Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Arrangement
2.2. Irrigation Treatment
2.3. Relative Water Content (RWC) and Photosynthetic Pigments Measurements
2.4. Determination of MDA, H2O2, Proline, and Total Soluble Carbohydrates Concentrations
2.5. Antioxidant Enzyme Activity
2.6. Stress Susceptibility Index
2.7. Statistical Analysis
3. Results and Discussion
3.1. Relative Water Content (RWC)
3.2. Chlorophyll-a, Chlorophyll-b, and Carotenoid Content
3.3. Leaf Proline and Total Soluble Carbohydrates (TSC)
3.4. Antioxidant Enzyme Activities
3.5. Malondialdehyde and Hydrogen Peroxide (H2O2) Analysis
3.6. Plant Height
3.7. Capsules and Seeds per Plant
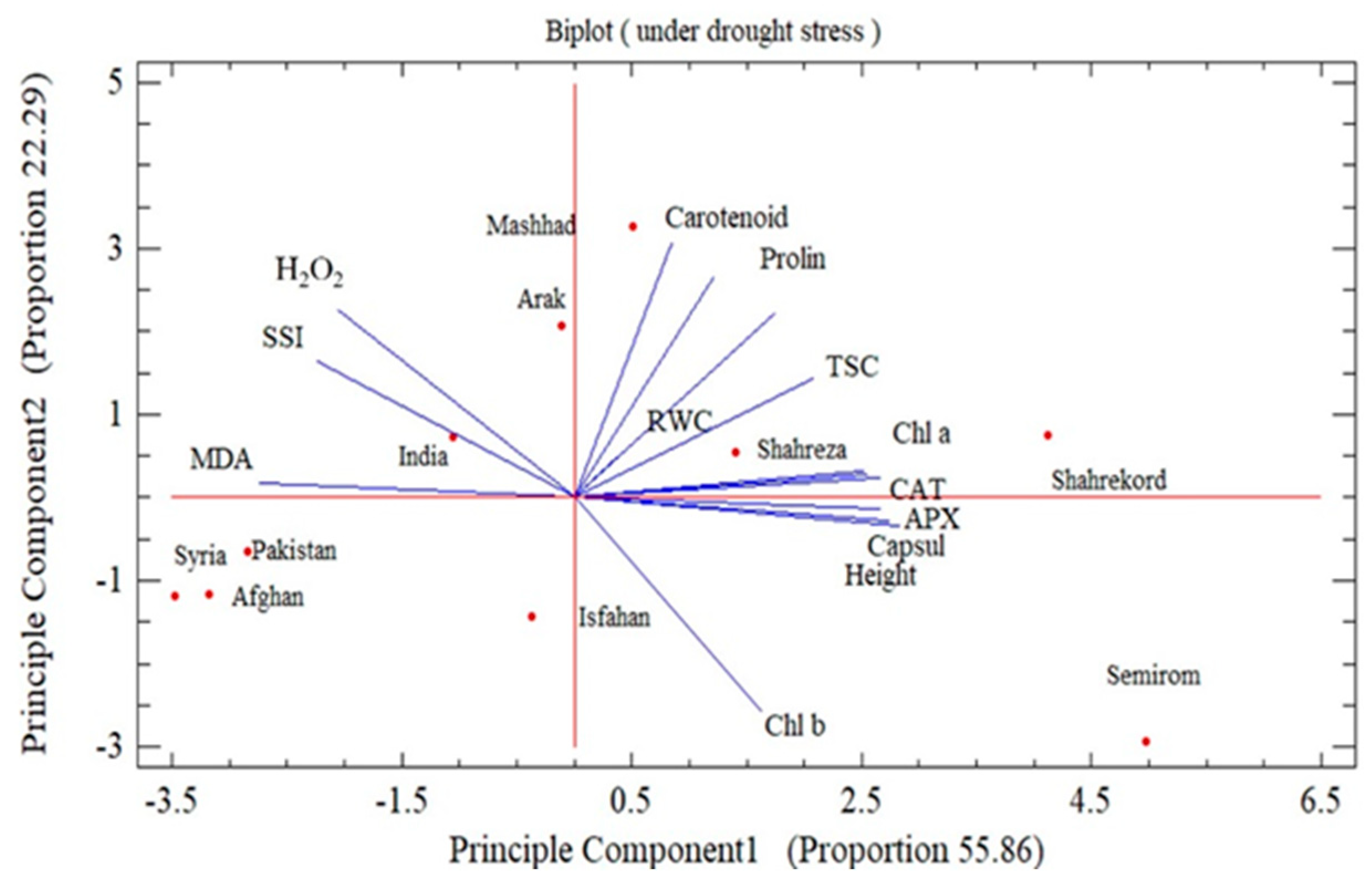
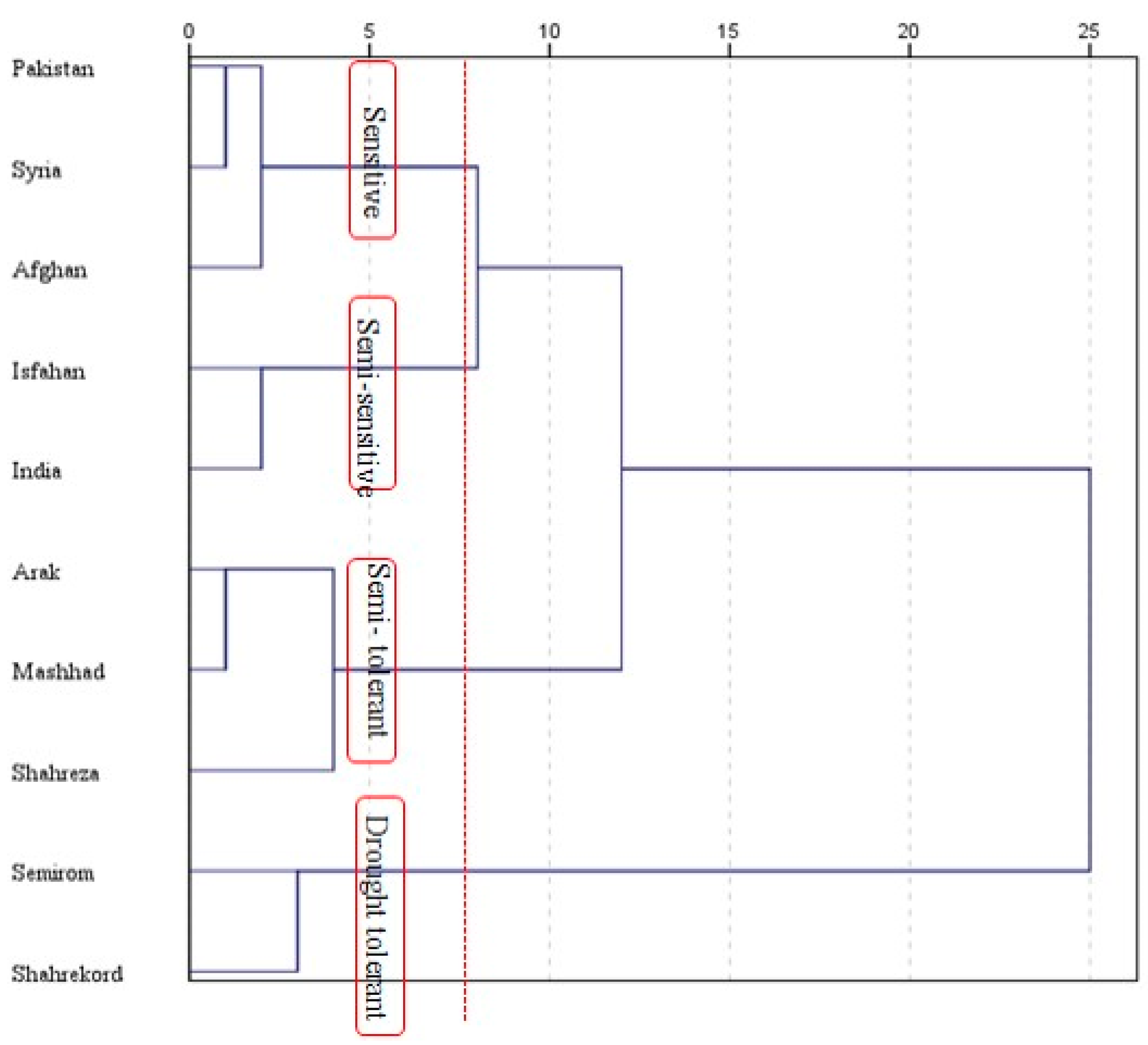
3.8. Stress Susceptibility Index and Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mirjahanmardi, H.; Ehsanzadeh, P. Iron supplement ameliorates drought-induced alterations in physiological attributes of fennel (Foeniculum vulgare). Nutr. Cycl. Agroecosyst. 2016, 106, 61–76. [Google Scholar] [CrossRef]
- Aghajanlou, F.; Mirdavoudi, H.; Shojaee, M.; Mac Sweeney, E.; Mastinu, A.; Moradi, P. Rangeland Management and Ecological Adaptation Analysis Model for Astragalus curvirostris Boiss. Horticulturae 2021, 7, 67. [Google Scholar] [CrossRef]
- Biareh, V.; Shekari, F.; Sayfzadeh, S.; Zakerin, H.; Hadidi, E.; Beltrao, J.G.T.; Mastinu, A. Physiological and Qualitative Response of Cucurbita pepo L. to Salicylic Acid under Controlled Water Stress Conditions. Horticulturae 2022, 8, 79. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [Green Version]
- Naservafaei, S.; Sohrabi, Y.; Moradi, P.; Mac Sweeney, E.; Mastinu, A. Biological Response of Lallemantia iberica to Brassinolide Treatment under Different Watering Conditions. Plants 2021, 10, 496. [Google Scholar] [CrossRef] [PubMed]
- Noryan, M.; Hervan, I.M.; Sabouri, H.; Kojouri, F.D.; Mastinu, A. Drought Resistance Loci in Recombinant Lines of Iranian Oryza sativa L. in Germination Stage. BioTech 2021, 10, 26. [Google Scholar] [CrossRef]
- Gharibvandi, A.; Karimmojeni, H.; Ehsanzadeh, P.; Maleki, M.R.; Mastinu, A. Weed management by allelopathic activity of Foeniculum vulgare essential oil. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2022, 1–15. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian, H.; Alizadeh, H.; Yousefi, A.R.; Gonzalez-Andujar, J.L.; Mac Sweeney, E.; Mastinu, A. Competitive Ability Effects of Datura stramonium L. and Xanthium strumarium L. on the Development of Maize (Zea mays) Seeds. Plants 2021, 10, 1922. [Google Scholar] [CrossRef]
