Spirulina platensis Foliar Spraying Curcuma longa Has Improved Growth, Yield, and Curcuminoid Biosynthesis Gene Expression, as Well as Curcuminoid Accumulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Spirulina platensis Aqueous Extract (SAE)
2.2. Analysis of Spirulina platensis Powder Compositions
2.3. Plant Materials and Growth Conditions
2.4. Chemical Analysis
2.4.1. Measurement of Photosynthetic Pigments
2.4.2. Mineral Composition of Plant Leaves and Powder of S. platensis
2.5. GC/MS Analysis
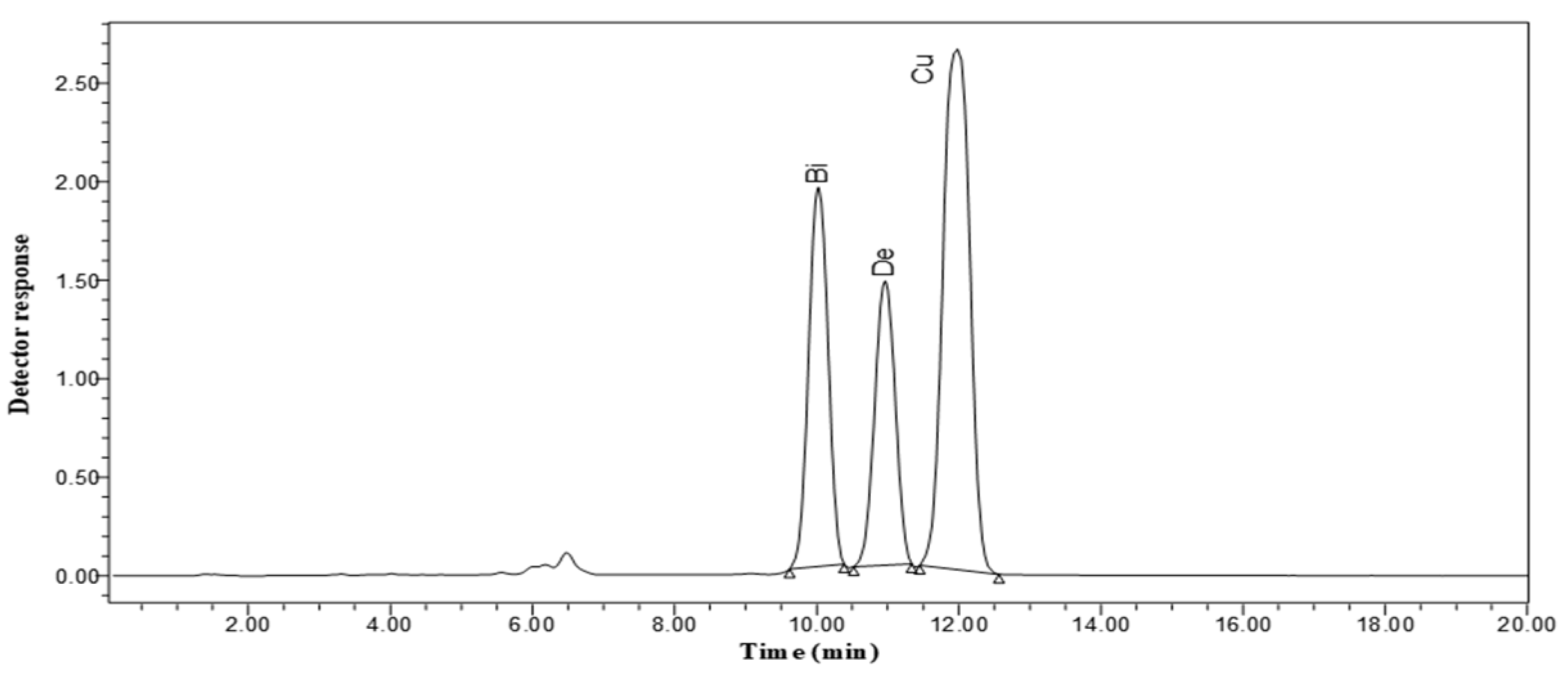
2.6. Determination of Curcumin, Bisdemethoxycurcumin and Demethoxycurcumin Contents by High-Performance Liquid Chromatography (HPLC)
2.6.1. Instrumentation
2.6.2. Preparation of Curcumin, Bisdemethoxycurcumin and Demethoxycurcumin Standard Curves
2.6.3. Preparation of C. longa Rhizome Ethanolic Extracts
2.6.4. HPLC Analysis Conditions
2.7. Analysis of CURS-1, -2, -3 and DCS Gene Expression by Real-Time Reverse Transcriptase Polymerase Chain Reaction (Real-Time RT-PCR)
2.7.1. Total RNA Preparation and cDNA Synthesis
2.7.2. Real-Time RT-PCR and Curcuminoid Gene Expression Analysis
2.8. Statistical Analysis
3. Results
3.1. Effect of SAE on Plant Development and Yield
3.2. Chemical Analysis
Photosynthetic Pigments and Mineral Contents
3.3. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis of Ethanolic Extract
3.4. HPLC Results
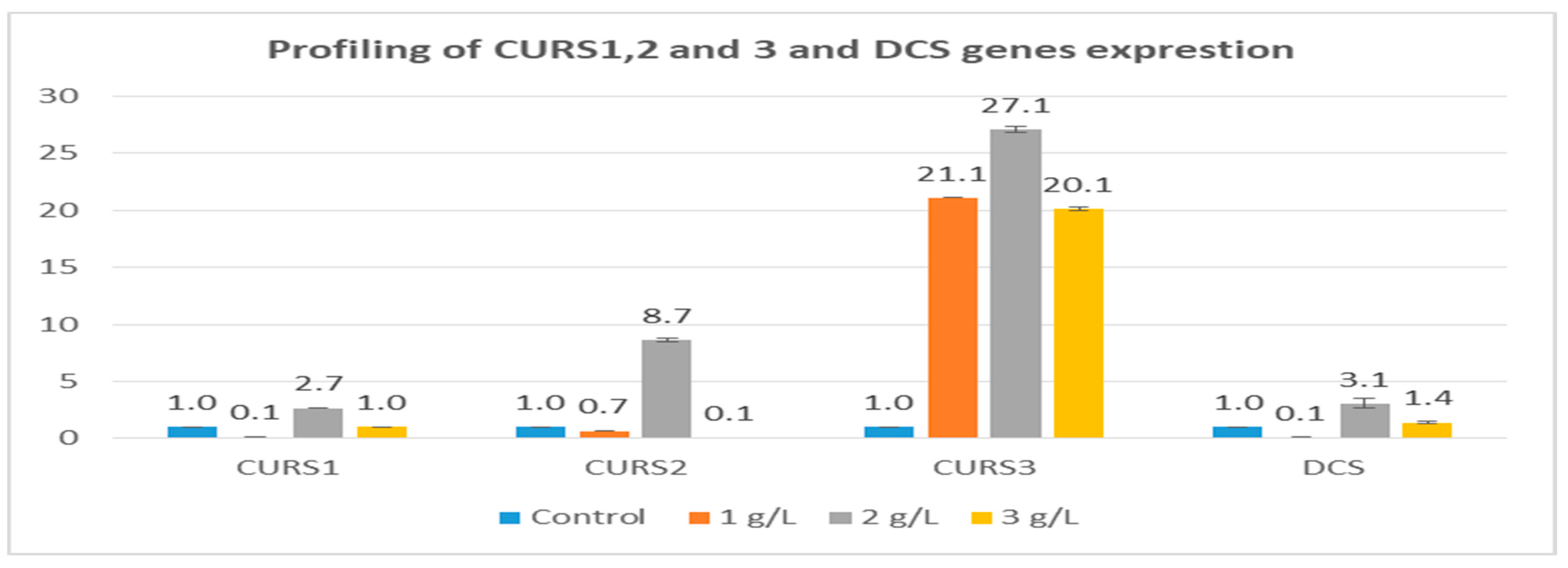
3.5. Effect of SAE on the Expression of Curcuminoid Biosynthesis Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López, I.; Martínez, L.; Pérez, G.; Reyes, Y.; Núñez, M.; Cabrera, J. Algae and their uses in agriculture. An update. Cultiv. Trop. 2020, 41, 1–10. [Google Scholar]
- Anitha, L.; Sai Bramari, G.; Kalpana, P. Effect of Supplementation of Spirulina platensis to Enhance the Zinc Status in Plants of Amaranthus gangeticus, Phaseolus aureus and Tomato. Adv. Biosci. Biotechnol. 2016, 7, 289–299. [Google Scholar] [CrossRef] [Green Version]
- El Sherif, F.; Albotnoor, N.; Yap, Y.K.; Meligy, A.; Khattab, S. Enhanced bioactive compounds composition in Lavandula officinalis in-vitro plantlets using NaCl and Moringa oleifera, Aloe vera and Spirulina platensis extracts. Ind. Crops Prod. 2020, 157, 112890. [Google Scholar] [CrossRef]
