Abstract
Autochthones grape variety of ‘Prokupac’ (Vitis vinifera L.) is being increasingly cultivated in the Republic of Serbia and is one of the predominant varieties in the vineyards of southern Serbia. ‘Prokupac’ grapes are used to produce red wine with specific and distinctive varietal aromatic characteristics. Medicinal herbs can be effectively combined in alcoholic beverages. The aim of this study was to evaluate the effect of medicinal herbs on the aroma profile and sensory characteristics of ‘Prokupac’ red wine. The analysis of the aromatic composition was conducted on ‘Prokupac’ wine (control) and ‘Prokupac’ wine aromatised with selected medicinal herbs: anise (Pimpinella anisum L.), cinnamon (Cinnamomum verum J. Presl.), wormwood (Artemisia absinthium L.) and licorice (Glycyrrhiza glabra L.). The analysis of volatile aromatic compounds in the wines, performed by the gas chromatography-mass spectrometry (GC/MS) method, identified 48 compounds that were classified in the following groups: alcohols, aldehydes, ketones, acids, ethyl esters and terpenes. Sensory analysis of wines was performed including visual, olfactory, gustatory and gustatory-olfactory perceptions. Terpenes were not identified in the ‘Prokupac’ control wine, while the highest content of all identified aromatic compounds was found in wines aromatised with anise, wormwood and cinnamon. The results indicated that selected medicinal herbs affected the composition and content of volatile aromatic compounds, as well as the sensory characteristics of analyzed wines. The unique aroma profile and pleasant taste of the wine aromatised with cinnamon contributed to its differentiation from other wines, and classification as very good wine.
1. Introduction
With the development of the wine industry globally and with increasing investment in the process of wine production, the main emphasis is placed on the production of high-quality wines obtained from autochthonous grape varieties with an authentic taste, and a balanced and unique varietal aroma. The aroma of the wine is a complex property that results from the interaction between the chemical compounds in the wine and the senses of smell and taste. Aroma consists of aromatic compounds from the grapes, as well as compounds that are formed during and after alcoholic fermentation. Wine aromas are created by the interactions of hundreds of compounds formed during alcoholic fermentation [1]. Aroma is the most complex chemical component of grapes and wine, consisting of a large number of compounds whose concentration varies under the influence of varieties and terroir [2,3,4]. The application of certain ampelotechnical measures, the moment of harvest, stressful growing conditions such as drought and cold [2,5,6,7,8], the yeast strain used during fermentation [9], the type of wood from which the vessel was made and the length of aging of the wine, have a large impact on the aroma of grapes [10,11,12,13].
According to origin, aromas are divided into: primary aroma derived from grapes; secondary aroma produced during primary processing, grape crushing and enzyme activity; the aroma produced during fermentation; and the tertiary aroma developed during aging and storage of the wine. The content of aromatic substances in wine ranges from 0.8–1.2 g/L, and half of them are alcohols that are produced during alcoholic fermentation. The odor detection threshold of aromatic substances is between 10−4 g/L to 10−12 [14]. Primary aromas are varietal aromas derived from grapes, found in pomace or must before fermentation and are the easiest to detect in young wines. Groups of fruity, floral and herbal aromas usually appear. The varietal character depends on the overall profile of the aroma-active compounds present in grapes and the corresponding wine [1]. Primary aromas that belong to the same grape variety may have different characteristics depending on some natural factors such as weather characteristics, soil type, fertilization, grapevine diseases and terroir, ripening period and harvesting method [15]. Secondary aromas are developed during the fermentation of must or pomace and they carry the main aromatic characteristics of the wine. During this period, complex biochemical and chemical reactions occur, as well as the synthesis of secondary aromatic compounds. Aromatic compounds such as higher alcohols, volatile acids, fatty acid ethyl esters and volatile phenols are formed. Fatty acid ethyl esters and acetate esters represent the most important esters in wine [11,16,17]. The loss of fruity and floral aromas of young wines is associated with the hydrolysis of esters [18]. Higher alcohols are produced and formed by the catabolism of amino acids by yeasts via the Ehrlich pathway [17]. Tertiary wine aromas are developed during wine maturation and aging and, depending on the method of aging, they make the wine bouquet.
Primary aromas are most pronounced in young red wines, whereas secondary aromas, as well as aromas due to wine aging, appear during the aging process [3,19,20,21]. According to the literature there are 30–40 of the most fragrantly active components, of about 800 in total, that are found in grapes and wine [21]. The most important aromatic compounds from the group of terpenoids, higher alcohols, esters, volatile phenols, benzene derivatives and C13-norisoprenoids appear in the form of aglycones.
The history of spiced wines and spirits dates back centuries. There is evidence that ancient Egyptians and Phoenicians added spices and healing herbs to wine [22]. Medicinal herbs can also affect the composition and sensory characteristic of various alcoholic beverages such as wine, brandies, distilled spirits, etc. [23,24]. Aromatic herbs are used for aromatization (obtaining aromatised wines), which gives the final product sensory characteristics that are different from wine. As defined in the International Code of Oenological Practice by the OIV, such a wine is obtained from at least 50% of the amount of wine with the addition of aromatic substances. The actual amount of alcohol is equal to or above 3.5 vol.% and less than 14.5 vol.%. The alcohol component comes exclusively from wine or special wine [25].
