Abstract
Changes in physicochemical traits, peel colour, and juice attributes of four blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) were evaluated during 180 days at 2 and 5 °C plus 2 days at 20 °C for shelf life. ‘Tarocco’ had the lowest weight and firmness losses at both temperatures during storage. Titratable acidity (TA) at 5 °C was higher than 2 °C, with ‘Sanguinello’ and ‘Tarocco’ showing the highest and lowest TA, respectively. Juice content decreased during storage at both temperatures, although ‘Sanguinello’ had the highest juice content among the tested cultivars. Peel colour parameters including L* (lightness), b*, hue angle (h°), and chroma (C*) decreased during cold storage, while a* and citrus colour index (CCI) increased in all cultivars at both temperatures. The order for CCI was ‘Tarocco’ > ‘Moro’ > ‘Sanguinello’ > ‘Sanguine’. Overall, prolonged storage at 5 °C was considered as optimum temperature for all cultivars, although ‘Sanguinello’ cultivar had a better aptitude for the citrus juice industry.
1. Introduction
Blood oranges (Citrus sinensis L. Osbeck), also called red oranges or pigmented sweet oranges, appeared as the result of a spontaneous bud mutation [1]. Blood oranges consist of different cultivars, accession, and hybrids that exhibit different ranges of anthocyanin pigment in the flesh under the same climate conditions and cultural practices [2]. Blood orange fruits synthesize anthocyanin pigment mainly in the flesh and sometimes in the peel even after harvesting at cold storage. Anthocyanin content has been considered as an important quality index of blood orange fruit [3]. The presence of anthocyanin has increased the popularity of blood orange fruit among consumers due to the antioxidant activity of this pigment and other bioactive compounds such as ascorbic acid, hydroxycinnamic acids, and flavonoids [4]. These compounds make blood orange fruit the most valuable citrus fruit for health-related properties by preventing some human diseases including cancer, arteriosclerosis, and cardiovascular disease [5].
Cold temperature is one of the most important tools of extending the storage life of citrus fruit, in which postharvest temperature management is an important technique for the citrus fruit industry [6]. A suitable storage period is useful for maintaining the quality and postharvest life of blood orange fruit [7]. In addition, the best fruit quality not only depends on the storage period but also the requirement for the appropriate storage temperature [8]. For example, some attributes of citrus fruit can be affected by storage period and temperatures including weight loss, firmness, biochemical attributes of juice, and peel colour [4]. Recently, it has been reported that some aroma volatile compounds of blood orange are affected during cold storage [9].
‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’ are the most important commercial cultivars of blood oranges. Besides the different levels of anthocyanin of these cultivars, fruit size, and peel thickness, there are distinct pomological characteristics at commercial maturity [7]. In addition, different physiological and biochemical attributes can influence fruit taste and flavour [4]. It has been reported that bioactive compounds, biochemical metabolism, and sensory attributes of blood orange cultivars were affected under low temperatures. For example, bioactive compounds, antioxidant activity, and nutritional quality of blood orange cultivars at two storage temperatures were different among cultivars [7]. In addition, a comparative study on chilling tolerance of blood orange cultivars showed that ‘Moro’ and ‘Sanguinello’ had the highest and the lowest chilling injury symptoms during 180 days of cold storage at 2 °C and 5 °C due to differences in osmoregulation and antioxidant enzyme activity [10]. In addition, fatty acid composition in the peel of these cultivars was correlated to chilling tolerance. ‘Moro’ as a cold-sensitive cultivar had the highest saturated fatty acid (SFA) content, the lowest unsaturated fatty acid (UFA) content, and UFA/SFA ratio, while ‘Sanguinello’ had the highest UFA content, UFA/SFA ratio, and the lowest SFA content [11]. The comparative evaluation of ‘Tarocco’ clonal selections showed that there were significant differences in physiological disorders (such as chilling injury and senescence), weight loss, firmness, total anthocyanin content, juice yield, total soluble solids (TSS), titratable acidity (TA), juice pH, and pulp and peel colour [12].
Information about physicochemical changes of these cultivars during prolonged cold storage still was not reported. With distinct pomological characteristics of blood orange cultivars, it is probable to have different postharvest physiological behaviour during cold storage and use this information for the management of their storability and postharvest life. This is the first report about changes in physicochemical attributes of four blood orange cultivars. Therefore, the objective of this study was the comparison of physicochemical attributes and their changes in ‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’ cultivars at 2 and 5 °C during long-term storage.
2. Materials and Methods
2.1. Plant Material and Storage Conditions
Blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) were harvested from a commercial citrus orchard of Dashtenaz company in Sari (36.5659° N, 53.0586° E), Mazandaran province, Iran. Trees were seven years old and grafted on ‘C-35′ citrange (Citrus sinensis L. Osbeck × Poncirus trifoliata L. Raf.) rootstock. All trees were grown under the same conditions and cultural practices. Fruit were immediately transported to the postharvest laboratory, checked for defects or rind injuries for each cultivar, disinfected with 2% sodium hypochlorite (NaOCl) solution, and rinsed with distilled water. Fruit were divided into sets of three replicates of five fruit for each replicate, placed in a polyethylene bag containing 16 holes, and separately stored 180 days at 2 and 5 °C and 90%) relative humidity (RH). The following parameters were measured at 0, 30, 60, 90, 120, 150, and 180 days of cold storage plus 2 days at 20 °C (shelf life).
2.2. Weight Loss
Fruit weight loss was determined by weighing before storage (W1) and at each sampling time (W2). Percentage of weight loss was reported with the following formula [4]:
2.3. Firmness
The firmness of each cultivar was determined with a texture analyser (TA-XT2, UK) equipped with a 3.5 mm diameter probe and equatorial area of fruit compressed 10% [13]. Data were reported as Newton (N).
2.4. Chemical Attributes of Juice
Total soluble solids (TSS) were measured with a hand-held refractometer (TI-RBX0032A, Singapore) and reported as a percentage (%). Titratable acidity (TA) was measured by titration with NaOH 0.1 N to pH 8.2 as an endpoint with a pH meter (Jenway 351, UK). Maturity index (TSS/TA) was expressed as the ratio of TSS to TA. Juice pH was measured by pH meter [14].
2.5. Juice Content
To measure the juice content, fruit was weighed with digital balance. Then, the juice of each cultivar was squeezed from hand-peeled fruit and weighed. Fruit juice content was calculated using the following formula [8]:
2.6. Peel Colour
The peel colour of cultivars was measured on two opposite sides of the equatorial area of 15 fruits using a colourimeter (CR400/4P, Minolta Camera Co., Japan) as L* (lightness, 0 = black to 100 = white), a* (green (−) to red (+)), and b* (blue (−) to yellow (+)) [15]. Hue angle (h°), chroma (C*), and citrus colour index (CCI) were calculated using the following formulas [16]:
2.7. Statistical Analysis
The experiment was conducted according to a completely randomized design (CRD) with three biological replicates. Data were analysed using three-factor (cultivar, temperature, and storage time) analysis of variance (ANOVA). Data analyses were performed with SAS software package v. 9.4 for Windows. Mean comparisons were done by the least significant difference (LSD) test (p < 0.05) with standard errors (SEs) of means.