- Yanni, Y.G.; Elashmouny, A.A.; Elsadany, A.Y. Differential Response of Cotton Growth, Yield and Fiber Quality to Foliar Application of Spirulina platensis and Urea Fertilizer. Asian J. Adv. Agric. Res. 2020, 12, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Godlewska, K.; Michalak, I.; Pacyga, P.; Baśladyńska, S.; Chojnacka, K. Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol. 2019, 35, 80. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, W.; Hasan, A.; Ansari, A.; Tarannum, T. Curcuma longa, Linn—A Review. Hippocrat. J. Unani Med. 2010, 5, 179–190. [Google Scholar]
- Sharifi-Rad, J.; El Rayess, Y.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 1021. [Google Scholar] [CrossRef]
- Itokawa, H.; Shi, Q.; Akiyama, T.; Morris-Natschke, S.L.; Lee, K.H. Recent advances in the investigation of curcuminoids. Chin. Med. 2008, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Katsuyama, Y.; Kita, T.; Funa, N.; Horinouchi, S. Curcuminoid biosynthesis by two type III polyketide synthases in the herb Curcuma longa. J. Biol. Chem. 2009, 284, 11160–11170. [Google Scholar] [CrossRef] [Green Version]
- Katsuyama, Y.; Kita, T.; Horinouchi, S. Identification and characterization of multiple curcumin synthases from the herb Curcuma longa. FEBS Lett. 2009, 583, 2799–2803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, A.; Mahajan, S.; Jaiswal, S.K.; Sharma, V.K. Genome sequencing of turmeric provides evolutionary insights into its medicinal properties. Commun. Biol. 2021, 4, 1193. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, I.S.; Das, S.; Nasim, N.; Mishra, A.; Acharya, L.; Joshi, R.K.; Nayak, S.; Mohanty, S. Differential expression of CURS gene during various growth stages, climatic condition and soil nutrients in turmeric (Curcuma longa): Towards site specific cultivation for high curcumin yield. Plant Physiol. Biochem. 2017, 118, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Amin, G.H.; Al-Gendy, A.A.; Yassin, M.E.A.; Abdel-Motteleb, A. Effect of Spirulina platensis extract on growth, phenolic compounds and antioxidant activities of Sisymbrium irio callus and cell suspension cultures. Aust. J. Basic Appl. Sci. 2009, 3, 2097–2110. [Google Scholar]
- Qian, H.; Sheng, M. Simultaneous determination of fat-soluble vitamins A, D and E and pro-vitamin D2 in animal feeds by one-step extraction and high-performance liquid chromatography analysis. J. Chromatogr. A 1998, 825, 127–133. [Google Scholar] [CrossRef]
