Abstract
This study investigates the sustainable production of Shiitake (Lentinula edodes) mushroom using agro-industrial wastes. The substrate of Shiitake (80% rice straw + 20% sugar cane bagasse) was moistened with 0 (freshwater as control), 50, and 100% concentrations of secondarily treated dairy plant and sugar mill wastewaters (DPW and SMW). After proper sterilization, the cultivation was carried out under controlled environmental conditions using the bag log method for 100 days. The results revealed that DPW and SMW moistening significantly (p < 0.05) increased the nutrient levels of the formulated substrate which later gave better mushroom yield. The highest Shiitake mycelial coverage (90.70 ± 1.47 and 88.65 ± 1.82%), yield (186.00 ± 3.10 and 176.09 ± 4.12 g/kg fresh substrate), biological efficiency (80.00 ± 0.58 and 75.73 ± 0.93%), total phenol (2.84 ± 0.03 and 2.69 ± 0.03 mg/g), ascorbic acid (0.34 ± 0.03 and 0.32 ± 0.02 mg/g), and β-carotene (2.48 ± 0.06 and 2.29 ± 0.02 μg/g) contents with the minimum time taken for spawn running (60 ± 1 days) was observed using a 50% concentration treatment of both DPW and SMW, respectively. Besides this, the kinetic studies using a first-order-based model showed acceptable accuracy in predicting the rate constant for substrate delignification and heavy metal uptake by Shiitake mushroom. These findings suggest a novel approach for sustainable mushroom cultivation using agro-industrial wastes. The concept can be used for the production of high-quality mushrooms for edible and medicinal purposes while contributing toward the United Nations’ Sustainable Development Goals (SDGs 12) on responsible consumption and production of superfoods.
1. Introduction
Nowadays, with the continuous growth of the industrial sector in developing countries, the large disposal of wastes results in a polluted and deplorable environment. Among these industries, agro-based factories are responsible for releasing a considerable amount of liquid and solid wastes, which in turn cause several environmental issues [1]. Wastewater could be defined as liquid waste, treated or not, disposed of by industrial factories or treatment plants [2]. According to the National Dairy Development Board [3], the production of the dairy industry accounts for more than 187.7 million tons, being one of the main economic sectors in India. The amount of dairy plant wastewaters (DPW) generated by this industry is estimated to be three times the volume of milk production [2]. The assessment of the use of dairy sludge in agriculture as a natural fertilizer for grasslands was reported by Shi et al. [4]. They observed wide variations in nutrient assimilation due to the continuous variation of dairy sludge properties. Besides this, the treatment of DPWs using microalgae has been reported to be effective [5,6]. However, discharging untreated or partially treated DPW onto the environment can cause environmental and health risks due to the high levels of biochemical oxygen demand (BOD: 40.000–48.000 mg/L) and chemical oxygen demand (COD: 500–800 mg/L) [7]. Furthermore, DPW contains high amounts of nutrients, heavy metals and organic matter [8,9], phosphorus [10], dissolved sugars, proteins, and fats [11], as well as casein, inorganic salts, detergents, and sanitizers [12]. Furthermore, considering the large and increasing amounts of DPW, these components are difficult to efficiently decompose prior to their final disposal.
On the other hand, the sugar industry ranks among the first leading industries in India; it generates large amounts of solid and liquid wastes [13,14]. The Ganga River is considered the national river of India; it has suffered catastrophic discharges of sugar mill wastewaters (SMW) from neighboring sugar factories [15]. According to the Department of Agriculture, India [16], and despite all strict rules and regulations imposed, SMWs are generated on an average of 2.347 × 1010 m3 annually and discharged into the Ganga River. Therefore, it is a must to pre-treat these wastewaters prior to their disposal [17]. Several efforts have been conducted to use sugar wastes and wastewaters in different applications such as biogas production [18] in order to mitigate the emerging water scarcity in India and reduce the negative disposal of wastewaters onto the environment. In this regard, SMWs have been applied as soil amendments for the production of several crops such as African marigold (Tagetes erecta L.), maize (Zea mays L.), common bean (Phaseolus vulgaris L.), eggplant (Solanum melongena L.), water hyacinth (Eichhornia crassipes Mart.) and okra (Abelmoschus esculentus (L.) Moench) [6,19,20,21,22]. However, SMWs contain high amounts of dissolved compounds such as chlorides, sulfates, nitrates, calcium, magnesium [20], nutrients, and heavy metals [23], besides high BOD (500–1000 mg/L) and COD (>2200 mg/L) amounts [24], which lead to potential antagonistic effects on plants and the rhizospheric environment [25].
Shiitake (Lentinula edodes (Berk) Pegler) is a saprophytic lignocellulosic fungus that grows naturally on the cambium of dried logs [26]. Shiitake mushroom possesses several nutritional and medicinal properties, and their flavor is widely appreciated throughout the world. The worldwide production of Shiitake mushrooms comprises nearly 22% of the mushroom’s overall production. This type of mushroom uses several enzymes (i.e., laccase and carboxymethylcellulose) to degrade complex carbon sources such as cellulose, hemicellulose, and lignin, which are essential for its nutrition [27]. The efficiency of mushroom production is closely related to the nutrient composition of the growing substrate [28,29,30] and the type of added supplements [31,32,33].
Mushroom cultivation has been applied as a bioremediation strategy to treat agro-industrial wastes containing hazardous amounts of heavy metals [34,35]. On the other hand, due to severe deforestation, governments have enforced strict prohibitions on the use of forest by-products in the cultivation of Shiitake mushroom [36]. These important restrictions prompted Shiitake growers to adopt several agro-industrial wastes as substrate substitutes such as sawdust [37], coffee husks [38], rice and wheat straws [39], as well as sugarcane bagasse alone or in combination with other supplements [40,41], depending on the regional availability.
The integration of mushroom production and agro-based wastewaters as a nutrient supply constitutes a sustainable and effective way for the treatment of the latter [42]. Moreover, the use of nutritional supplements in mushroom cultivation can provide the fungi with readily available nitrogen and carbon, thus promoting an earlier and larger mushroom production [35,43]. A few research works have reported the use of SMW as a substrate supplement in mushroom cultivation. The nutritional enrichment of rice straw with sugar effluents increased the production of milky mushrooms (Calocybe indica P&C), reduced the amounts of phosphates, nitrates, and heavy metals in the substrate, and improved the nutritional composition of the harvested mushrooms [44]. In another study, the supplementation of wheat straw and sugarcane bagasse substrates with treated sugar effluents increased the yield and medicinal properties of Agaricus bisporus ((J.E. Lange) Imbach) mushrooms [42]. On the other hand, there are just a couple of reports on the use of DPW for mushroom cultivation [1,45]. The addition of 25, 50, 75, and 100% of treated dairy effluents in A. bisporus cultivation resulted in larger mushroom yield and earlier production [1]. Gothwal et al. [45] reported that Pleurotus sajor-caju (Fr.) Fr. supplemented with 10% dairy effluents increased the biological efficiency and protein content of the product.