3. Results
3.1. Weight Loss
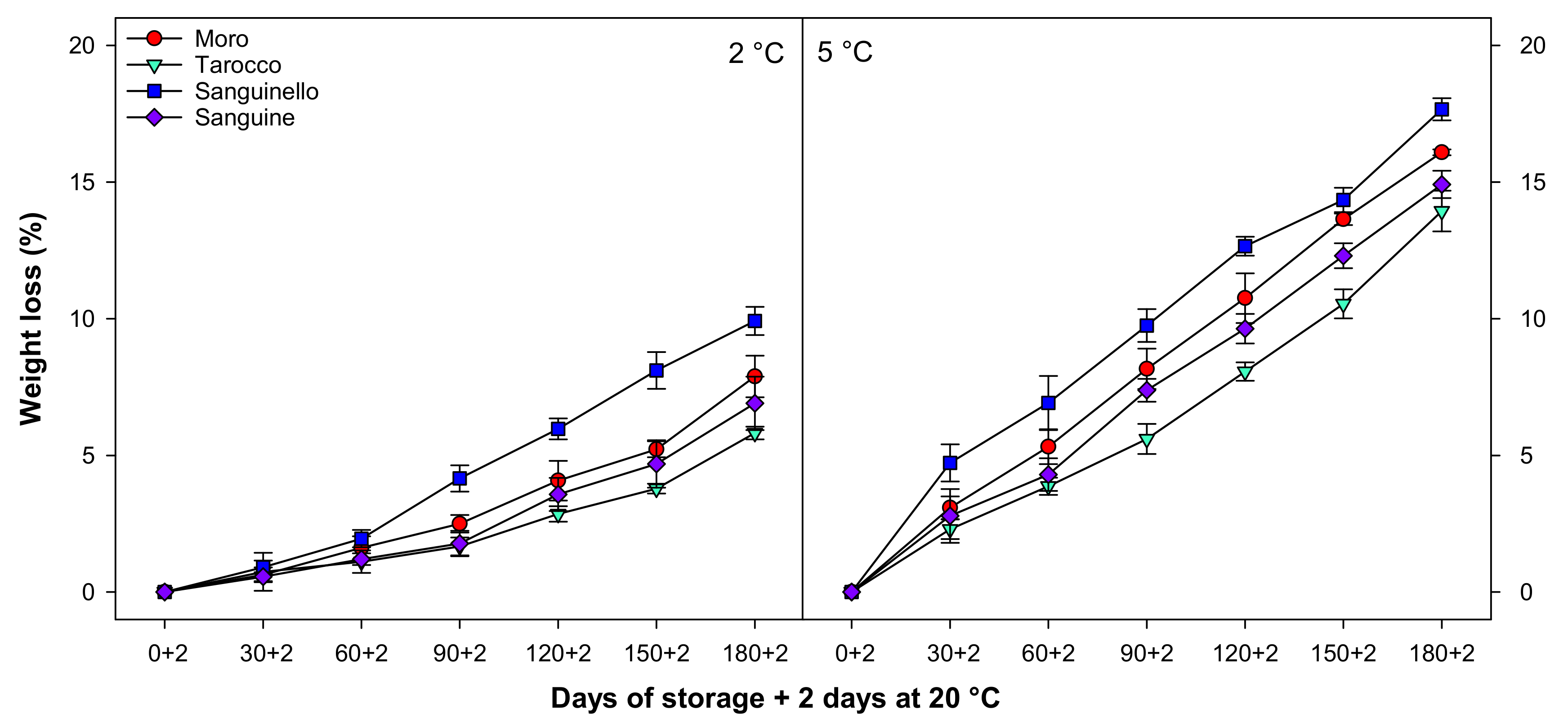
Fruit weight loss gradually increased in all cultivars at both temperatures (Figure 1). Weight loss was significantly higher at 5 than at 2 °C for all blood orange cultivars. The trend of weight loss for each cultivar was the same at both temperatures. ‘Sanguinello’ had significantly higher weight loss at the end of cold storage. However, the lowest weight loss was observed in ‘Tarocco’ cultivar at both temperatures. The order of weight loss was ‘Sanguinello’ > ‘Moro’ > ‘Sanguine’ > ‘Tarocco’.
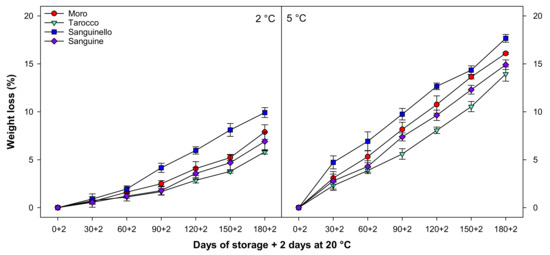
Figure 1.
Changes in weight loss of blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) during cold storage at 2 and 5 °C and 90% relative humidity (RH) plus 2 days at 20 °C. Vertical bars represent ± standard error (SE) of means. LSD (p < 0.05) value is 0.95.
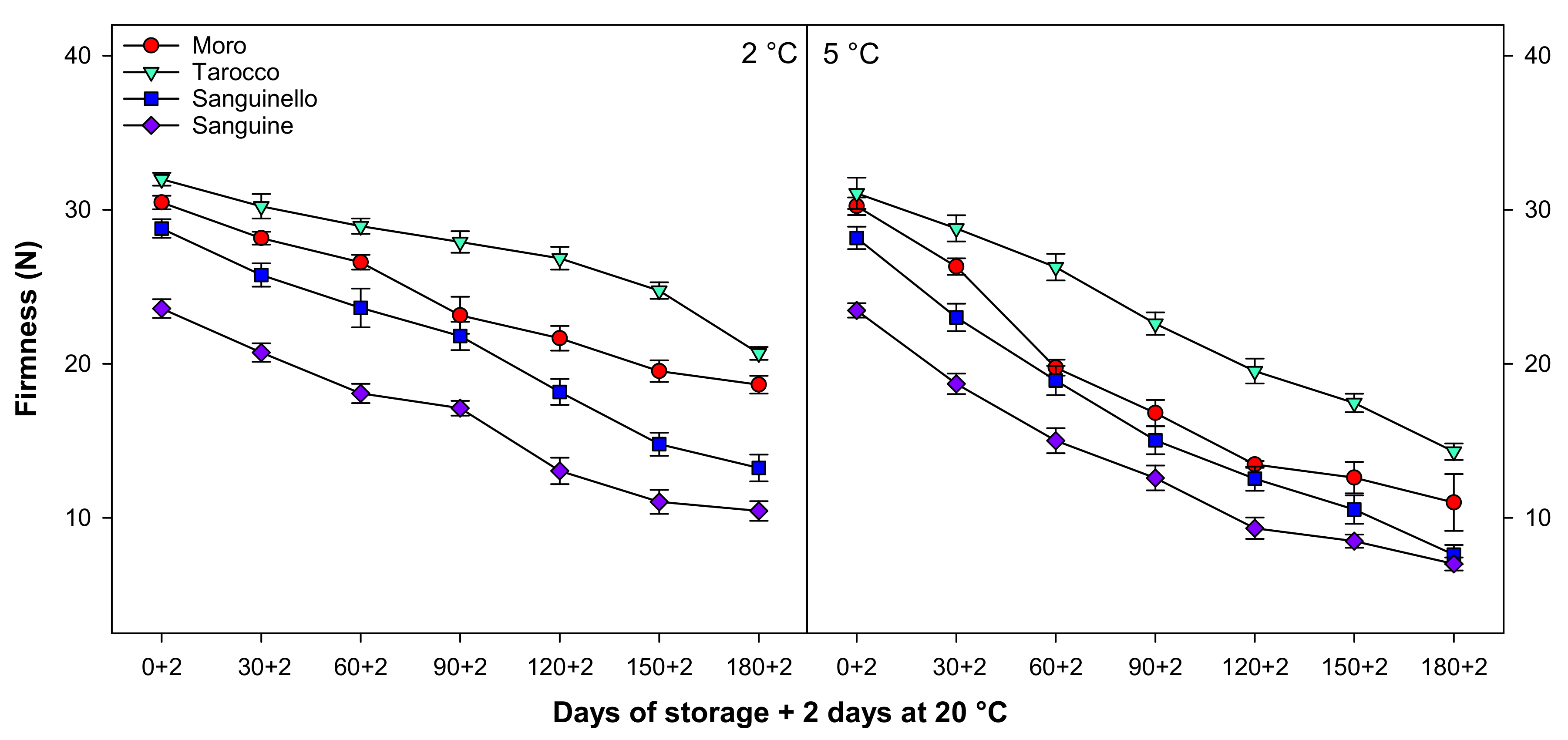
3.2. Firmness
Fruit firmness significantly decreased with a similar trend at both temperatures during cold storage (Figure 2). Firmness at 2 °C was 19.22% higher than at 5 °C, with ‘Tarocco’ having higher firmness than ‘Moro’, ‘Sanguinello’, and ‘Sanguine’ cultivars. The lowest firmness was observed in ‘Sanguine’ cultivar at both temperatures. ‘Tarocco’ had higher firmness than ‘Moro’ (9.81%), ‘Sanguinello’ (35.97%), and ‘Sanguine’ (49.46%) at 2 °C at the end of storage.