- Shindy, W.W.; Smith, O.E. Identification of Plant Hormones from Cotton Ovules. Plant Physiol. 1975, 55, 550–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, S.J.; Jørgensen, H.J.H.; Knudsen, K.E.B. The use of near-infrared spectroscopy (NIRS) to determine the energy value of individual feedstuffs and mixed diets for pigs. Anim. Feed Sci. Technol. 2022, 283, 115156. [Google Scholar] [CrossRef]
- González-Martín, I.; Álvarez-García, N.; González-Cabrera, J.M. Near-infrared spectroscopy (NIRS) with a fibre-optic probe for the prediction of the amino acid composition in animal feeds. Talanta 2006, 69, 706–710. [Google Scholar] [CrossRef]
- Buurman, P.; Van Lagen, B.; Velthorst, E.J. WfM Manual for Soil and Water Analysis; Backhuys Publishers: Leiden, The Netherlands, 1996. [Google Scholar]
- A.O.A.C. Official Method of Analysis of the Association of Official Chemist, 14th ed.; Official Methods of Analysis; Howitz, Ed.; AOAC International: Washington DC, USA, 1984. [Google Scholar]
- Piper, C.S. Soil and Plant Analysis; Hans Publishers: Wuhan, China, 1942. [Google Scholar]
- Jackson, M.L. Soil Chemica Analysis; Prentice Hall: New Delhi, India, 1967. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Mazumdar, B.; Majumder, K.; Mazumdar, B.C.; Majumder, K. Methods of Physiochemical Analysis of Fruits; Daya Publi. India, Daya Publishing House: Delhi, India, 2003. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis—Part 2: Chemical and Microbiological Properties, 9.2.2, 2nd ed.; Page, A.L., Ed.; American Society of Agronomy Inc. Publ.: Madison, WI, USA; SSSA: Madison, WI, USA, 1982; Volume 9, pp. 403–430. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- StatSoft STATISTICA for Windows, Version 6, 2300; StatSoft Inc.: Tulsa, OK, USA, 2001.
- Jufri, A.F.; Sudradjat; Sulistyono, E. Effects of Dry Spirulina platensis and Antitranspirant on Growth and Yield of Chili Pepper (Capsicum annuum L.). J. Agron. Indones. 2016, 44, 170–175. [Google Scholar] [CrossRef] [Green Version]
- Anvar, A.A.; Nowruzi, B. Bioactive Properties of Spirulina: A Review. Microb. Bioact. 2021, 4, 134–142. [Google Scholar] [CrossRef]
- Shalaby, T.A.; El-Ramady, H. Effect of foliar application of bio-stimulants on growth, yield, components, and storability of garlic (Allium sativum L.). Aust. J. Crop Sci. 2014, 8, 271–275. [Google Scholar]
