Effects of Exogenous Phthalic Acid on Seed Germination, Root Physiological Characteristics, and Mineral Element Absorption of Watermelon
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Design
2.3. Determination Method
2.4. Statistical Analysis
3. Results
3.1. Effects of Different Concentrations of Fatty Acid Esters on the Occurrence of Watermelon Root Border Cells
3.2. Effects of Exogenous Phthalic Acid on Germination Rate and Root Growth of Watermelon
3.3. Effects of Exogenous Phthalic Acid on Dehydrogenase Activity of Watermelon Roots
3.4. Effects of Exogenous Phthalic Acid on Antioxidant Enzyme Activity in Watermelon Roots
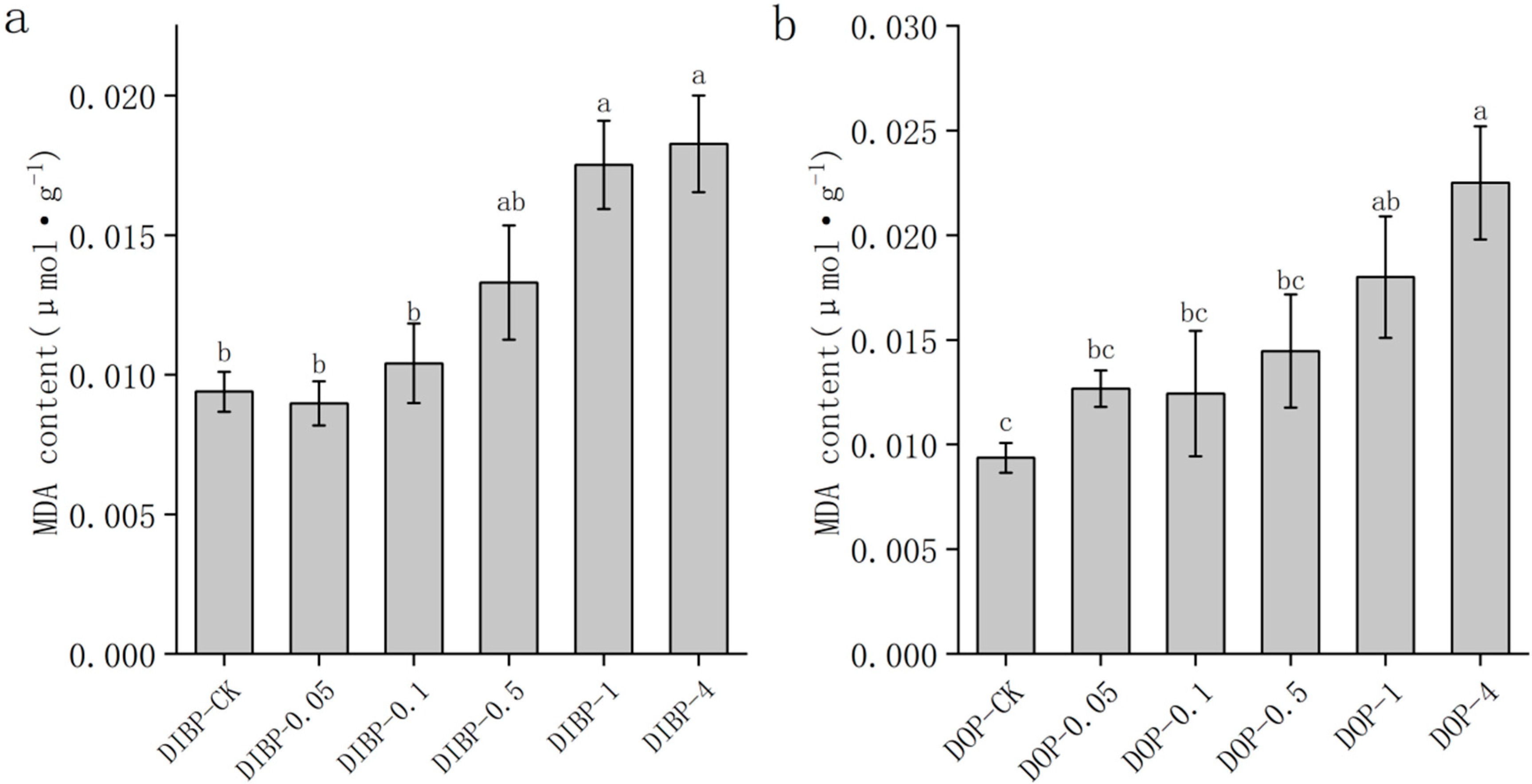
3.5. Effects of Exogenous Phthalic Acid on Malondialdehyde Content in Watermelon Roots
3.6. Effects of Exogenous Phthalic Acid on the Soluble Protein Content of Watermelon Roots
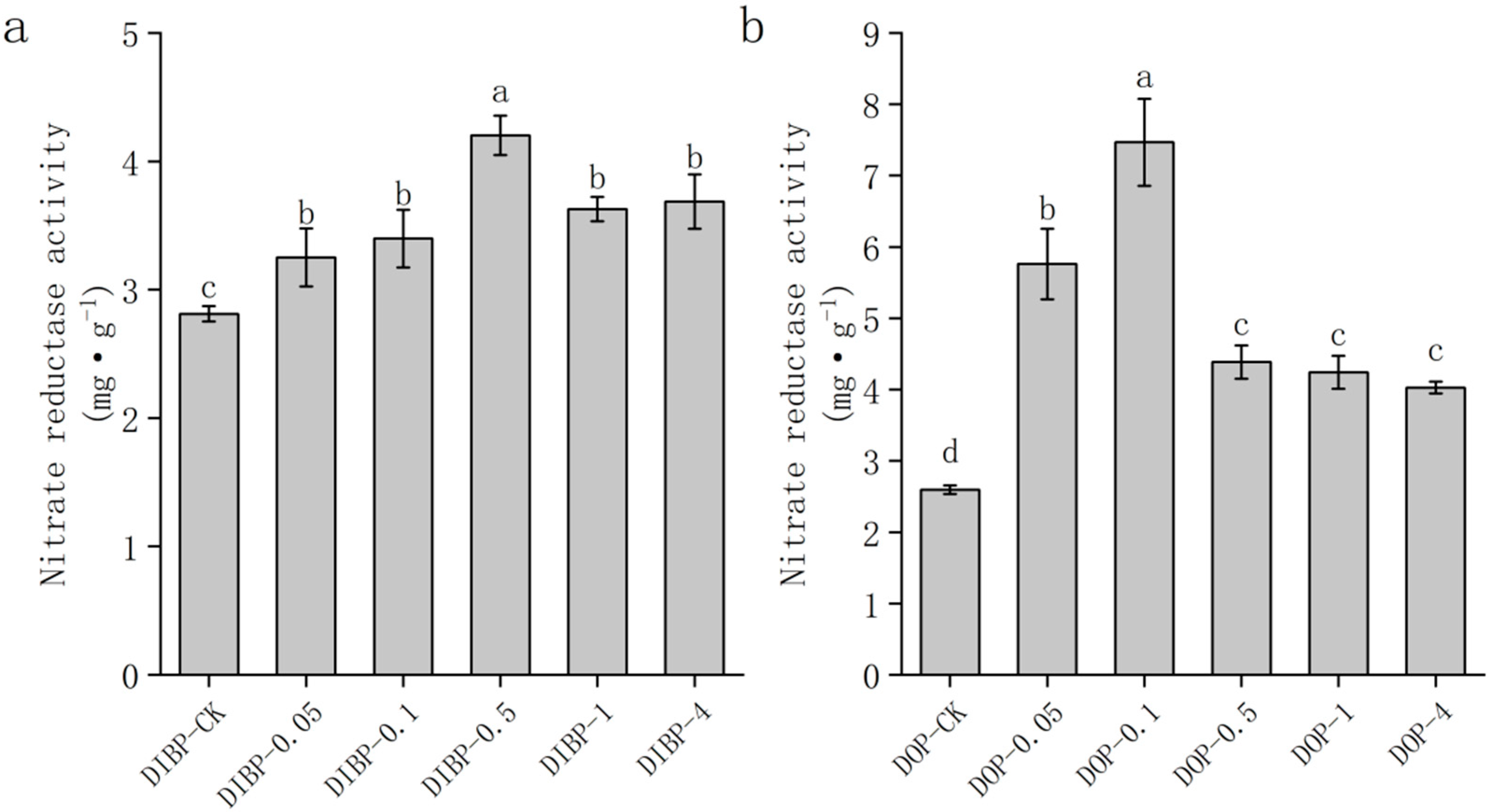
3.7. Effects of Exogenous Phthalic acid on Nitrate Reductase Activity in Watermelon Roots
3.8. Effects of Exogenous Phthalic Acid on Mineral Content in Watermelon Roots
4. Discussion
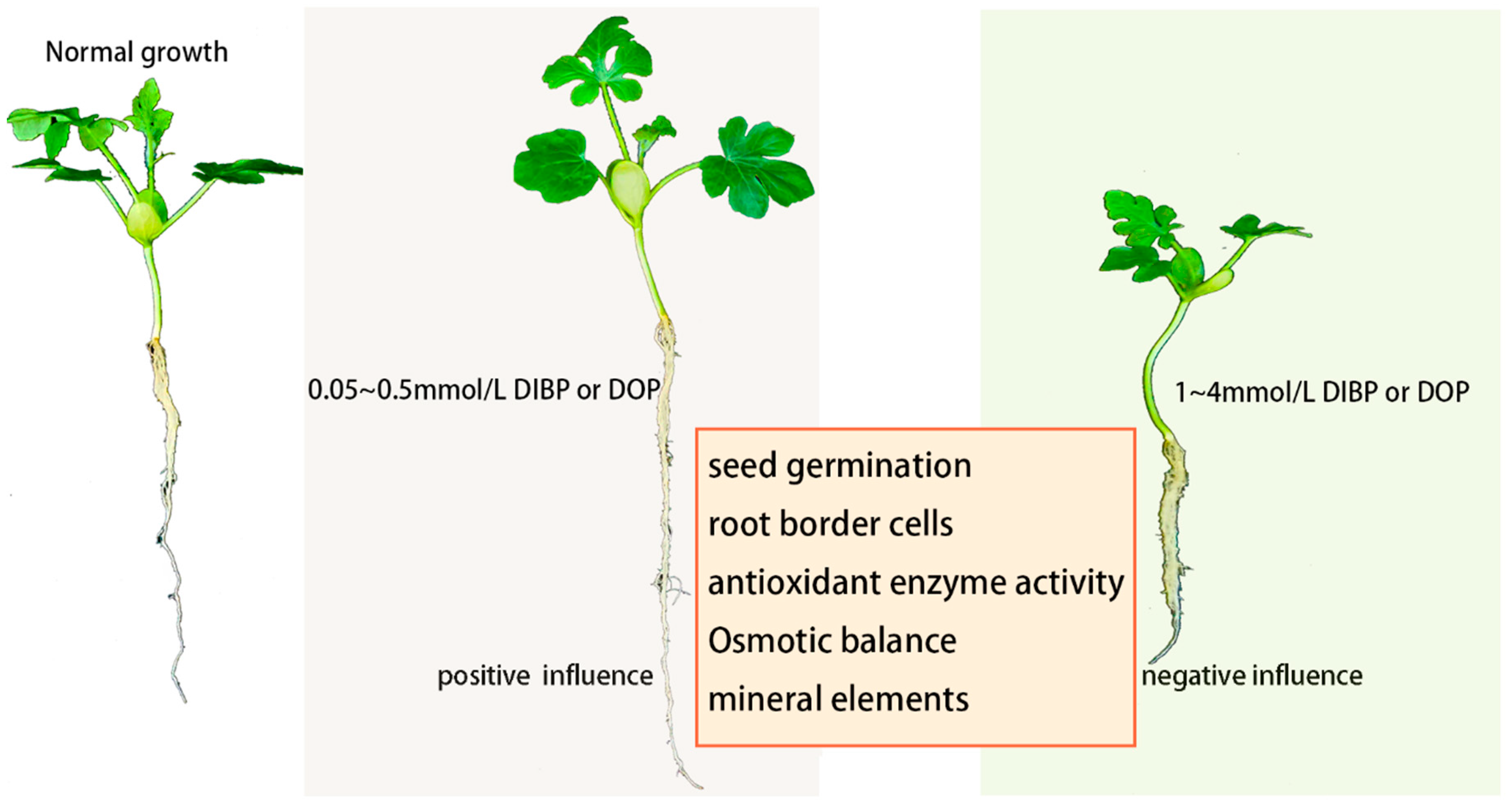
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Inderjit-Seastedt, T.R.; Callaway, R.M.; Pollock, J.L.; Kaur, J. Allelopathy and plant invasions: Traditional, congeneric, and bio-geographical approaches. Biol. Invasions 2008, 10, 875–890. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Sukcharevich, V.I. Allelopathic interactions between plants and icroorganisms in soil ecosystems. Biol. Bull. Rev. 2019, 9, 562–574. [Google Scholar] [CrossRef]
- Latif, S.; Chiapusio, G.; Weston, L.A. Allelopathy and the role of allelochemicals in plant defense. Adv. Bot. Res. 2017, 82, 19–54. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, X.; Wu, J.; Zhang, L.; Wang, J.; Wang-Pruski, G. Specific response mechanism to autotoxicity in melon (Cucumis melo L.) root revealed by physiological analyses combined with transcriptome profiling. Ecotoxicol. Environ. Saf. 2020, 200, 110779. [Google Scholar] [CrossRef]
