Individual and Interactive Effects of Elevated Ozone and Temperature on Plant Responses
Abstract
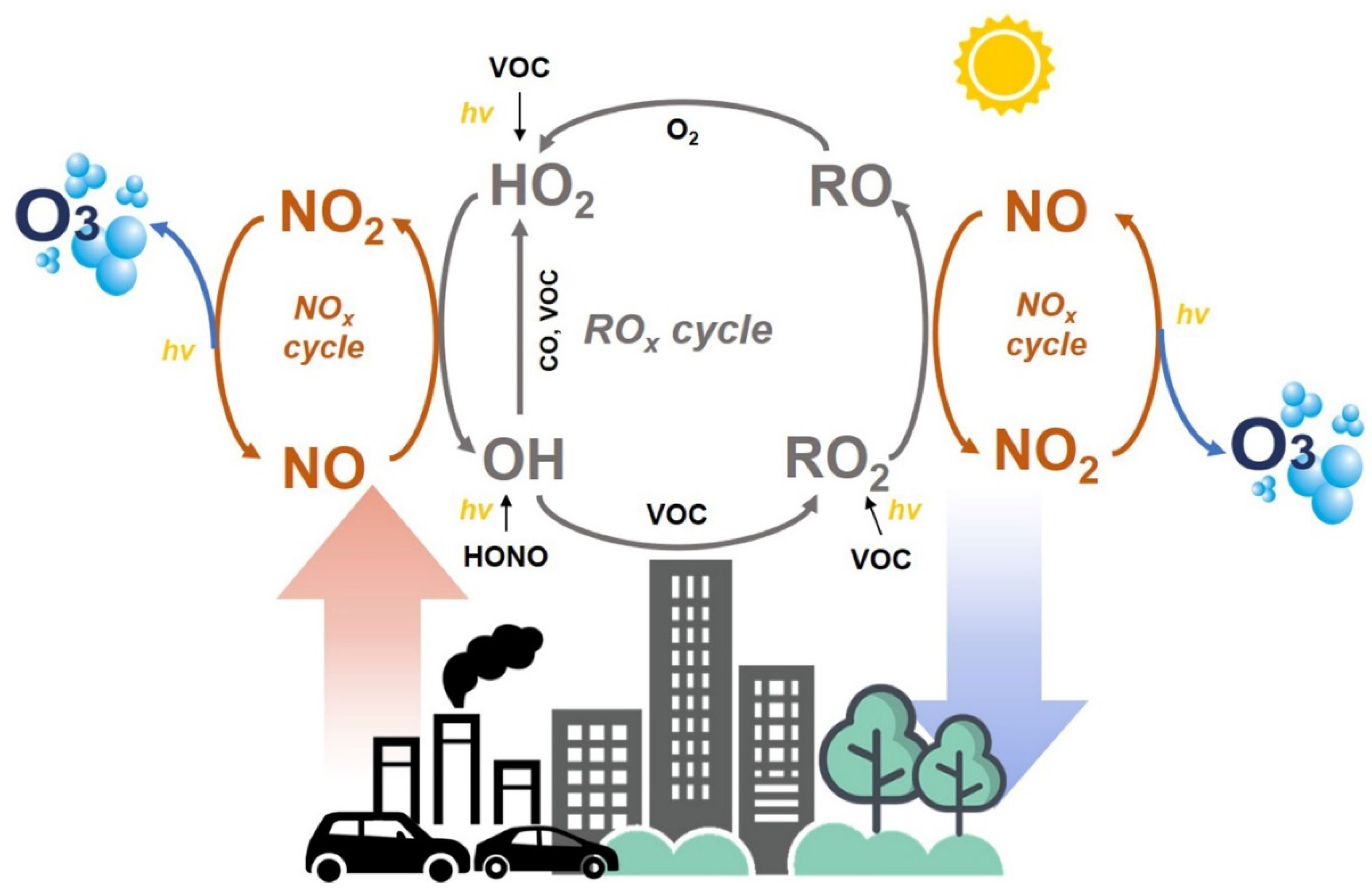
:1. Introduction
2. Plant Responses to Ozone
2.1. Visible Symptoms of Sensitive Plants to Ozone
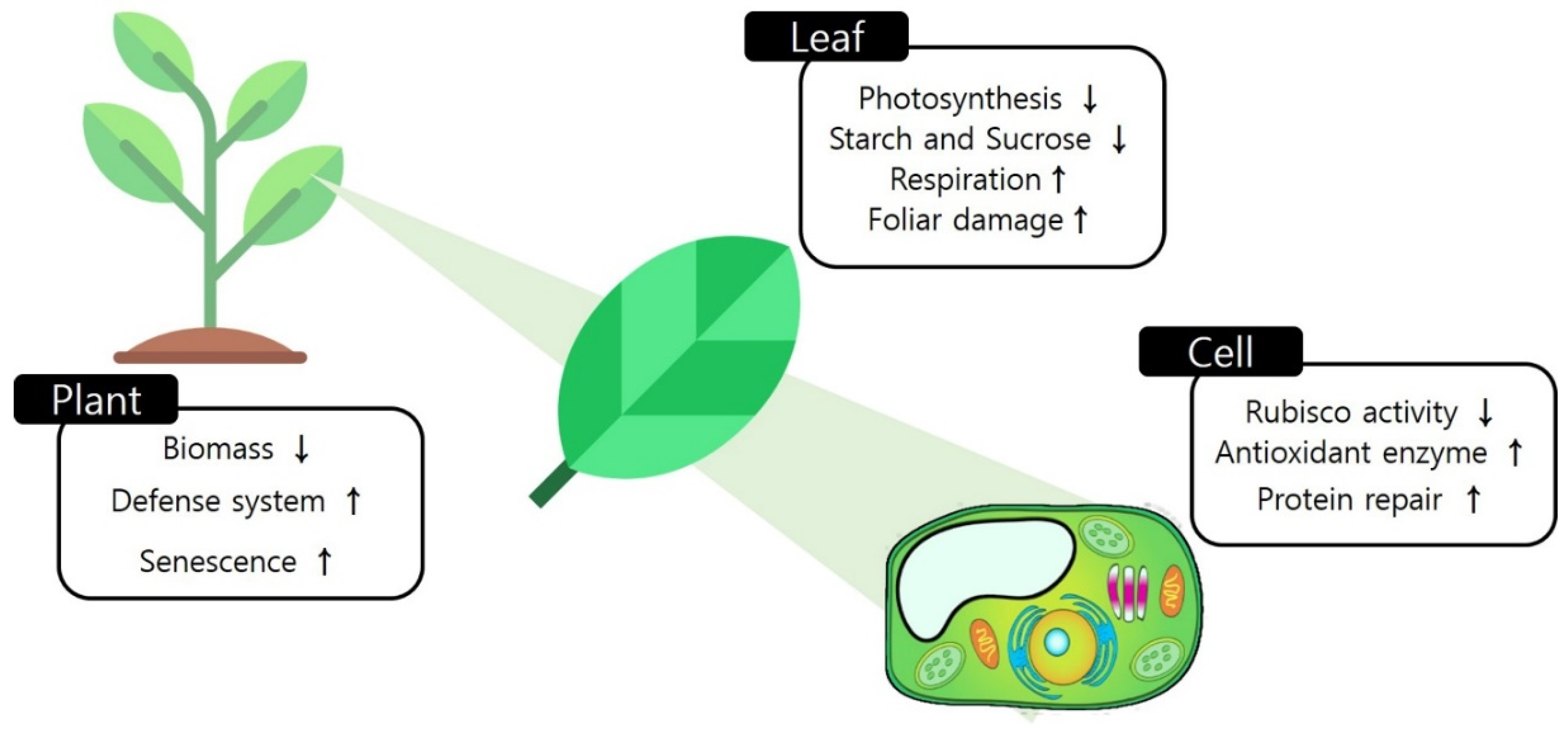
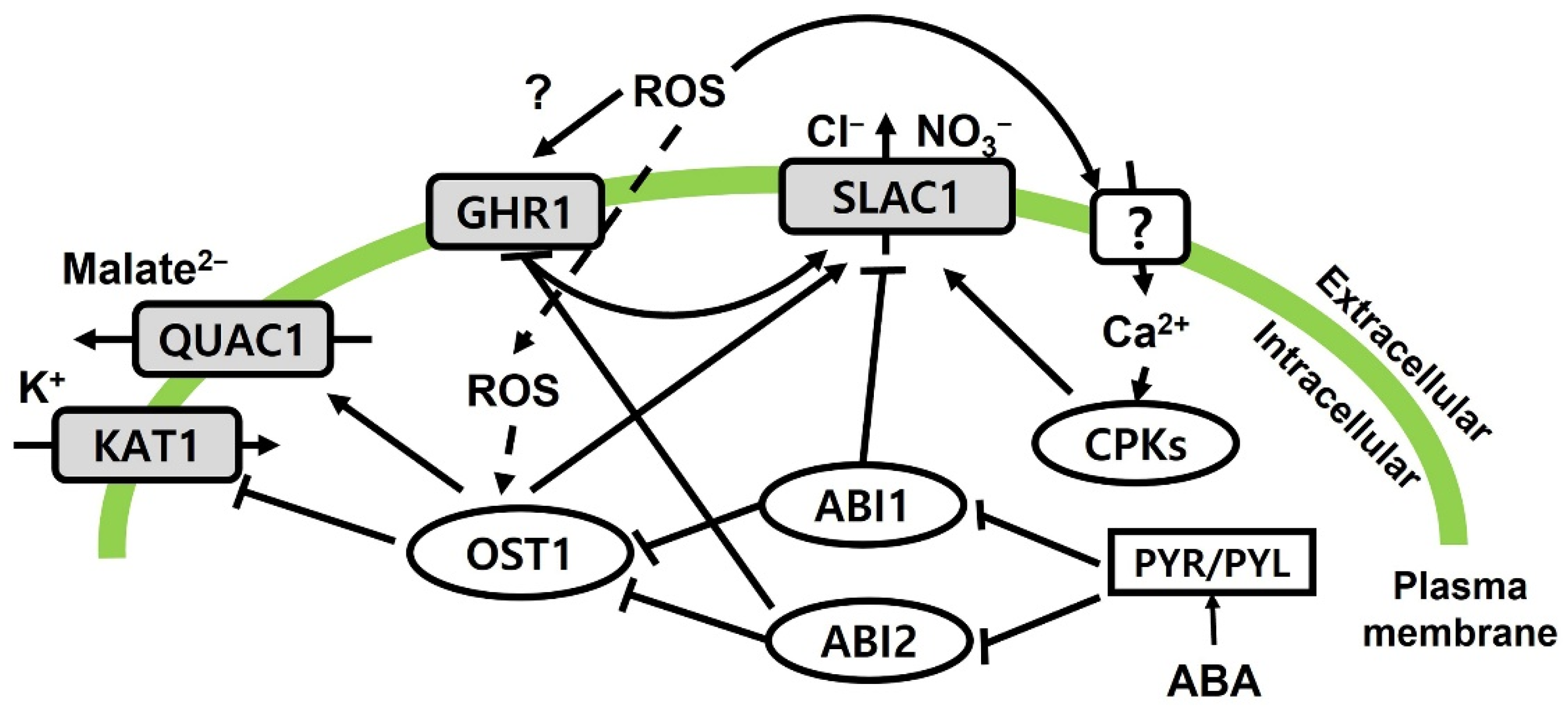
2.2. Physiological Changes in Response to Ozone
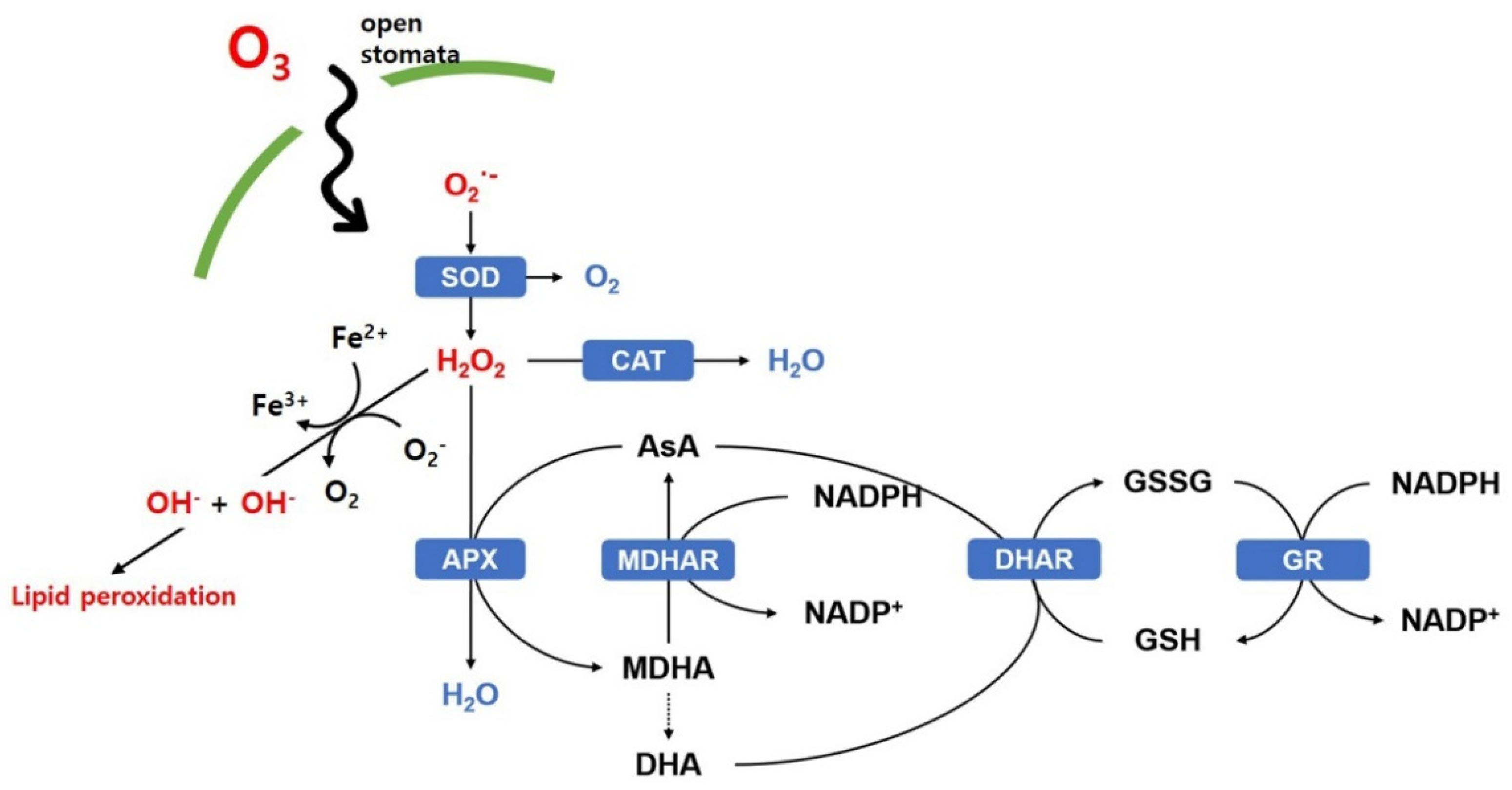
2.3. Biochemical Changes Caused by Ozone
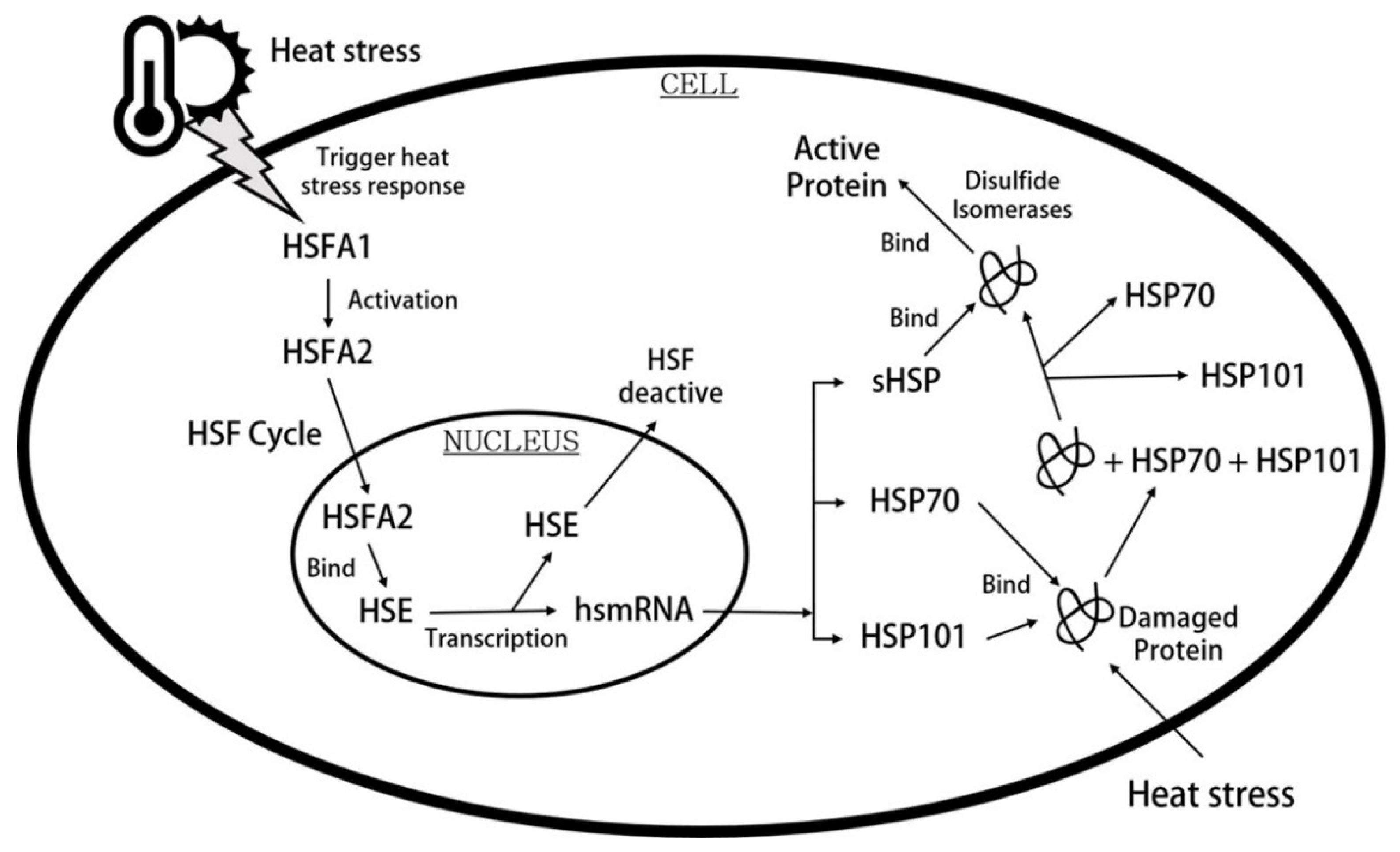
3. Plant Responses to Elevated Temperatures
3.1. Physiological Changes Caused by Elevated Temperatures
3.2. Biochemical Changes in Response to Elevated Temperatures
4. Plant Responses to Ozone under Elevated Temperatures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goldstein, A.; Turner, W.R.; Spawn, S.A.; Anderson-Teixeira, K.J.; Cook-Patton, S.; Fargione, J.; Gibbs, H.K.; Griscom, B.; Hewson, J.H.; Howard, J.F.; et al. Protecting irrecoverable carbon in Earth’s ecosystems. Nat. Clim. Chang. 2020, 10, 287–295. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Fuhrer, J. Ozone risk for crops and pastures in present and future climates. Naturwissenschaften 2009, 96, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Rogers, A.; Leakey, A.D.B. Targets for crop biotechnology in a future high-CO2 and high-O3 world. Plant Physiol. 2008, 147, 13–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Yendrek, C.R.; Sitch, S.; Collins, W.J.; Emberson, L.D. The effects of tropospheric ozone on net primary productivity and implications for climate change. Annu. Rev. Plant Biol. 2012, 63, 637–661. [Google Scholar] [CrossRef] [Green Version]
- Tammam, A.; Badr, R.; Abou-Zeid, H.; Hassan, Y.; Bader, A. Nickel and ozone stresses induce differential growth, antioxidant activity and mRNA transcription in Oryza sativa cultivars. J. Plant Interact. 2019, 14, 87–101. [Google Scholar] [CrossRef] [Green Version]
- Karnosky, D.F.; Pregitzer, K.S.; Zak, D.R.; Kubiske, M.E.; Hendrey, G.R.; Weinstein, D.; Nosal, M.; Percy, K.E. Scaling ozone responses of forest trees to the ecosystem level in a changing climate. Plant Cell Environ. 2005, 28, 965–981. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Cape, J.N. Surface ozone concentrations and ecosystem health: Past trends and a guide to future projections. Sci. Total Environ. 2008, 400, 257–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fitzky, A.C.; Sandén, H.; Karl, T.; Fares, S.; Calfapietra, C.; Grote, R.; Saunier, A.; Rewald, B. The Interplay between ozone and urban vegetation—BVOC emissions, ozone deposition, and tree ecophysiology. Front. For. Glob. Chang. 2019, 2, 50. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Martin, M.V.; Heald, C.L. Threat to future global food security from climate change and ozone air pollution. Nat. Clim. Chang. 2014, 4, 817–821. [Google Scholar] [CrossRef] [Green Version]