- Papenfus, H.B.; Kulkarni, M.G.; Stirk, W.A.; Finnie, J.F.; Van Staden, J. Effect of a commercial seaweed extract (Kelpak) and polyamines on nutrient-deprived (N, P and K) okra seedlings. Sci. Hortic. 2013, 151, 142–146. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Salem, S.M.M.; Hendi, D.M.G. Effect of Nitrogen Fertilizer Rates and Microalgae Application on the Vegetative Growth and Biochemical Constituents of Sweet Basil Plants. J. Hortic. Sci. Ornam. Plants 2019, 11, 244–251. [Google Scholar] [CrossRef]
- Akgül, F. Effect of Spirulina platensis (Gomont) Geitler Extract on Seed Germination of Wheat and Barley. Alınteri Zirai Bilimler Dergisi 2019, 34, 148–153. [Google Scholar] [CrossRef]
- Shaaban, M.M.; Abou-Zaid, E.A.A. Growth, yield and berries quality in Red Roomy grapevines improved under different foliar application of Spirulina algae, zinc and boron. Middle East J. Agric. Res. 2019, 8, 654–661. [Google Scholar]
- Dias, G.A.; Rocha, R.H.C.; Araújo, J.L.; De Lima, J.F.; Guedes, W.A. Growth, yield, and postharvest quality in eggplant produced under different foliar fertilizer (Spirulina platensis) treatments. Semin. Cienc. Agrar. 2016, 37, 3893–3902. [Google Scholar] [CrossRef] [Green Version]
- Morsy, N. Productivity and Quality of Kohlrabi Grown in a Newly Reclaimed Sandy Soil Using Organic and Mineral-N Fertilizer regimes with or without Spraying of Spirulina platensis Extract. Egypt. J. Hortic. 2019, 46, 169–178. [Google Scholar] [CrossRef] [Green Version]
- Geries, L.S.M.; Elsadany, A.Y. Maximizing growth and productivity of onion (Allium cepa L.) by Spirulina platensis extract and nitrogen-fixing endophyte Pseudomonas stutzeri. Arch. Microbiol. 2021, 203, 169–181. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.X.; Zhao, P.J.; Li, G.H. Advances in the study of metabolomics and metabolites in some species interactions. Molecules 2021, 26, 3311. [Google Scholar] [CrossRef] [PubMed]
- Amer, H.M.; Marrez, D.A.; Salama, A.B.; Wahba, H.E.; Khalid, K.A. Growth and chemical constituents of cardoon plant in response to foliar application of various algal extracts. Biocatal. Agric. Biotechnol. 2019, 21, 101336. [Google Scholar] [CrossRef]
- Yap, Y.K.; El-sherif, F.; Habib, E.S.; Khattab, S. Moringa oleifera leaf extract enhanced growth, yield and silybin content while mitigating salt-induced adverse effects on the growth of Silybum marianum. Agronomy 2021, 11, 2500. [Google Scholar] [CrossRef]
