Abstract
Chitosan (CS) is a natural polymer used in agriculture as a biostimulant that has been evaluated in different plant models. In this study, we evaluated the effect of the foliar application of chitosan–poly(acrylic acid) complexes (CS–PAA) and two nutrient solutions (A and B) on the parameters of growth and yield of two habanero pepper cultivars (Chichen Itza and Jaguar) in a greenhouse. Over the course of the experiment, eight foliar applications were carried out at 15-day intervals. Our results showed that foliar applications of CS–PAA complexes have a biostimulant effect on the habanero pepper crop by increasing the total dry biomass of the plant and the number of fruits of the two cultivars. Regarding nutrient solutions, the nutrient solution A increased the yield of the Chichen Itza cultivar; this effect was because it had a better balance of potassium and calcium compared to the nutrient solution B. These results provide advances on the use of CS–PAA complexes as a biostimulant and the management of nutrient solutions in the crop of habanero peppers.
1. Introduction
Chitosan is a natural polysaccharide derived from the deacetylation of chitin and is mainly obtained from the exoskeletons of crustaceans and insects [1,2]. The process of alkaline deacetylation is inexpensive compared to the enzymatic process due to its low-cost for industrial production; it uses NaOH at 40–50% to eliminate more than 80% of the acetyl groups to obtain N-acetyl-d-glucosamine in β-1,4-d-glucosamine (CS) [3]. In the search for alternatives to enable a move towards more sustainable agriculture, CS is a good option because it is biodegradable, biocompatible, and non-toxic to humans [4,5]. The wide availability of CS has meant that it has been tested for use in agriculture since the 1980s [3]. Currently, CS is being studied with regard to the administration of pesticides, fertilizers, and growth regulators in order to increase the efficiency and reduce the number of applications of these conventional products [4,6]. Due to its properties, CS promotes plant growth and protects plants from biotic and abiotic stress [7,8,9]. The chitosan signaling mechanism includes specific receptors and secondary messengers, such as reactive oxygen species (ROS), hydrogen peroxide, calcium, nitric oxide (NO), and phytohormones that induce physiological responses to mitigate biotic and abiotic stress, and promote plant growth [8]. The positive effects of CS on plants include improvements in physiological mechanisms and growth, as well as an increase in the shelf life of fruits and vegetables [9,10]. These effects have been demonstrated in model plants, including tomato, maize, wheat, cucumber, strawberry, and chili peppers, among others [5,9,10].
Poly(acrylic acid) is a synthetic polymer that is used as a chelating, dispersal, flocculating, and adhesive agent in agricultural soils [11]. It increases soil water availability to aid plant growth [12] and can be used in the remediation of heavy-metal contaminated soils [13]. In addition, it is used to form complexes with chitosan and has previously been applied to seeds, substrates, leaves, and on post-harvest fruits, showing a strong capacity for biostimulation [14]. In tomato plants, the application of chitosan-poly(acrylic acid) complexes increased yield [15]. In onion plants, chitosan-poly(acrylic acid) hydrogel nanoparticles improved growth rates and yield [16]. In lettuce plants, chitosan–poly(acrylic acid) complexes were shown to increase the biofortification with selenium [17]. Recently, biostimulants in crops have been used more frequently in order to improve productivity [18].
In Mexico, the production of habanero peppers has increased exponentially over the past two decades, increasing from 38.8 tons in 1999 to 20,829.6 tons in 2019 [19]. It is considered to be an economically important crop due to the current high demand for the consumption of fresh fruits, its use as an ingredient in sauces, as a natural colorant, and its medicinal uses. Habanero peppers are traditionally produced in soil, so information regarding nutrient solutions when grown in a greenhouse is scarce [20]. Few studies have evaluated different nutritional regimens used in hydroponic systems to produce habanero peppers in Mexico [20,21,22]. Therefore, it is important to carry out studies in Mexico, where findings will help to increase the productivity of the growth of habanero peppers; most of the published studies have focused on capsaicinoids [23]. Therefore, the objective of this study was to evaluate the effect of foliar applications of chitosan–poly(acrylic acid) complexes (CS–PAA) and two nutrient solutions on the growth and yield of two habanero pepper cultivars under greenhouse conditions.