Several studies have been conducted on the kinetics of heavy metals uptake by C. indica and A. bisporus [1,44]; however, to date, there are no reports on the kinetics of delignification and heavy metals uptake by Shiitake grown on substrates supplemented with both DPW and SMW. The objective of the present work was to optimize substrate formulation by using dairy and sugar industrial wastewaters as supplements in Shiitake cultivation to improve mushroom productivity and nutritional value.
2. Materials and Methods
2.1. Collection of Experimental Materials
The dairy plant wastewater (DPW) was collected from the treatment unit of Mohan Milk Processing and Dairy Plant, Bhagwanpur, Haridwar, India (29°58′24″ N and 77°46′38″ E), and the sugar mill wastewater (SMW) and sugar cane bagasse were collected from Rai Bahadur Narain Singh (RBNS) sugar mill situated in Laksar town of Haridwar, India (29°44′42.7″ N and 78°01′57.2″ E). A commercial strain of Shiitake (Lentinula edodes: DMRO-356) mushroom was procured from the Directorate of Mushroom Research (DMR), Indian Council of Agricultural Research (ICAR), Solan, Himachal Pradesh, India (30°55′18.5″ N and 77°06′09.4″ E). The spawn was stored at room temperature until its further inoculation on the respective substrates. For substrate preparation, rice straw waste was collected from Mirzapur village, Haridwar, India (29°52′30″ N and 78°06′16″ E).
2.2. Experimental Protocols for Shiitake Mushroom Cultivation
2.2.1. Substrate Preparation
The bag log method was adopted for the cultivation of Shiitake mushrooms from November 2020 to February 2021 [6]. A mixture containing 80% rice straw + 20% sugar cane bagasse was prepared, followed by chopping it into 2–3 cm long particles. Afterwards, 10 kg of dried substrate mixture was filled in plastic tubs along with 20 L of moistening liquid (supplied with 1 g carbendazim + 20 mL formalin solution), and the mixture was allowed to equilibrate for 24 h. The moistening liquid was prepared by adding borewell freshwater to the DPW and SMW. Different moistening liquid treatments were prepared separately: 0 (20 L freshwater as control), 50 (10 L freshwater + 10 L wastewater), and 100% (20 L wastewater). After moistening at room temperature, the substrate was removed and placed on a perforated tub to thoroughly drain the excess water (more than 60%), followed by the addition of 2% w/w gypsum. Subsequently, the substrate was filled in an aluminum container and moist-heat sterilized using a 7421PAD Equitron autoclave (Medica Instruments, Mumbai, India) at 121 °C and 15 psi for 1 h. Finally, the substrate was cooled down to room temperature and used for spawning. The experimental steps adopted for Shiitake cultivation are displayed in Figure 1.
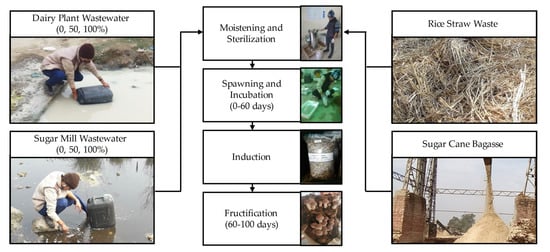
Figure 1.
Experimental steps for Shiitake mushroom cultivation using agro-industrial wastes.
2.2.2. Spawning, Spawn Running, and Incubation
A total of 2 kg of rice straw-based substrate was filled in sterile breathable polypropylene bags of 3 kg capacity while inserting 20 g of 24 h pre-acclimatized spawn distributed in four layers. The bags were closed and 5–6 small holes were made on the top using a needle. After that, each bag was allowed to set for spawn running at controlled environmental conditions: 25.0 ± 2.0 °C, 65.0 ± 5.0% substrate moisture, 70.0 ± 5.0% relative humidity, 4000 ppm of CO2, and 1000 lx of light intensity, without irrigation. The spawn running was carried out for 60 days with substrate samples collected at defined intervals of 20 days.
2.2.3. Induction and Fructification
After completion of the spawn running, the bags were subjected to iced water treatment (cold-shock) for 15 min. The substrate bag logs were completely sealed in another polyethylene bag to avoid the leaching of the moistened substrate during the socking process. Subsequently, the bags were removed and transferred to the mushroom cultivation chamber for the induction of fruiting bodies. The environmental and irrigation parameters for the induction stage were adjusted as follows: 14.0 ± 2.0 °C, 65.0 ± 5.0% substrate moisture, 82.0 ± 5.0% relative humidity, 1500 ppm of CO2, and 500 lx of light intensity, without auto-irrigation. After an induction period of 7 days, the fructification was triggered by applying the following conditions: 148.0 °C, 1100 ppm of CO2, and light intensity of 3000 lx. A total of 2 flushes were harvested by completing the induction and fructification stages in a period of 40 days; thus, the complete crop cycle of Shiitake mushroom had a duration of 100 days.
2.3. Analytical Methods
In the current study, the substrate and moistening sources (water supply and wastewaters) were analyzed for selected physicochemical and heavy metal parameters following standard protocols of AOAC [46] and APHA [47]. The substrate samples were taken by making small holes in the side of substrate bags, aseptically. After the sample collection, the holes were carefully closed using waterproof transparent sterile tape. A digital multimeter (ESICO 1611, India) was used for pH, EC, and TDS determinations. BOD5 was determined by measuring dissolved oxygen of water or wastewater samples using the 1801 digital DO meter (ESICO, India). COD was determined by acid-reflux digestion using a COD digester (Scientech, India) followed by spectrophotometric determination at 650 nm in a Cary 60 spectrophotometer (Agilent Technologies, Santa Clara, CA, USA). The contents of total Kjeldahl’s nitrogen (TKN), organic carbon (OC), and total phosphorus (TP) were determined by following Kjeldahl’s digestion-distillation, Walkley and Black, and spectrophotometric methods, respectively [44].
Substrate analysis was performed using a 1:10 suspension prepared in deionized water. In addition, heavy metals (Cd, Cr, Cu, Fe, Mn, and Zn) were analyzed using an A Analyst 800 atomic absorption spectroscopy (AAS, PerkinElmer, Waltham, Massachusetts, USA). The contents of Klason-lignin, cellulose, and hemicellulose in the mushroom substrate were determined by standard methods as previously reported by Gessner [48]. The harvested mushrooms were also characterized for selected biochemical parameters including total phenols (mg/g), ascorbic acid (mg/g), and β-carotene (µg/g). Total phenol (TP) content was estimated by using the acetone extraction method followed by the addition of sodium carbonate and the Folin–Ciocalteau reagent and spectrophotometric determination at 725 nm. The ascorbic acid content was determined by using a 5% metaphosphoric acid solution as an extraction reagent (absorbance peak 245 nm). Similarly, the contents of β-carotene were determined by using petroleum ether as an extraction agent and measuring the absorbance at 450 nm [1].