- Alkuwayti, M.A.; El-Sherif, F.; Yap, Y.K.; Khattab, S.; Alkuwayti, M.A. ESFYYK and KS. Foliar application of Moringa oleifera leaves extract altered stress-responsive gene expression and enhanced bioactive compounds composition in Ocimum basilicum. S. Afr. J. Bot. 2020, 129, 291–298. [Google Scholar] [CrossRef]
- Lan, T.T.P.; Huy, N.D.; Luong, N.N.; Quang, H.T.; Tan, T.H.; Thu, L.T.A.; Loc, N.H. Effect of salicylic acid and yeast extract on curcuminoids biosynthesis gene expression and curcumin accumulation in cells of Curcuma zedoaria. J. Plant Biotechnol. 2019, 46, 172–179. [Google Scholar] [CrossRef] [Green Version]
| Chemical Composition | Amount | Unit |
|---|---|---|
| Iron | 6000 | mg/100 g |
| Phosphorus | 2000 | mg/100 g |
| Manganese | 3000 | mg/100 g |
| Thiamin | 7000 | mg/100 g |
| Riboflavin | 5000 | mg/100 g |
| Vitamin B3 | 1000 | mg/100 g |
| Vitamin E | 1000 | mg/100 g |
| Fat | 2.4 | % |
| Fiber | 1.19 | % |
| Moisture | 10.23 | % |
| Protein | 58.51 | % |
| Starch | 1.81 | % |
| Ash | 5.48 | % |
| Characteristic | Value |
|---|---|
| Texture | Sandy |
| Sand % | 91.51 |
| Silt % | 5.74 |
| Clay % | 2.75 |
| Saturation % | 23 |
| pH | 7.5 |
| Electrical conductivity (EC) (dS/m) | 2.2 |
| Organic matter (OM) % | 0.05 |
| Total N % | 0.014 |
| Available P ppm | 3.9 |
| Available K ppm | 110 |
| Salinity Level (ppm) | Cations (meq/L) | Anions (meq/L) | Sodium Adsorption Ratio (SAR) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca2+ | Mg2+ | Na+ | K+ | CO32− | HCO3− | SO42− | Cl− | ||
| 864 | 5.72 | 2.02 | 7.27 | 0.38 | 0.28 | 2.68 | 4.03 | 8.4 | 3.43 |
| Gene | Primers Sequence | Amplicon Length (bp) | GenBank Accession Number | References |
|---|---|---|---|---|
| Diketide-CoA synthase (DCS) | 5′-GTGCTGTTCATCCTGGACGAG-3′ (forward primer) | 21 | AB495006.1 | [10] |
| 5′-CAACAGCACGCCCCAGTCGA-3′ (reverse primer) | 20 | |||
| Curcumin synthase 1 (CURS-1) | 5′-CATCATTGACGCCATCGAAGC-3′ (forward primer) | 21 | AB495007.1 | [10] |
| 5′-TCAGCTCATCCATCACGAAGTACAC-3′ (reverse primer) | 25 | |||
| Curcumin synthase 2 (CURS-2) | 5′-TCGGGATCAAGGACTGGAACAAC-3′ (forward primer) | 23 | AB506762.1 | [10] |
| 5′-TGTTGCCGAACTCGGAGAAGAC-3′ (reverse primer) | 22 | |||