‘Prokupac’ is a Serbian autochthonous grape variety (Vitis vinifera L.) and one of the predominant varieties in the vineyards of southern Serbia. It is characterised by strong vigour and large yielding capacity, while its bunch could be classified as medium-large, cylindrical or conical in shape, bearing medium compact, round or slightly oval berries with a dark blue epidermis [26]. ‘Prokupac’ grapes are used to produce red wine with specific and distinctive varietal aromatic characteristics.
The aim of this study was to determine the effect of the addition of aromatic herbs on the aromatic profile of ‘Prokupac’ wine and the sensory characteristics of the wine, that could meet demands of contemporary consumers. The following herbs were selected to aromatise the wines: anise (Pimpinella anisum L., syn. Anisum odoratum Raf.), cinnamon (Cinnamomum verum J. Presl), wormwood (Artemisia absinthium L.) and licorice (Glycyrrhiza glabra L., syn. Glycyrrhiza brachycarpa Boiss.). The obtained volatile wine components were determined by gas chromatography/mass spectrometry analysis. This is the first report of the aromatic profile of ‘Prokupac’ wines aromatised with medicinal herbs.
2. Materials and Methods
2.1. Wine Preparation
The grapes of the autochthonous variety ‘Prokupac’ (Vitis vinifera L.) were grown in Jug Bogdanovac vineyard, in the Toplica region (coordinates 43°13′ N/21°42′ E, at an altitude of 250–400 m). The grapes were harvested at the stage of full maturity (sugar content 19.29% and total acid content 6.2 g/L) and were immediately processed under laboratory microvinification conditions, as described in our previous paper [23]. Briefly, grapes were crushed (10 kg for each analysis) with a roller crusher and a stem removal attachment. The quantities of 120 mg/kg of SO2 and 5% (w/v) of potassium metabisulfite were added to the must. Alcoholic fermentation was performed with the yeast Saccharomyces cerevisiae Lalvin V1116 (0.3 g/L wider) (Lallemand, Montreal, QC, Canada) at a temperature of 22 °C. For the preparation of the aromatised wines with herbs, finely ground medicinal herbs were added to the must: anise (Pimpinella anisum L., syn. Anisum odoratum Raf.) seed, cinnamon (Cinnamomum verum J. Presl) bark, wormwood (Artemisia absinthium L.) leaf and licorice (Glycyrrhiza glabra L., syn. Glycyrrhiza brachycarpa Boiss.) root (Glycyrrhiza glabra) in the quantity of 1% (w/w) just before the beginning of the alcoholic fermentation. Upon completion of the fermentation, the wines were separated from the sediment, bottled and stored in a dark and cool place (5–6 °C). After 6 months, the chemical analysis of the wines and the gas chromatography/mass spectrometry (GC/MS) analysis of volatile compounds in the wines enriched with medicinal herbs were performed.
‘Prokupac’ wine labels were as follows: control—PW; wine with anise—PAW; wine with cinnamon—PCW; wine with wormwood—PWW; and wine with licorice—PLW.
2.2. Extraction of Volatile Compounds in Wines by Headspace-Solid Phase Microextraction (HS-SPME)
SPME manual holder and fused silica fiber coated with Carboxen®/Polydimetilsiloxane (CAR/PDMS) stationary phase (85 μm thickness) were purchased from Supelco (Bellefonte, PA, USA) and used for the extraction of the aroma compounds from the wine samples by HS–SPME. Before the first use, the fiber was preconditioned according to the manufacturer’s instruction. Twenty milliliters of the wine sample, 3 g of NaCl and a magnetic stirrer bar were placed in a 30 mL amber glass bottle, closed with rubber septum and sealed with parafilm. In order to achieve the equilibration state, the samples were heated at 55 °C and agitated with the magnetic stirrer for 15 min (pre-extraction). The SPME fiber was then inserted into the headspace of the sample and the volatiles were extracted for 35 min with constant heating and stirring at 55 °C. The fiber was than desorbed for 10 min in a split/splitless inlet set at 250 °C in 20:1 split mode and analyzed by GC/MS and GC/FID.
2.3. Gas Chromatography/Mass Spectrometry (GC/MS) Analysis and Gas Chromatography-Flame Ionization Detection (GC/FID) of Volatile Compounds in Wines
GC-MS analysis of the volatiles from wine samples was performed on an Agilent Technologies 7890B gas chromatograph, coupled with an inert, selective 5977A mass detector from the same company. The components were separated on a weakly polar, silica capillary column, HP-5MS (5% diphenyl- and 95% dimethyl-polysiloxane, 30 m × 0.25 mm, 0.25 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Helium was used as the carrier gas, at a constant flow rate of 1 mL/min. The GC oven temperature was held for 2 min at 40 °C, increased to 250 °C at the rate of 7 °C/min, and finally held at 250 °C for 2 min. The total run time was 34 min. Separated components were further analyzed by a mass spectrometer. Temperatures of the MSD transfer line, ion source and quadruple mass analyzer were set at 300 °C, 230 °C and 150 °C, respectively. The ionization voltage was 70 eV and mass detection was done in the scan mode, with a m/z range from 25 to 550.
