Genotype-Dependent Antioxidative Response of Four Sweet Pepper Cultivars to Water Deficiency as Affected by Drought-Tolerant Bacillus safensis SS-2.7 and Bacillus thuringiensis SS-29.2 Strains
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Selection and Characterization for Plant-Growth Promoting (PGP) Treats
2.2. Plant Material and Overall Experimental Design
2.3. Antioxidant Enzymes Activity Assays
2.4. Determination of Relative Water Content—RWC
2.5. Determination of Lipid Peroxidation and Hydrogen Peroxide Levels
2.6. Statistical Analysis
3. Results
3.1. Strain Selection and Plant Growth Promotion (PGP) Trait Analysis
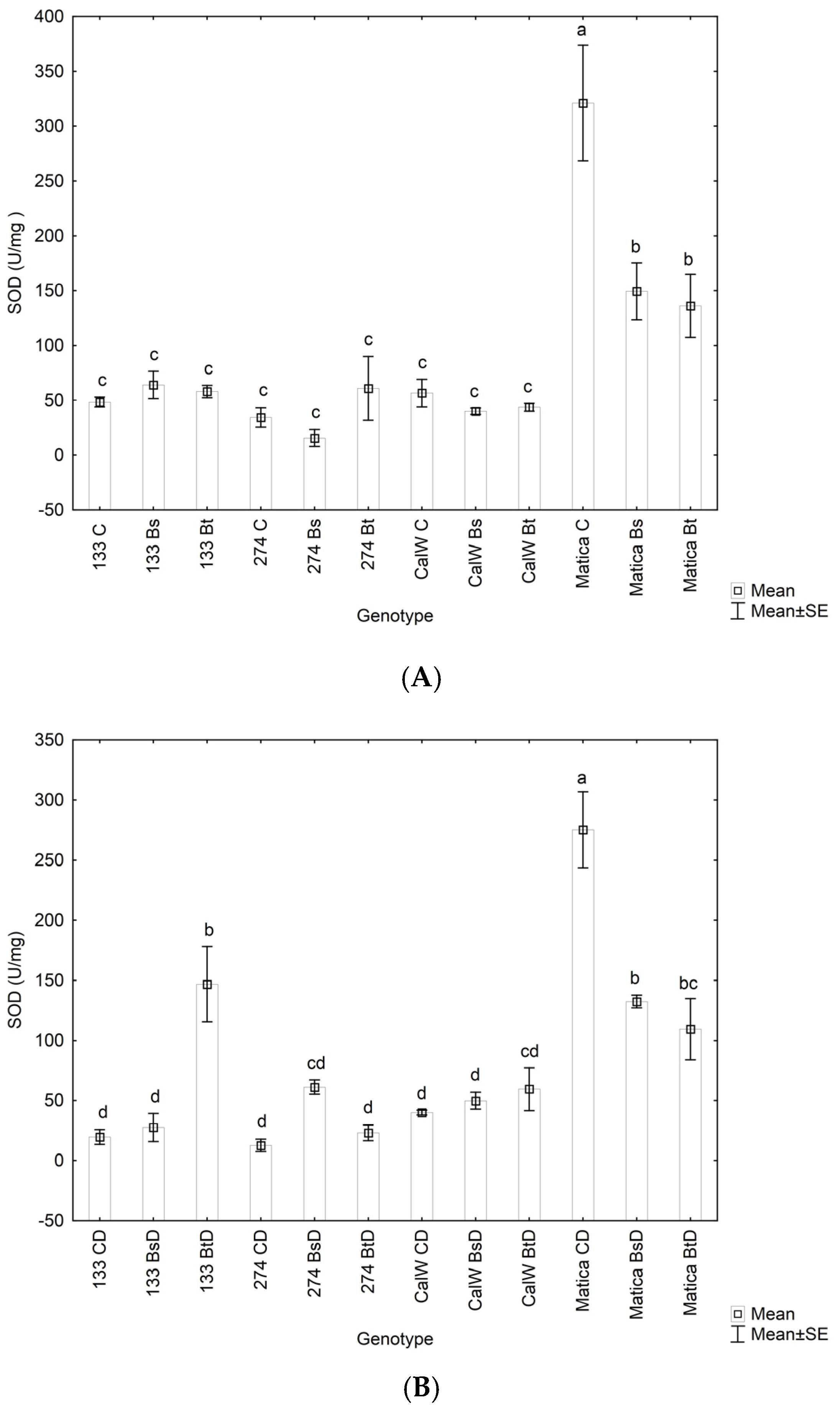
3.2. Antioxidant Enzyme Activities
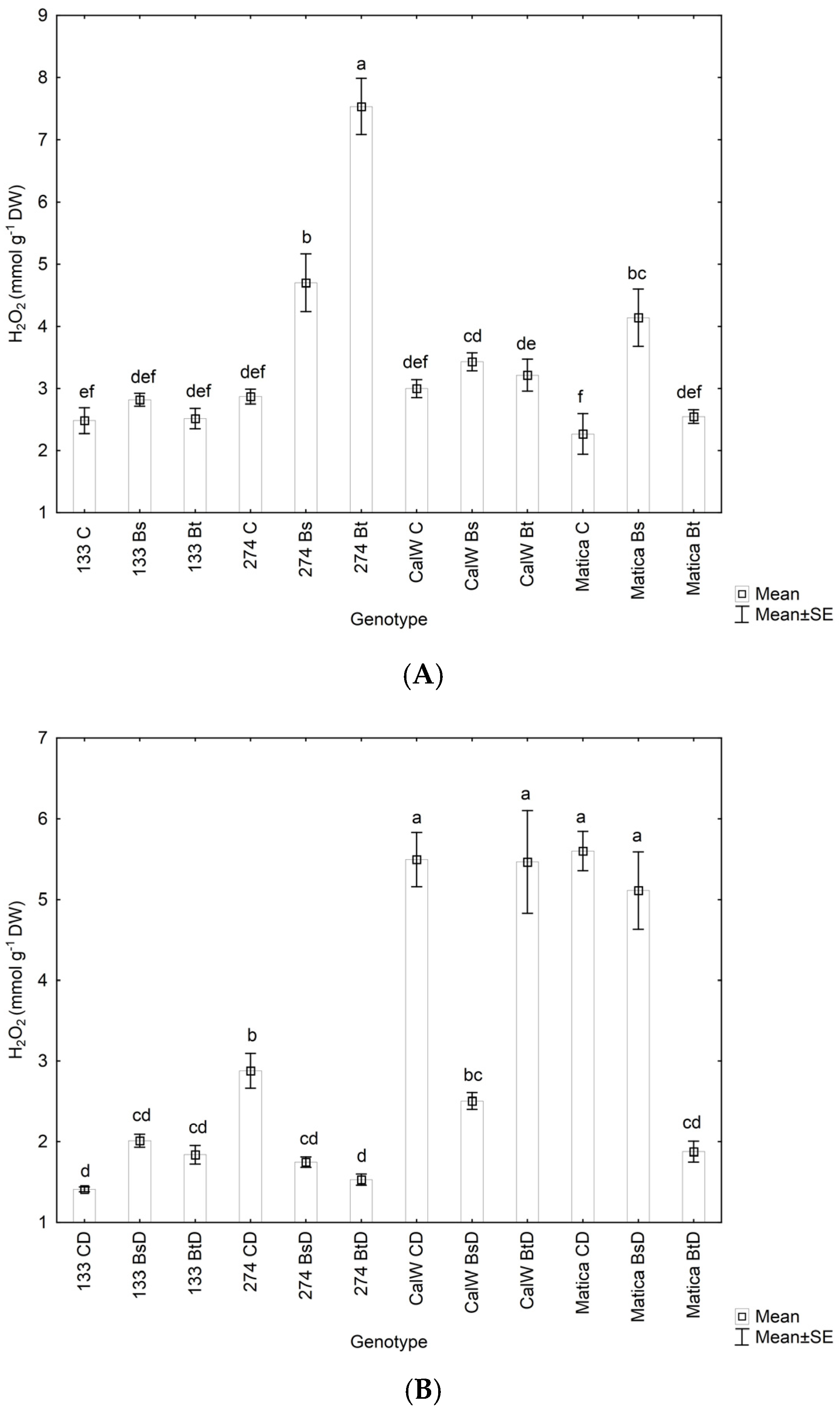
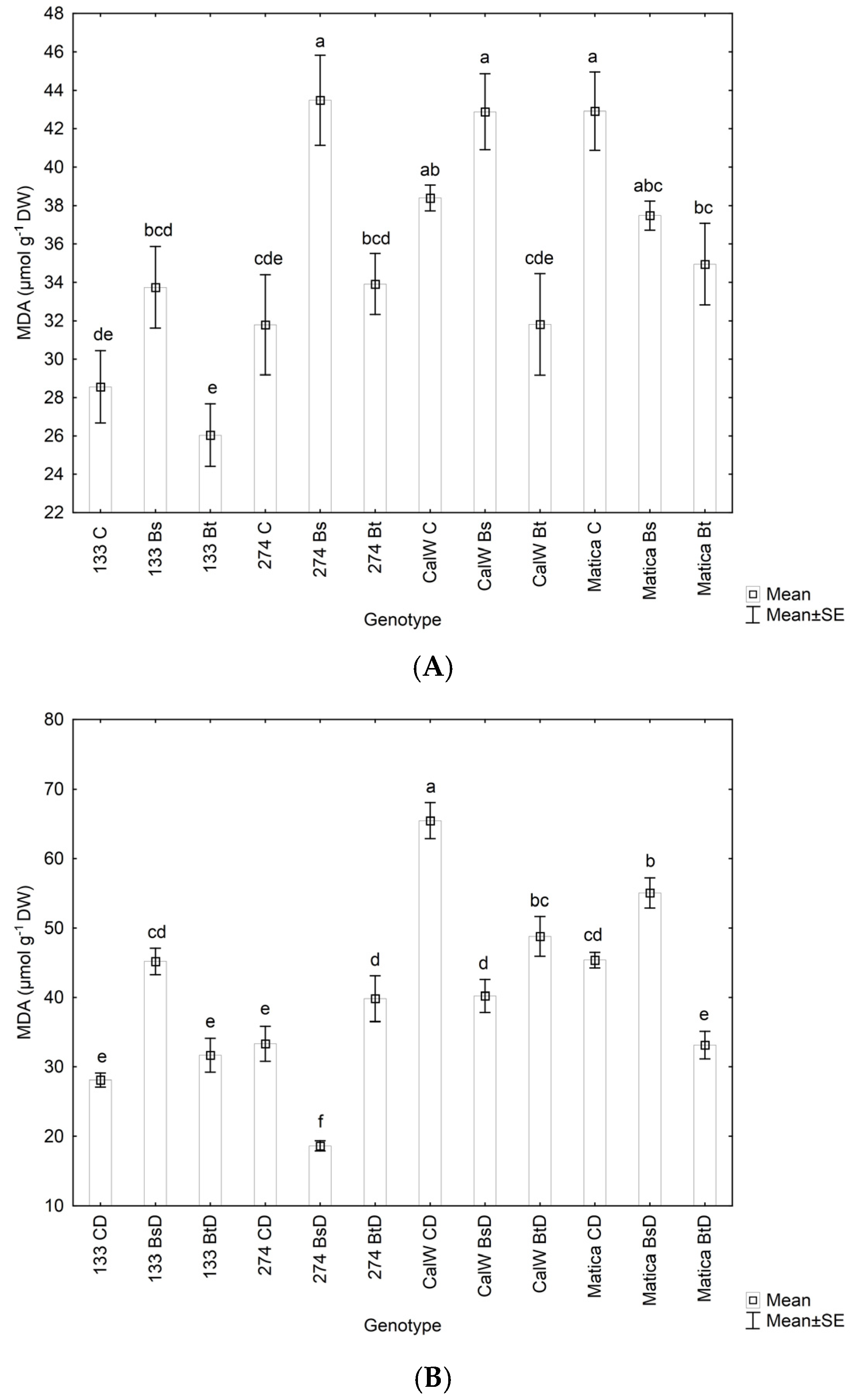
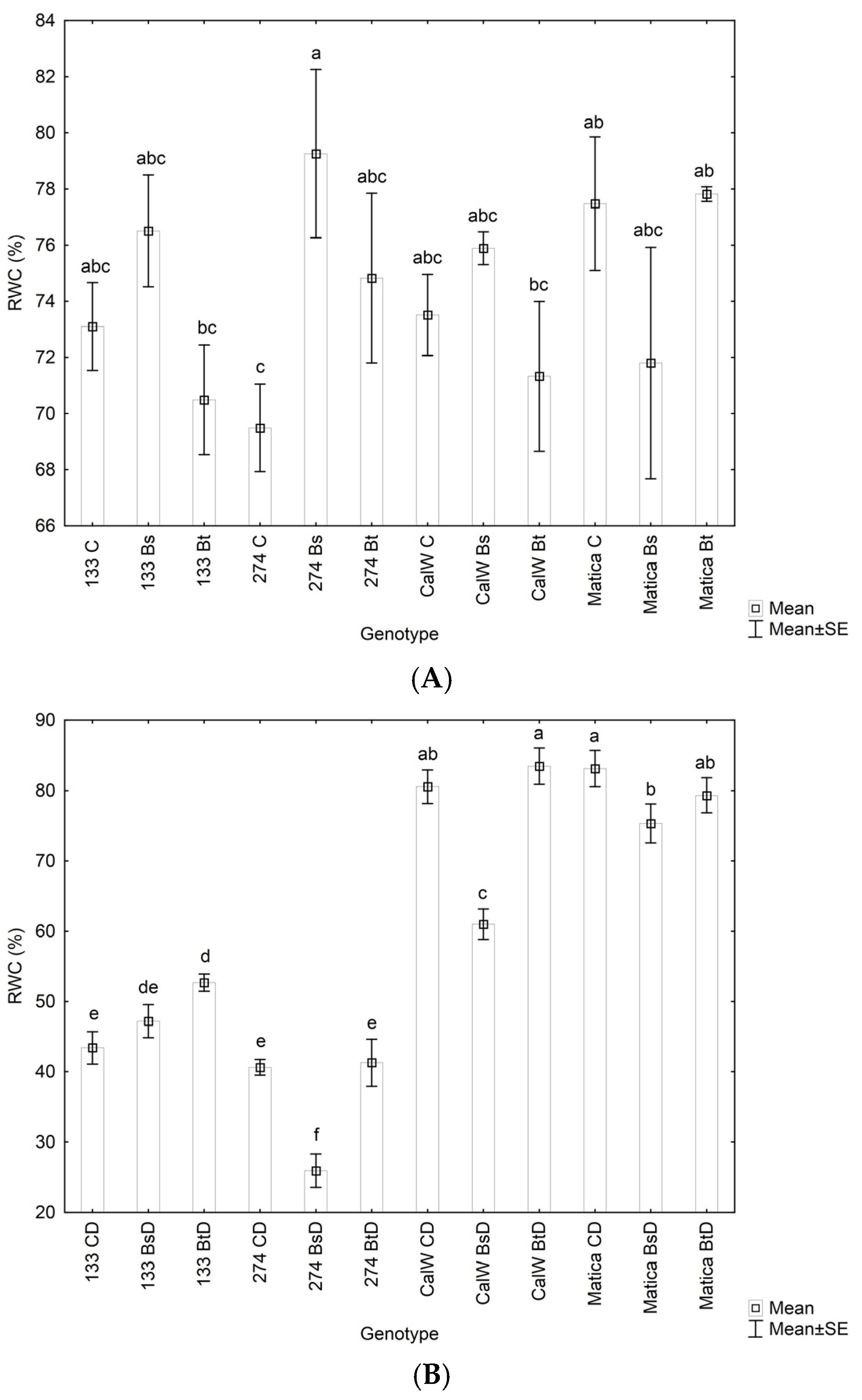
3.3. Effect of Bacterial Inoculation on Plant Hydrogen Peroxide Levels, Lipid Peroxidation under Drought Conditions, and Relative Water Contnt
3.4. Principal Component Analysis—PCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of action of plant growth promoting bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizaludin, M.S.; Stopnisek, N.; Raaijmakers, J.M.; Garbeva, P. The Chemistry of Stress: Understanding the ‘Cry for Help’ of Plant Roots. Metabolites 2021, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Zia, R.; Nawaz, M.S.; Siddique, M.J.; Hakim, S.; Imran, A. Plant survival under drought stress: Implications, adaptive responses, and integrated rhizosphere management strategy for stress mitigation. Microbiol. Res. 2021, 242, 126626. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, B.K.; Seong, E.S.; Lee, C.O.; Lee, J.G.