- Sage, R.F.; Kubien, D.S. The temperature response of C3 and C4 photosynthesis. Plant Cell Environ. 2007, 30, 1086–1106. [Google Scholar] [CrossRef]
- Booker, F.; Muntifering, R.; Mcgrath, M.; Burkey, K.; Decoteau, D.; Fiscus, E.; Manning, W.; Krupa, S.; Chappelka, A.; Grantz, D. The ozone component of global change: Potential effects on agricultural and horticultural plant yield, product quality and interactions with invasive species. J. Integr. Plant Biol. 2009, 51, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.; Sharps, K.; Simpson, D.; Pleijel, H.; Broberg, M.; Uddling, J.; Jaramillo, F.; Davies, W.J.; Dentener, F.; Van den Berg, M.; et al. Ozone pollution will compromise efforts to increase global wheat production. Glob. Chang. Biol. 2018, 24, 3560–3574. [Google Scholar] [CrossRef] [PubMed]
- Choquette, N.E.; Ainsworth, E.A.; Bezodis, W.; Cavanagh, A.P. Ozone tolerant maize hybrids maintain Rubisco content and activity during long-term exposure in the field. Plant. Cell Environ. 2020, 43, 3033–3047. [Google Scholar] [CrossRef]
- Feng, Z.; Sun, J.; Wan, W.; Hu, E.; Calatayud, V. Evidence of widespread ozone-induced visible injury on plants in Beijing, China. Environ. Pollut. 2014, 193, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, E.; Materassi, A.; Fasano, G.; Hoshika, Y.; Carriero, G.; Silaghi, D.; Badea, O. A new-generation 3D ozone FACE (Free Air Controlled Exposure). Sci. Total Environ. 2017, 575, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Günthardt-Goerg, M.S.; Vollenweider, P. Linking stress with macroscopic and microscopic leaf response in trees: New diagnostic perspectives. Environ. Pollut. 2007, 147, 467–488. [Google Scholar] [CrossRef]
- Baek, S.G.; Park, J.H.; Na, C.S.; Lee, B.; Cheng, H.C.; Woo, S.Y. The morphological characteristics of Pterocarpus indicus induced by elevated ozone under well-watered and drought conditions. Forest Sci. Technol. 2018, 14, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Pääkkönen, E. Ageing-related anatomical and ultrastructural changes in leaves of birch (Betula pendula Roth.) clones as affected by low ozone exposure. Ann. Bot. 1995, 75, 285–294. [Google Scholar] [CrossRef]
- Vollenweider, P.; Günthardt-Goerg, M.S.; Menard, T.; Baumgarten, M.; Matyssek, R.; Schaub, M. Macro- and microscopic leaf injury triggered by ozone stress in beech foliage (Fagus sylvatica L.). Ann. For. Sci. 2019, 76, 71. [Google Scholar] [CrossRef]