Data processing was performed using MSD ChemStation (revision F.01.00.1903) in combination with AMDIS (revision 2.70) and NIST MS Search (version 2.0 g) software (Agilent Technologies, Santa Clara, CA, USA). Retention indices of the components from the sample were experimentally determined using a homologous series of n-alkanes from C8-C20 as standards and were analyzed under identical GC/MS and GC/FID conditions. The identification of the compounds was based on the comparison of experimentally obtained linear retention indices with those found in the literature as well as on the comparison of their EI mass spectra with data from Willey 6, NIST11 and RTLPEST 3 mass spectra libraries [27].
2.4. Quantitative HS–SPME Analysis
Concentrations of volatiles (expressed in mg/L) in the wine samples were determined according to the procedure described in the literature [28]. The samples for quantification were prepared as explained, differing in the sense that the appropriate volume of standard solution, instead of being added in to the wine samples, was added in to a wine surrogate (12% ethanol and 4 g/L tartaric acid, pH 3.4 adjusted with sodium hydroxide solution).
where Areastd is the peak area of the analyte standard and Cstd is the concentration of the standard used. The mean of the RFs was calculated (RFmean) and used to quantify each of the analytes in the samples using the equation:
where CX is the concentration of the analyte in the sample, AreaX is the peak area of the analyte and RFmean is the mean response factor [28].
2.5. Sensory Analysis
The sensory analysis of the ‘Prokupac’ wines was carried out according to the literature [9,25,29,30]. It was performed by thirteen professional evaluators (seven men and five women, aged 30 to 51 years) holding a certificate issued by the Ministry of Agriculture, Forestry and Water Management of the Republic of Serbia for performing the sensory analysis of wine. Prior to the wine sensory evaluation, they had been trained in sensory description in an accredited laboratory [9]. The wines were evaluated for: visual perception (color and clarity), olfactory perception (purity, fineness and intensity), gustatory perception (purity, structure and harmony) and gustatory–olfactory perception (sustainability and concordance with the varietal characteristics) following the procedure given in a rule book [30]. Three sessions with reference wine were carried out to unify the scoring standard prior to the sensory evaluation, and five sessions were carried out to evaluate the wines. For each wine, three bottles were used in each session. The color intensity and color hue were determined using the spectrophotometric method [31]. A detailed description of methods is given in the Supplementary Materials, and results for color intensity and color hue of ‘Prokupac’ wines are presented in Table S1. The wine samples were evaluated in duplicate in random order, while the intensity of all features was assessed according to a 100-point scale; visual perception—20; olfactory perception—30; gustatory perception—30; gustatory–olfactory perception—20 (Table S2, Supplementary Materials).
2.6. Statistical Analysis
All measurements were done in triplicates and results were presented as mean ± standard deviation. Results for the aroma and color profile of the wine samples were analyzed using the one-way analysis of variance (ANOVA), followed by Tukey’s HSD post hoc test (SPSS Inc., Chicago, IL, USA). The threshold for statistical significance was set at p < 0.05. The principal component analysis (PCA) was used to explore the relationship between the addition of aromatic herbs and volatile aromatic compound profiles. PCA was performed by using Statistica 10 Software (StatSoft Inc., Tulsa, OK, USA, trial version).
3. Results
3.1. Aroma Composition of Wine Samples
The HS–SPME GC/MS analysis revealed the presence of 48 different compounds in the wine samples. Table 1 shows the volatile compounds of the ‘Prokupac’ wines (control and aromatised), grouped according to their chemical structures (alcohols, aldehydes, ketones, acids, esters and terpenes). Alcohols, ethyl esters and terpenes were the most abundant group of volatile compounds, with the exception of PW (control) where no terpenes were identified.

Table 1.
Volatile organic compounds (in mg/L) in studied ‘Prokupac’ wines and aroma descriptors.
Fourteen different alcohols were detected in the analyzed wines. The content of 3-methyl-1-butanol, 2-methyl-1-butanol, phenylethyl alcohol and 1-pentanol was the highest among the detected alcohols. In the sample PW, the most highly represented alcohol was 3-methyl-1-butanol with 168.48 mg/L. In comparison to PW, in aromatised wines increased content was observed for the following alcohols: 3-methyl-1-butanol, 3-methyl-1-pentanol (with the exception of PLW) and n-nonanol (with the exception of PCW and PWW). 1-propanol, 2-butanol, 2,3-butanediol, n-octanol, and hydrocinnamyl alcohol were not present in PW, but were present in most of the aromatised wines.
Among the carbonyl compounds, acetaldehyde was detected in all the wine samples and its concentration (in mg/L) ranged from 5.31 (PWW) to 10.20 (PCW). Benzaldehyde was found in the aromatised wines only (from trace to 2.86 mg/L) while methylolacetone was detected in the samples PW, PWW and PLW (1.70–6.86 mg/L).
The highest content of organic acids was found in the PLW sample, 44.44 mg/L, while the lowest was in PAW sample, 11.64 mg/L. Acetic acid was only detected in the control wine, PW, while in the aromatised wines it was found in trace amounts. The most abundant was octanoic (caprylic) acid in the sample PLW (31.66 mg/L).
The highest/lowest content of total esters were found in the samples PCW (179.63 mg/L) and PLW (76.71 mg/L), respectively. The most abundant ester in all the examined wines was ethyl octanoate with a concentration in the range of 37.11 mg/L (PAW) to 76.84 mg/L (PW), followed by ethyl acetate in PW (36.17 mg/L), and ethyl decanoate in PW (33.40 mg/L) and PAW (33.64 mg/L). Anisyl formate and hydrocinnamyl acetate were detected only in PAW (3.74 mg/L) and PCW (2.29 mg/L) samples, respectively.