2. Materials and Methods
2.1. Establishment of the Experiment
The experiment was carried out from August 2018 to February 2019 in a polyethylene-covered multi-tunnel-type greenhouse located on the Loma Bonita campus of the University of Papaloapan at coordinates N 18°05′52.8″ W 95°53′46.8″ at 38 masl. The average temperature and relative humidity inside the greenhouse were 25 °C and 79%, respectively. We used two habanero pepper cultivars: the Chichen Itza hybrid, acquired from the company Seminis®, and the Jaguar variety donated by the Las Huastecas experimental field of the National Institute of Forestry, Agricultural, and Livestock Research (Instituto Nacional de Investigaciones Forestales Agrícolas y Pecuarias; INIFAP). The seeds were germinated in 200-well polystyrene trays, and peat moss was used as the substrate. Seedlings were transplanted 45 days after sowing into 40 × 40 cm black polyethylene containers filled with river sand as a hydroponic substrate. A fertigation system was used, which consisted of drip irrigation with an emitter of 1 L per container. The planting density was three plants per square meter with a lateral separation of 1.3 m for each treatment. Each plant was pruned to the three main stems, lateral shoots were eliminated, and each plant was supported with a plastic ring and raffia ties suspended from the greenhouse structure. A factorial experiment (4 × 2 × 2) was established completely at random, considering four foliar applications (control, chitosan alone, poly(acrylic acid) and chitosan–poly(acrylic acid) complex; Table 1), two nutrient solutions (nutrient solution A and B; Table 2) and two cultivars (Chichen Itza and Jaguar) as factors. Each treatment consisted of 10 plants, considering each plant as a replicate.

Table 1.
Preparation of foliar applications.

Table 2.
Anion–cation proportions (meq L−1) in the NSA and NSB nutrient solutions.
2.2. Synthesis of the Non-Stoichiometric Interpolyelectrolyte CS–PAA Complexes and Foliar Applications
The complex was prepared at the Center for Applied Chemistry Research (Centro de Investigación en Química Aplicada; CIQA) using the methodology described by Ortega-Ortiz et al. [11]. To synthesize the non-stoichiometric interpolyelectrolyte CS–PAA complex, we used CS (polycation) from Meron Chemicals, India with a viscometric molecular weight (Mv) of 200,000 g/mol and degree of deacetylation of 86%, and PAA (polyanion) with an Mv of 45,000 g/mol acquired from Sigma-Aldrich, St. Louis, MO, USA. The concentration of CS and PAA was 0.04 M, which was then diluted by adding 50 mL per liter of water. The CS–PAA complex was diluted to 100 mL per liter of water (Table 1). These concentrations were determined based on previous works, as we observed that at low concentrations, polyions (CS and PAA) have a better inductive (or biostimulant) effect on plant tissues [11,14]. The foliar applications were control (water spray), chitosan alone (CS), poly(acrylic acid) (PAA), and the chitosan–poly(acrylic acid) complex (CS–PAA). Foliar applications were added with a manual sprayer eight times during the experiment at 15-day intervals after transplantation. The first application was made in the vegetative stage and the last application in the fruit development stage. The total volume of the foliar application was 120 mL per plant.
2.3. Nutrient Solutions
In the experiment two nutrient solutions were applied, the nutrient solution A (NSA) and the nutrient solution B (NSB) formulated with salt contents based on a modification of Steiner’s solution [24]. The nutrient solutions present a different relationship between potassium and calcium. The content of the nutrient solutions is shown in Table 2.
To prepare the NSA and NSB, we added the soluble fertilizers described in Table 3. In addition, we added a concentration of 0.05 g L−1 of the commercial product Ultrasol® Micromix as a source of micronutrients to both nutrient solutions (Fe-EDTA 7.5%, Mn-EDTA 3.7%, B 0.4%, Zn-EDTA 0.6%, Cu-EDTA 0.3%, and Mo 0.2%). The pH of the water was adjusted to between 5.8 and 6.2 with sulfuric acid. Each nutrient solution was applied at a different time to avoid mixture due to drainage. Both nutrient solutions were applied twice a day (morning and afternoon) with an irrigation volume of approximately 1 L per day each.

Table 3.
Sources of macronutrients used in the NSA and NSB nutrient solutions (g L−1).
2.4. Growth, Biomass and Yield Parameters
The growth, biomass and yield parameters of each treatment were measured 150 days after transplantation took place. The height of the plant was measured from the base of the stem to the growth apex using a measuring tape. The stem diameter was measured at the base of the stem with a digital caliper. The number of fruits was counted per plant. The dry weight of roots and leaves–stem per treatment was taken after dehydration in an oven at 80 °C for 72 h. The total dry weight was obtained from the sum of the dry weight of roots and leaves–stem. Only the yield per plant was obtained, determined by the accumulation of the fresh weight of the fruits harvested during the crop cycle.