2.4. Nutrient Removal and Kinetics Studies
The changes in Shiitake mushroom substrate were studied by using the nutrient removal efficiency index [44]. The formula for calculating the index is the following (Equation (1)):
where C and Ct are the initial and final values of substrate parameters after mushroom cultivation. Kinetic studies are helpful to understand the critical patterns of substrate degradation and nutrient utilization by mushrooms [49]. To perform the analyses, periodic samples were taken every 20 days from bags, without disturbing the rest of the mycelial growth, and analyzed for lignin, cellulose, hemicellulose, and six heavy metals (Cd, Cr, Cu, Fe, Mn, and Zn). The data were generated and analyzed using a first-order reaction model [50] according to the following equation (Equation (2)):
where [C] is the initial substrate content and [Ct] is the final substrate content after t days. A plot Log[Ct] vs. t was performed to derive the rate constant (k) of substrate degradation/utilization by the mushroom.
Removal efficiency (%) = [(C − Ct)/C × 100)]
Log[C] = Log[Ct] − kt
2.5. Data Analysis and Software
All the five experimental treatments (0 as control, DPW50%, DPW100%, SMW50%, and SMW100%) were performed using five replicates in a completely randomized block design (n = 5 × 5 = 25). The data generated in this study were analyzed using Microsoft Excel 2019 (Home and Student, Microsoft Corp., Redmond, Washington, USA) and OriginPro 2022 (Student Edition, OriginLab, Northampton, Massachusetts, USA) software packages. The results are expressed as the mean ± the standard deviation (SD) of five analyses (one sample from each treatment bag) and tested using a one-way Analysis of Variance (ANOVA) at a probability level of (p) < 0.05.
3. Results and Discussion
3.1. Properties of Water Supply and Wastewaters Used in This Study
Table 1 lists the values of the physicochemical and heavy metal parameters of the moistening sources (water supply and DPW/SMW). The DPW and SMW had significantly (p < 0.05) higher values of analyzed parameters as compared to the water supply used as a control treatment. Specifically, the values were reported to be higher in the case of the DPW as compared to SMW. Loads of heavy metals were similar for both wastewaters, except for Cd, which was higher in the case of SMW. On the other hand, the water supply was found to be below detectable limits for Cd and Cr, which might be due to the fact that this source is non-contaminated groundwater. High nutrient and heavy metal loads were detected in both treated wastewaters (DPW and SMW) indicating a poor secondary treatment by the plant/industry facility. In both industries, the presence of various heavy metals in wastewater is due to the use of animal and plant-based raw materials. In contrast, the levels of most of the pollutants in the wastewaters did not exceed the maximum permissible limits of the Bureau of Indian Standards (BIS) for surface discharge and the Central Pollution Control Board (CPCB) for inland irrigation, except for BOD, COD, TKN, TP, Fe, and Mn. In particular, the dairy plant utilizes animal fodder, feeds, milk preservatives, skim, and additives, with the release of animal excreta, as well as washing, bathing, and product manufacturing, processing wastewaters, etc. [51]. On the other hand, the sugar mill industry utilizes sugar cane as the primary raw material which is converted into commercial sugar through various physical and chemical processes [52]. In this study, DPW contains more “Fe” compared to SMW which might be due to the use of Fe-based coagulants in its secondary treatment step. In particular, wastewaters having high BOD, COD, TKN, and TP are considered very harmful to the environment since they may cause disturbances to the biota of receiving ecosystems. They may cause the accumulation of excess nutrients which results in the eutrophication of water bodies and thereby killing certain animal and plant species. Considering that most of the metal elements detected in this study are integral parts of the raw materials utilized in both industries, their presence in the industrial wastewaters was admissible. Previous studies have investigated the physicochemical characterization of DPWs and SMWs. Kumar et al. [44] showed that secondarily treated DPW was rich in several nutrient elements (Na, K, Ca, Mg, and P) and heavy metals (Cu, Fe, Mn, and Zn). Similarly, Kumar and Chopra [19] analyzed the heavy metal contents of SMW which was later used for safe inland irrigation purposes.

Table 1.
Properties of water supply and agro-industrial wastewaters used in the current study (mean ± SD of five analyses).
3.2. Effect of Water Supply/Wastewaters on Mushroom Substrate
Table 2 lists the changes in the physicochemical properties and heavy metal contents of Shiitake mushroom substrate in terms of the different wastewater moistening treatments tested in this work. The results showed that both DPW and SMW significantly (p < 0.05) increased the substrate properties (physicochemical and heavy metals) after the moistening step. In a similar way, the physicochemical and heavy metals properties increased with wastewater concentration from 50 to 100%. Overall, the DPW had a greater increasing effect on the physicochemical and heavy metal parameters than the SMW. The level of heavy metals was also increased due to their sorption from wastewater onto the mushroom substrate during the moistening process. However, no significant increment (p > 0.05) was observed after moistening in the cases of lignin, cellulose, and hemicellulose, which was probably due to the absence of their contents in the wastewaters. During commercial mushroom cultivation, substrate moistening is achieved using freshwater, in which many commercial fertilizers (urea, ammonium sulfate, superphosphate, potash), cereal brans, or animal-based manures are also added to increase the nutrient content of the substrate. Reports on the use of nutrient-rich wastewaters for mushroom substrate preparation are very limited. However, this approach provides a dual benefit, which is supplying moisture while increasing the nutrient content of the substrate. Previous studies have confirmed the effectiveness of the use of DPW and SMW for mushroom substrate preparation [1,50].

Table 2.
Changes in substrate properties (mean ± SD of five analyses) before and after Shiitake (L. edodes) mushroom cultivation.
3.3. Changes in Mushroom Substrate Properties after Shiitake (L. edodes) Mushroom Cultivation
The pre-and post-cultivation analysis of Shiitake mushroom substrate indicated significant (p < 0.05) changes in the selected parameters after the cultivation of 100 days (Table 2). As per the removal efficiency index, wastewater amended treatments showed a higher reduction in the substrate properties as compared to the control treatment (Figure 2). Specifically, DPW treated substrate showed better nutrient removal followed by SMW and control treatments. However, absolute wastewater treatments (100% concentration) showed relatively lesser removal efficiency which might be due to higher nutrient levels that might have inhibited the mycelial colonization. Thus, the highest removal efficiency (%) of OC (29.16), TKN (37.30), lignin (58.90), cellulose (35.10), hemicellulose (51.53), Cr (10.48), Cu (58.26), Fe (1.45), Mn (1.57), and Zn (21.40) was reported using 50% DPW. On the other hand, the highest removal efficiency (%) of Cd (44.83) was reported in the case of 50% SMW treatment bag logs. The reduction in pH is associated with the release of acidic enzymes by mushroom mycelia which further helped in substrate degradation. Similarly, the reduction in EC might be due to the breakdown and transportation of molecules out from the bag logs as the form of fruiting bodies that affect the conductance [53].