Terpenes were not identified in the control wine, PW, while in the aromatised wines their content was in the range from 16.48 mg/L (PLW) to 867.93 mg/L (PAW). Among the 13 terpenes identified in the sample PWW, the most abundant were terpinen-4-ol (53.47 mg/L), trans-thujone (45.13 mg/L) and (E)-anethole (34.01 mg/L). The samples PAW and PCW were characterised by the highest content of (E)-anethole (671.30 and 57.58 mg/L, respectively). Methyl chavicol was the second most abundant terpene. It was detected only in PAW and PWW samples, in concentrations of 137.12 mg/L and 3.73 mg/L, respectively. Borneol and (Z)-anethole were characteristic only for PCW and PWA wines, respectively.
3.2. PCA Analysis
Figure 1 illustrates a good separation of the ‘Prokupac’ wine samples according to aromatisation with medicinal herbs. The first two principal components accounted for more than 65% of the total variance. The PWW and PAW wine samples were located in the positive axis of PC2 and were strongly positively correlated with a greater abundance of terpenes, but these samples were separated from each other along the direction of PC1, mostly due to higher concentrations of a particular terpene. It should be noted that these compounds originated from the aromatic herb added to a certain sample. On the other hand, the location of PCW, PLW and PW indicates that this class of compounds is not present in these wine samples. The compounds 2-phenylethyl propanoate, hydrocinnamyl acetate and hydrocinnamyl alcohol were mainly responsible for PCW sample separation, while acetic acid was the main compound related to the control wine sample. Ethyl 3-methyl pentanoate and decanoic acid contributed most to the separation of the PLW wine sample.
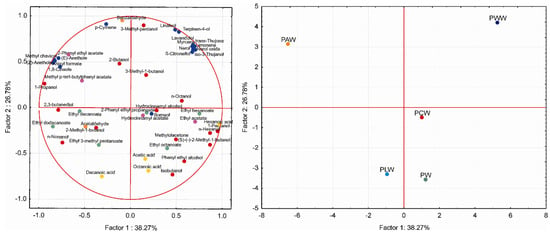
Figure 1.
Principal Component analysis (PCA) based on the volatile aroma compounds in the ‘Prokupac’ wine samples (control and aromatised with aromatic herbs).
3.3. Sensory Evaluation of Wines
Wines were evaluated based on visual perception, olfactory perception, gustatory perception and gustatory–olfactory perception. Results are summarized in Figure 2 and Table S2. ‘Prokupac’ wine aromatised with licorice (PLW) received a rating of 9.00 for visual perception (color and clarity) due to an undesirable yellow color, whereas the best rated was the wine aromatised with cinnamon (PCW), 16.70. Significant differences were observed between the samples for all studied color parameters except for the proportion of blue in intensity (p < 0.05). In all the tested wine samples, the color red was present in the highest percentage in the color of the wine (46.2–54.59%), with the exception of PLW where the color yellow was dominant (49.7%). The percentage of the color blue ranged from 9.34% (PLW) to 10.51% (PWW). Higher values of dA% indicated a higher proportion of red. The lowest value of dA was determined in the PLW (28.00%) and the same wine had the smallest/highest percentage of red and yellow, respectively. In other wine samples, dA ranged from 42.01% (PWW) to 58.51% (PCW), Table S1.
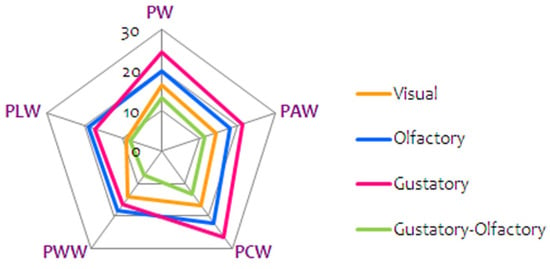
Figure 2.
Sensory characteristics of ‘Prokupac’ wines; control and aromatised with medicinal herbs (control—PW; anise—PAW; cinnamon—PCW; wormwood—PWW and licorice—PLW).
The highest number of points, 22.30, for olfactory perception (purity, fineness and intensity) was awarded to PCW, while the lowest rated was sample PAW with 18.00 points. For gustatory perception (purity, structure and harmony), the lowest number of points was awarded to PWW, 16.50, PW (control) received 24.50 points, and the highest number of points was awarded to PCW, 26.50. The lowest/highest number of points for gustatory–olfactory perception (sustainability and compliance with varietal characteristics of the wine) was awarded to PWW, (7.50) and PCW (13.20), respectively.
The overall assessments of sensory characteristics of ‘Prokupac’ wine aromatised with medicinal herbs are presented in Figure 3. The lowest overall rating was given to the samples PLW and PWW; 53.30 and 56.30 points, respectively. Those wines were classified as ‘acceptable’ wine. The sample PAW with 65.10 points was in the class of ‘average’ wine, whereas PW (control) was evaluated with 73.50 points, a ‘good wine’ class. The highest overall rating, 78.70 points, was given to the sample PCW, and it was classified as ‘very good’ wine (Table S2, Supplementary Materials).