- Cruz, O.R.; Anaya, A.L.; Hernandez-Bautista, B.E. Effects of allelochemical stress produced by sicyosdeppei on seedling root ultrastructure of Phaseolus valgaris and Cucubita ficifolia. J. Chem. Ecol. 1998, 24, 2039–2057. [Google Scholar] [CrossRef]
- Politycka, B. Free and glucosylatedphenolics, phenol β-glucosyltransferase activity and membrane permeability in cucumber roots affected by derivatives of cinnamic and benzoic acids. Acta Physiol. Plant. 1997, 19, 311–317. [Google Scholar] [CrossRef]
- Leslie, C.A.; Romani, R.J. Inhibition of ethylene biosynthesis by salicylic Acid. Plant Physiol. 1988, 88, 833–837. [Google Scholar] [CrossRef]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Yu, J.Q.; Ye, S.F.; Zhang, M.F.; Hu, W.H. Effects of root exudates and aqueous root extracts of cucumber (Cucumis sativus) and allelochemicals, on photosynthesis and antioxidant enzymes in cucumber. Biochem. Syst. Ecol. 2003, 31, 129–139. [Google Scholar] [CrossRef]
- Zhang, X.; Chang, H.N.; Li, H.Y. Allelopathic effects of water extracts from leaves of Koelreuteria paniculata on seedling growth of Ryegrass. North. Hortic. 2017, 2, 71–75. [Google Scholar]
- Ding, S.; Zhou, D.; Wei, H.; Wu, S.; Xie, B. Alleviating soil degradation caused by watermelon continuous cropping obstacle: Application of urban waste compost. Chemosphere 2021, 262, 128387. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yuan, G.; Zhu, C. Soil fumigation with ammonium bicarbonate or metam sodium under high temperature alleviates continuous cropping-induced Fusarium wilt in watermelon. Sci. Hortic. 2019, 246, 979–986. [Google Scholar] [CrossRef]
- Huang, Y.C.; Sun, J.Q.; Chen, Y.B. Effects of dibutyl phthalate on photosynthesis and Fusarium wilt of watermelon. North. Hortic. 2017, 2017, 111–115. [Google Scholar]
- Zheng, Y.X.; Lei, F.Y.; Qiu, S.; Zhao, S.M. Effects of fatty acid ester compounds on growth and physiological characteristics of water melon seedlings. J. Hunan Agric. Univ. 2020, 46, 297–302. [Google Scholar]