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Improved antioxidant activity in transgenic Perilla frutescens plants via overexpression of the γ-tocopherol methyltransferase (γ-tmt) gene. Protoplasma 2015, 252, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Unal, B.T.; García-Caparrós, P.; Khursheed, A.; Gul, A.; Hasanuzzaman, M. Osmoregulation and its actions during the drought stress in plants. Physiol. Plant. 2020, 172, 1321–1335. [Google Scholar] [CrossRef]
- Jeeatid, N.; Suriharna, B.; Techawongstiena, S.; Chanthaib, S.; Boslandc, P.W.; Techawongstiena, S. Evaluation of the effect of genotype-by-environment interaction on capsaicinoid production in hot pepper hybrids (Capsicum chinense Jacq.) under controlled environment. Sci. Hortic. Amst. 2018, 235, 334–339. [Google Scholar] [CrossRef]
- Sensoy, S.; Demir, S.; Turkmen, O.; Erdinc, C.; Savur, O.B. Responses of some different pepper (Capsicum annuum L.) genotypes to inoculation with two different arbuscular mycorrhizal fungi. Sci. Hortic. Amst. 2007, 113, 92–95. [Google Scholar] [CrossRef]
- Cisternas-Jamet, J.; Salvatierra-Martínez, M.; Vega-Gálvez, A.; Uribe, E.; Goñid, M.G.; Stoll, A. Root inoculation of green bell pepper (Capsicum annum) with Bacillus amyloliquefaciens BBC047: Effect on biochemical composition and antioxidant capacity. J. Sci. Food Agric. 2019, 99, 5131–5139. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.N.; Adhikari, A.; Al-Sadi, A.M.; Kang, S.-M.; Kim, L.-R.; Lee, I.-J. Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front. Plant. Sci. 2021, 12, 669693. [Google Scholar] [CrossRef]
