In Vitro Culture of Eremurus spectabilis (Liliaceae), a Rare Ornamental and Medicinal Plant, through Root Explants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Explant Disinfection
2.3. Culture Initiation and Shoot Regeneration
2.4. In Vitro Rooting
2.5. Acclimatization and Ex Vitro Conditions
2.6. Statistical Analysis
3. Results
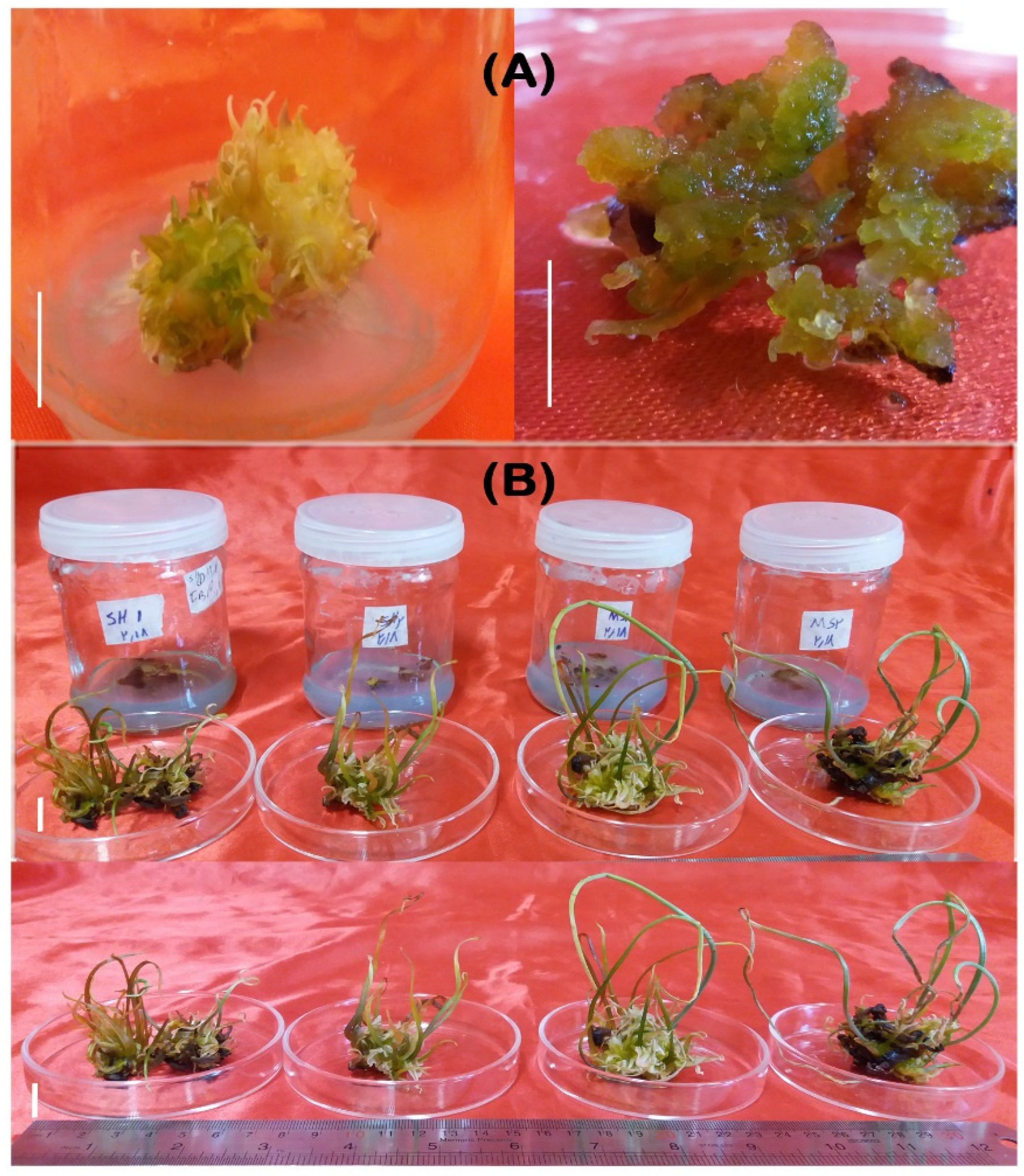
3.1. Callus Induction
3.2. Shoot Regeneration
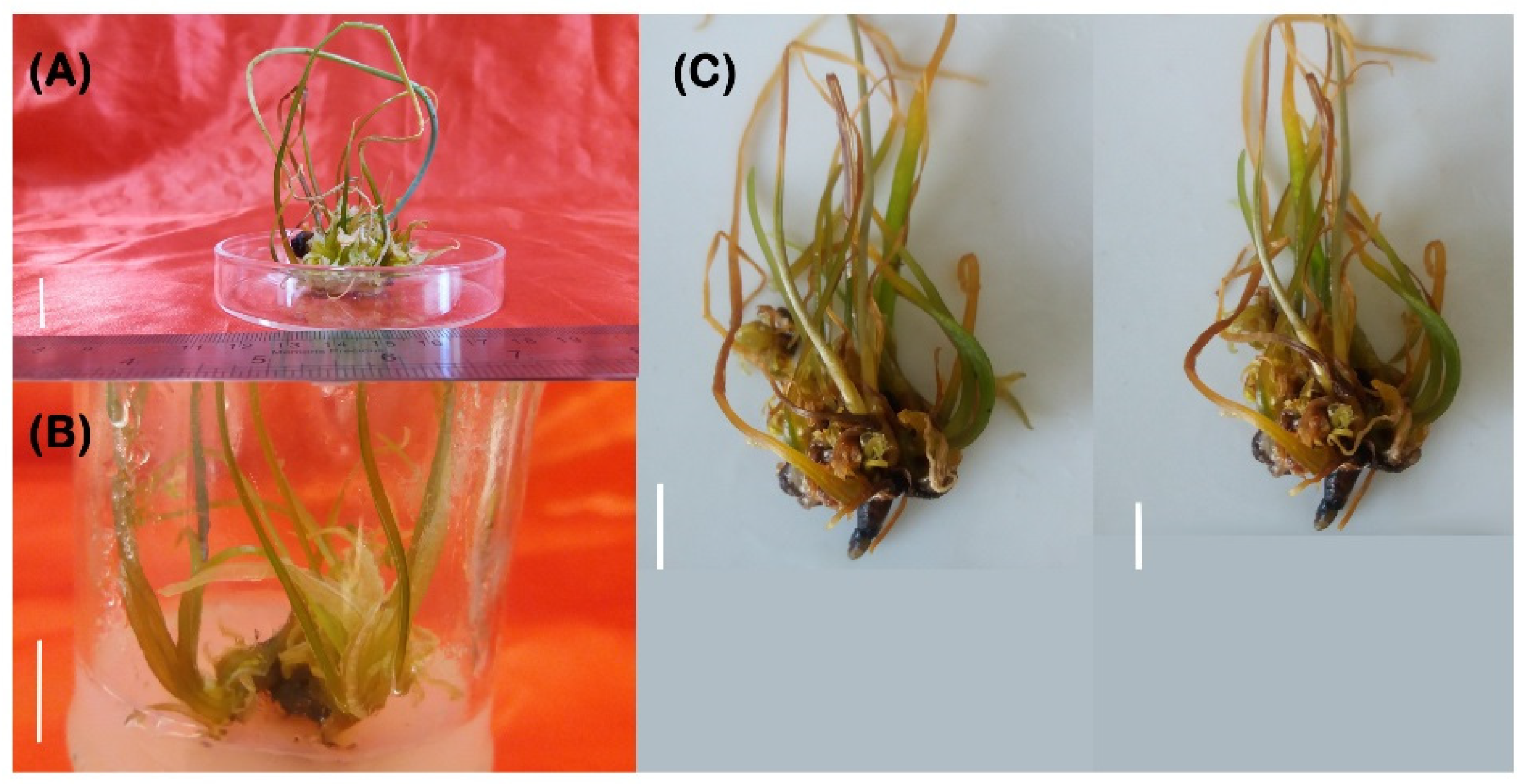
3.3. Root Regeneration
3.4. Acclimatization and Ex Vitro Transfer
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamenetsky, R.; Akhmetova, M. Floral development of Eremurus altaicus (Liliaceae). Isr. J. Plant Sci. 1994, 42, 227–233. [Google Scholar] [CrossRef]
- Tosun, M.; Ercisli, S.; Ozer, H.; Turan, M.; Polat, T.; Ozturk, E.; Padem, H.; Kilicgun, H. Chemical composition and antioxidant activity of Foxtail lily (Eremurus spectabilis). Acta Sci. Pol-Hortorum Cultus 2012, 11, 145–153. [Google Scholar]
- Tuzcu, Z.; Koclar, G.; Agca, C.A.; Aykutoglu, G.; Dervisoglu, G.; Tartik, M.; Sahin, K. Antioxidant, antimicrobial and anticancer effects of different extracts from wild edible plant Eremurus spectabilis leaves and roots. Int. J. Clin. Exp. Med. 2017, 10, 4787–4797. [Google Scholar]
- Altundag, E.; Ozturk, M. Ethnomedicinal studies on the plant resources of east Anatolia, Turkey. Proc. Soc. Behav. Sci. 2011, 19, 756–777. [Google Scholar] [CrossRef] [Green Version]
- Bircan, B.; Kirbag, S. Determination of antioxidant and antimicrobial properties of Eremurus spectabilis Bieb. Artvin Çoruh Univ. Orman. Fak. Derg. 2012, 16, 176–186. [Google Scholar] [CrossRef]
- Tuzlaci, E.; Dogan, A. Turkish folk medicinal plants, IX: Ovacık (Tunceli). Marmara. Pharm. J. 2012, 14, 136–143. [Google Scholar] [CrossRef]
- Pourfarzad, A.; Najafi, M.B.H.; Khodaparast, M.H.H.; Khayyat, M.H.; Malekpour, A. Fractionation of (Eremurus spectabilis) fructans by ethanol: Box–Behnken design and principal component analysis. Carbohydr. Polym. 2014, 106, 374–383. [Google Scholar] [CrossRef]
- Abubaker, S.R.; Hidayat, H.J. Anti-tumor potential of Local Aslerk (Eremurus spectabilis) leaf extracts by HPLC and applying on cancer cell lines in vitro. Iraqi J. Cancer Med. Genet. 2015, 8, 123–128. [Google Scholar]