- Zhou, B.L.; Chen, F.; Liu, N. Allelopathic effects of diisobutyl phthalate on verticillium wilt of eggplant and its seedling growth. Acta Agric. Boreali-Occident. Sin. 2010, 19, 179–183. [Google Scholar] [CrossRef]
- Sun, H.Y.; Wang, Y. Hollow fiber liquid-phase microextraction with in situ derivatization combined with gas chromatography−mass spectrometry for the determination of root exudate phenylamine compounds in hot pepper (Capsicum annuum L.). J. Agric. Food Chem. 2013, 61, 5494–5499. [Google Scholar] [CrossRef]
- Gao, Y.L.; Chang, J.; Wang, Y.H. Allelopathic effects of Stellerachamaejasme on seed germination and growth of three crops. Acta Prataculturae Sin. 2021, 10, 83–91. [Google Scholar] [CrossRef]
- Shandry, N.; Becker, C. Allelopathic plants: Models for studying plant-interkingdom interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef]
- Ma, J.; Feng, X.; Yang, X.; Cao, Y.; Zhao, W.; Sun, L. The leaf extract of crofton weed (Eupatorium adenophorum) inhibits primary root growth by inducing cell death in maize root border cells. Plant Divers. 2020, 42, 174–180. [Google Scholar] [CrossRef]
- Hou, S.W.; Jia, J.S. A simple method for counting plant protoplasts. Plant Physiol. Commun. 2002, 38, 57. [Google Scholar]
- Miyasaka, S.C.; Hawes, M.C. Possible role of root border cells in detection and avoidance of aluminum toxicity. Plant Physiol. 2001, 125, 1978–1987. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Kermode, A.R. An increase in pectin methyl esterase activity accompanies dormancy breakage and germination of yellow cedar seeds. Plant Physiol. 2000, 124, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.X.; Sun, M.G.; Xia, Y.; Li, G.L.; Zhang, J.F.; Zhang, L.Y. Effects of salt stress on root activity of melia Azedarach L. seedlings. Shandong Nongye Daxue Xue Bao 2005, 36, 9–38. [Google Scholar]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 99, 1161–1173. [Google Scholar] [CrossRef]