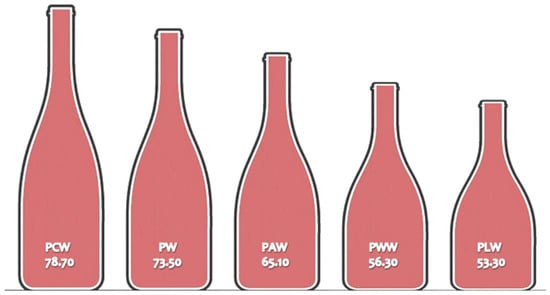
Figure 3.
Overall grades of sensory characteristics of ‘Prokupac’ wines (on a 100-point scale).
4. Discussion
4.1. Aroma Profile of Studied Wines
When it comes to the process of making wine, Saccharomyces cerevisiae has been the most popular yeast. It has a particular metabolism which helps regulate the production of volatile and aroma molecules [39]. The primary and secondary metabolites found in grapes, musts and wines are synthesised in the course of biosynthesis in grapes, yeast metabolism during the fermentation process and a few enzymatic and chemical reactions which happen in the time of aging. Furthermore, the quality of the wine’s aroma is influenced by some of these metabolites [40]. Floral tones of wines are attributed to terpenes [32,35]; esters and lactones give fruitiness [34,35,36], and higher alcohols and aldehydes give herbal tones [37].
According to the analysis of the studied wines, higher alcohols, ethyl esters, acetates and terpenes were measured in the highest concentrations of all volatile compounds detected. The most abundant alcohols in sample PW were 3-methyl-1-butanol, phenyl ethyl alcohol, 2-methyl-1-butanol and 1-pentanol. These results are supported by literature [9]. The presence of higher alcohols contributed to the complexity and fullness of the taste of PW, but also to its fruity–floral aroma. The most abundant alcohols were associated with cheese, fruity and balsamic aromas (3-methyl-1-butanol, 1-pentanol), floral, rose and honey aromas (phenyl–ethyl alcohol), while 2-methyl-1-butanol was associated with a whiskey, burnt aroma (Table 1). Higher concentrations of 3-methyl-1-butanol gave the wine an unpleasant note and a fuel-like odor [41,42,43]. On the other hand, alcohols such as 2-methyl-1-butanol, 3-methyl-1-butanol, 1-propanol, 1-butanol improved the perception of the solvent-like notes and reduced the perception of fruity and jammy aromas of some red wines [44]. Hydrocinnamyl alcohol with a recognizable aroma described as sweet, balsamic, hyacinth-like, spicy, green powdery and cinnamony was not detected in the sample PW, whereas its concentration was the highest in the sample PCW (Table 1).
Ethyl esters are the second group of compounds in terms of abundance in all the ‘Prokupac’ wines (Table 1). The type and relative contribution of ethyl esters depend on the yeast strain, as most of them are formed during the alcoholic fermentation. Ethyl esters positively affect the quality of wine by giving it fresh and fruity aromas [45,46]. Ethyl esters of medium-chain fatty acids (with C6-C12 atoms) were detected in all tested samples of the ‘Prokupac’ wine. The highest content of esters was found in the sample PW (control wine) and the most abundant were ethyl octanoate, ethyl decanoate and ethyl hexanoate which is in line with the literature [9,47]. The exception was 2-phenylethyl acetate, whose concentration was higher in the sample PCW which led to a pleasant floral aroma of the wine. Anisyl formate and hydrocinnamyl acetate were detected only in the samples PAW and PCW, respectively, indicating their origin from anis and cinnamon. The presence of esters in wine is common and desirable, because they give wine a pleasant fruity aroma [48].
Terpenes represent a group of compounds that are typical only for some grape varieties (Muscat Otonell, Muscat Hamburg, Muscat Frontignan, etc.) and give floral notes in the wine aroma [49,50]. Enzyme activity, as well as some procedures in wine production (maceration, soaking the must), can contribute to the increase of the content of terpenes in wine [51]. Terpenes were not identified in the ‘Prokupac’ control wine, which is consistent with the data found in literature [9]. In aromatised ‘Prokupac’ wines, terpenes were the most abundant in the sample PAW (61.3%) and the least in the sample PLW (3.5%). (E)-Anethole (in PAW) and limonene (in PWW) were measured in the highest and lowest concentrations, respectively. Moreover, (E)-anethole was the only terpene detected in all the aromatised wine samples (Table 1). In the PAW sample, the greatest influence on the aromatic profile had (E)-anethole, (Z)-anethole and methyl chavicol, which produced anise-like and licorice-like aromas, as well as 1,8-cineole, which contributed to the aroma of mint [32]. In the sample PCW, terpene compounds borneol and (E)-anethole were the most dominant. Terpinen-4-ol, linalool and 1,8-cineole were detected in lower concentrations. All together, these compounds contributed to the sweet, floral, camphor, nutmeg and mint aromas of the wine [32]. The sample PWW was rich in various terpenes. Among them, the highest content of terpinen-4-ol, trans-thujone, lavandulol, (E)-anethole and linalool was observed, which gave the aroma of nutmeg, anise-like and brought herbal tones, while β-citronellol and nerol influenced a floral, sweet and citrusy aroma [32,35]. In the sample PLW, only (E)-anethole was detected which contributed to licorice-like and anise-like aroma of the wine (Table 1).