2.5. Parameters Measured in Green and Ripe Fruits
The fruit parameters were evaluated to determine the pre-harvest effect of the CS–PAA complex [25] and the K+/Ca2+ ratio of the nutrient solutions [26], from two samples during the harvest period, 120 days and 140 days after transplantation. For each treatment, we selected 10 green fruits and 10 ripe fruits (100% orange) that showed no physical damage, spots, or rot. The total soluble solids were determined from the juice of the fruit using a digital refractometer (HI 96,801 Hanna Instruments® Woonsocket, RI, USA). The pH was determined with a potentiometer (HI 98,130 Hanna Instruments® Woonsocket, RI, USA). The titratable acid was determined using the AOAC method [27]; we took 10 mL of ground fruits, added 1% phenolphthalein, then titrated this with sodium hydroxide (0.1 N) until a pink color was obtained. The data obtained were expressed as the percentage of citric acid. We also determined the relationship between total soluble solids and titratable acid (TSS/TA).
2.6. Statistical Analysis
For each of the variables evaluated, we considered ten replicates per treatment. For the parameters measured in green and ripe fruits, we considered the two sampling times as covariate. A three-way analysis of variance was conducted and when significance was detected, at Fisher’s LSD test (p ≤ 0.05) was performed with InfoStat, version 2020 software (Córdoba, Argentina).
3. Results
3.1. Effect of Foliar Applications
Table 4 shows that the foliar applications of CS–PAA complexes increased the plant height, stem diameter, number of fruits, leaf-stem dry weight, total dry weight per plant, and yield of the habanero peppers compared to the control (13, 14, 53, 54, 43 and 44%, respectively). Meanwhile, foliar applications of CS increased the dry weight of the root compared to the control (17%). Table 5 shows that foliar applications of CS–PAA complexes increased the TSS value of the green and ripe fruits, although in the green fruits it did not differ significantly from the control. The titratable acidity, TSS/TA ratio, and pH of the fruits were not modified by the foliar applications.

Table 4.
Effect of the foliar applications (Control; CS = chitosan; PAA = poly(acrylic acid); and CS–PAA = chitosan–poly(acrylic acid) complexes); nutrient solution (NSA and NSB; see Table 3) and cultivars (Chichen Itza hybrid and Jaguar variety) as well as their interactions on plant height, stem diameter, number of fruits, leaf-stem dry weight, root dry weight, total dry weight, and yield per plant measured 150 days after transplantation.

Table 5.
Effect of the foliar applications (Control; CS = chitosan; PAA = poly(acrylic acid); and CS–PAA = chitosan–poly(acrylic acid) complexes), nutrient solution (NSA and NSB; see Table 3) and cultivars (Chichen Itza hybrid and Jaguar variety) as well as their interactions on total soluble solids, titratable acid, TSS/TA ratio and pH measured in green and ripe fruits.
3.2. Effect of Nutrient Solutions
The NSA increased the stem diameter and fruit yield of the habanero peppers compared to NSB (Table 4); however, the plant height, number of fruits, and dry biomass were not affected. For the TSS value, titratable acid, and pH of the green and ripe fruits of the habanero peppers, there were no significant differences between the nutrient solutions (Table 5).
3.3. Effect of Cultivars
The Chichen Itza hybrid presented higher results in plant height, fruit yield (Table 4), and the TSS value in the green fruits (Table 5), compared to the Jaguar variety. Meanwhile, the Jaguar variety presented a higher pH value in the green and ripe fruits compared to the Chichen Itza hybrid (Table 5).
3.4. Effect of the Interactions on the Growth, Biomass, and Yield of the Habanero Pepper
The interaction between the foliar applications and cultivars was significant for plant height, number of fruits, dry weight of the root, and total dry weight (Table 4). Foliar applications of the CS–PAA complexes and PAA only increased the plant height of the Chichen Itza hybrid but did not increase the height of the Jaguar variety (Figure 1A). In contrast, the foliar applications of the CS–PAA complexes increased the number of fruits and total dry weight per plant in both cultivars (Figure 1B,D). On the other hand, the foliar applications of CS in the Jaguar variety increased the dry weight of the root, although it was not significantly different with the control (Figure 1C). The interaction between the nutrient solutions and cultivars was significant for the fruit yield (Table 2). The NSA increased the yield of the Chichen Itza hybrid; however, it did not increase the yield of the Jaguar variety (Table 6). The interaction of the three factors studied (foliar applications, nutrient solutions, and cultivars) were significant for the dry weight of the root (Table 4). The highest root dry weight was obtained by foliar applications of CS–PAA complexes in the hybrid Chichen Itza and CS in the Jaguar variety irrigated with the NSA (Figure 2).