- Ghosh, D.; Sen, S.; Mohapatra, S. Modulation of proline metabolic gene expression in Arabidopsis thaliana under water-stressed conditions by a drought-mitigating Pseudomonas putida strain. Ann. Microbiol. 2017, 67, 655–668. [Google Scholar] [CrossRef]
- Sen, S.; Ghosh, D.; Mohapatra, S. Modulation of polyamine biosynthesis in Arabidopsis thaliana by a drought mitigating Pseudomonas putida strain. Plant. Physiol. Biochem. 2018, 129, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Draganić, V.; Lozo, J.; Biočanin, M.; Dimkić, I.; Garalejić, E.; Fira, D.; Stanković, S.; Berić, T. Genotyping of Bacillus spp. isolate collection from natural samples. Genetika 2017, 49, 445–456. [Google Scholar] [CrossRef]
- Berić, T.; Biočanin, M.; Stanković, S.; Dimkić, I.; Janakiev, T.; Fira, D.; Lozo, J. Identification and antibiotic resistance of Bacillus spp. isolates from natural samples. Arch. Biol. Sci. 2018, 70, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Belimov, A.A.; Hontzeas, N.; Safronova, V.I.; Demchinskaya, S.V.; Piluzza, G.; Bullitta, S.; Glick, B.R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 2005, 37, 241–250. [Google Scholar] [CrossRef]
- Stepanović, S.; Vuković, D.; Hola, V.; Di Bonaventura, G.; Djukić, S.; Cirković, I.; Ruzicka, F. Quantification of biofilm in microtiter plates: Overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS 2007, 115, 891–899. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant. Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Guilbault, G.G.; Kramer, D.N. 4-methoxy-α-naphthol as a spectrophotometric reagent substrate for measuring peroxidic activity. Anal. Chem. 1964, 36, 2494–2496. [Google Scholar] [CrossRef]