- Eghtedarnejad, N.; Mansouri, H.R. Building wooden panels glued with a combination of natural adhesive of tannin/Eremurus root (syrysh). Eur. J. Wood Prod. 2016, 74, 269–272. [Google Scholar] [CrossRef]
- Schiappacasse, F.; Szigeti, J.C.; Manzano, E.; Kamenetsky, R. Eremurus as a new cut flower crop in Aysen, Chile: Introduction from the Northern hemisphere. Acta Hortic. 2013, 1002, 115–121. [Google Scholar] [CrossRef]
- Ahmad, I.; Dole, J.M.; Schiappacasse, F.; Saleem, M.; Manzano, E. Optimal postharvest handling protocols for cut ‘Line Dance’ and ‘Tap Dance’ Eremurus inflorescences. Sci. Hortic. 2014, 179, 212–220. [Google Scholar] [CrossRef]
- Hesami, M.; Jones, A.M.P. Application of artificial intelligence models and optimization algorithms in plant cell and tissue culture. Appl. Microbiol. Biotechnol. 2020, 104, 9449–9485. [Google Scholar] [CrossRef] [PubMed]
- Baksha, R.; Jahan, M.A.A.; Khatun, R.; Munshi, J.L. Micropropagation of Aloe barbadensis Mill. through in vitro culture of shoot tip explants. Plant Tissue Cult. Biotechnol. 2005, 15, 121–126. [Google Scholar]
- Sun, L.; Jin, L. Studies on the in vitro rapid propagation of Lilium X Longliflorum. Liaoning Agric. Sci. 2011, 6, 9–12. [Google Scholar]
- Skoric, M.; Zivkovic, S.; Savic, J.; Siler, B.; Sabvoljevic, A.; Todorovic, S.; Grubisic, D. Efficient one-step tissue culture protocol for propagation of endemic plant, Lilium martagon var. Cattaniae Vis. Afr. J. Biotechnol. 2012, 11, 1862–1867. [Google Scholar]
- Monemi, M.B.; Kazemitabar, S.K.; Khaniki, G.B.; Yasari, E.; Sohrevardi, F.; Pourbagher, R. Tissue culture study of the medicinal plant Leek (Allium ampeloprasum L). Int. J. Mol. Cell. Med. 2014, 3, 118–125. [Google Scholar]
- Suh, S.Y.; Baskar, T.B.; Kim, H.H.; Al-Dhabi, N.A.; Park, S.U. Ethylene inhibitors promote shoot organogenesis of Aloe arborescens Miller. South Indian J. Biol. Sci. 2015, 1, 43–46. [Google Scholar] [CrossRef]
- Cakmak, D.; Karaoglu, C.; Aasim, M.; Sancak, C.; Ozcan, S. Advancement in protocol for in vitro seed germination, regeneration, bulblet maturation, and acclimatization of Fritillaria persica. Turk. J. Biol. 2016, 40, 878–888. [Google Scholar] [CrossRef]
- Pandey, A. Research on in-vitro clonal multiplication protocol in various cultivars of Chlorophytum. Tech Chron. Int. J. Emerg. Trends Sci. Eng. Technol. 2016, 1, 32–42. [Google Scholar]