- Wan, W.; Manning, W.J.; Wang, X.; Zhang, H.; Sun, X.; Zhang, Q. Ozone and ozone injury on plants in and around Beijing, China. Environ. Pollut. 2014, 191, 215–222. [Google Scholar] [CrossRef]
- Iyer, N.J.; Tang, Y.; Mahalingam, R. Physiological, biochemical and molecular responses to a combination of drought and ozone in Medicago truncatula. Plant Cell Environ. 2013, 36, 706–720. [Google Scholar] [CrossRef]
- Lee, J.K.; Woo, S.Y.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Park, J.H.; Lee, K.A. Effects of elevated temperature and ozone in Brassica juncea L.: Growth, physiology, and ROS accumulation. Forests 2020, 11, 68. [Google Scholar] [CrossRef] [Green Version]
- Sharps, K.; Hayes, F.; Harmens, H.; Mills, G. Ozone-induced effects on leaves in African crop species. Environ. Pollut. 2021, 268, 115789. [Google Scholar] [CrossRef]
- Fiscus, E.L.; Booker, F.L.; Burkey, K.O. Crop responses to ozone: Uptake, modes of action, carbon assimilation and partitioning. Plant Cell Environ. 2005, 28, 997–1011. [Google Scholar] [CrossRef]
- Wittig, V.E.; Ainsworth, E.A.; Long, S.P. To what extent do current and projected increases in surface ozone affect photosynthesis and stomatal conductance of trees? A meta-analytic review of the last 3 decades of experiments. Plant Cell Environ. 2007, 30, 1150–1162. [Google Scholar] [CrossRef]
- Feng, Z.; Kobayashi, K.; Ainsworth, E.A. Impact of elevated ozone concentration on growth, physiology, and yield of wheat (Triticum aestivum L.): A meta-analysis. Glob. Chang. Biol. 2008, 14, 2696–2708. [Google Scholar] [CrossRef]
- Ainsworth, E.A. Rice production in a changing climate: A meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob. Chang. Biol. 2008, 14, 1642–1650. [Google Scholar] [CrossRef]
- Paoletti, E.; Grulke, N.E. Does living in elevated CO2 ameliorate tree response to ozone? A review on stomatal responses. Environ. Pollut. 2005, 137, 483–493. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Sui, L.; Huang, Y.; Geng, C.; Yin, B. Physiological and visible injury responses in different growth stages of winter wheat to ozone stress and the protection of spermidine. Atmos. Pollut. Res. 2015, 6, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Kollist, T.; Moldau, H.; Rasulov, B.; Oja, V.; Rämma, H.; Hüve, K.; Jaspers, P.; Kangasjärvi, J.; Kollist, H. A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol. Plant. 2007, 129, 796–803. [Google Scholar] [CrossRef]