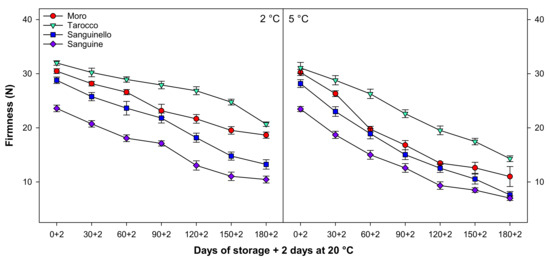
Figure 2.
Changes in firmness of blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) during cold storage at 2 and 5 °C and 90% relative humidity (RH) plus 2 days at 20 °C. Vertical bars represent ± standard error (SE) of means. LSD (p < 0.05) value is 2.03.
3.3. Chemical Attributes of Juice
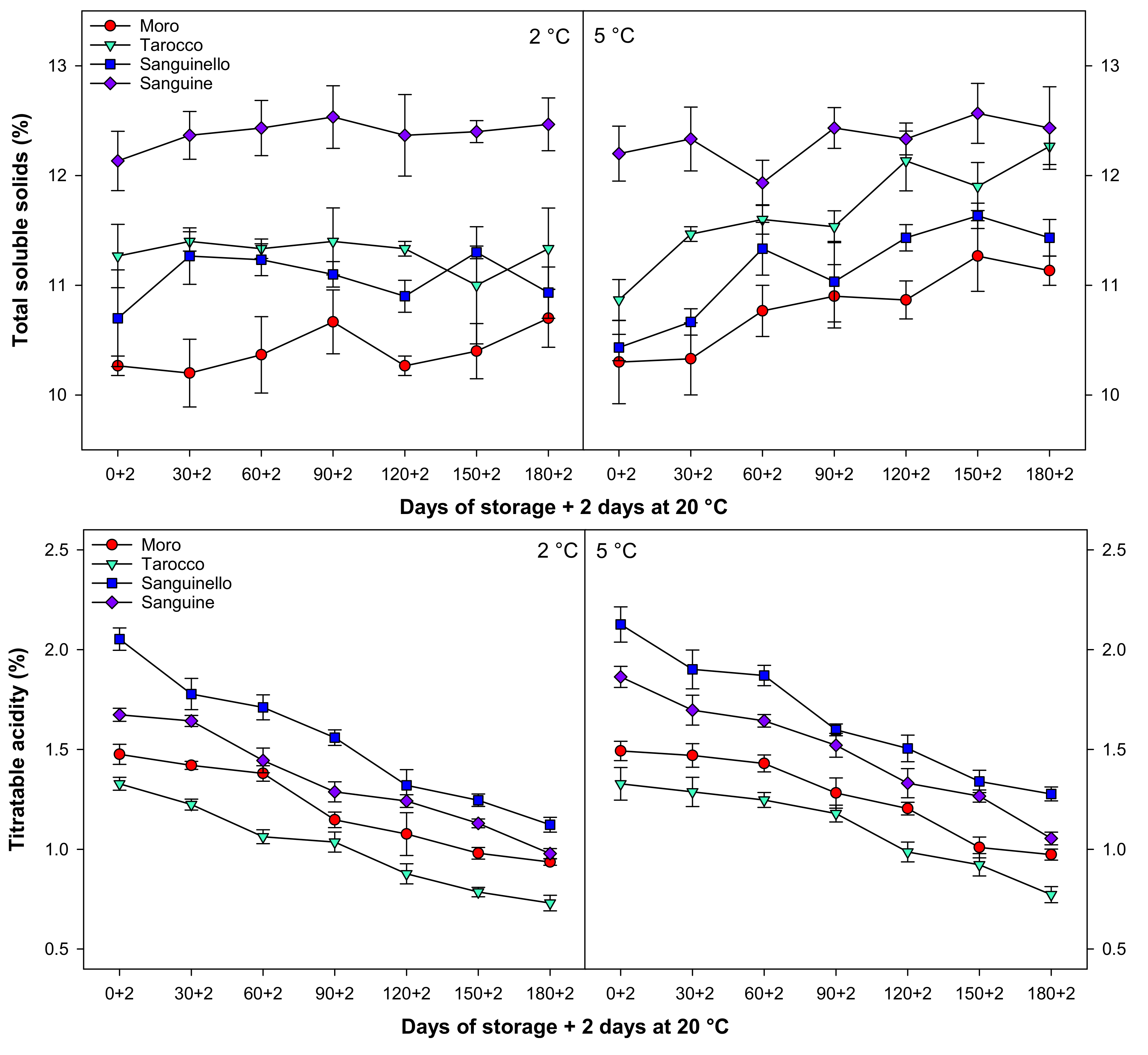
Total soluble solids (TSS) slightly increased during cold storage at both temperatures (Figure 3). ‘Sanguine’ had higher TSS at all sampling times at both temperatures, the highest TSS being found at 5 °C after 150 days of storage. The lowest TSS was observed in ‘Moro’ cultivar at both temperatures among cultivars.
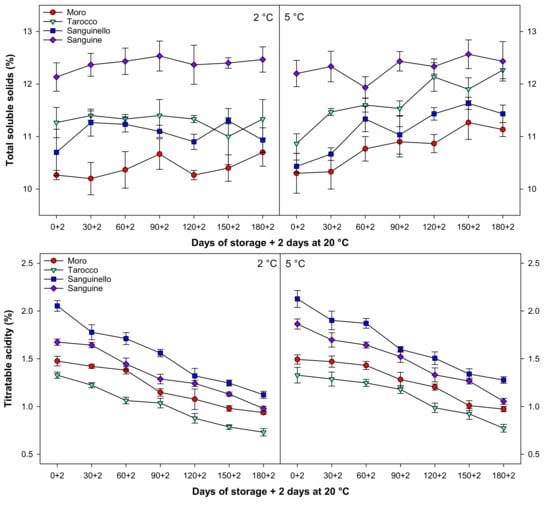
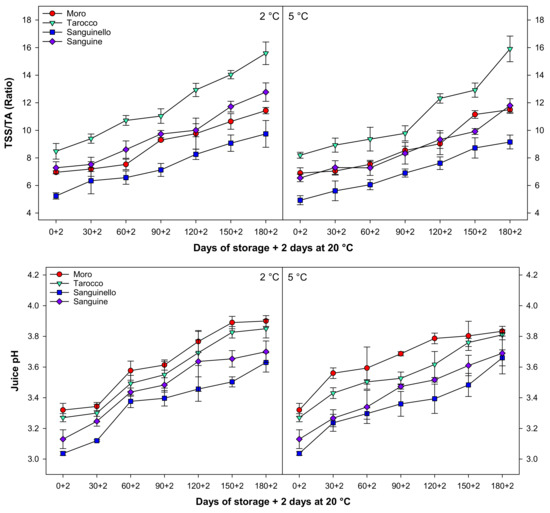
Figure 3.
Changes in total soluble solids (TSS, %), titratable acidity (TA, %), TSS/TA, and juice pH of blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) during cold storage at 2 and 5 °C and 90% relative humidity (RH) plus 2 days at 20 °C. Vertical bars represent ± standard error (SE) of means. LSD (p < 0.05) values are 0.87, 0.16, 1.51, and 0.17, respectively.