- Gururani, M.A.; Ganesan, M.; Song, I.J.; Han, Y.; Kim, J.I.; Lee, H.Y.; Song, P.S. Transgenic turfgrasses expressing hyperactive Ser599Ala Phytochromeamutant exhibit abiotic stress tolerance. J. Plant Growth Regul. 2016, 35, 11–21. [Google Scholar] [CrossRef]
- Cao, K.; Yu, J.; Xu, D.; Ai, K.; Bao, E. Exposure to lower red to far-red light ratios improve tomato tolerance to salt stress. BMC Plant Biol. 2018, 18, 92. [Google Scholar] [CrossRef]
- Leleu, O.; Vuylsteker, C. Unusual regulatory nitrate reductase activity in cotyledons of Brassica napus seedlings: Enhancement of nitrate reductase activity by ammonium supply. J. Exp. Bot. 2004, 55, 815–823. [Google Scholar] [CrossRef]
- Huang, C.J.; Wei, G.; Jie, Y.C.; Xu, J.J.; Zhao, S.Y.; Wang, L.C.; Anjum, S. Responses of gas exchange, chlorophyll synthesis and ROS-scavenging systems to salinity stress in two ramie Boehmerianivea. Photosynthetica 2015, 53, 455–463. [Google Scholar] [CrossRef]
- Yu, C.; Yang, J.M.; Liu, L.J. Changes of nitrogen, phosphorus and potassium in five-year-old Schisandra chinensis leaves at different growth stages. J. Anhui Agric. Sci. 2012, 40, 7055–7057. [Google Scholar]
- Nagayama, T.; Nakamura, A.; Yamaji, N.; Satoh, S.; Furukawa, J.; Iwai, H. Changes in the distribution of pectin in root border cells under aluminum stress. Front. Plant Sci. 2019, 10, 1216. [Google Scholar] [CrossRef]
- Ropitaux, M.; Bernard, S.; Schapman, D.; Follet-Gueye, M.L.; Vicré, M.; Boulogne, I.; Driouich, A. Root border cells and mucilage secretions of soybean, Glycine Max (Merr) L.: Characterization and role in interactions with the oomycete Phytophthora parasitica. Cells 2020, 9, 2215. [Google Scholar] [CrossRef] [PubMed]
- Hawes, M.C.; Gunawardena, U.; Miyasaka, S.; Zhao, X. The role of root border cells in plant defense. Trends Plant Sci. 2000, 5, 128–133. [Google Scholar] [CrossRef]
- Micheli, F. Pectin methylesterases: Cell wall enzymes with important roles in plant physiology. Trends Plant Sci. 2001, 6, 414–419. [Google Scholar] [CrossRef]