- Razib, M.A.; Mamun, A.N.; Kabir, M.H.; Al Din, S.M.; Hossain, M.E.; Firoz Alam, M. High frequency in vitro propagation of Aloe vera L. through shoot tip culture. Int. J. Appl. Biol. Pharm. 2016, 7, 28–32. [Google Scholar]
- Tuncer, B. Investigation of the in vitro regeneration of some medical and aromatic wild plant species. Appl. Ecol. Environ. Res. 2017, 15, 905–914. [Google Scholar] [CrossRef]
- Tuncer, B. In vitro germination and bulblet and shoot propagation for wild edible Eremurus spectabilis M.Bieb. Not. Bot. Horti. Agrobot. Cluj-Napoca 2020, 48, 814–825. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Hadizadeh, H.; Bahri, B.A.; Qi, P.; Wilde, H.D.; Devos, K.M. Intra-and interspecific diversity analyses in the genus Eremurus in Iran using genotyping-by-sequencing reveal geographic population structure. Hortic. Res. 2020, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schenk, R.V.; Hildebrandt, A.C. Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Barnes, J.D.; Balaguer, L.; Manrique, E.; Elvira, S.; Davison, A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophyll ‘a’ and ‘b’ in lichens and higher plants. Environ. Exp. Bot. 1992, 32, 85–90. [Google Scholar] [CrossRef]
- Alizadeh, M.; Singh, S.K.; Patel, V.B.; Deshmukh, P.S. In vitro clonal multiplication of two grape (Vitis spp.) rootstock genotypes. Plant Tiss. Cult. Biotechnol. 2018, 28, 1–11. [Google Scholar] [CrossRef]
- Dalila, Z.D.; Fahmi, A.B.M.; Nurkhalida, A.K.S. Optimization of sterilization method and callus induction of Cocos nucifera Linn. var. Matag from inflorescence. In Proceedings of the International Conference on Plant, Marine and Environmental Science, Kuala Lumpur, Malaysia, 1–2 January 2015. [Google Scholar]
- Allan, A. Plant cell culture. In Plant Cell and Tissue Culture; Stafford, A., Warren, G., Eds.; Open University Press: London, UK, 1991; pp. 1–24. [Google Scholar]
- Petersen, K.K.; Hansen, J.; Krogstrup, P. Significance of different carbon sources and sterilization methods on callus induction and plant regeneration of Miscanthus x ogiformis Honda ‘Giganteus’. Plant Cell Tissue Organ Cult. 1999, 58, 189–197. [Google Scholar] [CrossRef]