Titratable acidity (TA) was affected by cultivars, storage times, and temperatures (Figure 3). TA decreased in blood orange cultivars to the end of storage at both temperatures. However, TA at 5 °C was 7.58% higher than 2 °C. Among cultivars, ‘Sanguinello’ had the higher TA while the lowest TA was observed in ‘Tarocco’ at both temperatures. TA in ‘Sanguinello’ was 23.77, 39.48, and 17.46% higher than ‘Moro’, ‘Tarocco’, and ‘Sanguine’ cultivars, respectively, at 5 °C at the end of storage.
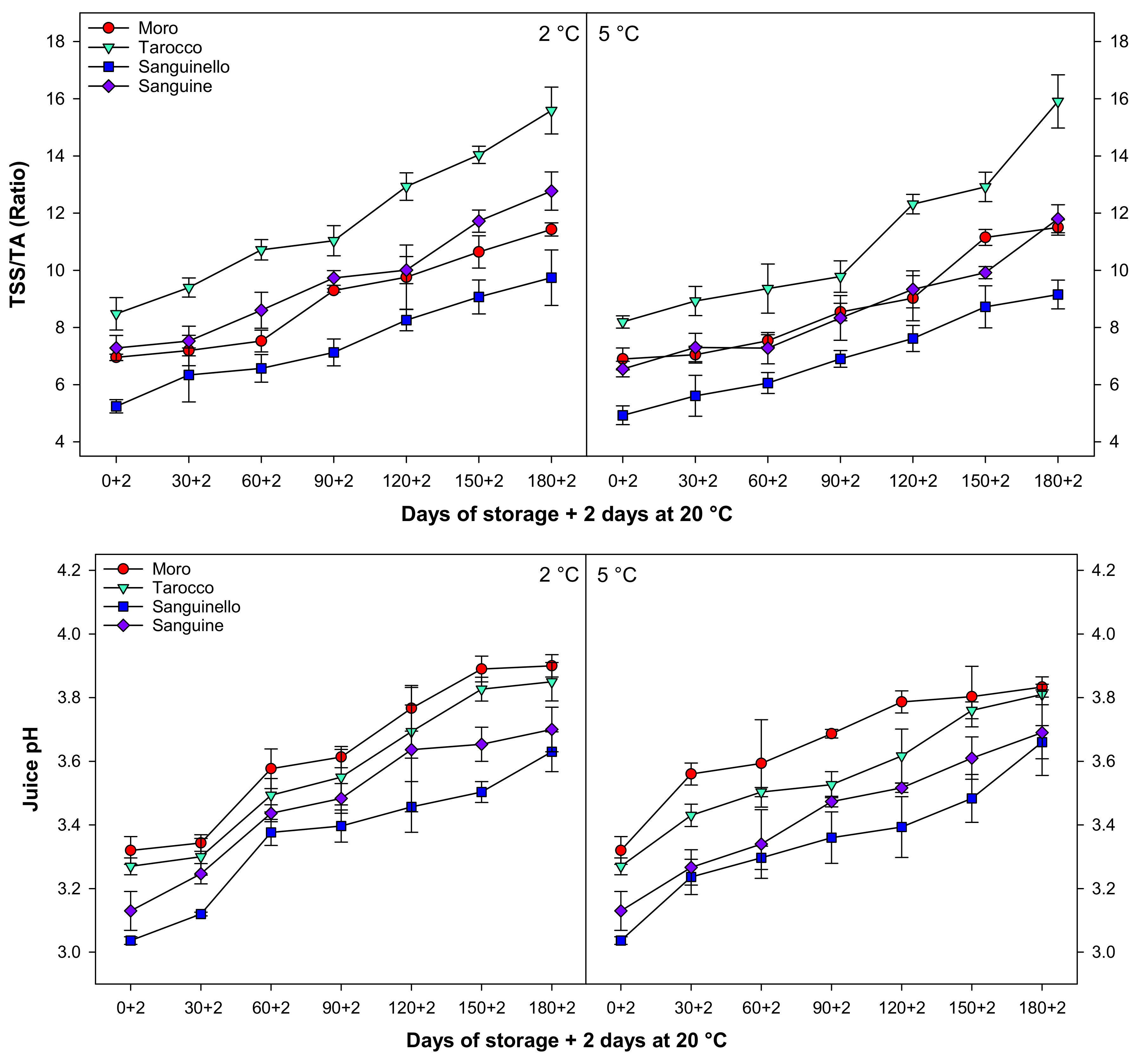
The ratio of TSS/TA significantly increased for all cultivars with a similar trend during cold storage at both temperatures (Figure 3). TSS/TA at 5 °C was 6.17% higher than 2 °C. ‘Tarocco’ had higher TSS/TA than ‘Moro’, ‘Sanguinello’, and ‘Sanguine’ cultivars. The lowest TSS/TA was observed in ‘Sanguinello’ cultivar at both temperatures. However, there was no significant difference between ‘Moro’ and ‘Sanguine’ for TSS/TA.
Juice pH gradually increased for all cultivars during cold storage with the same trend at both temperatures (Figure 3). The lowest and highest juice pH were in ‘Sanguinello’ and ‘Moro’, respectively. The order of juice pH in cultivars was ‘Moro’ > ‘Tarocco’ > ‘Sanguine’ > ‘Sanguinello’.
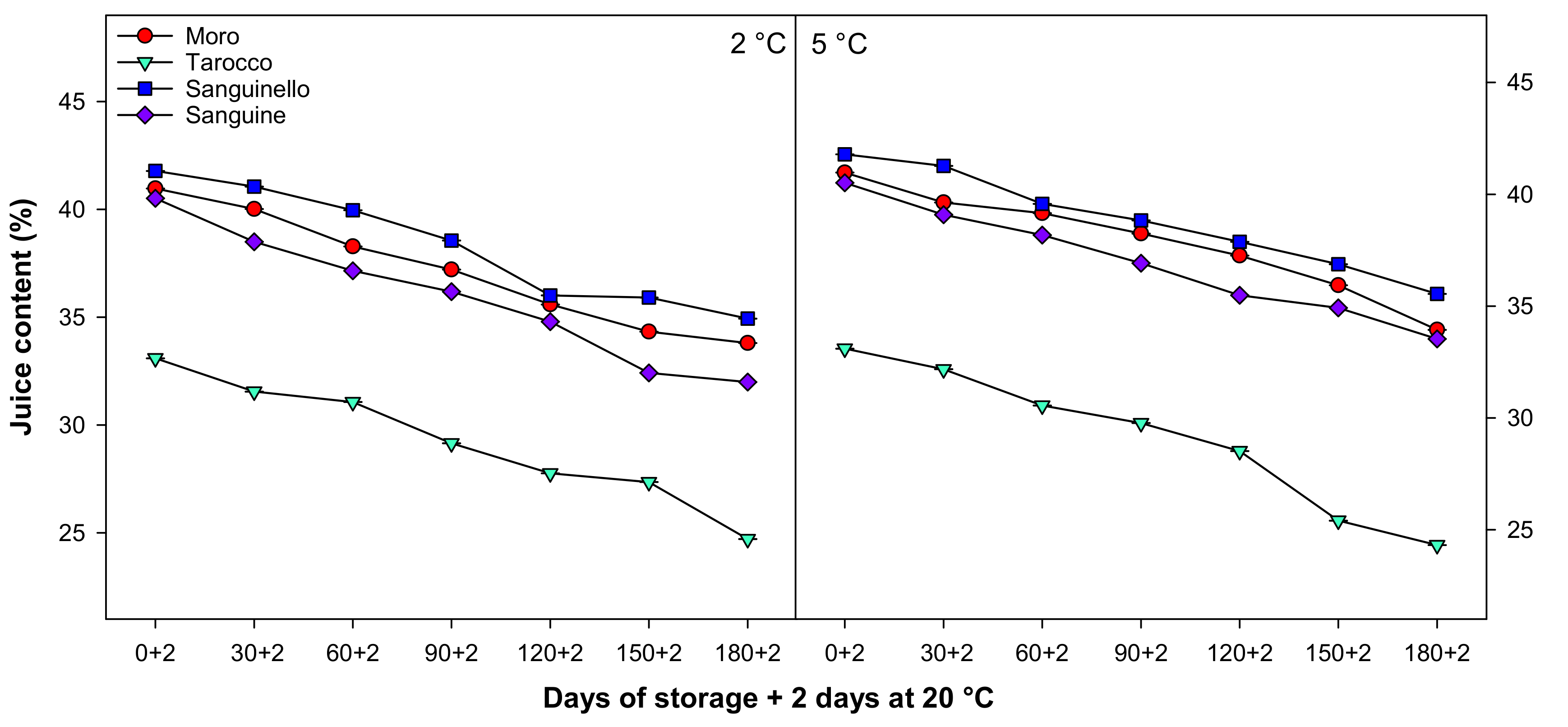
3.4. Juice Content
Juice content decreased during cold storage at both temperatures for all cultivars (Figure 4). The order of juice content was ‘Sanguinello’ > ‘Moro’ > ‘Sanguine’ > ‘Tarocco’, although juice content at 5 °C was higher than 2 °C, with ‘Tarocco’ having the lowest juice content at the end of cold storage.