- Abate, G.; Zhang, L.L.; Pucci, M.; Morbini, G.; Mac Sweeney, E.; Maccarinelli, G.; Ribaudo, G.; Gianoncelli, A.; Uberti, D.; Memo, M.; et al. Phytochemical Analysis and Anti-Inflammatory Activity of Different Ethanolic Phyto-Extracts of Artemisia annua L. Biomolecules 2021, 11, 975. [Google Scholar] [CrossRef]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Sabouri, H.; Kazerani, B.; Fallahi, H.A.; Dehghan, M.A.; Alegh, S.M.; Dadras, A.R.; Katouzi, M.; Mastinu, A. Association analysis of yellow rust, fusarium head blight, tan spot, powdery mildew, and brown rust horizontal resistance genes in wheat. Physiol. Mol. Plant Pathol. 2022, 118, 101808. [Google Scholar] [CrossRef]
- Moradi, P.; Aghajanloo, F.; Moosavi, A.; Monfared, H.H.; Khalafi, J.; Taghiloo, M.; Khoshzaman, T.; Shojaee, M.; Mastinu, A. Anthropic Effects on the Biodiversity of the Habitats of Ferula gummosa. Sustainability 2021, 13, 7874. [Google Scholar] [CrossRef]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, A.R.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Pucci, M.; Aria, F.; Premoli, M.; Maccarinelli, G.; Mastinu, A.; Bonini, S.; Memo, M.; Uberti, D.; Abate, G. Methylglyoxal affects cognitive behaviour and modulates RAGE and Presenilin-1 expression in hippocampus of aged mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2021, 158, 112608. [Google Scholar] [CrossRef] [PubMed]
- Zali, A.G.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crop Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Gupta, A.K.; Rather, M.A.; Jha, A.K.; Shashank, A.; Singhal, S.; Sharma, M.; Pathak, U.; Sharma, D.; Mastinu, A. Artocarpus lakoocha Roxb. and Artocarpus heterophyllus Lam. Flowers: New Sources of Bioactive Compounds. Plants 2020, 9, 1329. [Google Scholar] [CrossRef]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.L.; Lucini, L.; Memo, M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef]
- Zangani, E.; Afsahi, K.; Shekari, F.; Mac Sweeney, E.; Mastinu, A. Nitrogen and Phosphorus Addition to Soil Improves Seed Yield, Foliar Stomatal Conductance, and the Photosynthetic Response of Rapeseed (Brassica napus L.). Agriculture 2021, 11, 483. [Google Scholar] [CrossRef]
- Laribi, B.; Bettaieb, I.; Kouki, K.; Sahli, A.; Mougou, A.; Marzouk, B. Water deficit effects on caraway (Carum carvi L.) growth, essential oil and fatty acid composition. Ind. Crop Prod. 2009, 30, 372–379. [Google Scholar] [CrossRef]
- Zarrinabadi, I.G.; Razmjoo, J.; Mashhadi, A.A.; Mojeni, H.K.; Boroomand, A. Physiological response and productivity of pot marigold (Calendula officinalis) genotypes under water deficit. Ind. Crop Prod. 2019, 139. [Google Scholar] [CrossRef]
- Alinian, S.; Razmjoo, J.; Zeinali, H. Flavonoids, anthocynins, phenolics and essential oil produced in cumin (Cuminum cyminum L.) accessions under different irrigation regimes. Ind. Crop Prod. 2016, 81, 49–55. [Google Scholar] [CrossRef]