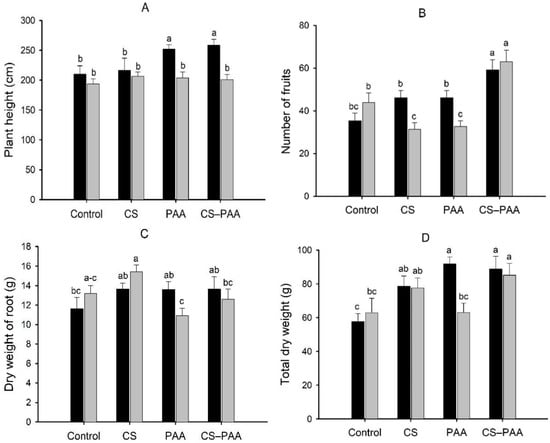
Figure 1.
Effect of the interaction of the foliar applications (Control; CS = chitosan; PAA = poly (acrylic acid); and CS–PAA = chitosan–poly(acrylic acid) complexes) and cultivars (black bars = Chichen Itza hybrid; grey bars = Jaguar variety) on plant height (A), number of fruits (B), dry weight of root (C), and total dry weight (D). Lower-case letters different between the bars indicate significant difference according to Fisher’s LSD test (p ≤ 0.05).

Table 6.
Effect of the interaction between nutrient solutions (NSA and NSB; see Table 3) and habanero pepper cultivars (Chichen Itza hybrid and Jaguar variety) on yield per plant.
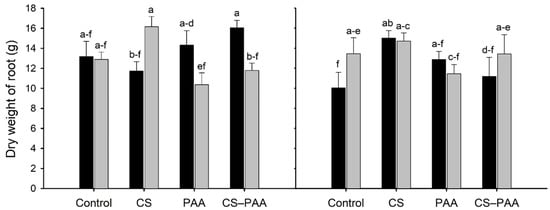
Figure 2.
Effect of the interaction of the foliar applications (Control; CS = chitosan; PAA = poly(acrylic acid); and CS–PAA = chitosan–poly(acrylic acid) complexes); cultivars (black bars = Chichen Itza hybrid; grey bars = Jaguar variety) and nutrient solutions (left = nutrient solution A; right = nutrient solution B) on root dry weight. Lower-case letters different between bars indicate significant difference according to Fisher’s LSD test (p ≤ 0.05).
3.5. Effect of the Interactions on the Green and Ripe Fruits of the Habanero Peppers
The interaction between the foliar applications and nutrient solutions was significant for the titratable acid and the TSS/TA ratio of the green fruits (Table 5). Foliar applications of the CS–PAA complexes increased the titratable acidity and TSS/TA ratio of the green fruits irrigated with the NSA and NSB, respectively; however, it did not differ significantly from the control (Figure 3A,B). The interaction between nutrient solutions and cultivars was significant for the TSS value of the green fruits and the pH of the ripe fruits (Table 5). The NSA increased the TSS value of the green fruits of the hybrid Chichen Itza; on the contrary, the pH of the ripe fruits decreased (Table 7). The interaction of the three factors studied (foliar applications, nutrient solutions, and cultivars) was significant for the total soluble solids of the green fruits and the titratable acid of the ripe fruits (Table 5). Foliar applications of the CS–PAA complexes increased the TSS value of the green fruits of the Jaguar variety irrigated with the NSA (Figure 4A). Meanwhile, the foliar applications of the PAA increased the titratable acid of the ripe fruits of the Jaguar variety irrigated with the NSB (Figure 4B).
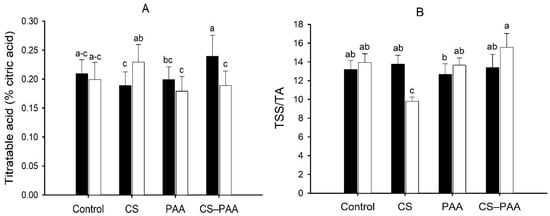
Figure 3.
Effect of the interaction of the foliar applications (Control; CS = chitosan; PAA = poly(acrylic acid); and CS–PAA = chitosan–poly(acrylic acid) complexes) and nutrient solutions (black bars = Nutrient Solution (A); white bars = Nutrient Solution (B)) on titratable acid of the green fruits of the habanero peppers. Lower-case letters different between bars indicate significant difference according to Fisher’s LSD test (p ≤ 0.05).

Table 7.
Effect of the interaction between nutrient solutions (NSA and NSB; see Table 3) and habanero pepper cultivars (Chichen Itza hybrid and Jaguar variety) on total soluble solids (green fruits) and pH (ripe fruits).