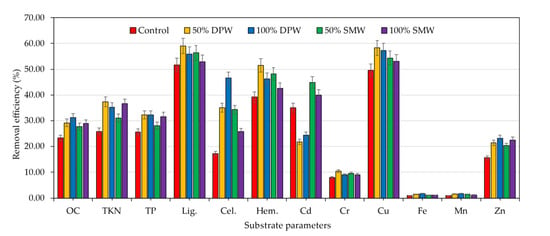
Figure 2.
Removal efficiency of the tested chemical components in the mushroom substrate after Shiitake cultivation.
The polysaccharides and the polymer lignin compounds from the substrate are breakdowns into simpler carbon molecules which are further taken by mycelia and help in mushroom growth [54]. the metabolism of nitrogenous compounds by fungi is essential for their proper growth and reproduction, mushrooms as macrofungi are capable of degrading and utilizing different forms of nitrogen. In this case, the GATA family of proteins is actively involved in mediating the nitrogen metabolism pathways [55,56]. ince Nitrogen helps in the cellular metabolism and synthesis of various amino acids which are the fundamental constituents of mushrooms making them economically and medicinally important [56]. Polysaccharides and the polymer lignin compounds from dead organic wastes are breakdowns into simpler carbon molecules which are further taken by mycelia and help in mushroom growth [54]. This leads to a decrease in the OC levels of mushroom substrate [57]. Fungal mycelia break the cell wall components during the spawn running phase and convert them into smaller molecular weight compounds which are later utilized as their energy source. While the heavy metal content is scarcely biologically utilized during this phase [1]. Phosphorus is essentially utilized by all organisms to build their genetic materials, while Zn and Cu act as essential micronutrients supporting the cellular metabolisms, transport, and virulence mechanisms. On the other hand, certain metal elements including Cu, Cr, Fe, Mn, and Zn are taken by mushrooms due to their essential role in growth, metabolism, virulence, etc. [1]. Thus, the heavy metals contents were reduced after the cultivation of Shiitake in the current study.
A report by Vane et al. [57] evaluated the changes in the carbon fraction of wheat straw substrate before and after the cultivation of Pleurotus ostreatus ((Jacq. ex Fr.) P.Kumm) mushroom. They found that the carbon content was reduced significantly from 45.68 to 37.62% after 63 days of P. ostreatus cultivation. They also found that the reduction of OC was lesser during the spawn running period (0–21 days) while it fluctuated rapidly from 22 to 42 days which again became stationary during the later growth (43–63 days). Similarly, Rizki and Tamai [58] evaluated the effect of different nitrogen levels on the yield performance of P. ostreatus. They applied the N-content dose of 0.35 to 2.09% which revealed that the yield performance of P. ostreatus increased up to 1.47% which reduced in 2.09% N treatment. Most recently, Kumar et al. [1] investigated the reduction in mushroom substrate parameters treated with DPW by A. bisporus. They observed that a 75% concentration showed the highest removal efficiency for the removal of 14 substrate parameters. Therefore, the findings of these studies are in line with the current experiment which supports the results.
3.4. Effect of Water Supply/Wastewater Addition on Shiitake (L. edodes) Mushroom Productivity
The maximum yields of Shiitake mushroom cultivated on DPW and SMW were 186.00 ± 3.10 and 176.09 ± 4.12 g/kg of the fresh substrate (FS) for the 50% concentration treatments, respectively. The biological efficiency is an index that indicates the amount of substrate conversion into fresh fruiting bodies, with an 80% biological efficiency considered economically feasible for the commercial cultivation of mushrooms [42]. In this study, the maximum biological efficiencies of Shiitake mushroom cultivated on DPW and SMW treated substrates were 80.00 ± 0.58 and 75.73 ± 0.93% as depicted in Table 3. The spawn running efficiency is determined by the coverage percentage of the mycelial network over the surface of the substrate. The maximum mycelium coverage (90.70 ± 1.47 and 88.65 ± 1.82%) and minimum running time (60 ± 1 day) were observed for both DPW and SMW wastewaters, respectively, using 50% concentration treatments. Thus, it can be concluded that the use of DPW and SMW increased the productivity parameters of the Shiitake mushroom.

Table 3.
Effect of agro-industrial wastewaters on yield and biochemical properties of cultivated Shiitake (L. edodes) mushroom.
The rapid spawn propagation in 50% of wastewater treatments is probably due to their supply of favorable nutrient amounts which supports efficient mycelial growth [59]. A regular practice to trigger the formation of fruiting bodies after complete spawn running consists of exposing the mushrooms to adverse environmental conditions such as nutrient-deficient soil/compost, cold-water treatment of bags, and temperature reduction of the cultivation room. These treatments cause a sudden shock to the mushroom mycelia which commences to form primordia and produce spores for its survival and preservation of future progenies [60]. In this work, the fruiting body formation was triggered by a cold-water treatment followed by lowering the room temperature and providing 1500 lx of light during pin-head formation [61].
Previous studies on the use of agro-industrial wastewaters as nutrient supplementation in mushroom cultivation have confirmed the effectiveness of these treatments. A report by Kumar et al. [44] studied the production of milky mushroom using rice straw substrate moistened with SMW and found that the yield and biological efficiency were increased while spawn running time was reduced significantly using a 50% SMW concentration. Similarly, Kalmis et al. [62] studied the role of olive mill wastewater in the production of P. ostreatus mushroom using wheat straw as the substrate. The authors reported that a 25% concentration of olive mill wastewater improved the yield of P. ostreatus and provides a means for the ecological disposal of this residue and a practical way for partly recovering its economic value.
3.5. Effect of Water Supply/Wastewater Addition on Biochemical Parameters of Shiitake (L. edodes) Mushroom
The mushrooms grown on DPW and SMW treated substrate presented a significant increase in total phenols, ascorbic acid, and β-carotene contents (Table 3). The highest contents of total phenols (2.84 ± 0.03 and 2.69 ± 0.03 mg/g) and ascorbic acid (0.34 ± 0.03 and 0.32 ± 0.02 mg/g) were obtained using 50% concentrations of DPW and SMW, whereas the concentrations of the bioactive compounds were lower when the absolute wastewater treatment (100%) was employed. Similarly, the maximum average contents of β-carotene in harvested L. edodes mushrooms were 2.48 ± 0.06 and 2.29 ± 0.02 μg/g using 50% moistening of DPW and SMW as compared to the control treatment (2.20 ± 0.04 μg/g). The nutritional properties of the substrate strongly determine the biochemical and antioxidant composition of the produced mushrooms, a process that is related to the synthesis of mushroom enzymes.