- Roy, A.; Ghosh, S.; Chaudhury, M.; Saha, P.K. Effect of different plant hormones on callus induction in Gymnema sylvestris R.Br. (Asclepiadaceae). Afr. J. Biotechnol. 2008, 7, 2209–2211. [Google Scholar]
- Osman, N.I.; Sidik, N.J.; Awal, A. Effects of variations in culture media and hormonal treatments upon callus induction potential in endosperm explant of Barringtonia racemosa L. Asian Pac. J. Trop. Biomed. 2016, 6, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Colgecen, H.; Koca, U.; Toker, G. Influence of different sterilization methods on callus initiation and production of pigmented callus in Arnebia densiflora Ledeb. Turk. J. Biol. 2009, 35, 513–520. [Google Scholar]
- Ehsandar, S.; Majd, A.; Choukan, R. Callus formation and regeneration of the first modified Iranian potato cultivar (Savalan). Adv. Crop. Sci. 2013, 3, 201–208. [Google Scholar]
- Kumlay, A.M.; Ercisli, S. Callus induction, shoot proliferation and root regeneration of potato (Solanum tuberosum L.) stem node and leaf explants under long-day conditions. Biotechnol. Equip. 2015, 29, 1074–1084. [Google Scholar]
- Hesami, M.; Jones, A.M.P. Modeling and optimizing callus growth and development in Cannabis sativa using random forest and support vector machine in combination with a genetic algorithm. Appl. Microbiol. Biotechnol. 2021, 105, 5201–5212. [Google Scholar] [CrossRef]
- Khatun, N.; Bari, M.A.; Islam, R.; Huda, S.; Siddque, N.A.; Rahman, M.A.; Mullah, M.U. Callus induction and regeneration from nodal segment of potato cultivar Diamant. J. Bio. Sci. 2003, 3, 1101–1106. [Google Scholar]
- Yasmin, S.; Nasiruddin, K.M.; Begum, R. Regeneration and establishment of potato plantlet through callus formation with BAP and NAA. Asian J. Plant Sci. 2003, 2, 936–940. [Google Scholar] [CrossRef]
- Carsono, N.; Yoshida, T. Identification of callus induction potential of 15 Indonesian rice genotypes. Plant Prod. Sci. 2006, 9, 65–70. [Google Scholar] [CrossRef]
- Benderradji, L.; Brini, F.; Kellou, K.; Ykhlef, N.; Djekoun, A.; Masmoudi, K.; Bouzerzour, H. Callus induction, proliferation, and plantlets regeneration of two bread wheat (Triticum aestivum L.) genotypes under saline and heat stress conditions. Int. Sch. Res. Notices 2011, 2012, 1–8. [Google Scholar]