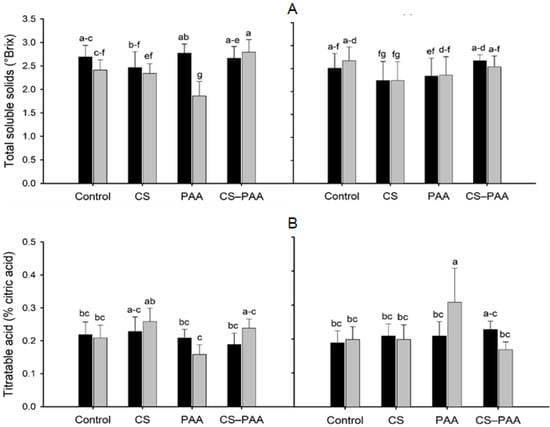
Figure 4.
Effect of the interaction of the foliar applications (Control; CS = chitosan; PAA = poly(acrylic acid); and CS–PAA = chitosan–poly(acrylic acid) complexes); cultivars (black bars = Chichen Itza hybrid; grey bars = Jaguar variety) and nutrient solutions (left = nutrient solution (A); right = nutrient solution (B)) on total soluble solids (green fruits) and titratable acid (ripe fruits). Lower-case letters different between bars indicate significant difference according to Fisher’s LSD test (p ≤ 0.05).
4. Discussion
Chitosan is used in horticultural crops to stimulate growth and productivity [9]. However, these responses depend on the degree of deacetylation, molecular weight, and the concentration of chitosan, as well as the plant species and the phenological stage of the plant [28]. This polymer is synthesized in combination with poly(acrylic acid) to form complexes that have been shown to have a biostimulant effects on plants [14]. In the present study, we demonstrated that the foliar applications of CS–PAA complexes increased growth and dry biomass parameters (except dry root biomass) of habanero peppers compared to the control. Meanwhile, the effect of the interactions showed that the applications of the CS–PAA complexes improved the height of the Chichen Itza hybrid and increased the total dry biomass of both cultivars. We did not find any published studies that discussed the effect of chitosan, poly(acrylic acid), or CS–PAA complexes on the growth of C. chinense. Similar results show that chitosan–poly(acrylic acid) hydrogel nanoparticles improved the growth of onion plants [16]. Of the few studies regarding Capsicum, Chookhongkha et al. [29] reported that the application of chitosan to the soil increased the plant height of Capsicum annuum cultivated in a greenhouse. Meanwhile, Esyanti et al. [30] showed that the foliar application of chitosan increased the plant height of C. annuum cultivated in a shade house. In C. frutescens cultivated in a greenhouse, the foliar application of oligochitosan increased the fresh and dry weight of the buds [31]. In addition, the foliar application of chitosan was shown to increase the growth of other species such as tomato, cucumber, strawberry, potato, maize, and wheat, as reported by Mukhtar Ahmed et al. [5] in their review.
Our results showed that the foliar applications of CS–PAA complexes increased fruit number, yield, and TSS value of ripe habanero pepper fruits compared to the control. Meanwhile, the effect of the interactions showed that the foliar applications of CS–PAA complexes increased the number of fruits of both cultivars. Furthermore, foliar applications of CS–PAA complexes improved the TSS value of the green fruits of the Jaguar variety when it was irrigated with the NSA. Some studies have reported that the application of chitosan–poly(acrylic acid) complexes increased the yield of tomato and onion [15,16]. Other authors, such as Chookhongkha et al. [29] reported that the application of chitosan to soil increased the number of fruits per plant and fresh weight of fruits of C. annuum cultivated in a greenhouse. Meanwhile, Mahmood et al. [32] reported that the foliar application of chitosan increased the yield and average weight of fruits of C. annuum cultivated in open fields. Regarding C. frutescens cultivated in a greenhouse, the foliar application of oligochitosan was shown to increase the fresh weight of fruits [31]. Meanwhile, He et al. [25] demonstrated that the pre-harvest application of chitosan oligosaccharides increased the total soluble solids values of strawberry fruits as shown in the results reported here.
The biostimulant effect of CS–PAA complexes is probably due to the involvement of chitosan in the regulation of nitrogen and carbon metabolism [33,34]. This is supported by previous studies that report that chitosan increases the activity of enzymes such as fructose-1,6-bisphosphatase, sucrose phosphate synthase, sucrose synthase, phosphoenolpyruvate carboxylase, pyruvate dehydrogenase, malate dehydrogenase, nitrate reductase, glutamate synthase, glutamine synthetase, among others [5,33,34,35,36]. Similarly, El-Tanahy et al. [37] and Dwivany Fenny et al. [38] propose that the biostimulating effect of chitosan on plant growth is due to the primary aliphatic amino groups of the polymer, which provide a source of nitrogen. This corresponds with the findings of Ravi Kumar [39], who reported that the structure of this polymer can contain up to 6.89% nitrogen. Another hypothesis is that chitosan increases the net photosynthetic rate, which leads to better plant growth and development [5,36]. Whether chitosan-specific receptors exist or not is unknown as they have not been clearly elucidated [33]. So far it is known that the chitosan signaling pathways in plants are calcium, NO, ROS, phytohormones and hydrogen peroxide [8].