- Kollist, H.; Moldau, H.; Mortensen, L.; Rasmussen, S.K.; Jørgensen, L.B. Ozone flux to plasmalemma in barley and wheat is controlled by stomata rather than by direct reaction of ozone with cell wall ascorbate. J. Plant Physiol. 2000, 156, 645–651. [Google Scholar] [CrossRef]
- Pell, E.J.; Eckardt, N.; Enyedi, A.J. Timing of ozone stress and resulting status of ribulose bisphosphate carboxylase/oxygenase and associated net photosynthesis. New Phytol. 1992, 120, 397–405. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Shomali, A.; Azad, N.; Hassani, B.; Lastochkina, O.; Li, T. Calcium signaling and salt tolerance are diversely entwined in plants. Plant Signal. Behav. 2019, 14, 1665455. [Google Scholar] [CrossRef]
- Held, A.A.; Mooney, H.A.; Gorham, J.N. Acclimation to ozone stress in radish: Leaf demography and photosynthesis. New Phytol. 1991, 118, 417–423. [Google Scholar] [CrossRef]
- Droutsas, I.; Challinor, A.J.; Arnold, S.R.; Mikkelsen, T.N.; Hansen, E.M.Ø. A new model of ozone stress in wheat including grain yield loss and plant acclimation to the pollutant. Eur. J. Agron. 2020, 120, 126125. [Google Scholar] [CrossRef]
- Agrawal, G.K.; Rakwal, R.; Yonekura, M.; Kubo, A.; Saji, H. Proteome analysis of differentially displayed proteins as a tool for investigating ozone stress in rice (Oryza sativa L.) seedlings. Proteomics 2002, 2, 947–959. [Google Scholar] [CrossRef]
- Cho, K.; Shibato, J.; Kubo, A.; Kohno, Y.; Satoh, K.; Kikuchi, S.; Agrawal, G.K.; Sarkar, A.; Rakwal, R. Genome-wide mapping of the ozone-responsive transcriptomes in rice panicle and seed tissues reveals novel insight into their regulatory events. Biotechnol. Lett. 2013, 35, 647–656. [Google Scholar] [CrossRef]
- Hoshika, Y.; Haworth, M.; Watanabe, M.; Koike, T. Interactive effect of leaf age and ozone on mesophyll conductance in Siebold’s beech. Physiol. Plant. 2020, 170, 172–186. [Google Scholar] [CrossRef]
- Di Baccio, D.; Castagna, A.; Paoletti, E.; Sebastiani, L.; Ranieri, A. Could the differences in O3 sensitivity between two poplar clones be related to a difference in antioxidant defense and secondary metabolic response to O3 influx? Tree Physiol. 2008, 28, 1761–1772. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Sun, P.; Feng, H.; Zhou, H.; Wang, R.; Liang, Y.; Lu, J.; Zhu, W.; Zhang, J.; Fang, J. Reactive oxygen species in a non-thermal plasma microjet and water system: Generation, conversion, and contributions to bacteria inactivation—An analysis by electron spin resonance spectroscopy. Plasma Process. Polym. 2012, 9, 417–424. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Halliwell, B. The presence of glutathione and glutathione reductase in chloroplasts: A proposed role in ascorbic acid metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Vainonen, J.P.; Kangasjärvi, J. Plant signalling in acute ozone exposure. Plant Cell Environ. 2015, 38, 240–252. [Google Scholar] [CrossRef]