- Gamborge, O.L. Media preparation. In Plant Tissue Culture Manual; Lindsey, K., Ed.; Springer: New York, NY, USA, 1991; Volume A1, pp. 1–24. [Google Scholar]
- George, E.F. Plant Propagation by Tissue Culture; Exegetics Ltd.: Edington, UK, 1993; p. 574. [Google Scholar]
- Deswiniyanti, N.W.; Dwipayani Lestari, N.K. In vitro propagation of Lilum longiflorum using NAA and BAP plant growth regulator treatment. KnE Life Sci. 2020, 5, 6437. [Google Scholar] [CrossRef]
- Ozcan, S.; Yildiz, M.; Sancak, C.; Ozgen, M. Adventitious shoot regeneration in sainfoin (Onobrychis viciifolia Scop.). Turk. J. Bot. 1996, 20, 497–501. [Google Scholar]
- Yıldız, M.; Saglik, C.; Telci, C.; Erkilic, E.G. The effect of in vitro competition on shoot regeneration from hypocotyl explants of Linum usitatissimum. Turk. J. Bot. 2011, 35, 211–218. [Google Scholar]
- Iqbal, M.; Jaskani, J.M.; Rafique, R.; Hassan, S.Z.; Iqbal, M.S.; Raheed, M.; Mushtaq, S. Effect of plant growth regulators on callus formation in potato. J. Sci. Food Agric. 2014, 2, 77–81. [Google Scholar]
- Irvani, N.; Solouki, M.; Omidi, M.; Zare, A.R.; Shahnazi, S. Callus induction and plant regeneration in Dorem ammoniacum D., an endangered medicinal plant. Plant Cell Tissue Organ Cult. 2010, 100, 293–299. [Google Scholar] [CrossRef]
- Huang, W.; Ratkowsky, D.A.; Hui, C.; Wang, P.; Su, J.; Shi, P. Leaf fresh weight versus dry weight: Which is better for describing the scaling relationship between leaf biomass and leaf area for broad-leaved plants? Forests 2019, 10, 256. [Google Scholar] [CrossRef] [Green Version]
- Simon, S.; Petrášek, P. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef] [Green Version]
- Kulus, D. Influence of growth regulators on the development, quality, and physiological state of in vitro-propagated Lamprocapnos spectabilis (L.) Fukuhara. In Vitro Cell. Dev. Biol. Plant 2020, 56, 447–457. [Google Scholar] [CrossRef]
- Overvoorde, P.; Fukaki, H.; Beeckman, T. Auxin control of root development. Cold Spring Harb. Perspect. Biol. 2010, 2, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paek, K.Y.; Murthy, H.N. High frequency of bulblet regeneration from bulb scale sections of Fritillaria thunbergii. Plant Cell Tissue Organ Cult. 2002, 68, 247–252. [Google Scholar] [CrossRef]