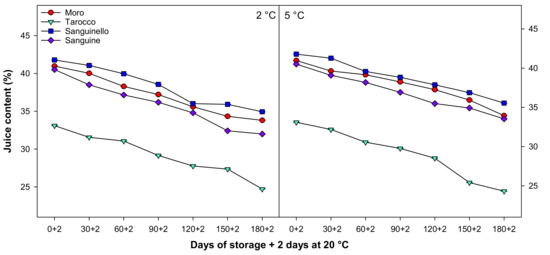
Figure 4.
Changes in juice content of blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) during cold storage at 2 and 5 °C and 90% relative humidity (RH) plus 2 days at 20 °C. Vertical bars represent ± standard error (SE) of means. LSD (p < 0.05) value is 1.13.
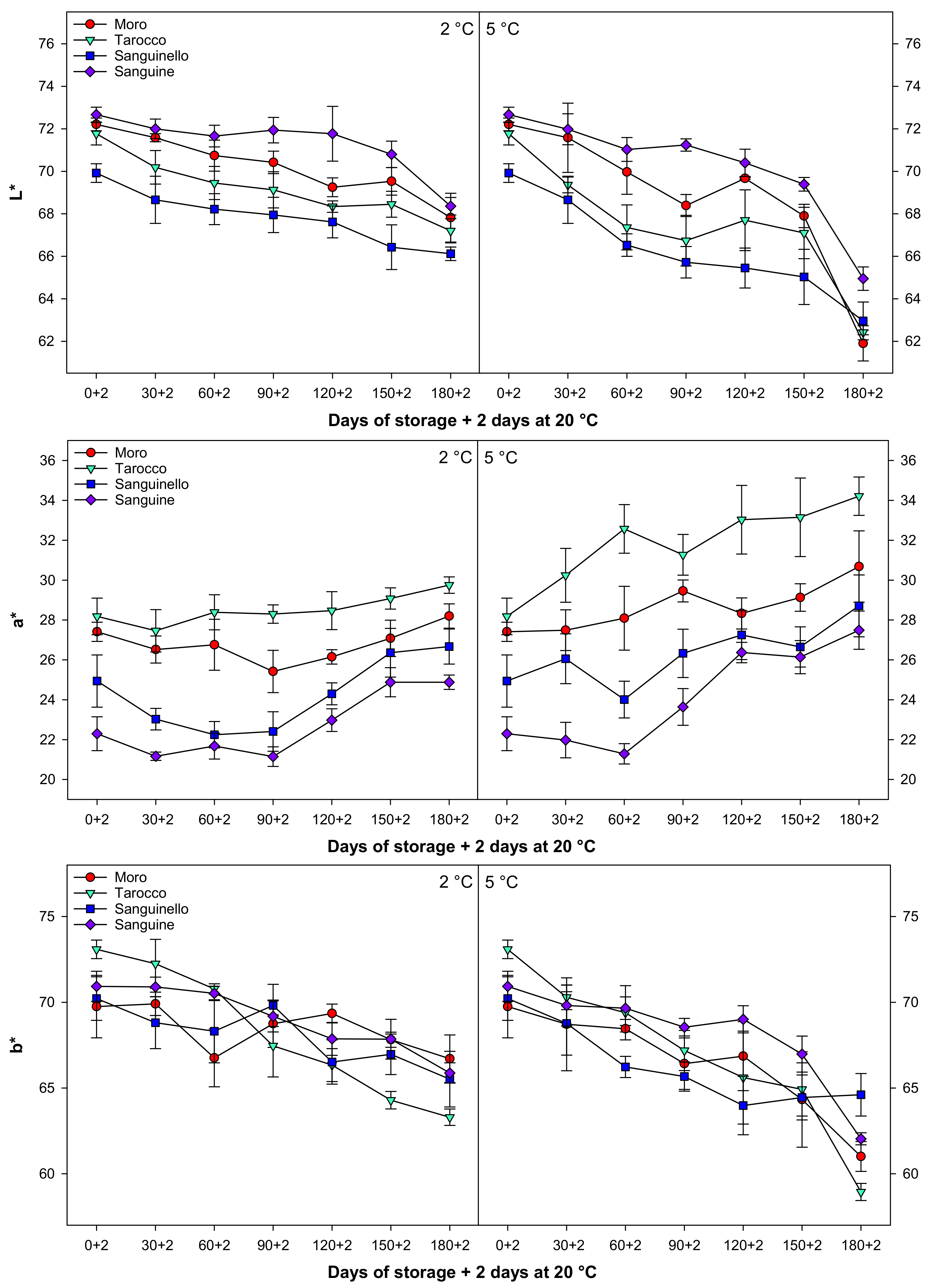
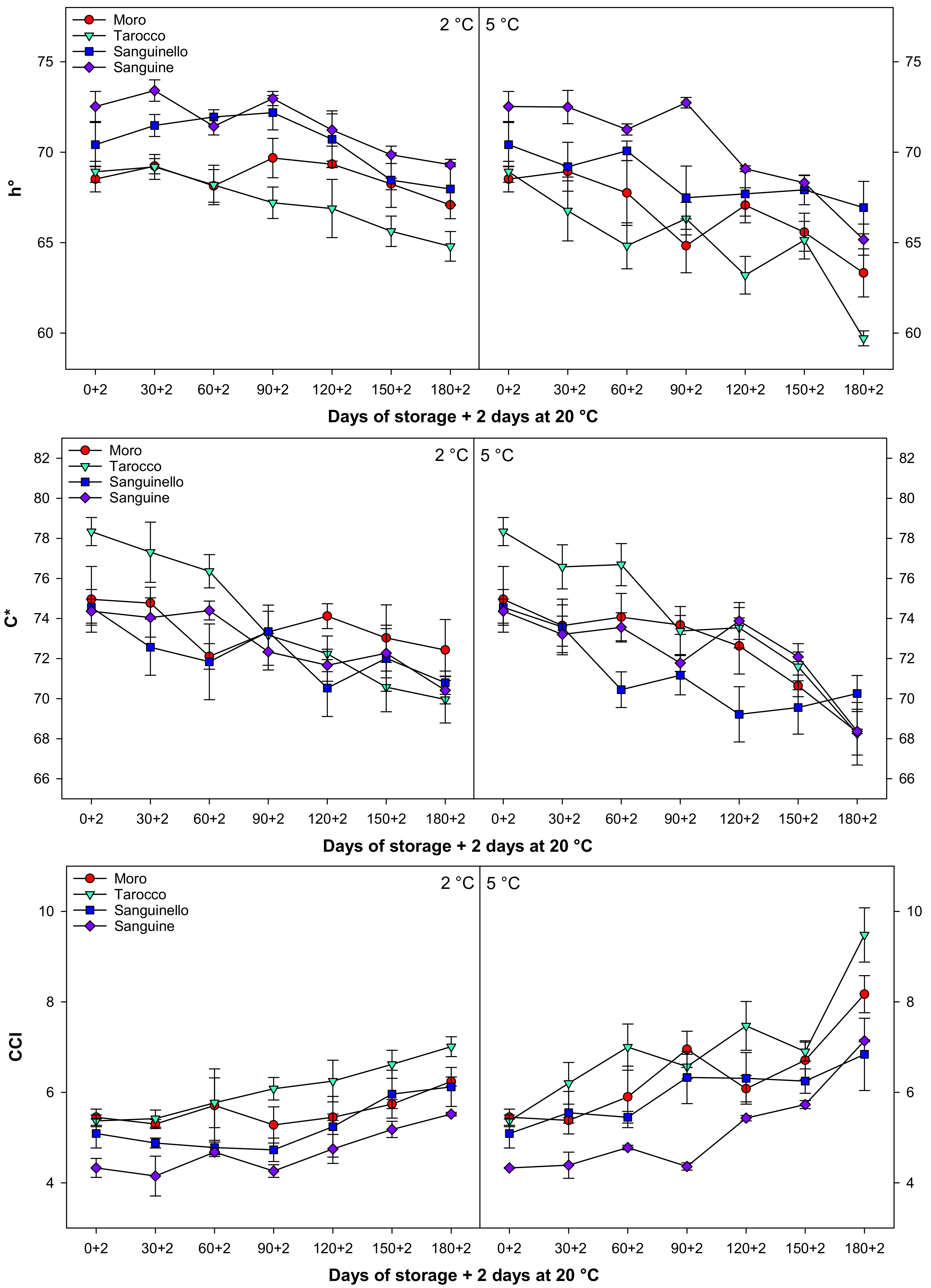
3.5. Peel Colour
Colour parameters, L*, a* b*, h°, C*, and CCI changed during cold storage at both temperatures (Figure 5 and Figure 6). In this study, L*, b*, h°, and C* decreased during cold storage, while a* increased for all cultivars at both temperatures. Colour parameter L* value decreased along with storage by the same trends at both temperatures for all cultivars, the reduction of L* at 5 °C being higher than at 2 °C. The b* decreased at both temperatures, and this reduction was more pronounced in ‘Tarocco’ (Figure 5). The h° value decreased during storage, and ‘Sanguine’ and ‘Tarocco’ had the highest and the lowest h° value, respectively, at both temperatures. CCI increased during cold storage at both temperatures for all cultivars (Figure 6). The increase of CCI at 5 °C was 11.8% higher than at 2 °C. Overall, the order of CCI was ‘Tarocco’ > ‘Moro’ > ‘Sanguinello’ > ‘Sanguine’.
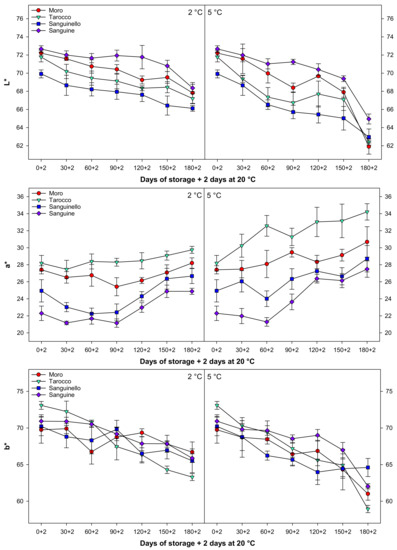
Figure 5.
Changes in L*, a*, and b* of blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) during cold storage at 2 and 5 °C and 90% relative humidity (RH) plus 2 days at 20 °C. Vertical bars represent ± standard error (SE) of means. LSD (p < 0.05) values are 1.45, 1.26, and 1.33, respectively.
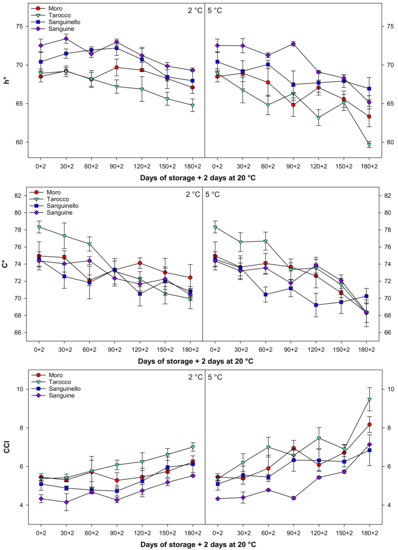
Figure 6.
Changes in hue angle (h°), chroma (C*), and citrus colour index (CCI) of blood orange cultivars (‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’) during cold storage at 2 and 5 °C and 90% relative humidity (RH) plus 2 days at 20 °C. Vertical bars represent ± standard error (SE) of means. LSD (p < 0.05) values are 1.43, 1.24, and 0.27, respectively.
4. Discussion
Changes in physicochemical attributes of blood orange cultivars would provide useful information for the evaluation of their storability based on differences in weight loss, firmness, peel colour, and chemical attributes of the juice during long-term cold storage. This information can create some knowledge for postharvest management of blood orange cultivars. In this study, weight loss increased for all cultivars at both temperatures, although weight loss at 2 °C was lower than at 5 °C. Water is the main component of fruit peel, and reducing moisture loss is effective for maintaining moisture content during cold storage. The loss of moisture is mainly due to water diffusion through the