- Foyer, C.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Barrs, H.D.; Weatherley, P.E. A re-examination of the relative turgidity techniques for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Junglee, S.; Urban, L.; Sallanon, H.; Lopez-Lauri, F. Optimized assay for hydrogen peroxide determination in plant tissue using potassium iodide. Am. J. Anal. Chem. 2014, 5, 730–736. [Google Scholar] [CrossRef] [Green Version]
- Matus, I.; Gonzales, M.I.; Del Pozo, A. Evaluation of phenotypic variation in a Chilean collection of garlic (Allium sativum L.) clones using multivariate analysis. Plant Gen. Res. News 1999, 117, 31–36. [Google Scholar]
- Shukla, P.R.; Skea, J.; Calvo Buendia, E.; Masson-Delmotte, V.; Portner, H.O.; Roberts, D.C.; Zhai, P.; Slade, R.; Connors, S.; van Diemen, R.; et al. Climate Change and Land: An IPCC Special Report on Climate Change, Desertification, Land Degradation, Sustainable Land Management, Food Security, and Greenhouse Gas Fluxes in Terrestrial Ecosystems; IPCC: Geneva, Switzerland, 2019. [Google Scholar]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd_Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Glick, B.R.; Santoyo, G. ACC deaminase in plant growth-promoting bacteria (PGPB): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef]
- Vílchez, J.I.; Niehaus, K.; Dowling, D.N.; González-López, J.; Manzanera, M. Protection of pepper plants from drought by Microbacterium sp. 3J1 by modulation of the plant’s glutamine and α-ketoglutarate content: A comparative metabolomics approach. Front. Microbiol. 2018, 9, 284. [Google Scholar] [CrossRef]