- Short, E.F.; North, K.A.; Roberts, M.R.; Hetherington, A.M.; Shirras, A.D.; McAinsh, M.R. A stress-specific calcium signature regulating an ozone-responsive gene expression network in Arabidopsis. Plant J. 2012, 71, 948–961. [Google Scholar] [CrossRef]
- Kollist, H.; Jossier, M.; Laanemets, K.; Thomine, S. Anion channels in plant cells. FEBS J. 2011, 278, 4277–4292. [Google Scholar] [CrossRef] [Green Version]
- Joshi-Saha, A.; Valon, C.; Leung, J. A brand new START: Abscisic acid perception and transduction in the guard cell. Sci. Signal. 2011, 4, re4. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Wang, C.; He, J.; Liao, H.; Duan, Y.; Zhu, Z.; Guo, Y.; Chen, Z.; Gong, Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 2012, 24, 2546–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, F.K.; Menezes-Benavente, L.; Galvão, V.C.; Margis-Pinheiro, M. Multigene families encode the major enzymes of antioxidant metabolism in Eucalyptus grandis L. Genet. Mol. Biol. 2005, 28, 529–538. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Posch, B.C.; Kariyawasam, B.C.; Bramley, H.; Coast, O.; Richards, R.A.; Reynolds, M.P.; Trethowan, R.; Atkin, O.K. Exploring high temperature responses of photosynthesis and respiration to improve heat tolerance in wheat. J. Exp. Bot. 2019, 70, 5051–5069. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [Green Version]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Effects of temperature and watering regime on growth, gas exchange and abscisic acid content of canola (Brassica napus) seedlings. Environ. Exp. Bot. 2012, 75, 107–113. [Google Scholar] [CrossRef]
- Wise, R.R.; Olson, A.J.; Schrader, S.M.; Sharkey, T.D. Electron transport is the functional limitation of photosynthesis in field-grown Pima cotton plants at high temperature. Plant Cell Environ. 2004, 27, 717–724. [Google Scholar] [CrossRef]
- Camejo, D.; Rodríguez, P.; Morales, M.A.; Dell’Amico, J.M.; Torrecillas, A.; Alarcón, J.J. High temperature effects on photosynthetic activity of two tomato cultivars with different heat susceptibility. J. Plant Physiol. 2005, 162, 281–289. [Google Scholar] [CrossRef]
- Liu, X.; Huang, B. Heat stress injury in relation to membrane lipid peroxidation in creeping bentgrass. Crop Sci. 2000, 40, 503–510. [Google Scholar] [CrossRef]
- Medina, E.; Kim, S.-H.; Yun, M.; Choi, W.-G. Recapitulation of the function and role of ROS generated in response to heat stress in plants. Plants 2021, 10, 371. [Google Scholar] [CrossRef]