- Moinuddin, G.; Devi, K.; Khajuria, D.K. Evaluation of the anti-depressant activity of Myristica fragrans (Nutmeg) in male rats. Avicenna J. Phytomed. 2012, 2, 72–78. [Google Scholar]
- Askari, E.; Ehsanzadeh, P. Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol. Plant. 2015, 37, 1–14. [Google Scholar] [CrossRef]
- Askari, E.; Ehsanzadeh, P. Osmoregulation-mediated differential responses of field-grown fennel genotypes to drought. Ind. Crop Prod. 2015, 76, 494–508. [Google Scholar] [CrossRef]
- Baghalian, K.; Abdoshah, S.; Khalighi-Sigaroodi, F.; Paknejad, F. Physiological and phytochemical response to drought stress of German chamomile (Matricaria recutita L.). Plant Physiol. Biochem. 2011, 49, 201–207. [Google Scholar] [CrossRef]
- Bahreininejad, B.; Razmjoo, J.; Mirza, M. Effect of Water Stress on Productivity and Essential Oil Content and Composition ofThymus carmanicus. J. Essent. Oil Bear. Plants 2014, 17, 717–725. [Google Scholar] [CrossRef]
- Jacob, B.; Narendhirakannan, R.T. Role of medicinal plants in the management of diabetes mellitus: A review. 3 Biotech 2019, 9, 4. [Google Scholar] [CrossRef]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharm. Res. 2015, 95–96, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Mohammad Reza Haj Seyed, H. Effects of irrigation treatment and Azospirillum inoculation on yield and yield component of black cumin (Nigella sativa L.). J. Med. Plants Res. 2012, 6, 4553–4561. [Google Scholar] [CrossRef]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 2002, 28, 350–356. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Frebort, I.; Haviger, A.; Jilek, M. A Kinetic Method for Assay of Plant Peroxidase and Catalase Activities by Chemiluminescence. Phytochem. Anal. 1992, 3, 55–60. [Google Scholar] [CrossRef]
- Kruger, N.J. The Bradford Method For Protein Quantitation. In The Protein Protocols Handbook; Springer: Berlin/Heidelberg, Germany, 2009; pp. 17–24. [Google Scholar] [CrossRef]
- Fischer, R.A.; Maurer, R. Drought Resistance in Spring Wheat Cultivars. 1. Grain-Yield Responses. Aust. J. Agric. Res. 1978, 29, 897–912. [Google Scholar] [CrossRef]
- Maghsoodi, M.; Razmjoo, J. Identify Physiological Markers for Drought Tolerance in Alfalfa. Agron. J. 2015, 107, 149–157. [Google Scholar] [CrossRef]
- Menconi, M.; Sgherri, C.L.M.; Pinzino, C.; Navariizzo, F. Activated Oxygen Production and Detoxification in Wheat Plants Subjected to a Water-Deficit Program. J. Exp. Bot. 1995, 46, 1123–1130. [Google Scholar] [CrossRef]