Mushrooms are rich in phenolic compounds which can be classified into three major groups: simple phenols (gallic, benzoic, syringic, chlorogenic acids, etc.), and polyphenols. These phenolic compounds have many biological and pharmacological properties including the inhibition of hydrolytic and oxidative enzymes. Ascorbic acid is a water-soluble antioxidant agent that aids in pathogen inactivation, body detoxification, collagen formation in body tissues, and prevention of various diseases such as scurvy. Mushrooms constitute an excellent source of ascorbic acid, an attribute which makes them medicinally important foods [63]. Some phenolic compounds can chelate different metal ions including heavy metals [64]. On the other hand, β-carotene is a pigment found in many fruits, plants, and fungi, including mushrooms. After its consumption, β-carotene is converted into vitamin A which helps in proper body functioning. More specifically, β-carotene can help older people to maintain healthy lungs and protect them from free radicals [65]. There are certain factors such as high N-content, and metabolic feedback inhibitions by the fungal mycelia which affect the pH of the substrate resulting in a slowdown of the fungal network. Therefore, with the results obtained in this study, it was concluded that substrate moistening using agro-industrial wastewaters was helpful to increase the contents of bioactive compounds in harvested Shiitake mushrooms.
In previous reports, Barros et al. [65] studied the total phenols, ascorbic acid, and β-carotene contents of Leucopaxillus giganteus (Sowerby) Singer, Sarcodon imbricatus (L.) P.Karst., and Agaricus arvensis Schaeff. mushrooms. Nevertheless, they discovered that L. giganteus had superior antioxidant characteristics than both S. imbricatus or A. arvensis, which would be consistent with the first species’ higher phenol content. Moreover, Keleş et al. [63] also analyzed 25 wild mushroom species for their antioxidant and biochemical compounds and found that A. bisporus had the highest total phenolic compounds while ascorbic acid was below detectable limits. They concluded that wood-loving mushrooms had higher contents of total phenols and ascorbic acid as compared to broadleaf substrate-loving mushrooms.
3.6. Kinetics of Delignification and Heavy Metals Uptake by Shiitake (L. edodes) Mushroom
Table 4 lists the contents of heavy metals in Shiitake mushroom cultivated on substrates supplemented with agro-industrial wastewaters (DPW and SMW). The results suggested that the heavy metal levels significantly increased with wastewater concentration in the moistening liquid. The increasing order of heavy metal concentrations was the following: Cd < Cr < Mn < Cu < Zn < Fe. The concentration of heavy metals in fruiting bodies did not exceed the maximum permissible limits stipulated by the WHO/FAO [66] and Indian Standards [67]. In this regard, kinetic studies on microelement uptake by fungi help to characterize heavy metal bioaccumulation and uptake rate. The kinetics of delignification and heavy metal uptake by Shiitake mushroom was conducted using a first-order rate equation (Table 5), from which the rate constant (k) and R2 values were calculated. From the results, it was evidenced that the highest delignification and heavy metal uptake rates were obtained with a 50% concentration of both DPW and SMW. The high R2 values indicated a good data fit for the developed model, which can be used to simulate substrate utilization time-course by Shiitake mushroom (Figure 3).

Table 4.
Heavy metal contents in Shiitake (L. edodes) mushroom fruiting bodies cultivated on agro-industrial wastewaters treated substrate.

Table 5.
Kinetic parameters of delignification and heavy metals uptake by Shiitake (L. edodes) mushroom cultivated on agro-industrial wastewaters treated substrate.
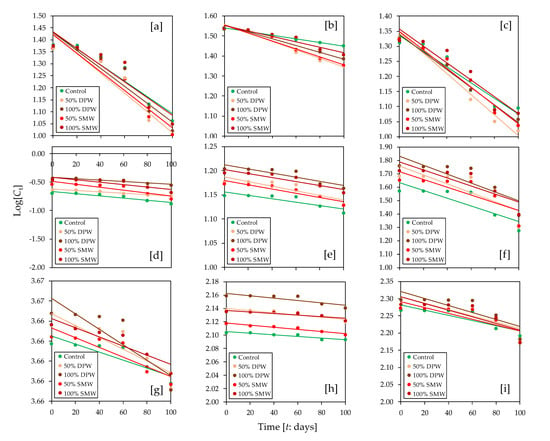
Figure 3.
Time-course fitness plots (Log[Ct] vs. time [t]) using a first-order kinetic model for reduction of substrate parameters by Shiitake (L. edodes) mushroom [(a): lignin; (b): cellulose; (c): hemicellulose; (d): Cd; (e): Cu; (f): Cr; (g): Fe; (h): Mn; (i): Zn].
The degradation of the substrate by mushrooms is mediated by certain extracellular and intracellular enzymes, which are responsible for substrate catabolism and nutrient absorption into the fungal mycelia for the later transportation of nutrients to the fruiting bodies. Mushrooms accumulate a very little amount of nutrients during the spawn running phases; however, nutrient accumulation is greatly accelerated during the formation of fruiting bodies [68]. During this growth phase of the mushroom, the main carbon sources, lignin, cellulose, and hemicellulose are consumed by fungal mycelia.
Several micronutrients are essential for the growth and development of mushroom mycelia and fruiting bodies. The hyphae’s cell wall is capable to allow the passage of molecules with sizes larger than 4500 Da. However, heavy metal uptake by the mushroom greatly depends on the selectivity and pore size distribution of the cell wall [69]. Certain metals such as Cu, Cr, Fe, Mn, and Zn are helpful in fungal metabolism and virulence, and thus, are assimilated by mycelia. The microelement Cd is not essentially utilized by mushrooms; however, due to its affinity towards the fungal cell wall, it is partially absorbed by mushrooms, which are later translocated to edible parts. The presence of Cd in the substrate is not toxic for mushrooms up to a certain level (1–2 mg/Kg d.w.), as recommended by Australian Standards [70].
On the other hand, very limited studies are available on the implementation of kinetic models on micronutrient uptake by mushrooms. Kumar et al. [44] evaluated the kinetics of micronutrient uptake by C. indica mushroom grown on SMW treated rice straw substrate and found that the uptake process followed a quadratic trend which had a good model fitness (R2 > 0.80) for Cu, Fe, Mn, and Zn. Similarly, Ertugay and Bayhan [71] studied the kinetics of Cu uptake by A. bisporus mushroom. The authors studied the thermodynamics of Cu absorption and concluded that the Langmuir, Freundlich, Dubinin–Radushkevich, and Temkin isotherms were best fitted to the experimental data.
4. Conclusions
The findings of this study concluded that the use of dairy plant and sugar mill wastewaters (DPW and SMW) was helpful to increase the nutritional value of the substrate used to produce Shiitake mushroom. A 50% concentration of both DPW and SMW in the moistening liquid resulted in the highest Shiitake mushroom mycelial coverage, yield, biological efficiency, bioactive compound contents, as well as the minimum time for spawn running. The kinetic studies on delignification and heavy metals uptake also suggested the highest rate of substrate utilization for the 50% treatments of both DPW and SMW wastewaters. The levels of heavy metals did not exceed the safety limits, which suggested the safe consumption of cultivated Shiitake mushroom. Furthermore, it is recommended that the spent mushroom substrate should be used for secondary mushroom cultivation, such as Agaricus mushrooms or/and plant cultivation. Furthermore, probable feed for husbandry animals such as pigs, cows, and poultry would help in perfectly closing the sustainability chain.