4.2. Sensory Analysis
Color is an important feature of red wine, which is directly related to its quality; it is the first characteristic that wine consumers perceive. The basic color of tested wines was red and blue tones were observed, which is a characteristic of a young wine. Color of red wines is attributed to a very complex matrix made up of a wide range of compounds extracted from grapes and metabolites released by yeast during the fermentation process [52]. Among tested wines, only in the sample PLW the color yellow was dominant which contributed to the lowest visual perception rating. When the value of dA is below 40%, the color red of the wine is dark and atypical, whereas higher values of dA% indicate a higher proportion of red [53]. The proportion of the red component is attributed to free anthocyanins in the form of the flavylium cation and the combination of anthocyanins and tannins in older wines, while the proportion of yellow is attributed to the presence of tannins and the products of anthocyanin degradation. The proportion of blue is attributed to free anthocyanins in the form of a quinoidal base and a combination of tannins and anthocyanins [53].
The addition of medicinal herbs has led to a change in the composition of volatile components of wine samples and thus to a change in the sensory characteristics of wines. In respect to visual, olfactory, gustatory and gustatory–olfactory perceptions the best sensory characteristics were the samples PCW and PW. The control wine, PW, was ruby in color and clear, its aroma was discreet, with a fruity taste reminiscent of cherries. The wine PCW was intensely ruby in color and clear. The aroma of cinnamon was present; it had a sweet taste, it was moderately full and with a moderate constancy of the aroma. The wines PAW and PWW were also ruby in color. The PAW sample had a discreet anise-like aroma, and its taste was moderately full and rounded. On the other hand, the aroma of PWW was discreetly spicy and it had a bitter taste. This wine had the lowest rating in respect to gustatory perception. The color of PLW was ruby and clear, but due to the dominance of yellow and the low value of dA% the wine had an undesirable color of red wine. It had a very pronounced tone of onion and a sweet licorice-like aroma. The taste was sweet and there was a lack of acid that would make the wine more harmonious. All of the above contributed to the lowest rating for PLW in term of sensory characteristics (Figure 2 and Figure 3).
5. Conclusions
Consumer requirements and the modern wine industry require constant improvement in the quality of wine and enrichment of the aromatic profile of wine in order to have new and fresh wine aromas and balanced tastes. Wines obtained from regional autochthonous grape varieties are gaining more and more in their specificity and are increasingly appreciated. That is the reason we chose the autochthonous variety ‘Prokupac’, so as to complete its aroma by aromatisation with aromatic herbs.
The results obtained in this study on volatile aromatic compounds in wines aromatised with medicinal herbs indicated significant differences in the number of aromatic compounds and their relative content. Considering the same conditions of fermentation of ‘Prokupac’ must, the differences in the aromatic profiles could be attributed to the medicinal herbs. This was most pronounced in terms of terpenes, as this class of compound was not detected in the control wine (PW). Aromatisation with anise, wormwood and liqorice did not contribute to the sensory characteristics and overall quality of ‘Prokupac’ wine. The respected wines were classified as acceptable (PLW and PWW) and average (PAW) wines. In contrast, cinnamon enhanced the sensory characteristics and contributed to the fruity, sweet and floral aroma of the wine. The addition of cinnamon increased not only the content of some esters (2-phenylethyl acetate) and alcohols (hydrocinnamyl alcohol), but also distinguished this wine in terms of specific esters and terpenes such as hydrocinnamyl acetate and borneol, respectively. The PCW was classified as a very good wine that may appeal to consumers.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8040277/s1, Table S1: Color characteristics of ‘Prokupac’ wines, Table S2: Sensory characteristics evaluation of ‘Prokupac’ wines. References [30,31,53] are cited in the supplementary materials
Author Contributions
Conceptualization, M.L.L. and S.H.L.; methodology, M.L.L., S.H.L., I.T.K. and R.J.; software, I.T.K.; validation, D.J.C.; formal analysis, I.T.K. and D.J.C.; investigation, S.H.L.; resources, S.H.L.; data curation, I.T.K.; writing—original draft preparation, S.H.L., J.B.P.-D. and R.J.; writing—review and editing, J.B.P.-D.; visualization, J.B.P.-D.; supervision, I.T.K., M.L.L. and J.B.P.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
This work was carried out within the agreements for scientific research work in 2022 between the Faculty of Agriculture, University of Belgrade and Faculty of Technology, University of Niš, and the Ministry of Education, Science, and Technological Development of the Republic of Serbia (Nos. 451-03-68/2022-14/200116 and 451-03-68/2022-14/200133).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Sanchez-Palomo, E.; Diaz-Maroto, H.M.C.; Gonzales-Vinas, M.A.; Perez-Coelo, M.S. Aroma enchacement in wines from different grape varieties usin exogenous glucosidases. Food Chem. 2005, 92, 627–635. [Google Scholar] [CrossRef]