- Khawar, K.M.; Cocu, S.A.; Parmaksiz, I.; Sarihan, E.O.; Ozcan, S. Mass proliferation of Madona Lilly (Lilium candidum L.) under in vitro conditions. Pak. J. Bot. 2005, 37, 243–248. [Google Scholar]
- Priyakumari, I.; Sheela, V.L. Micropropagation of gladiolus cv. ‘Peach Blossom’ through enhanced release of axillary buds. J. Trop. Agric. 2005, 43, 47–50. [Google Scholar]
- Ozel, C.A.; Khawar, K.M.; Unal, F. Factors affecting efficient in vitro micropropagation of Muscari muscarimi Medikus using twin bulb scale. Saudi J. Biol. Sci. 2015, 22, 132–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, U.; Kataria, V.; Shekhawat, N.S. In vitro propagation, ex vitro rooting and leaf micromorphology of Bauhinia racemosa Lam.: A leguminous tree with medicinal values. Physiol. Mol. Biol. Plant 2017, 23, 969–977. [Google Scholar] [CrossRef] [PubMed]


| Media | Basal Medium | Growth Regulators (mgL−1) | Other Modifications |
|---|---|---|---|
| MS | NAA (0.1) + BAP (5.0) | - | |
| Culture initiation (callus induction) | NAA (0.1) + BAP (10) | - | |
| SH | NAA (0.1) + BAP (5.0) | - | |
| NAA (0.1) + BAP (10) | - | ||
| MS | IBA (0.1) + BAP (2.0) | AC 1 (200 mgL−1) | |
| Shoot regeneration | IBA (0.1) + BAP (5.0) | AC (200 mgL−1) | |
| SH | IBA (0.1) + BAP (2.0) | AC (200 mgL−1) | |
| IBA (0.1) + BAP (5.0) | AC (200 mgL−1) | ||
| MS | IBA (2.0) + NAA (1.0) | - | |
| Root regeneration | SH | IBA (2.0) + NAA (1.0) | - |
| ½ MS | IBA (2.0) | 4.0% sucrose + 200 mgL−1 AC | |
| ½ SH | IBA (2.0) | 4.0% sucrose + 200 mgL−1 AC |
| Medium | Growth Regulators (mgL−1) | Sterilization Method | C | DW | Chl a | Chl b | Ctn | Cf | CI | CC |
|---|---|---|---|---|---|---|---|---|---|---|
| NAA (0.1) + BAP (5.0) | NaClO, 40% for 15 min | 36.67 e | 6.21 bc | 4.06 c | 6.01 b | 1.53 c | 3.33 bc | 1.25 bc | YG | |
| MS | HgCl2, 0.1% for 7 min | 25.00 f | 5.19 cd | 0.16 e | 0.33 c | 0.55 d | 2.00 f | 1.00 c | B | |
| NAA (0.1) + BAP (10) | NaClO, 40% for 15 min | 76.67 a | 10.25 a | 10.23 a | 19.81 a | 6.50 a | 3.97 a | 2.83 a | G | |
| HgCl2, 0.1% for 7 min | 55.00 b | 8.16 ab | 3.18 d | 10.07 b | 3.50 b | 3.00 cd | 1.25 bc | YG | ||
| NAA (0.1) + BAP (5.0) | NaClO, 40% for 15 min | 56.67 b | 6.03 bc | 4.82 bc | 10.29 b | 2.84 b | 3.67 ab | 1.33 b | YG | |
| SH | HgCl2, 0.1% for 7 min | 46.67 cd | 4.15 cd | 0.27 e | 0.60 c | 0.20 d | 2.67 de | 1.17 bc | B | |