peel surface which can affect fruit weight loss by respiration and transpiration processes [17]. In addition, decreasing the natural wax of fruit during cold storage can increase transpiration and affect the gradient of water vapour pressure between fruit and the surrounding air. These blood orange cultivars had different fruit sizes and rind thicknesses [10]. Therefore, fruit surface/volume ratio, epidermis, and cuticle structure can show differences in the weight loss of cultivars, as has been reported in four plum cultivars [13]. In this study, ‘Tarocco’ had the lowest weight loss at the end of cold storage.
The firmness is one of the most important variables which allows for determining the acceptability of fruit [4]. Fruit firmness decreased during cold storage at both temperatures in all cultivars, although firmness loss at 2 °C was lower than at 5 °C. It seems that lower temperatures decreased metabolic activity and cell-wall-degrading enzymes of fruit. Softening is a result of the pectin depolymerization by the activity of cell-wall-degrading enzymes including polygalacturonase, pectin lyase, pectin methylesterase, and cellulose. Therefore, these changes in cell wall composition are contributing to firmness losses during cold storage [17]. In this study, the highest and the lowest firmness was found in ‘Tarocco’ and ‘Sanguine’ cultivars, respectively, at both temperatures. These differences among cultivars probably are associated with the activity of cell-wall-degrading enzymes as reported for plum cultivars [13]. In addition, the peel thickness of these cultivars was different which can affect fruit firmness [10]. Fruit firmness of sixteen ‘Tarocco’ clonal selections decreased during cold storage, and there was a significant difference among them [12].
Cellular metabolism of citrus fruit during cold storage needs to provide energy through glycolysis and the Krebs cycle. On the other hand, these processes can change the sugars and organic acids of citrus fruit by biosynthesis and catabolism [18]. TSS refer to the total amount of soluble constituents of the citrus fruit juice. TSS is an excellent index to the sugar content of citrus fruit since roughly 80% of their TSS are sugars (mainly sucrose, glucose, and fructose), 10% organic acids (mainly citric, malic, and ascorbic acids), and other components consist of vitamins, proteins, free amino acids, and glucosides [19]. In this study, ‘Sanguine’ and ‘Moro’ cultivars had the highest and the lowest TSS, respectively. Conversion of organic acids to sugars by glycolytic enzymes can increase the TSS [20]. However, in this study, TSS slightly increased at initial storage time and then remained unchanged in cultivars probably due to the reduction of sugars.