Author Contributions
Funding acquisition, E.M.E., A.A.A.-H. and I.Š.; Investigation, P.K.; Methodology, P.K., R.V.-B. and M.G.; Project administration, E.M.E. and A.A.A.-H.; Resources, V.K.; Software, P.K. and B.A.; Supervision, E.M.E. and V.K.; Validation, P.K., I.Š., B.A., S.A.F., R.V.-B., M.G., F.O.A. and K.S.C.; Visualization, P.K.; Writing—original draft, P.K. and S.A.F.; Writing—review & editing, E.M.E., A.A.A.-H., I.Š., B.A., R.V.-B., M.G., F.O.A., K.S.C. and V.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by King Khalid University (grant number RGP.1/7/43), Abha, Saudi Arabia; and Princess Nourah bint Abdulrahman University Researchers Supporting Project (PNURSP 2022R93), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to their host institutes for providing the necessary facilities to conduct this study. E.M.E. extends his appreciation to King Khalid University for funding this work through the Research Group Project under grant number RGP. 1/7/43, King Khalid University, Abha, Saudi Arabia; A.A.A. express her gratitude to Princess Nourah bint Abdulrahman University Researchers Supporting Project (PNURSP 2022R93), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. All individuals included in this section have consented to the acknowledgement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kumar, P.; Kumar, V.; Goala, M.; Singh, J.; Kumar, P. Integrated use of treated dairy wastewater and agro-residue for Agaricus bisporus mushroom cultivation: Experimental and kinetics studies. Biocatal. Agric. Biotechnol. 2021, 32, 101940. [Google Scholar] [CrossRef]
- Kaur, V.; Sharma, G. Impact of Dairy Industrial Effluent of Punjab (India) on Seed Germination and Early Growth of Triticum aestivum. Indian J. Sci. Technol. 2017, 10, 1–9. [Google Scholar] [CrossRef]
- National Dairy Development Board. Milk Production in India. Available online: https://www.nddb.coop/information/stats/milkprodindia (accessed on 24 February 2022).
- Shi, W.; Healy, M.G.; Ashekuzzaman, S.M.; Daly, K.; Leahy, J.J.; Fenton, O. Dairy processing sludge and co-products: A review of present and future re-use pathways in agriculture. J. Clean. Prod. 2021, 314, 128035. [Google Scholar] [CrossRef]
- Hena, S.; Fatimah, S.; Tabassum, S. Cultivation of algae consortium in a dairy farm wastewater for biodiesel production. Water Resour. Ind. 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Patel, A.; Dixit, G.; Shah, E. Comprehensive evaluation of microalgal based dairy effluent treatment process for clean water generation and other value added products. Int. J. Phytoremediat. 2019, 21, 519–530. [Google Scholar] [CrossRef]
- Kushwaha, J.P.; Srivastava, V.C.; Mall, I.D. An overview of various technologies for the treatment of dairy wastewaters. Crit. Rev. Food Sci. Nutr. 2011, 51, 442–452. [Google Scholar] [CrossRef]
- Farizoglu, B.; Uzuner, S. The investigation of dairy industry wastewater treatment in a biological high performance membrane system. Biochem. Eng. J. 2011, 57, 46–54. [Google Scholar] [CrossRef]
- Goala, M.; Yadav, K.K.; Alam, J.; Adelodun, B.; Choi, K.S.; Cabral-Pinto, M.M.S.; Hamid, A.A.; Alhoshan, M.; Ali, F.A.A.; Shukla, A.K. Phytoremediation of dairy wastewater using Azolla pinnata: Application of image processing technique for leaflet growth simulation. J. Water Process Eng. 2021, 42, 102152. [Google Scholar] [CrossRef]
- Boczkaj, G.; Fernandes, A. Wastewater treatment by means of advanced oxidation processes at basic pH conditions: A review. Chem. Eng. J. 2017, 320, 608–633. [Google Scholar] [CrossRef]
- Perle, M.; Kimchie, S.; Shelef, G. Some biochemical aspects of the anaerobic degradation of dairy wastewater. Water Res. 1995, 29, 1549–1554. [Google Scholar] [CrossRef]
- Raghunath, B.V.; Punnagaiarasi, A.; Rajarajan, G.; Irshad, A.; Elango, A.; Mahesh Kumar, G. Impact of Dairy Effluent on Environment—A Review. In Integrated Waste Management in India; Prashanthi, M., Sundaram, R., Eds.; Environmental Science and Engineering; Springer: Cham, Switzerland, 2016; pp. 239–249. [Google Scholar] [CrossRef]
- Balakrishnan, M.; Batra, V.S. Valorization of solid waste in sugar factories with possible applications in India: A review. J. Environ. Manag. 2011, 92, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, A.; Kesari, K.K.; Shurpali, N. Multidisciplinary Approaches to Handling Wastes in Sugar Industries. Water Air Soil Pollut. 2016, 227, 11. [Google Scholar] [CrossRef]
- Dwivedi, S.; Mishra, S.; Tripathi, R.D. Ganga water pollution: A potential health threat to inhabitants of Ganga basin. Environ. Int. 2018, 117, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Jha, P. Increase in Area Coverage under Sugarcane. Available online: https://www.chinimandi.com/area-coverage-under-sugarcane-increases/ (accessed on 24 February 2022).