| NAA (0.1) + BAP (10) | NaClO, 40% for 15 min | 50.00 bc | 5.04 cd | 5.35 b | 9.91 b | 3.07 b | 3.33 bc | 1.33 b | YG | |
| HgCl2, 0.1% for 7 min | 40.00 de | 3.28 d | 0.36 e | 0.84 c | 0.49 d | 2.33 ef | 1.00 c | B | ||
| Source of variation | ||||||||||
| Medium (M) | 25.00 ns | 95.99 ** | 35.02 ** | 159.03 ** | 22.49 ** | 0.19 ns | 1.69 ** | |||
| Growth regulator (G) | 2408.33 ** | 19.81 * | 72.28 ** | 410.96 ** | 53.30 ** | 1.02 * | 2.08 ** | |||
| Sterilization method (S) | 2133.33 ** | 34.24 ** | 315.09 ** | 875.43 ** | 63.43 ** | 15.19 ** | 4.08 ** | |||
| M × G | 5208.33 ** | 58.92 ** | 55.04 ** | 420.50 ** | 41.25 ** | 4.69 ** | 3.00 ** | |||
| M × S | 133.33 * | 0.21 ns | 1.48 ns | 8.35 ns | 1.17 * | 0.19 ns | 1.33 ** | |||
| G × S | 75.00 ns | 0.66 ns | 9.74 ** | 8.88 ns | 2.83 ** | 0.02 ns | 1.69 ** | |||
| M × G × S | 75.00 ns | 1.10 ns | 9.91 ** | 16.37 ns | 3.23 ** | 0.02 ns | 1.02 ** | |||
| Error | 45.83 | 3.33 | 0.55 | 15.49 | 0.33 | 0.19 | 0.06 |
| Medium | Growth Regulator | Callus Type | SP | Ln | Ll | Chl a | Chl b | Ctn |
|---|---|---|---|---|---|---|---|---|
| MS | IBA (0.1) + BAP (2.0) | Intact | 6.33 a | 39.17 abc | 20.45 a | 9.96 a | 18.78 a | 6.12 a |
| Divided | 5.17 ab | 41.83 ab | 16.42 ab | 9.71 ab | 16.82 ab | 6.36 a | ||
| IBA (0.1) + BAP (5.0) | Intact | 3.67 bcd | 47.17 a | 5.83 c | 7.45 c | 13.64 bc | 4.2 b | |
| Divided | 4.17 bc | 33.33 bc | 6.28 c | 8.33 bc | 13.32 bc | 4.53 b | ||
| SH | IBA (0.1) + BAP (2.0) | Intact | 4.33 ab | 36.67 abc | 16.45 ab | 7.47 c | 14.34 bc | 4.63 b |
| Divided | 3.17 bcd | 37.33 abc | 12.42 b | 7.09 cd | 12.75 dc | 4.87 b | ||
| IBA (0.1) + BAP (5.0) | Intact | 1.83 d | 42.17 ab | 2.17 c | 4.96 e | 9.19 d | 2.71 c | |
| Divided | 2.16 cd | 28.33 c | 2.78 c | 5.81 de | 9.21 d | 3.04 c | ||
| Source of variation | ||||||||
| Medium (M) | 46.02 ** | 216.75 ns | 172.52 ** | 76.68 ** | 218.62 ** | 26.65 ** | ||
| Growth regulator (G) | 38.52 ** | 12.00 ns | 1776.33 ** | 44.29 ** | 224.98 ** | 71.53 ** | ||
| Callus type (C) | 1.69 ns | 444.08 * | 36.75 ns | 0.90 ns | 11.08 ns | 0.99 ns | ||
| M × G | 0.02 ns | 6.75 ns | 0.52 ns | 0.008 ns | 0.003 ns | 0.00 ns | ||
| M × C | 0.02 ns | 3.00 ns | 0.02 ns | 0.02 ns | 0.38 ns | 0.00 ns | ||