- Ulrich, D.E.M.; Sevanto, S.; Ryan, M.; Albright, M.B.N.; Johansen, R.B.; Dunbar, J.M. Plant-microbe interactions before drought influence plant physiological responses to subsequent severe drought. Sci. Rep. UK 2019, 9, 249. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Salazar, J.; Suárez, R.; Caballero-Mellado, J.; Iturriaga, G. Trehalose accumulation in Azospirillum brasilense improves drought tolerance and biomass in maize plants. FEMS Microbiol. Lett. 2009, 296, 52–59. [Google Scholar] [CrossRef] [Green Version]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought stress impacts on plants and different approaches to alleviate its adverse effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
- Nadarajah, K.K. ROS Homeostasis in Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 5208. [Google Scholar] [CrossRef] [PubMed]
- Sahitya, U.L.; Krishna, M.S.R.; Deepthi, R.S.; Prasad, G.S.; Kasim, D.P. Seed antioxidants interplay with drought stress tolerance indices in Chilli (Capsicum annuum L) seedlings. BioMed. Res. Inter. 2018, 15, 1605096. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Genotype | RL (cm) | SL (cm) | TW (cm) | G (%) | V.I. |
|---|---|---|---|---|---|
| 133C | 1.418 ± 0.056 e | 1.245 ± 0.099 e | 0.29 ± 0.086 c | 80 ± 4.082 def | 212.8 ± 8.914 g |
| 133Bs | 1.295 ± 0.045 e | 1.44 ± 0.059 de | 0.322 ± 0.043 c | 95 ± 4.082 ab | 259.84 ± 11.725 fg |
| 133Bt | 1.36 ± 0.069 e | 2.508 ± 0.465 b | 0.282 ± 0.025 c | 96.25 ± 4.787 ab | 371.79 ± 39.513 de |
| 274C | 2.55 ± 0.244 c | 2.488 ± 0.172 b | 0.508 ± 0.065 b | 82.5 ± 13.229 cde | 414.46 ± 66.879 cd |
| 274Bs | 3.87 ± 0.243 a | 3.56 ± 0.422 a | 0.81 ± 0.055 a | 91.25 ± 4.787 abc | 678.1 ± 65.946 b |