- Prosen, H.; Janeš, L.; Strlič, M.; Rusjan, D.; Kočar, D. Analysisi of free and bound aroma compounds in grape berries using headspace solid-phase microextraction with GC-MS and a preliminary study of solid-phase extraction with LC-MS. Acta. Chim. Slov. 2007, 54, 25–32. [Google Scholar]
- Miklosy, E.I.; Kereny, Z. Comparison of the volatile aroma components in noble rotted grape berries from two different locations of the Tokaj wine district in Hungary. Anal. Chim. Acta 2004, 513, 177–181. [Google Scholar] [CrossRef]
- Bureau, S.M.; Baumes, R.L.; Razungles, A.J. Effects of vine or bunch shading on the glycosiladed flavour precursors in grapes of Vitis vinifera L. cv. Syrah. J. Agric. Food Chem. 2000, 48, 1290–1297. [Google Scholar] [CrossRef]
- Darriet, P.; Bouchilloux, P.; Poupot, C.; Bugatet, Y.; Clerjeau, M.; Sauris, P.; Medina, B.; Dubourdieu, D. Effects of copper fungicide sprying on volatile thiols of the varietal aroma of Sauvignon blanc, Cabernet sauvignon and Merlot wines. Vitis 2001, 40, 93–99. [Google Scholar]
- Gachons, C.P.; Leuwen, C.; Tominaga, T.; Soyer, J.P.; Gaudillere, J.P.; Dubourdieu, D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L. cv Sauvignon blanc in field conditiona. J. Sci. Food. Agric. 2005, 85, 73–85. [Google Scholar] [CrossRef]
- Lutskova, V.; Martirosyan, I. Influence of harvest date and grape variety on sensory attributes and aroma compounds in experimental ice wines of Ukraine. Fermentation 2021, 7, 7. [Google Scholar] [CrossRef]
- Karabegović, I.T.; Malićanin, M.; Danilović, B.; Stanojević, J.; Stamenković Stojanović, S. Potential of non-Saccharomyces yeast for improving the aroma and sensory profile of Prokupac red wine. OENO ONE 2021, 2, 181–195. [Google Scholar] [CrossRef]
- Spillman, P.J.; Sefton, M.A.; Gawel, R. The contributiom of volatile compounds derived during oak barrel maturation to the aroma of a Chardonay and Cabernet sauvignon wine. Aust. J. Grape Wine Res. 2004, 10, 227–235. [Google Scholar] [CrossRef]
- Francis, I.L.; Newton, J.L. Determining wine aroma from compositional data. Aust. J. Grape. Wine Res. 2005, 11, 114–126. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolation from Saccharomyces cerevisae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Košmerl, T.; Jakončič, M.; Kralj-Cigić, I.; Strlič, M.; Prosen, H. Aroma compound in “sur lies” produced and aged Chardonay wines. In Proceedings of the 31th Congesso Mondiale Della Vigna E Del Vino, 6a Assemblea Generale Dell OIV, Verona, Italy, 15–20 June 2008. [Google Scholar]
- Rusjan, D. Aromas in grape and wine. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 411–442. [Google Scholar]
- Pereira, A.G.; Fraga, M.; Garcia-Oliveira, P.; Carpena, M.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Barros, L.; Ferreira, I.C.F.R.; Prieto, M.A.; Simal-Gandara, J. Management of wine aroma compounds: Principal basis and future perspectives. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; Cosme, F., Nunes, F.M., Filipe-Ribeir, L., Eds.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Ferreira, V.; López, R.; Cacho, J.F. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agric. 2000, 80, 1659–1667. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape. Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Ubeda, C.; Kania-Zelada, I.; del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, Á. Study of the changes in volatile compounds, aroma and sensory attributes during the production process of sparkling wine by traditional method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef]
- Castro-Vasquez, L.; Perez-Coello, M.S.; Cabezudo, M.D. Effects of enzyme treatments and skin extraction on varietal volatile in Spanish wines made from Chardonay, Muscat, Airen and Macabeo grapes. Anal. Chim. Acta 2002, 458, 39–44. [Google Scholar] [CrossRef]
- Schneider, R.; Razungles, A.; Augier, C.; Baumes, R. Monoterpenic and norisoprenoidic glycoconjugates of Vitis vinifera L. cv MerlotB as precursors of odorant in Muscated wines. J. Chromatogr. A 2001, 936, 145–157. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Varietal aroma. In Handbook of Enology: The Chemistry of Wine Stabilization and Treatments, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2006; pp. 205–230. [Google Scholar]
- Martínez-Francés, V.; Rivera, D.; Obon, C.; Alcaraz, F.; Ríos, S. Medicinal plants in traditional herbal wines and liquors in the East of Spain and the Balearic Islands. Front. Pharmacol. 2021, 12, 713414. [Google Scholar] [CrossRef]
- Lakićević, S.H.; Popović Djordjević, J.B.; Pejin, B.; Djordjević, A.S.; Matijašević, S.M.; Lazić, M.L. An insight into chemical composition and bioactivity of ‘Prokupac’ red wine. Nat. Prod. Res. 2020, 34, 1542–1546. [Google Scholar] [CrossRef]
- Karabegović, I.T.; Vukosavljević, P.V.; Novaković, M.M.; Gorjanović, S.Z.; Džamić, A.M.; Lazić, M.L. Influence of the storage on bioactive compounds and sensory attributes of herbal liqueur. Dig. J. Nanomater. Biostruct. 2012, 7, 1587–1598. [Google Scholar]
- International Code of Oenological Practices (OIV), International Organisation of Vine and Wine, OIV Code Sheet—Issue 2021/01. Available online: https://www.oiv.int/public/medias/7713/en-oiv-code-2021.pdf (accessed on 14 March 2022).