Regarding nutrient solutions evaluated, the NSA increased the stem diameter and the yield of habanero peppers compared to the NSB. Meanwhile, the effect of the interactions showed that the NSA increased the yield and the TSS value of the green fruits of the Chichen Itza hybrid. These responses are due to the relationship between potassium and calcium in the nutrient solution. Potassium and calcium are two cations that present antagonism [40], so an unbalanced ratio of these cations in the nutrient solution can affect crop yield. Hernández-Pérez et al. [26] reported that the relationship between potassium and calcium in the nutrient solution is associated with higher yield and sugar concentration in the tomato fruits with the optimal balance being between 0.82 and 0.85, while if this value is above 1.0, yield the sugar concentration then decreases drastically. This coincides with our results as the NSA and NSB present a relationship between potassium and calcium with values of 0.83 and 1.2, respectively. Among the cultivars evaluated, the Chichen Itza hybrid obtained a higher fruit yield than the Jaguar variety. This shows the genetic potential per se of the hybrid compared to the improved variety. The use of hybrid seeds is a good alternative to improve crop yields due to the exploitation of heterosis [41].
5. Conclusions
Our results showed that the foliar applications of CS–PAA complexes improved plant height, stem diameter, total dry biomass, number of fruits, and yield of habanero peppers compared to the control. Meanwhile, the effect of the interactions showed that foliar applications of CS–PAA complexes increased the total dry biomass and the number of fruits per plant of both cultivars. Regarding nutrient solutions, nutrient solution A increased the yield of the Chichen Itza hybrid compared to the nutrient solution B, this was due to it presenting a better balance of potassium and calcium. Meanwhile, the best cultivar was the Chichen Itza hybrid, because it obtained a higher yield than the Jaguar variety, this was due to the exploitation of heterosis. This research offers advances in the use of CS–PAA as a biostimulant to improve the genetic potential of habanero pepper cultivars. It also provides information on the management of nutrient solutions in the crop of habanero peppers in greenhouses, which is very scarce. This could give guidelines for more researchers to address this research topic.
Author Contributions
Conceptualization, H.H.-H. and R.E.P.-T.; methodology, H.O.-O.; formal analysis, J.A.Y.-T. and A.A.-M.; investigation, A.S.-C. and A.R.R.-S.; resources, H.O.-O.; synthesis of the complexes, H.H.-H. and J.A.Y.-T.; writing—original draft preparation, R.E.P.-T.; visualization, H.H.-H.; writing—review and editing, H.H.-H. and A.J.-M.; supervision, H.H.-H.; funding acquisition, M.R.-O., J.A.Y.-T., A.A.-M. and A.J.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
All of the authors thank the researcher Moisés Ramírez Meraz from INIFAP for the donation of seeds of the Jaguar variety of habanero peppers.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Özüsağlam, M.A.; Çakmak, Y.S. Physicochemical Comparison of Chitin and Chitosan Obtained from Larvae and Adult Colorado Potato Beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Cakmak, Y.S.; Baran, T.; Asan-Ozusaglam, M.; Mentes, A.; Tozak, K.O. New Chitin, Chitosan, and O-Carboxymethyl Chitosan Sources from Resting Eggs of Daphnia Longispina (Crustacea); with Physicochemical Characterization, and Antimicrobial and Antioxidant Activities. Biotechnol. Bioprocess Eng. 2014, 19, 58–69. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan Nanoparticle Based Delivery Systems for Sustainable Agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and Its Oligosaccharides, a Promising Option for Sustainable Crop Production—A Review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, M.; Khawar, K.M.; Camara, M.C.; Carvalho, L.B.; Fraceto, L.F.; Morsi, R.E.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Ullah, H.; et al. Chitosan-Based Delivery Systems for Plants: A Brief Overview of Recent Advances and Future Directions. Int. J. Biol. Macromol. 2020, 154, 683–697. [Google Scholar] [CrossRef]