- Azizpour, K.; Shakiba, M.R.; Sima, N.A.K.K.; Alyari, H.; Mogaddam, M.; Esfandiari, E.; Pessarakli, M. Physiological Response of Spring Durum Wheat Genotypes to Salinity. J. Plant Nutr. 2010, 33, 859–873. [Google Scholar] [CrossRef]
- Singh, A.K.; Dubey, R.S. Changes in Chlorophyll-a and Chlorophyll-B Contents and Activities of Photosystem-1 and Photosystem-2 in Rice Seedlings Induced by Nacl. Photosynthetica 1995, 31, 489–499. [Google Scholar]
- Kaiser, W.M.; Kaiser, G.; Schoner, S.; Neimanis, S. Photosynthesis under Osmotic-Stress-Differential Recovery of Photosynthetic Activities of Stroma Enzymes, Intact Chloroplasts, Protoplasts, and Leaf Slices after Exposure to High Solute Concentrations. Planta 1981, 153, 430–435. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y.; Wang, L.C.; Saleem, M.F.; Man, C.; Lei, W. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Mohammadkh, N.; Heidari, R. Effects of Water Stress on Respiration, Photosynthetic Pigments and Water Content in Two Maize Cultivars. Pak. J. Biol. Sci. 2007, 10, 4022–4028. [Google Scholar] [CrossRef] [Green Version]
- Raal, A.; Orav, A.; Nesterovitsch, J.; Maidla, K. Analysis of Carotenoids, Flavonoids and Essential Oil of Calendula officinalis Cultivars Growing in Estonia. Nat. Prod. Commun. 2016, 11, 1157–1160. [Google Scholar] [CrossRef] [Green Version]
- Waqas, M.A.; Kaya, C.; Riaz, A.; Farooq, M.; Nawaz, I.; Wilkes, A.; Li, Y. Potential Mechanisms of Abiotic Stress Tolerance in Crop Plants Induced by Thiourea. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- Golldack, D.; Li, C.; Mohan, H.; Probst, N. Tolerance to drought and salt stress in plants: Unraveling the signaling networks. Front. Plant Sci. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Moghadam, H.R.T.; Zahedi, H.; Ghooshchi, F. Qualidade De Óleo De Cultivares De Canola Em Resposta Ao Estresse HÍdrico E AplicaÇÃo De PolÍmero Hidroabsorvente. Pesqui. Agropecuária Trop. 2011, 41. [Google Scholar] [CrossRef]
- Silvente, S.; Sobolev, A.P.; Lara, M. Metabolite Adjustments in Drought Tolerant and Sensitive Soybean Genotypes in Response to Water Stress. PLoS ONE 2012, 7, e38554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, G.; Asthir, B. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Dien, D.C.; Mochizuki, T.; Yamakawa, T. Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod. Sci. 2019, 22, 530–545. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Arabzadeh, N. The Effect of Drought Stress on Soluble Carbohydrates (Sugars) in Two Species of Haloxylon persicum and Haloxylon aphyllum. Asian J. Plant Sci. 2011, 11, 44–51. [Google Scholar] [CrossRef]