| Curcumin synthase 3 (CURS-3) | 5′-TGGAGCCCTCCTTCGACGACC-3′ (forward primer) | 21 | AB506763.1 | [10] |
| 5′-CCCATTCCTTGATCGCCTTTTCC-3′ (reverse primer) | 23 | |||
| Actin | 5′-GGATATGCTCTTCCTCATGCT-3′ (forward primer) | 21 | CP002686.1 AK118354.1 AY087740.1 | [10] |
| 5′-TCTGCTGTGGTGGTGAATGA-3′ (reverse primer) | 20 |
| SAE (g/L) | Plant Height(cm) | No. of Leaves (n) | No. of Roots (n) | Root Length (cm) | Leaves Dried Weight (g) | Roots Dried Weight (g) |
|---|---|---|---|---|---|---|
| Control | 97.5 b * | 9.50 b | 19.25 b | 12.25 b | 0.825 b | 9.60 b |
| SAE (1) | 145 a | 13.67 ab | 65.00 a | 21.67 a | 3.63 a | 38.77 a |
| SAE (2) | 147 a | 19.00 a | 52.00 a | 18.17 a | 3.10 a | 46.63 a |
| SAE (3) | 155 a | 13.25 ab | 56.50 a | 20.50 a | 3.35 a | 42.15 a |
| SAE (g/L) | No. of Rhizomes (n) | Rhizome Dried Weight (g) | Rhizome Diameters (mm) |
|---|---|---|---|
| Control | 3.25 b * | 11.35 b | 14.58 b |
| SAE (1) | 12.67 a | 14.57 ab | 18.11 a |
| SAE (2) | 19.00 a | 21.27 a | 19.46 a |
| SAE (3) | 14.75 a | 16.18 ab | 17.93 a |
| SAE (g/L) | Chl a (mg/100 g F.W.) | Chl b (mg/100 g F.W.) | Carotenoids (mg/100 g F.W.) |
|---|---|---|---|
| Control | 62.23 b * | 28.34 b | 78.99 b |
| SAE (1) | 69.96 b | 28.97 b | 79.54 b |
| SAE (2) | 82.59 a | 33.62 ab | 94.94a b |
| SAE (3) | 87.09 a | 38.44 a | 100.31 a |
| SAE (g/L) | N% | P (ppm) | K (ppm) |
|---|---|---|---|
| Control | 16.14 a * | 0.0399 a | 11.45 a |
| SAE (1) | 13.66 a | 0.0199 b | 9.57 bc |
| SAE (2) | 18.90 a | 0.0245 b | 10.65 ab |
| SAE (3) | 15.55 a | 0.0205 b | 9.41 c |
| Phytochemical | Composition (Area %) | |||
|---|---|---|---|---|
| Control | SEA (1 g) | SEA (2 g) | SEA (3 g) | |
| (-)-alpha-cedrene | ND | ND | ND | 1.33 |
| (-)-Zingiberene | ND | 0.27 | 0.40 | ND |
| (1,1-Dimethyldodecyl)benzene | ND | ND | ND | 7.12 |
| 2,4,4-Trimethyl-3-(3-methylbutyl)cyclohex-2-enone | 1.69 | 1.83 | 2.30 | 4.00 |
| 3-Methyl-but-2-enoic acid, 1,7,7-trimethyl-bicyclo[2.2.1]hept-2-yl ester | 0.18 | ND | ND | ND |
| 4-Fluorophenol | 0.39 | 0.16 | ND | ND |
| 4-Hydroxy-3-methylacetophenone | 0.09 | 3.48 | 3.63 | ND |
| 4-Propylphenol | ND | 0.07 | ND | ND |
| alpha.-Curcumene | 1.01 | 1.35 | 2.08 | 1.13 |
| Ar-tumerone | 0.69 | 49.29 | 43.52 | 42.2 |
| Benzene, (1,1-dimethylnonyl)- | ND | 1.49 | ND | 1.70 |
| Benzene, 1,4-dimethyl-2-(2-methylpropyl)- | ND | 2.03 | ND | ND |