- Ott, M.; Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria, oxidative stress and cell death. Apoptosis 2007, 12, 913–922. [Google Scholar] [CrossRef]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A review. Agron. Sustain. Dev. 2017, 37, 37. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Savicka, M.; Škute, N. Effects of high temperature on malondialdehyde content, superoxide production and growth changes in wheat seedlings (Triticum aestivum L.). Ekologija 2010, 56, 26–33. [Google Scholar] [CrossRef]
- Balogi, Z.; Multhoff, G.; Jensen, T.K.; Lloyd-Evans, E.; Yamashima, T.; Jäättelä, M.; Harwood, J.L.; Vígh, L. Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 2019, 74, 18–30. [Google Scholar] [CrossRef]
- Scharf, K.D.; Berberich, T.; Ebersberger, I.; Nover, L. The plant heat stress transcription factor (Hsf) family: Structure, function and evolution. Biochim. Biophys. Acta Gene Regul. Mech. 2012, 1819, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Van Montagu, M.; Verbruggen, N. Small heat shock proteins and stress tolerance in plants. Biochim. Biophys. Acta Gene Struct. Expr. 2002, 1577, 1–9. [Google Scholar] [CrossRef]
- Asthir, B. Mechanisms of heat tolerance in crop plants. Biol. Plant. 2015, 59, 620–628. [Google Scholar] [CrossRef]
- Qu, A.L.; Ding, Y.F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef]
- Hu, W.; Hu, G.; Han, B. Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. Plant Sci. 2009, 176, 583–590. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Schöffl, F.; Prändl, R.; Reindl, A. Regulation of the heat-shock response. Plant Physiol. 1998, 117, 1135–1141. [Google Scholar] [CrossRef] [Green Version]
- Larkindale, J.; Hall, J.D.; Knight, M.R.; Vierling, E. Heat stress phenotypes of Arabidopsis mutants implicate multiple signaling pathways in the acquisition of thermotolerance. Plant Physiol. 2005, 138, 882–897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Port, M.; Tripp, J.; Zielinski, D.; Weber, C.; Heerklotz, D.; Winkelhaus, S.; Bublak, D.; Scharf, K.D. Role of Hsp17.4-CII as coregulator and cytoplasmic retention factor of tomato heat stress transcription factor HsfA2. Plant Physiol. 2004, 135, 1457–1470. [Google Scholar] [CrossRef] [Green Version]
- Cotrozzi, L.; Remorini, D.; Pellegrini, E.; Landi, M.; Massai, R.; Nali, C.; Guidi, L.; Lorenzini, G. Variations in physiological and biochemical traits of oak seedlings grown under drought and ozone stress. Physiol. Plant. 2016, 157, 69–84. [Google Scholar] [CrossRef] [Green Version]