- Gutiérrez-Hernández, R.F.; Nájera-Aguilar, H.A.; Araiza-Aguilar, J.A.; Martínez-Salinas, R.I.; García-Lara, C.M.; González-Vázquez, U.; Cruz-Salomón, A. Novel Treatment of Sugar Mill Wastewater in a Coupled System of Aged Refuse Filled Bioreactors (ARFB): Full-Scale. Processes 2021, 9, 516. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, J.; Nadeem, M.; Kumar, P.; Pathak, V.V. Experimental and Kinetics Studies for Biogas Production Using Water Hyacinth (Eichhornia crassipes [Mart.] Solms) and Sugar Mill Effluent. Waste Biomass Valoriz. 2020, 11, 109–119. [Google Scholar] [CrossRef]
- Vaithiyanathan, T.; Sundaramoorthy, P. Analysis of sugar mill effluent and its influence on germination and growth of African marigold (Tagetes erecta L.). Appl. Water Sci. 2017, 7, 4715–4723. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K. Ferti-irrigational impact of sugar mill effluent on agronomical characteristics of Phaseolus vulgaris (L.) in two seasons. Environ. Monit. Assess. 2014, 186, 7877–7892. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K. Effects of sugarcane pressmud on agronomical characteristics of hybrid cultivar of eggplant (Solanum melongena L.) under field conditions. Int. J. Recycl. Org. Waste Agric. 2016, 5, 149–162. [Google Scholar] [CrossRef]
- Kumar, V.; Chopra, A.K.; Srivastava, S.; Singh, J.; Thakur, R.K. Irrigating okra with secondary treated municipal wastewater: Observations regarding plant growth and soil characteristics. Int. J. Phytoremediat. 2017, 19, 490–499. [Google Scholar] [CrossRef]
- Güven, G.; Perendeci, A.; Tanyolaç, A. Electrochemical treatment of simulated beet sugar factory wastewater. Chem. Eng. J. 2009, 151, 149–159. [Google Scholar] [CrossRef]
- Sahu, O. Assessment of sugarcane industry: Suitability for production, consumption, and utilization. Ann. Agrar. Sci. 2018, 16, 389–395. [Google Scholar] [CrossRef]
- Ayyasamy, P.M.; Yasodha, R.; Rajakumar, S.; Lakshmanaperumalsamy, P.; Rahman, P.K.S.M.; Lee, S. Impact of sugar factory effluent on the growth and biochemical characteristics of terrestrial and aquatic plants. Bull. Environ. Contam. Toxicol. 2008, 81, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, S.Y.; Han, J.G.; Kong, W.S.; Jung, M.Y.; Kim, S.H. Fatty acids and stable isotope ratios in shiitake mushrooms (Lentinula edodes) indicate the origin of the cultivation substrate used: A preliminary case study in Korea. Foods 2020, 9, 1210. [Google Scholar] [CrossRef] [PubMed]
- de Andrade, M.C.N.; Sales-Campos, C.; de Carvalho, C.S.M.; de Aguiar, L.B.; de Almeida Minhoni, M.T. Uso de resíduos madeireiros da Amazônia brasileira no cultivo in vitro de Lentinus strigosus/Use of wood waste from the Brazilian Amazon in the in vitro cultivation of Lentinus strigosus. Rev. Ambiência 2013, 9, 189–196. [Google Scholar] [CrossRef]
- Sassine, Y.N.; Naim, L.; El Sebaaly, Z.; Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H. Nano urea effects on Pleurotus ostreatus nutritional value depending on the dose and timing of application. Sci. Rep. 2021, 11, 5588. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; Alsanad, M.A.; Yordanova, M.H.; El Sebaaly, Z.; Najjar, R.; Sassine, Y.N. Effect of olive pruning residues on substrate temperature and production of oyster mushroom (Pleurotus ostreatus). In Proceedings of the IV International Symposium on Horticulture in Europe-SHE2021, Stuttgart, Germany, 8–12 March 2021; pp. 245–252. [Google Scholar] [CrossRef]
- Hoa, H.T.; Wang, C.L.; Wang, C.H. The effects of different substrates on the growth, yield andnutritional composition of two oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 2015, 43, 423–434. [Google Scholar] [CrossRef]
- Parola, S.; Chiodaroli, L.; Orlandi, V.; Vannin, C.; Panno, L. Lentinula edodes and Pleurotus ostreatus: Functional food with antioxidant—Antimicrobial activity and an important source of Vitamin D and medicinal compounds. Funct. Foods Health Dis. 2017, 7, 773. [Google Scholar] [CrossRef]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Upadhyay, R.C.; Verma, R.N.; Singh, S.K.; Yadav, M.C. Effect of organic nitrogen supplementation in Pleurotus species. Mushroom Biol. Mushroom Prod. 2002, 105, 225–232. [Google Scholar]
- Kumar, P.; Kumar, V.; Adelodun, B.; Bedeković, D.; Kos, I.; Širić, I.; Alamri, S.A.M.; Alrumman, S.A.; Eid, E.M.; Abou Fayssal, S.; et al. Sustainable Use of Sewage Sludge as a Casing Material for Button Mushroom (Agaricus bisporus) Cultivation: Experimental and Prediction Modeling Studies for Uptake of Metal Elements. J. Fungi 2022, 8, 112. [Google Scholar] [CrossRef]
- Abou Fayssal, S.; El Sebaaly, Z.; Alsanad, M.A.; Najjar, R.; Bohme, M.; Yordanova, M.H.; Sassine, Y.N. Combined effect of olive pruning residues and spent coffee grounds on Pleurotus ostreatus production, composition, and nutritional value. PLoS ONE 2021, 16, e0255794. [Google Scholar] [CrossRef]
- Xu, S.; Wang, F.; Fu, Y.; Li, D.; Sun, X.; Li, C.; Song, B.; Li, Y. Effects of mixed agro-residues (corn crop waste) on lignin-degrading enzyme activities, growth, and quality of: Lentinula edodes. RSC Adv. 2020, 10, 9798–9807. [Google Scholar] [CrossRef]
- Yamanaka, K. Cultivation of Mushrooms in Plastic Bottles and Small Bags. In Edible and Medicinal Mushrooms: Technology and Applications; Zied, D.C., Pardo-Giménez, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 309–338. [Google Scholar] [CrossRef]
- Murthy, P.S.; Manonmani, H.K. Bioconversion of Coffee Industry Wastes with White Rot Fungus Pleurotus florida. Res. J. Environ. Sci. 2008, 2, 145–150. [Google Scholar] [CrossRef][Green Version]
- Royse, D.J.; Sanchez, J.E. Ground wheat straw as a substitute for portions of oak wood chips used in shiitake (Lentinula edodes) substrate formulae. Bioresour. Technol. 2007, 98, 2137–2141. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, L.C.; Bolaños Rojas, A.C.; Chaurra Arboleda, A.M.; Zuñiga Escobar, O. Changes in macronutrients and physical prpoerties during the growth of Lentinula edodes and Pleurotus ostreatus in a composed based on sugarcane bagasse agricultural waste. Chil. J. Agric. Anim. Sci. 2021, 37, 301–312. [Google Scholar] [CrossRef]
- Philippoussis, A.; Diamantopoulou, P.; Zervakis, G. Correlation of the properties of several lignocellulosic substrates to the crop performance of the shiitake mushroom Lentinula edodes. World J. Microbiol. Biotechnol. 2003, 19, 551–557. [Google Scholar] [CrossRef]
- Kumar, V.; Goala, M.; Kumar, P.; Singh, J.; Kumar, P. Integration of treated agro-based wastewaters (TAWs) management with mushroom cultivation. Environ. Degrad. Causes Remediat. Strateg. 2020, 6, 63–75. [Google Scholar] [CrossRef]
- Naim, L.; El Sebaaly, Z.; Abou Fayssal, S.; Alsanad, M.A.; Najjar, R.; Yordanova, M.H.; Sassine, Y.N. The use of nitrogen-rich nano-supplements affects substrate temperature, delays the production cycle, and increases yield of Pleurotus ostreatus. In Proceedings of the IV International Symposium on Horticulture in Europe-SHE2021, Stuttgart, Germany, 8–12 March 2021; pp. 831–840. [Google Scholar] [CrossRef]
- Kumar, V.; Valadez-Blanco, R.; Kumar, P.; Singh, J.; Kumar, P. Effects of treated sugar mill effluent and rice straw on substrate properties under milky mushroom (Calocybe indica P&C) production: Nutrient utilization and growth kinetics studies. Environ. Technol. Innov. 2020, 19, 101041. [Google Scholar] [CrossRef]
- Gothwal, R.; Gupta, A.; Kumar, A.; Sharma, S.; Alappat, B.J. Feasibility of dairy waste water (Dww) and distillery spent wash (dsw) effluents in increasing the yield potential of pleurotus flabellatus (pf 1832) and pleurotus sajor-caju (ps 1610) on bagasse. 3 Biotech. 2012, 2, 249–257. [Google Scholar] [CrossRef][Green Version]
- AOAC Official Methods of Analysis, 21st Edition (2019)—AOAC International. Available online: https://www.aoac.org/official-methods-of-analysis-21st-edition-2019/ (accessed on 15 November 2021).
