis reveals that antioxidation mechanisms contribute to cold tolerance in plantain (Musa paradisiaca L.; ABB Group) seedlings. Mol. Cell. Proteom. 2012, 11, 1853–1869. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Lai, Z.X.; Lin, Y.L.; Lai, G.T.; Lian, C.L. Genome-wide identification and characterization of the superoxide dismutase gene family in Musa acuminata cv. Tianbaojiao (AAA group). BMC Genom. 2015, 16, 823. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Yang, H.; Tie, W.W.; Yan, Y.; Ding, Z.H.; Liu, Y.; Wu, C.L.; Wang, J.S.; Reiter, R.J.; Tan, D.X.; et al. Natural variation in banana varieties highlights the role of melatonin in postharvest ripening and quality. J. Agric. Food Chem. 2017, 65, 9987–9994. [Google Scholar] [CrossRef]
- Li, T.T.; Wu, Q.X.; Zhu, H.; Zhou, Y.J.; Jiang, Y.; Gao, H.J.; Yun, Z. Comparative transcriptomic and metabolic analysis reveals the effect of melatonin on delaying anthracnose incidence upon postharvest banana fruit peel. BMC Plant Biol. 2019, 19, 289. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.L.; Xu, H.; Li, D.; Gao, X.; Li, T.L.; Wang, R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica 2018, 56, 884–892. [Google Scholar] [CrossRef]
- Cheng, C.Z.; Li, D.; Qi, Q.; Sun, X.L.; Anue, M.R.; David, B.M.; Zhang, Y.Y.; Hao, X.; Zhang, Z.H.; Lai, Z.X. The root endophytic fungus Serendipita indica improves resistance of banana to Fusarium oxysporum f. sp. cubense tropical race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Cheng, C.Z.; Liu, F.; Sun, X.L.; Wang, B.; Liu, J.P.; Ni, X.T.; Hu, C.H.; Deng, G.M.; Tong, Z.; Zhang, Y.; et al. Genome-wide identification of FAD gene family and their contributions to the temperature stresses and mutualistic and parasitic fungi colonization responses in banana. Int. J. Biol. Macromol. 2022, 204, 661–676. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhong, H.Y.; Kuang, J.F.; Li, J.G.; Lu, W.J.; Chen, J.Y. Validation of reference genes for RT-qPCR studies of gene expression in banana fruit under different experimental conditions. Planta 2011, 234, 377. [Google Scholar] [CrossRef] [PubMed]
- Aghdam, M.S.; Jannatizadeh, A.; Nojadeh, M.S.; Ebrahimzadeh, A. Exogenous melatonin ameliorates chilling injury in cut anthurium flowers during low temperature storage. Postharvest Biol. Technol. 2019, 148, 184–191. [Google Scholar] [CrossRef]
- Hayat, F.; Sun, Z.X.; Ni, Z.J.; Iqbal, S.; Xu, W.Y.; Gao, Z.H.; Qiao, Y.S.; Tufail, M.A.; Jahan, M.S.; Khan, U.; et al. Exogenous melatonin improves cold tolerance of strawberry (Fragaria × ananassa Duch.) through modulation of DREB/CBF-COR pathway and antioxidant defense system. Horticulturae 2022, 8, 194. [Google Scholar] [CrossRef]
- Ehlert, B.; Hincha, D.K. Chlorophyll fluorescence imaging accurately quantifies freezing damage and cold acclimation re-sponses in Arabidopsis leaves. Plant Methods 2008, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Oxborough, K. Imaging of chlorophyll a fluorescence: Theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. J. Exp. Bot. 2004, 55, 1195–1205. [Google Scholar] [CrossRef]
- Barbagallo, R.P.; Oxborough, K.; Pallett, K.E.; Baker, N.R. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiol. 2003, 132, 485–493. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Sun, Q.Q.; Zhang, H.J.; Cao, Y.Y.; Weeda, S.; Ren, S.X.; Guo, Y.D. Roles of melatonin in abiotic stress resistance in plants. J. Exp. Bot. 2015, 66, 647–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, D.X.; Manchester, L.C.; Di Mascio, P.; Martinez, G.R.; Prado, F.M.; Reiter, R.J. Novel rhythms of N1-acetyl-N2-formyl-5-methoxykynuramine and its precursor melatonin in water hyacinth: Importance for phytoremediation. FASEB J. 2007, 21, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.H.; Liang, D.; Li, C.; Ma, F.W.; Yue, Z.Y. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef]
- Zhang, H.M.; Zhang, Y.Q. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014, 57, 131–146. [Google Scholar] [CrossRef]
- Khazaei, M.; Maali Amiri, R.; Taleei, A.; Ramezanpour, S. Differential transcript accumulation of dhydrin and beta-glucosidase genes to cold-induced oxidative stress in chickpea. J. Agric. Sci. Technol. Iran 2015, 17, 725–734. [Google Scholar]
- Fan, J.B.; Hu, Z.R.; Xie, Y.; Chan, Z.L.; Chen, K.; Amombo, E.; Chen, L.; Fu, J.M. Alleviation of cold damage to photosystem II and metabolisms by melatonin in bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Chang, J.J.; Chen, H.J.; Wang, Z.Y.; Gu, X.R.; Wei, C.H.; Zhang, Y.; Ma, J.; Yang, J.X.; Zhang, X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Zhao, H.L.; Ye, L.; Wang, Y.P.; Zhou, X.T.; Yang, J.W.; Wang, J.W.; Cao, K.; Zou, Z.R. Melatonin increases the chilling tolerance of chloroplast in cucumber seedlings by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. Front. Plant Sci. 2016, 7, 1814. [Google Scholar] [CrossRef] [Green Version]
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Wu, H.; Wang, B.; Zhang, Y.; Wang, J.; Cheng, C.; Huang, Y. Exogenous Melatonin Enhances Cold Resistance by Improving Antioxidant Defense and Cold-Responsive Genes’ Expression in Banana. Horticulturae 2022, 8, 260. https://doi.org/10.3390/horticulturae8030260
Liu J, Wu H, Wang B, Zhang Y, Wang J, Cheng C, Huang Y. Exogenous Melatonin Enhances Cold Resistance by Improving Antioxidant Defense and Cold-Responsive Genes’ Expression in Banana. Horticulturae. 2022; 8(3):260. https://doi.org/10.3390/horticulturae8030260
Chicago/Turabian StyleLiu, Jiapeng, Huan Wu, Bin Wang, Yongyan Zhang, Jiashui Wang, Chunzhen Cheng, and Yuji Huang. 2022. "Exogenous Melatonin Enhances Cold Resistance by Improving Antioxidant Defense and Cold-Responsive Genes’ Expression in Banana" Horticulturae 8, no. 3: 260. https://doi.org/10.3390/horticulturae8030260
APA StyleLiu, J., Wu, H., Wang, B., Zhang, Y., Wang, J., Cheng, C., & Huang, Y. (2022). Exogenous Melatonin Enhances Cold Resistance by Improving Antioxidant Defense and Cold-Responsive Genes’ Expression in Banana. Horticulturae, 8(3), 260. https://doi.org/10.3390/horticulturae8030260