| 274Bt | 3.765 ± 0.261 ab | 3.86 ± 0.214 a | 0.872 ± 0.048 a | 98.75 ± 2.5 a | 753.34 ± 57.587 a |
| CalWC | 1.55 ± 0.191 e | 1.15 ± 0.058 e | 0.525 ± 0.096 b | 83.75 ± 7.5 cde | 225.62 ± 15.445 g |
| CalWBs | 3.475 ± 0.33 b | 1.9 ± 0.141 c | 0.9 ± 0.141 a | 87.5 ± 6.455 bcd | 470.62 ± 57.098 c |
| CalWBt | 3 ± 0.182 c | 1.75 ± 0.129 cd | 0.85 ± 0.058 a | 75 ± 12.247 ef | 354.38 ± 45.668 de |
| Matica C | 1.525 ± 0.263 e | 0.75 ± 0.129 f | 0.4 ± 0.141 bc | 55 ± 4.082 g | 125.75 ± 28.619 h |
| Matica Bs | 3.6 ± 0.258 ab | 1.625 ± 0.126 cd | 0.9 ± 0.141 a | 71.25 ± 4.787 f | 373 ± 39.151 de |
| Matica Bt | 3.465 ± 0.299 b | 1.7 ± 0.081 cd | 0.775 ± 0.096 a | 60 ± 7.071 g | 307.25 ± 38.413 ef |
| Genotype | 133C | 133Bs | 133Bt | 133CD | 133BsD | 133BtD | CalWC | CalWBs | CalWBt | CalWCD | CalWBsD | CalWBtD |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| APX | 78.828 ± 3.165 j | 97.134 ± 4.367 ij | 101.334 ± 8.118 i | 257.015 ± 5.288 d | 766.799 ± 11.141 a | 183.64 ± 10.254 ef | 198.965 ± 17.505 e | 158.312 ± 5.816 gh | 81.087 ± 8.353 j | 151.613 ± 16.995 h | 108.082 ± 3.776 i | 328.368 ± 13.999 c |
| POD | 14.552 ± 1.487 g | 19.238 ± 1.546 g | 39.118 ± 2.931 d | 50.296 ± 5.006 c | 114.565 ± 3.413 a | 79.33 ± 7.401 b | 18.835 ± 1.747 g | 13.884 ± 0.051 g | 14.351 ± 1.952 g | 18.388 ± 1.675 g | 32.411 ± 3.625 e | 16.333 ± 2.833 g |
| Genotype | 274C | 274Bs | 274Bt | 274CD | 274BsD | 274BtD | MaticaC | MaticaBs | MaticaBt | MaticaCD | MaticaBsD | MaticaBtD |
| APX | 251.913 ± 12.5 d | 241.743 ± 14.565 d | 248.444 ± 30.719 d | 175.355 ± 2.028 fg | 141.605 ± 10.789 h | 392.05 ± 20.501 b | 3.682 ± 0.935 k | 0.579 ± 0.104 k | 0.637 ± 0.214 k | 2.451 ± 0.138 k | 3.38 ± 0.238 k | 6.189 ± 0.293 k |