Titratable acidity (TA) decreased for all cultivars during storage at both temperatures, although fruit stored at 2 °C had lower TA in comparison with 5 °C. TA is related to the concentrations of organic acids. Organic acids are important components of citrus fruit juice, and their amount is different among species and cultivars of citrus fruit [21]. It has been reported that citric and malic acids are the main organic acids in blood orange fruit [4]. The different TA of cultivars at initial sampling time could be related to the H+-ATPase pump that can accumulate additional organic acid in citrus fruit especially citric acid [21]. The consumption of organic acids for the synthesis of sugars and ATP production can reduce TA during cold storage [22]. In addition, the senescence of citrus fruit at prolonged storage is related to the reduction of organic acids [7]. In this study, the reduction of organic acids at 2 °C was greater than 5 °C probably due to alcoholic fermentation at a lower temperature [20].
In this study, there was a difference among blood orange cultivars for TSS/TA ratio, and it increased during cold storage. Sugar and organic acid ratio levels are not only used as a harvest maturity indicator of citrus fruit but also to determine the taste and flavour index during cold storage [23]. Accordingly, ‘Tarocco’ and ‘Sanguinello’ cultivars had the highest and the lowest TSS/TA, respectively. Citric and malic acid levels are responsible for the acidity of citrus fruit, and conversion of organic acids during fruit respiration can change the TSS and TA ratio resulting in the different quality among citrus fruit cultivars [24]. Therefore, different changes in sugars and organic acids can be associated with the TSS/TA ratio of blood orange cultivars which had a different range. Overall, TSS and TA contents were sensitive to storage temperatures, and these changes could affect the TSS/TA ratio and flavour of cultivars.
In this study, juice pH increased during storage at both temperatures. The pH increasing is due to the biochemical activity of fruit, which causes the organic acid change to sugar products in the fruit. Citric acid is the main substrate in respiration and the dominant acid in citrus fruit, which is gradually reduced during the respiratory process and increases the pH of fruit juice [20]. Therefore, an increasing pH of fruit juice can be indicative of the consumption of organic acids during cold storage. In addition, enzyme activity of vacuolar ATPase (V-ATPase) and proton-pumping pyrophosphatase (H+-PPase) or vacuolar-type inorganic pyrophosphatase (V-PPas) pump protons across vacuole during ripening and fruit senescence, and there is a close correlation between the activities of these enzymes and pH. Therefore, the cold stress can slightly increase the gene expression of V-ATPase and H+-PPase or V-PPas, which can be related to the pH, which can enhance the pH at a lower temperature [25]. In addition, TA reduction is associated with an increase in juice pH [14].
Juice content is an important index for the assessment of citrus fruit quality [8]. In this study, juice content decreased in all cultivars during cold storage at both temperatures. Juice content at 5 °C was higher than at 2 °C. This is probably due to inducing vesicle drying at a colder temperature. The vesicle drying in ‘Satsuma’ and ‘Ponkan’ mandarins stored at a relatively higher temperature (>10 °C) did not happen [26]. In addition, juice content in ‘Ponkan’ mandarin was affected at different temperatures, transportation, and shelf-life conditions [8]. In this study, the difference of reduction in the juice content of cultivars probably was due to the different ability of maintenance of cell membrane integrity which can prevent drying of fruit juice vesicles by preventing the damage of sac-juice membranes under a low temperature [4].
Peel colour is one of the most important quality attributes of citrus fruit as a key factor for consumer acceptance. Peel colour in citrus species and cultivars is the result of three main group pigments including chlorophylls, carotenoids, and anthocyanins. The third group of pigments is anthocyanins providing red colour in blood oranges and mainly restricted to the flesh. Therefore, the differential accumulation of these pigments can create colour diversity in citrus fruit [27]. For example, citrus species and cultivars have diverse peel colour such as red in some grapefruit and shaddocks, intense orange in mandarins and oranges, yellow in lemons, and white in grapefruit [28]. The relationship between changes in colour and the storage temperature has only been performed in a few citrus species and cultivars.
Although citrus fruits are non-climacteric and they have negligible ethylene production and a low respiration rate during maturity and ripening, fruit peel does not follow this process, and peel colour changes can be continued after harvesting during cold storage [29]. In this study, peel colour in all cultivars changed at both temperatures. Lightness (L*) refers to the proportion of light reflected from the object on a scale of zero (black) to 100 (white). The L* value decreased at both temperatures in all cultivars, and ‘Sanguine’ had the highest L* value at 2 °C. The a* value is between green (−) and red (+). In this study, a* value had a positive value in blood orange cultivars and increased at both temperatures. The b* value is between blue (−) to yellow (+). The b* decreased at both temperatures. The reduction of b* value probably was due to increasing a* or fruit senescence after long-term storage [30]. The reduction of b* at 2 °C was lower than 5 °C. The change of colour and expression of carotenoid genes in ‘Navelina’ orange fruit was greater at 12 °C than 2 °C. Higher temperature increased total carotenoid content in both flesh and peel. Meanwhile, colour and carotenoid content remained almost unchanged in fruit stored at 2 °C. The authors suggested that enhancement in peel colour was associated with the increased expression of carotenoid genes during storage at 12 °C [28].
In this study, the h° value slightly decreased during storage at both temperatures. The h° value exhibits actual perceived colour including orange or green and is the primary variable in changes in orange colour. The h° is considered as the colour index with value ranges of red (0 or 360°), yellow (90°), green (180°), and blue (270°) colours [30]. In this study, h° values were between 60 and 70°. This colour range is orange-yellow to yellow as evaluated in all cultivars. ‘Sanguine’ and ‘Tarocco’ had the highest and the lowest h° value during storage at both temperatures. Chroma (C*) or saturation index quantifies the intensity or saturation of colour [30]. The C* decreased during cold storage. CCI increased in all cultivars at both temperatures. The CCI in ‘Ponkan’ mandarin increased at different storage temperatures, and the increment of CCI was higher than at 15 °C in comparison with 5 °C during 4 months of storage [8]. In addition, peel colour in the ‘Satsuma’ mandarin remained unchanged or reduced at 5 °C, while carotenoid accumulation was induced at temperatures between 8 and 15 °C [31]. In this study, the changes in peel colour at 5 °C were higher than 2 °C. It seems that lower temperature reduced the biosynthesis or degradation of blood orange pigments.
5. Conclusions
This is the first comparative study of changes in physicochemical attributes of ‘Moro’, ‘Tarocco’, ‘Sanguinello’, and ‘Sanguine’ blood orange cultivars at different storage temperatures. In this study, lower temperature (2 °C) reduced weight loss, firmness loss, and peel colour changes. ‘Tarocco’ had the highest firmness at both temperatures. However, lower temperature was efficient on physical parameters, but the chemical attributes of juice were better at 5 °C. In addition, our study created a cultivar-specific protocol for the selection of the best storage duration based on physiochemical attributes for fresh consumption or juice processing after long-term storage.