- Lopez-Moya, F.; Suarez-Fernandez, M.; Lopez-Llorca, L. Molecular Mechanisms of Chitosan Interactions with Fungi and Plants. Int. J. Mol. Sci. 2019, 20, 332. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of Chitosan on Plant Responses with Special Reference to Abiotic Stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [CrossRef]
- Sharif, R.; Mujtaba, M.; Ur Rahman, M.; Shalmani, A.; Ahmad, H.; Anwar, T.; Tianchan, D.; Wang, X. The Multifunctional Role of Chitosan in Horticultural Crops; A Review. Molecules 2018, 23, 872. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Advances of Chitosan Applications in Plants. Polymers 2018, 10, 118. [Google Scholar] [CrossRef]
- Ortega-Ortíz, H.; Benavides-Mendoza, A.; Flores-Olivas, A.; Ledezma-Pérez, A. Use of the Interpolyelectrolyte Complexes of Poly(Acrylic Acid)-Chitosan as Inductors of Tolerance Against Pathogenic Fungi in Tomato (Lycopersicon esculentum Mill. Var. Floradade). Macromol. Biosci. 2003, 3, 566–570. [Google Scholar] [CrossRef]
- Bai, M.; Wilske, B.; Buegger, F.; Esperschütz, J.; Bach, M.; Frede, H.-G.; Breuer, L. Relevance of Nonfunctional Linear Polyacrylic Acid for the Biodegradation of Superabsorbent Polymer in Soils. Environ. Sci. Pollut. Res. 2015, 22, 5444–5452. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Zhang, K.; Chen, J.; Shi, X.; Li, X.; Ma, Y.; Fang, G.; Xu, S. Changes in Heavy Metal Mobility and Availability in Contaminated Wet-Land Soil Remediated Using Lignin-Based Poly(Acrylic Acid). J. Hazard. Mater. 2019, 368, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-De la Fuente, M.; Ortega-Ortiz, H.; Juárez-Maldonado, A.; Sandoval-Rangel, A.; González-Morales, S.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Use of Chitosan-Polyacrylic Acid (CS–PAA) Complex, Chitosan-Polyvinyl Alcohol (CS-PVA) and Chitosan Hydrogels in Greenhouses as a Carrier for Beneficial Elements, Nanoparticles, and Microorganisms. Acta Hortic. 2020, 1296, 1153–1160. [Google Scholar] [CrossRef]
- Benavides-Mendoza, A.; Burgos-Limón, D.; Ortega-Ortiz, H.; Ramírez, H. Benzoic Acid and Poly(Acrylic Acid)-Chitosan in Tomato Quality and Yield in Calcareous Soil. Terra Latinoam. 2007, 25, 261–268. [Google Scholar]
- Abd El-Aziz, M.E.; Morsi, S.M.M.; Salama, D.M.; Abdel-Aziz, M.S.; Abd Elwahed, M.S.; Shaaban, E.A.; Youssef, A.M. Preparation and Characterization of Chitosan/Polyacrylic Acid/Copper Nanocomposites and Their Impact on Onion Production. Int. J. Biol. Macromol. 2019, 123, 856–865. [Google Scholar] [CrossRef]
- Leija-Martínez, P.; Benavides-Mendoza, A.; Cabrera-De La Fuente, M.; Robledo-Olivo, A.; Ortega-Ortíz, H.; Sandoval-Rangel, A.; González-Morales, S. Lettuce Biofortification with Selenium in Chitosan-Polyacrylic Acid Complexes. Agronomy 2018, 8, 275. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural Uses of Plant Biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Food and Agriculture Information Service (SIAP). Statistical Yearbook of Agricultural Production. 2020. Available online: https://nube.siap.gob.mx/cierreagricola/ (accessed on 21 February 2021).
- López-Gómez, J.D.; Sotelo-Nava, H.; Villegas-Torres, O.G.; Andrade-Rodríguez, M. Yield and quality of habanero chili in response to driving pruning and nutritional regime. Rev. Mex. Cienc. Agríc. 2020, 11, 315–325. [Google Scholar] [CrossRef][Green Version]
- Tucuch-Haas, C.J.; Alcántar-González, G.; Santizo-Rincón, J.A.; Ordaz-Chaparro, V.M.; Larqué-Saavedra, A. Production and quality of habanero pepper (Capsicum Chinense Jacq.) with different NH4+/NO3− ratios and size of substrate particles. Terra Latinoam. 2012, 30, 9–15. [Google Scholar]
- Tapia-Vargas, M.; Larios-Guzmán, A.; Díaz-Sánchez, D.D.; Ramírez-Ojeda, G.; Hernández-Pérez, A.; Vidales-Fernández, I.; Guillén-Andrade, H. Black habanero pepper (Capsicum chinense Jacq.) hydroponics production. Rev. Fitotec. Mex. 2016, 39, 241–245. [Google Scholar] [CrossRef]