| POD | 25.923 ± 4.373 f | 26.788 ± 4.31 f | 24.786 ± 2.516 f | 53.281 ± 3.474 c | 50.119 ± 6.727 c | 38.76 ± 2.841 d | 0 ± 0 h | 0 ± 0 h | 0 ± 0 h | 0 ± 0 h | 0 ± 0 h | 0 ± 0 h |
| Genotype | 6th Day | 9th Day | 14th Day | 17th Day | 19th Day | 22nd Day | 28th Day * | 30th Day | 33rd Day | 35th Day |
|---|---|---|---|---|---|---|---|---|---|---|
| 133CD | - | + | ++ | ++ | +++ | +++ | ++++/4 | 3 | 2 | 1 |
| 133BsD | - | + | ++ | ++ | +++ | +++ | ++++/4 | 3 | 2 | 1 |
| 133BtD | - | + | ++ | ++ | +++ | +++ | ++++/4 | 3 | 2 | 1 |
| 274CD | + | + | ++ | ++ | +++ | ++++ | ++++/4 | 2 | 1 | 0 |
| 274BsD | + | + | ++ | ++ | +++ | ++++ | ++++/4 | 2 | 1 | 0 |
| 274BtD | + | + | ++ | ++ | +++ | ++++ | ++++/4 | 2 | 1 | 0 |
| CalWCD | - | - | + | + | ++ | +++ | ++++/4 | 3 | 2 | 2 |
| CalWBsD | - | - | + | + | ++ | +++ | ++++/4 | 3 | 2 | 2 |
| CalWBtD | - | - | + | + | ++ | +++ | ++++/4 | 3 | 2 | 2 |
| Matica CD | - | - | - | + | ++ | +++ | ++++/4 | 3 | 2 | 2 |
| Matica BsD | - | - | - | + | ++ | +++ | ++++/4 | 3 | 2 | 2 |
| Matica BtD | - | - | - | + | ++ | +++ | ++++/4 | 3 | 2 | 2 |
| Trait | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| SOD | 0.683 | 0.467 | −0.359 |
| GR | 0.509 | 0.468 | −0.647 |
| MDA | 0.437 | −0.686 | −0.348 |
| H2O2 | 0.603 | −0.574 | −0.205 |
| RWC | 0.804 | −0.363 | 0.245 |
| APX | −0.721 | −0.480 | −0.386 |
| POD | −0.852 | −0.040 | −0.440 |
| Eigenvalue | 3.173 | 1.601 | 1.114 |
| Total variance % | 45.323 | 22.870 | 15.917 |
| Cumulative variance % | 45.323 | 68.193 | 84.110 |
| Variable | H2O2 | RWC | SOD | GR |
|---|---|---|---|---|
| MDA | 0.518 ** | 0.466 ** | 0.142 | 0.057 |