- Tunc-Ozdemir, M.; Miller, G.; Song, L.H.; Kim, J.; Sodek, A.; Koussevitzky, S.; Misra, A.N.; Mittler, R.; Shintani, D. Thiamin Confers Enhanced Tolerance to Oxidative Stress in Arabidopsis. Plant Physiol. 2009, 151, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Caverzan, A.; Passaia, G.; Rosa, S.B.; Ribeiro, C.W.; Lazzarotto, F.; Margis-Pinheiro, M. Plant responses to stresses: Role of ascorbate peroxidase in the antioxidant protection. Genet. Mol. Biol. 2012, 35, 1011–1019. [Google Scholar] [CrossRef] [Green Version]
- Shigeoka, S.; Ishikawa, T.; Tamoi, M.; Miyagawa, Y.; Takeda, T.; Yabuta, Y.; Yoshimura, K. Regulation and function of ascorbate peroxidase isoenzymes. J. Exp. Bot. 2002, 53, 1305–1319. [Google Scholar] [CrossRef]
- Dhanda, S.S.; Sethi, G.S.; Behl, R.K. Indices of drought tolerance in wheat genotypes at early stages of plant growth. J. Agron. Crop Sci. 2004, 190, 6–12. [Google Scholar] [CrossRef]
- Gawel, S.; Wardas, M.; Niedworok, E.; Wardas, P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad. Lek. 2004, 57, 453–455. [Google Scholar] [PubMed]
- Garg, N.; Manchanda, G. ROS generation in plants: Boon or bane? Plant Biosyst. 2009, 143, 81–96. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Tanveer, M.; Khan, I.; Hussain, S.; Shahzad, B.; Zohaib, A.; Abbas, F.; Saleem, M.F.; Ali, I.; et al. Drought Induced Changes in Growth, Osmolyte Accumulation and Antioxidant Metabolism of Three Maize Hybrids. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Al-Suhaibani, N.; Ali, N.; Akmal, M.; Alotaibi, M.; Refay, Y.; Dindaroglu, T.; Abdul-Wajid, H.H.; Battaglia, M.L. Drought Stress Impacts on Plants and Different Approaches to Alleviate Its Adverse Effects. Plants 2021, 10, 259. [Google Scholar] [CrossRef]
| Sources of Changes | DF | Chl-a | Chl-b | Chl-a/b | Chl-a+b | Cars | Proline | TSC | RWC | H2O2 | MDA | CAT | APX |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| I | 2 | 0.3050 ** | 0.2527 ** | 9.053 ** | 1.1108 ** | 0.0243 ** | 27.10 ** | 12.534 ** | 1114.42 ** | 2.117 ** | 1377.4 ** | 0.1271 ** | 4.67 ** |
| Y | 1 | 0.0081 ns | 0.0028 ns | 0.0144 * | 0.0207 ns | 0.000004 ns | 0.011 ns | 0.524 * | 8.97 ns | 0.0061 ns | 2.52 ns | 0.0005 ns | 0.015 ns |
| I × Y | 2 | 0.0023 ns | 0.0014 ns | 0.1309 ns | 0.0012 ns | 0.00003ns | 0.004 ns | 0.201 ns | 3.59 ns | 0.001 ns | 0.2290 ns | 0.0020 ns | 0.061 ns |
| Error | 12 | 0.0022 | 0.0022 | 0.2613 | 0.0050 | 0.0001 | 0.4376 | 0.143 | 9.50 | 0.003 | 2.21 | 0.0015 | 0.055 |
| G | 9 | 0.0993 ** | 0.0095 ** | 1.1318 ** | 0.1550 ** | 0.0034 ** | 8.52 ** | 6.098 ** | 125.77 ** | 0.168 ** | 302.18 ** | 0.0860 ** | 1.307 ** |
| G × Y | 9 | 0.0205 ns | 0.0017 ns | 0.7795 ns | 0.0021 ns | 0.0001 ** | 0.0565 ns | 0.096 ns | 13.53 ns | 0.0008 ns | 7.52 ns | 0.0018 ns | 0.029 ns |
| G × I | 18 | 0.0087 ** | 0.0033 ** | 0.3848 ns | 0.0185 ** | 0.0002 ** | 0.5589 ** | 0.350 ** | 17.39 ** | 0.015 ** | 8.927 ** | 0.0061 ** | 0.144 ** |