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association: Washington, DC, USA, 2012. [Google Scholar] [CrossRef]
- Gessner, M.O. Proximate Lignin and Cellulose. In Methods to Study Litter Decomposition; Manuel, A.S., Graça, F.B., Mark, O.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–120. [Google Scholar]
- Dhiman, N.; Chaudhary, S.; Singh, A.; Chauhan, A.; Kumar, R. Sustainable degradation of pharmaceutical waste using different fungal strains: Enzyme induction, kinetics and isotherm studies. Environ. Technol. Innov. 2022, 25, 102156. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Singh, J.; Kumar, P. Kinetics of nutrients remediation from sugar industry effluent—Treated substrate using Agaricus bisporus: Mushroom yield and biochemical potentials. 3 Biotech. 2021, 11, 164. [Google Scholar] [CrossRef]
- Stasinakis, A.S.; Charalambous, P.; Vyrides, I. Dairy wastewater management in EU: Produced amounts, existing legislation, applied treatment processes and future challenges. J. Environ. Manag. 2022, 303, 114152. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, M.; Natarajan, N. Towards achieving sustainable bioplastics production and nutrient recovery from wastewater—A comprehensive overview on polyhydroxybutyrate. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Afanasevna, I.L.; Andreevich, F.; Alekseevna, C.L.; Pavlovna, K.T. Overview of Mycelial Fungi—Lignin Destructors. KnE Life Sci. 2022, 2022, 175–180. [Google Scholar] [CrossRef]
- Mäkelä, M.R.; Donofrio, N.; De Vries, R.P. Plant biomass degradation by fungi. Fungal Genet. Biol. 2014, 72, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Marzluf, G.A. Genetic regulation of nitrogen metabolism in the fungi. Microbiol. Mol. Biol. Rev. 1997, 61, 17–32. [Google Scholar] [CrossRef]
- Carrasco-Cabrera, C. The role of nitrogen sources and caffeine for growth of Pleurotus ostreatus (oyster mushroom). Ph.D. Thesis, Faculty of Science, School of Life and Environmental Sciences, The University of Sydney, Sydney, Australia, 2018. [Google Scholar]
- Vane, C.H.; Martin, S.C.; Snape, C.E.; Abbott, G.D. Degradation of lignin in wheat straw during growth of the oyster mushroom (Pleurotus ostreatus) using off-line thermochemolysis with tetramethylammonium hydroxide and solid-state 13C NMR. J. Agric. Food Chem. 2001, 49, 2709–2716. [Google Scholar] [CrossRef]
- Rizki, M.; Tamai, Y. Effects of different nitrogen rich substrates and their combination to the yield performance of oyster mushroom (Pleurotus ostreatus). World J. Microbiol. Biotechnol. 2011, 27, 1695–1702. [Google Scholar] [CrossRef]
- Pardo, A.; de Juan, A.; Alvarez-Ortí, M.; Pardo, J.E. Screening of Agaricus bisporus (Lange, Imbach) strains and the casing variables for quality mushroom production in Spain. HortScience 2010, 45, 231–235. [Google Scholar] [CrossRef]
- El Sebaaly, Z.; Assadi, F.; Sassine, Y.N.; Shaban, N. Substrate types effect on nutritional composition of button mushroom (Agaricus bisporus). Agric. For. 2019, 65, 73–80. [Google Scholar] [CrossRef]
- Sharma, V.P.; Kumar, S.; Sharma, S. Technologies Developed by ICAR-DMR for Commercial Use; ICAR-Directorate of Mushroom Research: Solan, India, 2020. [Google Scholar]
- Kalmis, E.; Azbar, N.; Yildiz, H.; Kalyoncu, F. Feasibility of using olive mill effluent (OME) as a wetting agent during the cultivation of oyster mushroom, Pleurotus ostreatus, on wheat straw. Bioresour. Technol. 2008, 99, 164–169. [Google Scholar] [CrossRef]
- Keleş, A.; Koca, I.; Gençcelep, H. Antioxidant Properties of Wild Edible Mushrooms. J. Food Process. Technol. 2011, 2, 2–6. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Barros, L.; Ferreira, M.J.; Queirós, B.; Ferreira, I.C.F.R.; Baptista, P. Total phenols, ascorbic acid, β-carotene and lycopene in Portuguese wild edible mushrooms and their antioxidant activities. Food Chem. 2007, 103, 413–419. [Google Scholar] [CrossRef]
- FAO/WHO. Report of the Thirty Eight Session of the Codex Committee on Food Hygiene. In Proceedings of the FAO/WHO Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission 13th Session, Houston, TX, USA, 2007; ALINORM 07/30/13. Available online: http://files.eacce.org.ma/pj/1417757518.pdf (accessed on 11 January 2022).
- Awashthi, S.K. Prevention of Food Adulteration Act No. 37 of 1954; Central and State Rules as Amended for 1999; Ashoka Law House: New Delhi, India, 2000. [Google Scholar]
- Damodaran, D.; Balakrishnan, R.M.; Shetty, V.K. The Uptake Mechanism of Cd(II), Cr(VI), Cu(II), Pb(II), and Zn(II) by Mycelia and Fruiting Bodies of Galerina vittiformis. BioMed Res. Int. 2013, 2013, 149120. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.T.; Miles, P.G. Edible mushrooms and their cultivation. In Edible Mushrooms and Their Cultivation; Change, S.T., Miles, P.G., Eds.; CRC Press: Boca Raton, FL, USA, 1989; pp. 189–223. [Google Scholar]
- As 4454-2003; Australian Standard: Composts, Soil Conditioners and Mulches. Standards Australia: Sydney, Australia, 2012.
- Ertugay, N.; Bayhan, Y.K. The removal of copper (II) ion by using mushroom biomass (Agaricus bisporus) and kinetic modelling. Desalination 2010, 255, 137–142. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).