| G × C | 7.52 ns | 720.75 * | 62.56 * | 4.11 ns | 7.92 ns | 0.02 ns | ||
| M × G × C | 0.02 ns | 3.00 ns | 0.02 ns | 0.008 ns | 0.002 ns | 0.00 ns | ||
| Error | 3.35 | 128.87 | 13.49 | 1.92 | 9.42 | 0.59 |
| Medium | Rl | Bv | Ln | Ll | Chl a | Chl b | Ctn |
|---|---|---|---|---|---|---|---|
| MS + 2.0 mgL−1 IBA + 1.0 mgL−1 NAA | 0 c | 37.30 a | 16.77 a | 4.70 b | 8.92 a | 15.19 ab | 5.65 a |
| SH + 2.0 mgL−1 IBA + 1.0 mgL−1 NAA | 0 c | 37.70 a | 6.71 c | 4.30 b | 7.23 b | 12.60 bc | 3.79 b |
| ½ MS + 2.0 mgL−1 IBA + 4.0% sucrose + 200 mgL−1 AC | 1.11 a | 41.00 a | 18.95 a | 6.40 a | 8.58 a | 16.53 a | 3.41 b |
| ½ SH + 2.0 mgL−1 IBA + 4.0% sucrose + 200 mgL−1 AC | 0.39 b | 41.50 a | 11.93 b | 5.10 ab | 5.85 c | 11.00 c | 2.04 c |
| Source of variation | |||||||
| Medium | 2.73 ** | 47.56 ns | 296.44 ** | 8.29 * | 19.62 ** | 62.20 ** | 22.23 ** |
| Error | 0.062 | 140.35 | 17.21 | 2.65 | 1.60 | 12.38 | 1.03 |
| Nutrient Solutions | Rl | Ln | Ll | Bv |
|---|---|---|---|---|
| ½ MS | 1.14 a | 32.60 a | 13.66 a | 5.80 a |
| ½ SH | 0.66 b | 27.80 a | 6.98 b | 3.70 b |
| Source of variation | ||||
| Nutrient solution | 1.15 * | 115.20 ns | 223.11 ** | 22.05 * |
| Error | 0.18 | 76.78 | 20.25 | 2.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiri, Y.; Etemadi, N.; Alizadeh, M.; Alizargar, J. In Vitro Culture of Eremurus spectabilis (Liliaceae), a Rare Ornamental and Medicinal Plant, through Root Explants. Horticulturae 2022, 8, 202. https://doi.org/10.3390/horticulturae8030202
Basiri Y, Etemadi N, Alizadeh M, Alizargar J. In Vitro Culture of Eremurus spectabilis (Liliaceae), a Rare Ornamental and Medicinal Plant, through Root Explants. Horticulturae. 2022; 8(3):202. https://doi.org/10.3390/horticulturae8030202
Chicago/Turabian StyleBasiri, Yeganeh, Nematollah Etemadi, Mahdi Alizadeh, and Javad Alizargar. 2022. "In Vitro Culture of Eremurus spectabilis (Liliaceae), a Rare Ornamental and Medicinal Plant, through Root Explants" Horticulturae 8, no. 3: 202. https://doi.org/10.3390/horticulturae8030202
APA StyleBasiri, Y., Etemadi, N., Alizadeh, M., & Alizargar, J. (2022). In Vitro Culture of Eremurus spectabilis (Liliaceae), a Rare Ornamental and Medicinal Plant, through Root Explants. Horticulturae, 8(3), 202. https://doi.org/10.3390/horticulturae8030202