| I × Y × G | 18 | 0.0026 ns | 0.0006ns | 0.1745 ns | 0.0038 ns | 0.00003 ns | 0.0715 ns | 0.114 ns | 9.81 ns | 0.001 ns | 6.545 ns | 0.0026 ** | 0.053 ns |
| Error | 108 | 0.0043 | 0.0020 | 0.0057 | 0.3529 | 0.00006 | 0.231 | 0.124 | 6.67 | 0.007 | 4.330 | 0.0009 | 0.062 |
| Cars (mg.g−1FW) | RWC (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. | I1 | I2 | I3 | Avg. |
| Afghanistan | 0.23 k † | 0.26 gh (+15) ‡ | 0.26 hi (+12) | 0.256 F | 71.53 a–d | 68.66 a–e (−5) | 64.81 d–f (−10) | 68.33 AB |
| Pakistan | 0.23 k | 0.26 gh (+15) | 0.26 hi (+13) | 0.256 F | 70.60 a–d | 62.00 e–g (−12) | 54.84 g (−24) | 62.48 C |
| Syria | 0.23 k | 0.26 g–i (+14) | 0.26 i (+12) | 0.255 F | 71.17 a–d | 65.14 c–e (−9) | 59.78 fg (−16) | 65.37 BC |
| India | 0.26 hi | 0.28 ef (+15) | 0.27 fg (+5) | 0.266 DE | 73.02 a–c | 67.49 a–e (−8) | 65.03 b–g (−10) | 68.49 AB |
| Arak | 0.26 hi | 0.30 b (+16) | 0.29 cd (+12) | 0.286 B | 75.40 a | 68.23 a–e (−10) | 67.46 a–e (−11) | 70.36 A |
| Isfahan | 0.23 k | 0.26 g–i (+14) | 0.26 hi (+12) | 0.256 F | 73.29 ab | 68.43 a–e (−7) | 65.10 b–g (−12) | 68.94 AB |
| Semirom | 0.24 jk | 0.27 fg (+14) | 0.26 g–i (+11) | 0.261 EF | 73.30 a–c | 70.66 a–d (−4) | 68.28 a–e (−7) | 70.74 A |
| Shahreza | 0.24 j | 0.28 e (+14) | 0.30 bc (+22) | 0.277 C | 72.96 a–c | 68.60 a–e (−7) | 62.19 l–n (−15) | 68.91 AB |
| Shahrekord | 0.24 j | 0.27 ef (+13) | 0.29 d (+20) | 0.272 CD | 74.02 ab | 72.48 a–d (−3) | 67.48 a–f (−9) | 71.24 A |
| Mashhad | 0.26 hi | 0.30 bc (+14) | 0.31 a (+18) | 0.294 A | 74.75 ab | 67.48 a–f (−10) | 65.37 b–g (−13) | 69.20 A |
| Avg. | 0.245 B | 0.279 A | 0.280 A | 73.02 A | 67.89 B | 64.33 C | ||
| Chl-a (mg g−1 FW) | Chl-a+b (mg g−1FW) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. | I1 | I2 | I3 | Avg. |
| Afghanistan | 0.410 mn † | 0.479 k–l (−14) ‡ | 0.384 n (−19) | 0.424 C | 0.683 i–n † | 0.707 j–i (−4) ‡ | 0.554 p (−11) | 0.648 C |
| Pakistan | 0.545 d–h | 0.449 lm (−17) | 0.411 mn (−25) | 0.668 C | 0.796 f–i | 0.660 l–n (−17) | 0.590 n–p (−25) | 0.682 C |
| Syria | 0.508 i–l | 0.399 mn (−21) | 0.374 n (−26) | 0.427 C | 0.786 g–i | 0.624 m–o (−20) | 0.530 p (−32) | 0.647 C |
| India | 0.662 b–d | 0.537 g–k (−18) | 0.497 j–l (−24) | 0.553 B | 0.918 de | 0.769 h–j (−16) | 664 l–n (−28) | 0.784 B |
| Arak | 0.739 a | 0.584 d–g (−20) | 0.505 i–l (−31) | 0.610 AB | 1.093 ab | 0.841 f–g (−23) | 0.649 l–n (−40) | 0.861 A |
| Isfahan | 0.641 b–d | 0.550 e–j (−14) | 0.522 h–k (−18) | 0.571 AB | 0.952 cd | 0.799 f–i (−16) | 0.708 j–l (−25) | 0.820 AB |
| Semirom | 0.611 c–e | 0.581 d–h (−5) | 0.533 j–k (−13) | 0.575 AB | 0.955 cd | 0.866 ef (−9) | 0.762 ij (−20) | 0.861 A |