- Meneses-Lazo, R.E.; Garruña, R. The Habanero Pepper (Capsicum Chinense Jacq.) as a Study Plant Mothe in Mexico. Trop. Subtrop. Agroecosyst. 2020, 23, 1–17. [Google Scholar]
- Steiner, A.A. A Universal Method for Preparing Nutrient Solutions of a Certain Desired Composition. Plant Soil 1961, 15, 134–154. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-Harvest Treatment of Chitosan Oligosaccharides Improved Strawberry Fruit Quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef]
- Hernández-Pérez, O.I.; Valdez-Aguilar, L.A.; Alia-Tejacal, I.; Cartmill, A.D.; Cartmill, D.L. Tomato Fruit Yield, Quality, and Nutrient Status in Response to Potassium: Calcium Balance and Electrical Conductivity in the Nutrient Solution. J. Soil Sci. Plant Nutr. 2020, 20, 484–492. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the AOAC, Methods 932.06, 925.09, 985.29, 923.03; Association of Official Analytical Chemists: Arlington, VA, USA, 1990. [Google Scholar]
- Pichyangkura, R.; Chadchawan, S. Biostimulant Activity of Chitosan in Horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Chookhongkha, N.; Miyagawa, S.; Jirakiattikul, Y.; Photchanachai, S. Chili Growth and Seed Productivity as Affected by Chitosan. In Proceedings of the International Conference on Agriculture Technology and Food Sciences (ICATFS’2012), Manila, Philippines, 17–18 November 2012; pp. 146–149. [Google Scholar]
- Esyanti, R.R.; Dwivany, F.M.; Mahani, S.; Nugrahapraja, H.; Meitha, K. Foliar Application of Chitosan Enhances Growth and Modulates Expression of Defense Genes in Chilli Pepper (Capsicum annuum L.). Aust. J. Crop Sci. 2019, 13, 55–60. [Google Scholar] [CrossRef]
- Dzung, P.D.; Phu, D.V.; Du, B.D.; Ngoc, L.S.; Duy, N.N.; Hiet, H.D.; Nghia, D.H.; Thang, N.T.; Le, B.V.; Hien, N.Q. Effect of Foliar Application of Oligochitosan with Different Molecular Weight on Growth Promotion and Fruit Yield Enhancement of Chili Plant. Plant Prod. Sci. 2017, 20, 389–395. [Google Scholar] [CrossRef]
- Mahmood, N.; Abbasi, N.A.; Hafiz, I.A.; Ali, I.; Zakia, S. Effect of Biostimulants on Growth, Yield and Quality of Bell Pepper Cv. Yolo Wonder. Pak. J. Agric. Sci. 2017, 54, 311–317. [Google Scholar] [CrossRef]
- Li, K.; Xing, R.; Liu, S.; Li, P. Chitin and Chitosan Fragments Responsible for Plant Elicitor and Growth Stimulator. J. Agric. Food Chem. 2020, 68, 12203–12211. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Xing, R.; Liu, S.; Li, P. Metabolite Profiling of Wheat Seedlings Induced by Chitosan: Revelation of the Enhanced Carbon and Nitrogen Metabolism. Front. Plant Sci. 2017, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Hasanuzzaman, M.; Rahman, M.; Khan, M.A.R.; Bhowmik, P.; Mahmud, N.U.; Tanveer, M.; Islam, T. Mechanism of Plant Growth Promotion and Disease Suppression by Chitosan Biopolymer. Agriculture 2020, 10, 624. [Google Scholar] [CrossRef]
- Zhang, X.; Li, K.; Xing, R.; Liu, S.; Chen, X.; Yang, H.; Li, P. MiRNA and MRNA Expression Profiles Reveal Insight into Chitosan-Mediated Regulation of Plant Growth. J. Agric. Food Chem. 2018, 66, 3810–3822. [Google Scholar] [CrossRef] [PubMed]
- El-Tanahy, A.M.M.; Mahmoud, A.R.; Abde-Mouty, M.M.; Ali, A.H. Effect of Chitosan Doses and Nitrogen Sources on the Growth, Yield and Seed Quality of Cowpea. Aust. J. Basic. Appl. Sci. 2012, 6, 115–121. [Google Scholar]
- Dwivany Fenny, M.; Karlia, M.; Kuswati, K.; Esyanti Rizkita, R.; Husna, N. Chitosan Improving Growth in Chili (Capsicum annuum L.) Plants and Acting through Distinct Gene Regulation between Cultivars. Res. J. Biotechnol. 2021, 16, 87–92. [Google Scholar]
- Ravi Kumar, M.N.V. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Jakobsen, S.T. Interaction between Plant Nutrients: III. Antagonism between Potassium, Magnesium and Calcium. Acta Agric. Scand. B Soil Plant Sci. 1993, 43, 1–5. [Google Scholar] [CrossRef]
- Labroo, M.R.; Studer, A.J.; Rutkoski, J.E. Heterosis and Hybrid Crop Breeding: A Multidisciplinary Review. Front. Genet. 2021, 12, 234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).