- Marković, N.; Atanacković, Z. Fertility variation of Prokupac cultivar under influence of different rootstocks. Agro-Knowl. J. 2013, 14, 171–178. [Google Scholar] [CrossRef][Green Version]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Co.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sparkman, D.O.; Penton, Z.E.; Fulton, K.G. Gas Chromatography and Mass Spectrometry: A Practical Guide, 2nd ed.; Elsevier Inc.: Oxford, MS, USA, 2011. [Google Scholar]
- ISO 6658; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- RULEBOOK on the Procedure and Methods of Sensory Evaluation of Wine, the Method of Training and Checking the Professional Competence of Sensory Evaluators. Official Gazette of RS: 2015; No. 93. Available online: https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/ministarstva/pravilnik/2015/93/6/reg (accessed on 15 March 2022).
- Guidelines for the Characterization of Wine Yeasts of the Genus Saccharomyces Isolated from Vitivinicultural Environments; International Organisation of Vine and Wine: Paris, France, 2012.
- Acree, T.; Arn, H. Flavornet and Human Odor Space. Available online: http://www.flavornet.org/flavornet.html (accessed on 20 January 2022).
- Odor & Flavor Detection Thresholds in Water (In Parts per Billion). Available online: http://www.leffingwell.com/odorthre.htm (accessed on 28 January 2022).
- Farenzena, S.; Tombesi, N. Volatile profile of Malbec wine from Buenos Aires province (Argentina). Int. Food Res. J. 2015, 22, 2691–2696. [Google Scholar]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile profile characterization of Croatian commercial sparkling wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast derivatives (extracts and autolysates) in winemaking: Release of volatile compounds and effects on wine aroma volatility. Food Chem. 2006, 99, 217–230. [Google Scholar] [CrossRef]
- Tufariello, M.; Capone, S.; Siciliano, P. Volatile components of Negroamaro red wines produced in Apulian Salento area. Food Chem. 2012, 132, 2155–2164. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef]
- Abreu, T.; Perestrelo, R.; Bordiga, M.; Locatelli, M.; Daniel Coïsson, J.; Câmara, J.S. The flavor chemistry of fortified wines—A comprehensive approach. Foods 2021, 10, 1239. [Google Scholar] [CrossRef]
- Callejon, R.M.; Clavijo, A.; Ortigueira, P.; Troncoso, A.M.; Paneque, P.; Morales, M.L. Volatile and sensory profile of organic red wines produced by different selected autochthonous and commercial Saccharomyces cerevisiae strains. Anal. Chim. Acta 2010, 660, 68–75. [Google Scholar] [CrossRef]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Pérez-Coello, M.S.; Briones Pérez, A.I.; Ubeda Iranzo, J.F.; Martin Alvarez, P.J. Characteristics of wines fermented with different Saccharomyces cerevisiae strains isolated from the La Mancha region. Food Microbiol. 1999, 16, 563–573. [Google Scholar] [CrossRef]
- Cameleyre, M.; Lytra, G.; Tempere, S.; Barbe, J. Olfactory impact of higher alcohols on red wine fruity ester aroma expression in model solution. J. Agric. Food Chem. 2015, 63, 9777–9788. [Google Scholar] [CrossRef] [PubMed]
- García-Carpintero, E.G.; Sánchez-Palomo, E.; Gallego, M.A.G.; González-Viñas, M.A. Volatile and sensory characterization of red wines from cv. Moravia Agria minority grape variety cultivated in La Mancha region over five consecutive vintages. Food Res. Int. 2011, 44, 1549–1560. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Luan, Y.; Duan, C.Q.; Yan, G.L. Use of Torulaspora delbrueckii co-fermentation with two Saccharomyces cerevisiae strains with different aromatic characteristic to improve the diversity of red wine aroma profile. Front. Microbiol. 2018, 9, 606. [Google Scholar] [CrossRef]
- Renault, P.E.; Albertin, W.; Bely, M. An innovative tool reveals interaction mechanisms among yeast populations under oenological conditions. Appl. Microbiol. Biotechnol. 2013, 97, 4105–4119. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Jin, G.J.; Mei, W.C.; Lim, T.; Tao, Y.S. Increase of medium-chain fatty acid ethyl ester content in mixed H. uvarum/S. cerevisiae fermentation leads to wine fruity aroma enhancement. Food Chem. 2018, 15, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Bayonove, C.L.; Baumes, R.L.; Crouzet, J.; Günata, Z. Aromas. In Enología: Fundamentos Científicos y Tecnológicos, 1st ed.; Flanzy, C., Ed.; Mundi-Prensa: Madrid, Spain, 2003; pp. 137–176. [Google Scholar]
- Matijašević, S.; Popović-Djordjević, J.; Ristić, R.; Ćirković, D.; Ćirković, B.; Popović, T. Volatile aroma compounds of brandy ‘Lozovača’ produced from Muscat table grapevine cultivars (Vitis vinifera L.). Molecules 2019, 24, 2485. [Google Scholar] [CrossRef]
- Cheynier, V.; Schneider, R.; Salmon, J.M.; Fulcrand, H. Chemistry of wine. In Comprehensive Natural Products II, 1st ed.; Liu, H.W., Mander, L., Eds.; Elsevier: Amsterdam, The Netherlands, 2010; pp. 1119–1172. [Google Scholar]
- de Freitas, V.A.P.; Fernandes, A.; Oliveira, J.; Teixeira, N.; Mateus, N. A review of the current knowledge of red wine colour. OENO One 2017, 51, 1–15. [Google Scholar] [CrossRef]
- Glories, Y. La coleur des vins rouges. 2. Partie: Mesure, origine et interpretation. Conn. Vigne Vin. 1984, 18, 253–273. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).