| Shahreza | 0.673 bc | 0.562 e–i (−17) | 0.509 i–l (−24) | 0.581 AB | 0.973 cd | 0.811 f–i (−17) | 0.710 j–l (−28) | 0.277 C |
| Shahrekord | 0.683 ab | 0.592 d–g (−13) | 0.520 h–k (−24) | 0.598 AB | 1.020 bc | 0.848 e–g (−16) | 0.742 j–k (−27) | 0.870 A |
| Mashhad | 0.742 a | 0.606 d–f (−18) | 0.504 i–l (−32) | 0.618A | 1.100 a | 0.852 e–g (−18) | 0.651 l–n (−32) | 0.868 A |
| Avg. | 0.618 A | 0.534 B | 0.476 C | 0.928 A | 0.787 B | 0.656 C | ||
| Chl-a (mg g−1 FW) | Chl-b (mg g−1 FW) | Chl-a/b (mg g−1 FW) | Chl-a+b (mg g−1 FW) | Cars (mg g−1 FW) | |
|---|---|---|---|---|---|
| Irrigation regime (I) | |||||
| I1 | 0.618 a | 0.309 a | 2.003 b | 0.928 a | 0.245 b |
| I2 | 0.534 b (−14) | 0.243 b (−17) | 2.260 a (+11) | 0.778 b (−16) | 0.279 a (+14) |
| I3 | 0.476 c (−23) | 0.181 c (−33) | 2.792 a (+37) | 0.656 b (−28 | 0.280 a (+14) |
| Genotypes | |||||
| Afghanistan | 0.424 c | 0.232 c–e | 2.046 c | 0.648 c | 0.256 f |
| Pakistan | 0.468 b | 0.214 e | 2.253 a–c | 0.682 c | 0.256 f |
| Syria | 0.427 c | 0.219 de | 2.081 bc | 0.647 c | 0.255 f |
| India | 0.553 b | 0.230 c–e | 2.549 ab | 0.784 b | 0.266 de |
| Arak | 0.610 ab | 0.251 bc | 2.490 c | 0.861 a | 0.286 b |
| Isfahan | 0.571 ab | 0.248 b–d | 2.315 a–c | 0.820 ab | 0.256 cd |
| Semirom | 0.575 ab | 0.286 a | 2.088 bc | 0.861 a | 0.261 ef |
| Shahreza | 0.581 ab | 0.250 b–d | 2.484 a–c | 0.832 ab | 0.277 c |
| Shahrekord | 0.598 ab | 0.271 ab | 2.260 a–c | 0.870 a | 0.272 cd |
| Mashhad | 0.618 a | 0.249 b–d | 2.738 a | 0.868 a | 0.294 a |
| TSC (mg/FW) | Proline (μmol/g) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. | I1 | I2 | I3 | Avg. |
| Afghanistan | 1.51 n–p † | 1.80 l–n (+20) ‡ | 2.66 c–f (+70) | 1.99 D | 1.78 n † | 2.09 m–n (+18) | 2.41 l–n (+36) | 2.10 E |
| Pakistan | 1.17 p | 1.36 op (+16) | 1.48 n–p (+25) | 1.33 E | 2.51 l–m | 3.63 g–k (+45) | 4.44 b–d (+76) | 3.53 C |
| Syria | 1.16 p | 1.37 op (+18) | 1.65 m–o (+42) | 1.39 E | 2.02 m–n | 3.54 h–k (+74) | 3.73 f–j (+85) | 3.15 D |
| India | 1.93 j–m | 2.54 e–h (+31) | 2.64 c–h (+37) | 2.37 C | 2.54 l–m | 3.53 h–k (+39) | 3.86 d–g (+52) | 3.31 CD |
| Arak | 2.21 h–k | 2.73 c–f (+24) | 3.33 b (+50) | 2.76 B | 2.59 l–m | 3.83 e–g (+48) | 4.99 c–i (+95) | 3.50 C |
| Isfahan | 1.36 op | 1.59 m–o (+14) | 1.75 m–o (+28) | 1.56 E | 2.50 l–m | 3.35 j–k (+34) | 3.41 i–k (+37) | 3.09 D |
| Semirom | 2.14 i–l | 2.79 c–e (+31) | 3.07 bc (+44) | 2.67 B | 3.69 f–k | 3.97 d–j (+8) | 4.51 f–i (+22) | 4.06 B |
| Shahreza | 1.82 k–n | 2.33 f–j (+28) | 2.65 c–g (+45) | 2.27 C | 3.02 k–l | 3.50 h–k (+16) | 4.30 b–g (+43) | 3.61 C |
| Shahrekord | 2.40 e–i | 2.97 b–d (+24) | 4.22 a (+75) | 3.19 A | 4.15 c–h | 4.65 a–c (+12) | 5.26 a (+26) | 4.69 A |