| beta.-Sesquiphellandrene | 1.64 | ND | ND | ND |
| Caryophyllene | 49.05 | 0.22 | 0.29 | 0.16 |
| Cedr-8(15)-ene | ND | 1.45 | 2.14 | ND |
| cis oleic acid | ND | ND | 0.56 | 1.35 |
| cis-Linoleic acid | ND | 0.77 | 0.72 | 0.90 |
| Coumaran | 0.86 | 1.28 | 1.75 | 1.64 |
| cuminone | ND | ND | ND | 0.26 |
| Curlone | 5.35 | 27.92 | 21.28 | 22.25 |
| Cyclohexanecarboxylic acid, 3-phenylpropyl ester | 22.02 | ND | ND | ND |
| Germacron | 2.66 | ND | ND | ND |
| Humulane-1,6-dien-3-ol | ND | ND | 1.19 | ND |
| Linoleic acid ethyl ester | ND | ND | 0.70 | 0.97 |
| M-cymene | 4.30 | ND | ND | ND |
| Oleic Acid | ND | 0.68 | ND | ND |
| Oleic acid, ethyl ester | ND | 0.56 | 0.30 | 0.70 |
| Palmetic acid | ND | 2.70 | ND | ND |
| Pentadecanoic acid | ND | 1.52 | ND | 2.72 |
| Thymol | ND | 0.09 | 0.21 | 0.04 |
| Tumerone | 3.49 | 1.06 | 7.12 | 5.33 |
| Vanillin | 3.07 | 0.78 | 0.89 | 0.87 |
| SAE (g/L) | Bisdemethoxycurcumin (ug/mL) | Demethoxycurcumin (ug/mL) | Curcumin (ug/mL) |
|---|---|---|---|
| Control | 210.392 d * | 156.008 d | 559.867 d |
| SAE (1) | 558.227 b | 358.859 b | 1190.234 b |
| SAE (2) | 651.199 a | 422.195 a | 1300.827 a |
| SAE (3) | 276.799 c | 231.947 c | 857.027 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Dayel, M.F.; El Sherif, F. Spirulina platensis Foliar Spraying Curcuma longa Has Improved Growth, Yield, and Curcuminoid Biosynthesis Gene Expression, as Well as Curcuminoid Accumulation. Horticulturae 2022, 8, 469. https://doi.org/10.3390/horticulturae8060469
Al Dayel MF, El Sherif F. Spirulina platensis Foliar Spraying Curcuma longa Has Improved Growth, Yield, and Curcuminoid Biosynthesis Gene Expression, as Well as Curcuminoid Accumulation. Horticulturae. 2022; 8(6):469. https://doi.org/10.3390/horticulturae8060469
Chicago/Turabian StyleAl Dayel, Munirah F., and Fadia El Sherif. 2022. "Spirulina platensis Foliar Spraying Curcuma longa Has Improved Growth, Yield, and Curcuminoid Biosynthesis Gene Expression, as Well as Curcuminoid Accumulation" Horticulturae 8, no. 6: 469. https://doi.org/10.3390/horticulturae8060469
APA StyleAl Dayel, M. F., & El Sherif, F. (2022). Spirulina platensis Foliar Spraying Curcuma longa Has Improved Growth, Yield, and Curcuminoid Biosynthesis Gene Expression, as Well as Curcuminoid Accumulation. Horticulturae, 8(6), 469. https://doi.org/10.3390/horticulturae8060469