- Pelloux, J.; Rustérucci, C.; Mellerowicz, E.J. New insights into pectin methylesterase structure and function. Trends Plant Sci. 2007, 12, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Su, W.Z.; Li, G.X.; Zhu, Q.L.; Zhang, Y.; Wang, Q.; Wu, C.J. Effects of phthalic acid on seed germination and seedling growth of Pepper. North. Hortic. 2021, 24, 8–15. [Google Scholar]
- Ning, K.Z.; Li, G.S.; Yuan, X.S.; Yang, Z.R.; Hao, L.Z.; Zhang, F.L. Effects of phthalic acid and cinnamic acid on growth and protective enzyme activities of Arabidopsis thaliana. J. Northwest A F Univ. 2021, 49, 97–104. [Google Scholar]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef]
- Axa, B.; Xi, A.; Hui, J.A. The accumulation of reactive oxygen species in root tips caused by autotoxicallelochemicals—A significant factor for replant problem of Angelica sinensis (Oliv.) Diels. Ind. Crops Prod. 2019, 138, 111432. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 2017, 122, 21–27. [Google Scholar] [CrossRef]
- Del, R.D.; Stewart, A.J.; Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef]
- Wang, C.; Liu, M.; Xu, N.; Dou, H.M.; Liu, X.H. Effects of root exudates on growth and physiological characteristics of Cucumber seedlings. North. Hortic. 2015, 10, 39–42. [Google Scholar]
- Abdulghader, K.; Nojavan, M.; Naghshbandi, N. Chemical stress induced by heliotrope (Heliotropiumeuropaeum L.) allelochemicals and increased activity of antioxidant enzymes. Pak. J. Biol. Sci. 2008, 11, 915–919. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K.; Yadav, S.S. Caffeic acid affects early growth, and morphogenetic response of hypocotyl cuttings of mung bean (Phaseolus aureus). J. Plant Physiol. 2007, 165, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, D.; Yu, J.; Huang, B. Metabolic effects of acibenzolar-Smethyl for im-proving heat or drought stress in creeping bentgrass. Front. Plant Sci. 2017, 8, 1224. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Sakihama, Y. Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: In Vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett. 2000, 468, 89–92. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhao, H.L.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and Plant Allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Jing, Q.Y.; Matsui, Y. Effects of Root Exudates of Cucumber (Cucumissativus) and Allelochemicals on Ion Uptake by Cucumber Seedlings. J. Chem. Ecol. 1997, 23, 817–827. [Google Scholar] [CrossRef]
- Guo, W.; Sun, H.Y.; Wang, Y. Effects of N-phenyl-2-naphthylamine and phthalic acid on antioxidant system and mineral nutrient absorption of Lettuce. Plant Physiol. J. 2017, 53, 71–78. [Google Scholar]
- Abenavoli, M.R.; Lupini, A.; Oliva, S. Allelochemical effects on net nitrate uptake and plasma membrane H+-ATPase activity in maize seedlings. Biol. Plant. 2010, 54, 149–153. [Google Scholar] [CrossRef]
- Glass, D.M. Inhibition of phosphate uptake in barley roots by hydroxy-benzoic acids. Pergamon 1975, 14, 117–125. [Google Scholar] [CrossRef]
- Geng, G.D.; Zhang, S.Q.; Cheng, Z.H. Allelopathy of phthalic acid on Lettuce and its mechanism. J. Hunan Agric. Univ. 2008, 34, 656–659. [Google Scholar]



| Treatment | Survival Rate of RBCs | PME Activity | Number of RBCs | |
|---|---|---|---|---|
| (mmol·L−1) | (%) | (μmol·h−1) | (pcs) | |
| DIBP | 0 | 85.19 ± 1.30 b | 0.033 ± 0.003 d | 5761.09 ± 257.96 e |
| 0.05 | 81.56 ± 2.88 b | 0.068 ± 0.005 c | 10,920.38 ± 257.96 d | |
| 0.1 | 82.66 ± 1.35 b | 0.147 ± 0.003 a | 13,242.03 ± 547.77 c | |
| 0.5 | 91.76 ± 1.41 a | 0.094 ± 0.004 b | 16,853.50 ± 1240.12 a | |
| 1 | 83.80 ± 0.70 b | 0.077 ± 0.005 c | 15,460.50 ± 1186.62 b | |
| 4 | 80.5 ± 0.80 b | 0.029 ± 0.002 d | 17,778.13 ± 694.13 a | |
| DOP | 0 | 85.19 ± 1.30 ab | 0.033 ± 0.003 d | 5761.09 ± 257.96 d |
| 0.05 | 86.78 ± 0.27 ab | 0.087 ± 0.002 b | 13,465.39 ± 1256.64 a | |
| 0.1 | 89.45 ± 1.86 ab | 0.157 ± 0.003 a | 14,904.45 ± 1302.17 a | |
| 0.5 | 94.51 ± 1.68 a | 0.153 ± 0.005 a | 13,872.61 ± 1007.67 a | |
| 1 | 84.8 ± 4.35 ab | 0.075 ± 0.003 c | 10,401.68 ± 650.57 b | |