- Juráň, S.; Grace, J.; Urban, O. Temporal changes in ozone concentrations and their impact on vegetation. Atmosphere 2021, 12, 82. [Google Scholar] [CrossRef]
- Li, K.; Jacob, D.J.; Liao, H.; Shen, L.; Zhang, Q.; Bates, K.H. Anthropogenic drivers of 2013–2017 trends in summer surface ozone in China. Proc. Natl. Acad. Sci. USA 2019, 116, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Peltola, H.; Kilpeläinen, A.; Kellomäki, S. Diameter growth of Scots pine (Pinus sylvestris) trees grown at elevated temperature and carbon dioxide concentration under boreal conditions. Tree Physiol. 2002, 22, 963–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kivimäenpää, M.; Sutinen, S.; Valolahti, H.; Häikiö, E.; Riikonen, J.; Kasurinen, A.; Ghimire, R.P.; Holopainen, J.K.; Holopainen, T. Warming and elevated ozone differently modify needle anatomy of Norway spruce (Picea abies) and Scots pine (Pinus sylvestris). Can. J. For. Res. 2017, 47, 488–499. [Google Scholar] [CrossRef] [Green Version]
- Agathokleous, E.; Feng, Z.; Oksanen, E.; Sicard, P.; Wang, Q.; Saitanis, C.J.; Araminiene, V.; Blande, J.D.; Hayes, F.; Calatayud, V.; et al. Ozone affects plant, insect, and soil microbial communities: A threat to terrestrial ecosystems and biodiversity. Sci. Adv. 2020, 6, eabc1176. [Google Scholar] [CrossRef] [PubMed]
- Wieser, G.; Matyssek, R. Linking ozone uptake and defense towards a mechanistic risk assessment for forest trees. New Phytol. 2007, 174, 7–9. [Google Scholar] [CrossRef]
- Lee, J.K.; Kwak, M.J.; Park, S.H.; Kim, H.D.; Lim, Y.J.; Jeong, S.G.; Choi, Y.S.; Woo, S.Y. Ozone response of leaf physiological and stomatal characteristics in Brassica juncea L. at supraoptimal temperatures. Land 2021, 10, 357. [Google Scholar] [CrossRef]
- Kasurinen, A.; Biasi, C.; Holopainen, T.; Rousi, M.; Mäenpää, M.; Oksanen, E. Interactive effects of elevated ozone and temperature on carbon allocation of silver birch (Betula pendula) genotypes in an open-air field exposure. Tree Physiol. 2012, 32, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, O.; Klem, K.; Ač, A.; Havránková, K.; Holišová, P.; Navrátil, M.; Zitová, M.; Kozlová, K.; Pokorný, R.; Šprtová, M.; et al. Impact of clear and cloudy sky conditions on the vertical distribution of photosynthetic CO2 uptake within a spruce canopy. Funct. Ecol. 2012, 26, 46–55. [Google Scholar] [CrossRef]
- Juráň, S.; Šigut, L.; Holub, P.; Fares, S.; Klem, K.; Grace, J.; Urban, O. Ozone flux and ozone deposition in a mountain spruce forest are modulated by sky conditions. Sci. Total Environ. 2019, 672, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Jurán, S.; Edwards-Jonášová, M.; Cudlín, P.; Zapletal, M.; Šigut, L.; Grace, J.; Urban, O. Prediction of ozone effects on net ecosystem production of Norway spruce forest. iForest 2018, 11, 743. [Google Scholar] [CrossRef] [Green Version]