| H2O2 | 0.580 ** | 0.098 | 0.194 | |
| RWC | 0.312 ** | 0.058 | ||
| SOD | 0.621 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozo, J.; Danojević, D.; Jovanović, Ž.; Nenadović, Ž.; Fira, D.; Stanković, S.; Radović, S. Genotype-Dependent Antioxidative Response of Four Sweet Pepper Cultivars to Water Deficiency as Affected by Drought-Tolerant Bacillus safensis SS-2.7 and Bacillus thuringiensis SS-29.2 Strains. Horticulturae 2022, 8, 236. https://doi.org/10.3390/horticulturae8030236
Lozo J, Danojević D, Jovanović Ž, Nenadović Ž, Fira D, Stanković S, Radović S. Genotype-Dependent Antioxidative Response of Four Sweet Pepper Cultivars to Water Deficiency as Affected by Drought-Tolerant Bacillus safensis SS-2.7 and Bacillus thuringiensis SS-29.2 Strains. Horticulturae. 2022; 8(3):236. https://doi.org/10.3390/horticulturae8030236
Chicago/Turabian StyleLozo, Jelena, Dario Danojević, Živko Jovanović, Željka Nenadović, Djordje Fira, Slaviša Stanković, and Svetlana Radović. 2022. "Genotype-Dependent Antioxidative Response of Four Sweet Pepper Cultivars to Water Deficiency as Affected by Drought-Tolerant Bacillus safensis SS-2.7 and Bacillus thuringiensis SS-29.2 Strains" Horticulturae 8, no. 3: 236. https://doi.org/10.3390/horticulturae8030236
APA StyleLozo, J., Danojević, D., Jovanović, Ž., Nenadović, Ž., Fira, D., Stanković, S., & Radović, S. (2022). Genotype-Dependent Antioxidative Response of Four Sweet Pepper Cultivars to Water Deficiency as Affected by Drought-Tolerant Bacillus safensis SS-2.7 and Bacillus thuringiensis SS-29.2 Strains. Horticulturae, 8(3), 236. https://doi.org/10.3390/horticulturae8030236