| 4 | 82.45 ± 5.37 b | 0.034 ± 0.004 d | 8159.23 ± 1004.01 c | |
| Treatment | SOD Activity | POD Activity | CAT Activity | |
|---|---|---|---|---|
| (mmol·L−1) | (U·g−1 FW) | (U·g−1 FW) | (U·g−1 FW) | |
| DIBP | 0 | 28.41 ± 1.26 c | 1296.28 ± 40.48 b | 0.17 ± 0.07 c |
| 0.05 | 32.38 ± 2.98 c | 1460.60 ± 7.45 a | 0.88 ± 0.13 b | |
| 0.1 | 34.62 ± 4.09 c | 1387.27 ± 56.41 ab | 1.77 ± 0.19 a | |
| 0.5 | 96.48 ± 2.19 a | 1076.11 ± 26.31 c | 0.76 ± 0.05 b | |
| 1 | 77.44 ± 2.74 b | 1074.69 ± 27.71 c | 1.09 ± 0.11 b | |
| 4 | 75.06 ± 5.05 b | 1015.01 ± 34.49 c | 1.00 ± 0.04 b | |
| DOP | 0 | 28.40 ± 1.26 e | 1262.95 ± 7.38 bc | 0.17 ± 0.01 d |
| 0.05 | 58.46 ± 4.99 cd | 1312.87 ± 14.36 b | 1.45 ± 0.05 c | |
| 0.1 | 121.44 ± 7.08 a | 1417.76 ± 31.07 a | 3.33 ± 0.18 a | |
| 0.5 | 93.01 ± 2.58 b | 1210.78 ± 47.16 bcd | 2.26 ± 0.17 b | |
| 1 | 63.85 ± 1.41 c | 1196.88 ± 46.29 cd | 1.30 ± 0.19 c | |
| 4 | 46.73 ± 5.52 d | 1115.62 ± 20.46 d | 1.26 ± 0.05 c | |
| Treatment | P Content | K Content | Ca Content | Mg Content | Fe Content | Cu Content | Zn Content | |
|---|---|---|---|---|---|---|---|---|
| (mmol·L−1) | (mg·g−1) | (mg·g−1) | (mg·g−1) | (mg·g−1) | (mg·g−1) | (mg·g−1) | (mg·g−1) | |
| DIBP | 0 | 280.21 ± 22.22 c | 100.39 ± 3.06 c | 1.65 ± 0.02 cd | 0.68 ± 0.04 a | 1.38 ± 0.07 bc | 3.38 ± 0. 3 b | 0.076 ± 0.005 bc |
| 0.05 | 395.89 ± 5.32 ab | 194.27 ± 4.31 a | 2.25 ± 0.01 ab | 0.38 ± 0.02 b | 1.27 ± 0.06 cd | 4.70 ± 0. 04 a | 0.087 ± 0.009 ab | |
| 0.1 | 444.21 ± 7.19 a | 161.88 ± 6.15 b | 1.56 ± 0.06 d | 0.36 ± 0.01 b | 1.17 ± 0.04 cd | 4.68 ± 0. 1 a | 0.099 ± 0.001 a | |
| 0.5 | 433.52 ± 52.82 a | 51.58 ± 8.04 d | 2.48 ± 0.22 a | 0.61 ± 0.07 a | 1.06 ± 0.06 bd | 4.28 ± 0. 25 a | 0.054 ± 0.002 d | |
| 1 | 342.86 ± 9.88 bc | 142.59 ± 8.81 b | 1.98 ± 0.10 bc | 0.63 ± 0.07 a | 1.45 ± 0.12 b | 3.59 ± 0. 13 b | 0.061 ± 0.003 cd | |
| 4 | 348.11 ± 9.77 bc | 149.87 ± 7.27 b | 1.41 ± 0.09 d | 0.57 ± 0.04 a | 2.14 ± 0.01 a | 2.63 ± 0. 16 c | 0.076 ± 0.003 bc | |