Author Contributions
Conceptualization, F.H. and D.V.; methodology, F.H.; software, F.H.; validation, D.V., M.S., and F.G.; formal analysis, F.H.; investigation, F.H.; resources, D.V.; data curation, D.V., M.S., and F.G.; writing—original draft preparation, F.H.; writing—review and editing, D.V. and M.S.; visualization, D.V. and M.S.; supervision, D.V.; project administration, F.H. and D.V.; funding acquisition, D.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Shiraz University and University Miguel Hernández (UMH) for the sabbatical opportunity of Fariborz Habibi. In addition, we thank Dashtenaz company for providing blood orange cultivars.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Butelli, E.; Licciardello, C.; Zhang, Y.; Liu, J.; Mackay, S.; Bailey, P.; Reforgiato-Recupero, G.; Martin, C. Retrotransposons Control Fruit-Specific, Cold-Dependent Accumulation of Anthocyanins in Blood Oranges. Plant Cell 2012, 24, 1242–1255. [Google Scholar] [CrossRef] [Green Version]
- Piero, A.R.L. The State of the Art in Biosynthesis of Anthocyanins and Its Regulation in Pigmented Sweet Oranges [(Citrus sinensis) L. Osbeck]. J. Agric. Food Chem. 2015, 63, 4031–4041. [Google Scholar] [CrossRef]
- Rapisarda, P.; Bellomo, S.E.; Intelisano, S. Storage Temperature Effects on Blood Orange Fruit Quality. J. Agric. Food Chem. 2001, 49, 3230–3235. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Blood oranges maintain bioactive compounds and nutritional quality by postharvest treatments with γ-aminobutyric acid, methyl jasmonate or methyl salicylate during cold storage. Food Chem. 2020, 306, 125634. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Galvano, F.; Mistretta, A.; Marventano, S.; Nolfo, F.; Calabrese, G.; Buscemi, S.; Drago, F.; Veronesi, U.; Scuderi, A.G. Red Orange: Experimental Models and Epidemiological Evidence of Its Benefits on Human Health. Oxidative Med. Cell. Longev. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Habibi, F.; Ramezanian, A.; Rahemi, M.; Eshghi, S.; Guillén, F.; Serrano, M.; Valero, D. Postharvest treatments with γ -aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J. Sci. Food Agric. 2019, 99, 6408–6417. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Castillo, S.; Serrano, M.; Valero, D. Changes in Bioactive Compounds, Antioxidant Activity, and Nutritional Quality of Blood Orange Cultivars at Different Storage Temperatures. Antioxidants 2020, 9, 1016. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Wang, C.; Xu, S.; Chen, Y.; Wang, Y.; Li, X.; Sun, C. The effects of transportation temperature on the decay rate and quality of postharvest Ponkan (Citrus reticulata Blanco) fruit in different storage periods. Sci. Hortic. 2019, 247, 42–48. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Serrano, M.; Valero, D. Effect of Various Postharvest Treatment on Aroma Volatile Compounds of Blood Orange Fruit Exposed to Chilling Temperature After Long-Term Storage. Food Bioprocess Technol. 2020, 13, 2054–2064. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A.; Guillén, F.; Martínez-Romero, D.; Serrano, M.; Valero, D. Susceptibility of Blood Orange Cultivars to Chilling Injury Based on Antioxidant System and Physiological and Biochemical Responses at Different Storage Temperatures. Foods 2020, 9, 1609. [Google Scholar] [CrossRef]
- Habibi, F.; García-Pastor, M.E.; Guillén, F.; Serrano, M.; Valero, D. Fatty acid composition in relation to chilling susceptibility of blood orange cultivars at different storage temperatures. Plant Physiol. Biochem. 2021, 166, 770–776. [Google Scholar] [CrossRef]
- Strano, M.C.; Di Silvestro, S.; Allegra, M.; Russo, G.; Caruso, M. Effect of cold storage on the postharvest quality of different Tarocco sweet orange clonal selections. Sci. Hortic. 2021, 285, 110167. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of alginate edible coating on preserving fruit quality in four plum cultivars during postharvest storage. Postharvest Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Habibi, F.; Ramezanian, A. Vacuum infiltration of putrescine enhances bioactive compounds and maintains quality of blood orange during cold storage. Food Chem. 2017, 227, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moscoso-Ramírez, P.A.; Palou, L. Effect of ethylene degreening on the development of postharvest penicillium molds and fruit quality of early season citrus fruit. Postharvest Biol. Technol. 2014, 91, 1–8. [Google Scholar] [CrossRef]
- Zhou, J.-Y.; Sun, C.-D.; Zhang, L.-L.; Dai, X.; Xu, C.-J.; Chen, K.-S. Preferential accumulation of orange-colored carotenoids in Ponkan (Citrus reticulata) fruit peel following postharvest application of ethylene or ethephon. Sci. Hortic. 2010, 126, 229–235. [Google Scholar] [CrossRef]
- Valero, D.; Serrano, M. Postharvest Biology and Technology for Preserving Fruit Quality, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Sun, X.-H.; Xiong, J.-J.; Zhu, A.-D.; Zhang, L.; Ma, Q.-L.; Xu, J.; Cheng, Y.-J.; Deng, X.-X. Sugars and organic acids changes in pericarp and endocarp tissues of pumelo fruit during postharvest storage. Sci. Hortic. 2012, 142, 112–117. [Google Scholar] [CrossRef]
- Lado, J.; Rodrigo, M.J.; Zacarías, L. Maturity indicators and citrus fruit quality. Stewart Postharvest Rev. 2014, 10, 1–6. [Google Scholar]
- Rapisarda, P.; Bianco, M.L.; Pannuzzo, P.; Timpanaro, N. Effect of cold storage on vitamin C, phenolics and antioxidant activity of five orange genotypes [Citrus sinensis (L.) Osbeck]. Postharvest Biol. Technol. 2008, 49, 348–354. [Google Scholar] [CrossRef]
- Asencio, A.D.; Serrano, M.; Martínez, S.G.; Pretel, M.T. Organic acids, sugars, antioxidant activity, sensorial and other fruit characteristics of nine traditional Spanish Citrus fruits. Eur. Food Res. Technol. 2018, 244, 1497–1508. [Google Scholar] [CrossRef]
- Habibi, F.; Serrano, M.; Zacarías, L.; Valero, D.; Guillén, F. Postharvest Application of 24-Epibrassinolide Reduces Chilling Injury Symptoms and Enhances Bioactive Compounds Content and Antioxidant Activity of Blood Orange Fruit. Front. Plant Sci. 2021, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Lado, J.; Gambetta, G.; Zacarias, L. Key determinants of citrus fruit quality: Metabolites and main changes during maturation. Sci. Hortic. 2018, 233, 238–248. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; He, W.; Zheng, W.; Tan, Q.; Xie, Z.; Zheng, C.; Hu, C. Fruit sugar and organic acid were significantly related to fruit Mg of six citrus cultivars. Food Chem. 2018, 259, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Marsh, K.; Attanayake, S.; Walker, S.; Gunson, A.; Boldingh, H.; MacRae, E. Acidity and taste in kiwifruit. Postharvest Biol. Technol. 2004, 32, 159–168. [Google Scholar] [CrossRef]
- Cao, J.; Kang, C.; Chen, Y.; Karim, N.; Wang, Y.; Sun, C. Physiochemical changes in Citrus reticulata cv. Shatangju fruit during vesicle collapse. Postharvest Biol. Technol. 2020, 165, 111180. [Google Scholar] [CrossRef]
- Rodrigo, M.J.; Alquezar, B.; Alos, E.; Lado, J.; Zacarias, L. Biochemical bases and molecular regulation of pigmentation in the peel of Citrus fruit. Sci. Hortic. 2013, 163, 46–62. [Google Scholar] [CrossRef]
- Carmona, L.; Zacarías, L.; Rodrigo, M.J. Stimulation of coloration and carotenoid biosynthesis during postharvest storage of ‘Navelina’ orange fruit at 12 °C. Postharvest Biol. Technol. 2012, 74, 108–117. [Google Scholar] [CrossRef]
- Ramezanian, A.; Dadgar, R.; Habibi, F. Postharvest Attributes of “Washington Navel” Orange as Affected by Preharvest Foliar Application of Calcium Chloride, Potassium Chloride, and Salicylic Acid. Int. J. Fruit Sci. 2017, 18, 68–84. [Google Scholar] [CrossRef]
- Van Wyk, A.A.; Huysamer, M.; Barry, G.H. Extended low-temperature shipping adversely affects rind colour of ‘Palmer Navel’ sweet orange [Citrus sinensis (L.) Osb.] due to carotenoid degradation but can partially be mitigated by optimising post-shipping holding temperature. Postharvest Biol. Technol. 2009, 53, 109–116. [Google Scholar] [CrossRef]
- Matsumoto, H.; Ikoma, Y.; Kato, M.; Nakajima, N.; Hasegawa, Y. Effect of Postharvest Temperature and Ethylene on Carotenoid Accumulation in the Flavedo and Juice Sacs of Satsuma Mandarin (Citrus unshiu Marc.) Fruit. J. Agric. Food Chem. 2009, 57, 4724–4732. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).