- Matyssek, R.; Bytnerowicz, A.; Karlsson, P.E.; Paoletti, E.; Sanz, M.; Schaub, M.; Wieser, G. Promoting the O3 flux concept for European forest trees. Environ. Pollut. 2007, 146, 587–607. [Google Scholar] [CrossRef]
- Barnes, J.D.; Reiling, K.; Davison, A.W.; Renner, C.J. Interaction between ozone and winter stress. Environ. Pollut. 1988, 53, 235–254. [Google Scholar] [CrossRef]
- Kostaki, K.-I.; Coupel-Ledru, A.; Bonnell, V.C.; Gustavsson, M.; Sun, P.; McLaughlin, F.J.; Fraser, D.P.; McLachlan, D.H.; Hetherington, A.M.; Dodd, A.N.; et al. Guard cells integrate light and temperature signals to control stomatal aperture. Plant Physiol. 2020, 182, 1404–1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, G.; Hayes, F.; Simpson, D.; Emberson, L.; Norris, D.; Harmens, H.; Büker, P. Evidence of widespread effects of ozone on crops and (semi-)natural vegetation in Europe (1990–2006) in relation to AOT40- and flux-based risk maps. Glob. Chang. Biol. 2011, 17, 592–613. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, S.; Zhang, W.; Li, Y.; Wang, N.; He, X.; Chen, W. Responses of growth, photosynthesis and related physiological characteristics in leaves of Acer ginnala Maxim. to increasing air temperature and/or elevated O3. Plant Biol. 2021, 23, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Changey, F.; Bagard, M.; Souleymane, M.; Lerch, T.Z. Cascading effects of elevated ozone on wheat rhizosphere microbial communities depend on temperature and cultivar sensitivity. Environ. Pollut. 2018, 242, 113–125. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.K.; Kwak, M.J.; Jeong, S.G.; Woo, S.Y. Individual and Interactive Effects of Elevated Ozone and Temperature on Plant Responses. Horticulturae 2022, 8, 211. https://doi.org/10.3390/horticulturae8030211
Lee JK, Kwak MJ, Jeong SG, Woo SY. Individual and Interactive Effects of Elevated Ozone and Temperature on Plant Responses. Horticulturae. 2022; 8(3):211. https://doi.org/10.3390/horticulturae8030211
Chicago/Turabian StyleLee, Jong Kyu, Myeong Ja Kwak, Su Gyeong Jeong, and Su Young Woo. 2022. "Individual and Interactive Effects of Elevated Ozone and Temperature on Plant Responses" Horticulturae 8, no. 3: 211. https://doi.org/10.3390/horticulturae8030211
APA StyleLee, J. K., Kwak, M. J., Jeong, S. G., & Woo, S. Y. (2022). Individual and Interactive Effects of Elevated Ozone and Temperature on Plant Responses. Horticulturae, 8(3), 211. https://doi.org/10.3390/horticulturae8030211