| DOP | 0 | 280.21 ± 22.22 c | 100.39 ± 3.06 d | 1.65 ± 0.01 b | 0.68 ± 0.05 a | 1.38 ± 0.08 bc | 3.38 ± 0. 3 ab | 0.076 ± 0.005 ab |
| 0.05 | 291.89 ± 2.78 c | 183.11 ± 3.28 c | 1.65 ± 0.01 b | 0.43 ± 0.02 b | 1.30 ± 0.06 bc | 3.62 ± 0. 24 ab | 0.074 ± 0.011 ab | |
| 0.1 | 380.62 ± 27.68 b | 227.78 ± 8.02 ab | 2.55 ± 0.34 a | 0.48 ± 0.04 b | 1.23 ± 0.02 c | 3.13 ± 0. 33 ab | 0.059 ± 0.005 b | |
| 0.5 | 377.81 ± 16.90 b | 268.34 ± 26.7 a | 1.96 ± 0.07 b | 0.44 ± 0.05 b | 1.58 ± 0.13 b | 4.29 ± 0. 05 a | 0.093 ± 0.002 a | |
| 1 | 389.44 ± 17.68 b | 217.11 ± 4.62 bc | 1.90 ± 0.08 b | 0.13 ± 0.02 c | 1.47 ± 0.01 c | 3.98 ± 0. 59 a | 0.096 ± 0.013 a | |
| 4 | 453.07 ± 1.50 a | 193.05 ± 3.60 bc | 2.68 ± 0.12 a | 0.02 ± 0.01 c | 2.08 ± 0.11 a | 2.56 ± 0. 16 b | 0.099 ± 0.004 a | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Xiao, J.; Lei, F.; Zheng, K.; Lu, W.; Ma, J.; He, M.; Zheng, Y. Effects of Exogenous Phthalic Acid on Seed Germination, Root Physiological Characteristics, and Mineral Element Absorption of Watermelon. Horticulturae 2022, 8, 235. https://doi.org/10.3390/horticulturae8030235
Li M, Xiao J, Lei F, Zheng K, Lu W, Ma J, He M, Zheng Y. Effects of Exogenous Phthalic Acid on Seed Germination, Root Physiological Characteristics, and Mineral Element Absorption of Watermelon. Horticulturae. 2022; 8(3):235. https://doi.org/10.3390/horticulturae8030235
Chicago/Turabian StyleLi, Mengyao, Jiachang Xiao, Fengyun Lei, Kaimin Zheng, Wei Lu, Junying Ma, Maolin He, and Yangxia Zheng. 2022. "Effects of Exogenous Phthalic Acid on Seed Germination, Root Physiological Characteristics, and Mineral Element Absorption of Watermelon" Horticulturae 8, no. 3: 235. https://doi.org/10.3390/horticulturae8030235
APA StyleLi, M., Xiao, J., Lei, F., Zheng, K., Lu, W., Ma, J., He, M., & Zheng, Y. (2022). Effects of Exogenous Phthalic Acid on Seed Germination, Root Physiological Characteristics, and Mineral Element Absorption of Watermelon. Horticulturae, 8(3), 235. https://doi.org/10.3390/horticulturae8030235