| Mashhad | 2.26 g–j | 2.56 d–h (+14) | 3.07 bc (+35) | 2.63 B | 2.66 l–m | 4.34 b–d (+64) | 4.96 ab (+86) | 3.98 B |
| Avg. | 1.79 C | 2.21 B | 2.65 A | 2.54 | 3.5 | 4.05 | ||
| CAT (Unit mg−1 Protein) | APX (Unit mg−1 Protein) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. | I1 | I2 | I3 | Avg. |
| Afghanistan | 0.143 p † | 0.229 i–o (+58) ‡ | 0.217 j–o (+52) | 0.196 EF§ | 1.23 k–n † | 1.50 h–k (+21) | 1.64 g–i (+33) | 1.46 C–E |
| Pakistan | 0.203 k–p | 0.256 f–l (+26) | 0.282 e–i (+38) | 0.247 CD | 1.21 mn | 1.47 i–n (+21) | 1.57 g–j (+28) | 1.42 DE |
| Syria | 0.145 p | 0.169 op (+16) | 0.187 m–p (+28) | 0.167 F | 1.19 n | 1.38 i–n (+14) | 1.49 i–l (+25) | 1.36 E |
| India | 0.195 l–p | 0.213 j–o (+9) | 0.226 i–o (+16) | 0.211 DE | 1.34 j–n | 1.60 g–j (+23) | 1.79 e–g (+32) | 1.57 B–D |
| Arak | 0.267 f–j | 0.299 d–g (+12) | 0.334 de (+25) | 0.300 B | 1.33 j–n | 1.66 f–i (+23) | 1.82 d–g (+35) | 1.42 DE |
| Isfahan | 0.151 p | 0.173 op (+15) | 0.180 n–p (+19) | 0.168 F | 1.22 l–n | 1.48 i–m (+21) | 1.58 g–j (+30) | 1.60 BC |
| Semirom | 0.257 f–k | 0.408 bc (+60) | 0.433 ab (+69) | 0.366 A | 1.54 g–j | 2.07 cd (+34) | 2.77 a (+79) | 2.13 A |
| Shahreza | 0.214 j–o | 0.309 d–f (+44) | 0.346 d (+62) | 0.346 B | 1.49 i–m | 1.78 e–h (+20) | 1.94 c–f (+31) | 1.73 B |
| Shahrekord | 0.236 h–n | 0.347 cd (+47) | 0.429 a (+81) | 0.290 B | 1.57 g–j | 2.17 c (+38) | 2.74 b (+75) | 2.07 A |
| Mashhad | 0.216 j–o | 0.242 g–m (+15) | 0.294 d–g (+37) | 0.250 C | 1.44 i–n | 1.65 g–i (+15) | 2.03 c–e (+40) | 1.70 B |
| Avg. | 0.203 B | 0.264 A | 0.293 A | 1.35 C | 1.67 B | 1.91 A | ||
| H2O2 (μmol•g−1 FW) | MDA (nmol•g−1 FW) | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. | I1 | I2 | I3 | Avg. |
| Afghanistan | 0.3916 lm † | 0.5526 d–g (+41) ‡ | 0.7148 bc (+82) | 0.552 CD § | 28.86 d–j † | 35.67 ac (+24) | 40.07 (+39) | 34.87 A |
| Pakistan | 0.4033 h–m | 0.5688 d–f (+40) | 0.6871 bc (+70) | 0.553 CD | 26.03 f–l | 31.17 b–g (+20) | 35.97 a–c (+38) | 31.06 B |
| Syria | 0.3536 ml | 0.5405 d–g (+54) | 0.6990 bc (+95) | 0.531 D | 23.04 i–m | 30.27 b–h (+32) | 36.42 ab (+58) | 29.91 BC |
| India | 0.4516 g–l | 0.6420 cd (+42) | 0.7036 bc (+55) | 0.599 BC | 23.27 i–m | 29.22 d–i (+26) | 34.48 a–d (+48) | 28.99 BC |
| Arak | 0.4848 f–j | 0.6310 c–e (+32) | 0.7886 ab (+62) | 0.634 B | 26.82 d–l | 32.76 b–e (+22) | 37.95 b (+42) | 32.01 AB |
| Isfahan | 0.3488 ml | 0.3783 j–m (+10) | 0.5346 e–g (+55) | 0.420 E | 27.30 e–l | 34.99 a–d (+28) | 35.60 a–c (+31) | 32.63 AB |
| Semirom | 0.3253 m | 0.3543 k–m (+8) | 0.4081 h–m (+25) | 0.362 F | 21.12 k–m | 26.02 f–l (+23) | 29.83 d–h (+41) | 25.65 D |
| Shahreza | 0.3705 k–m | 0.4636 f–k (+27) | 0.5351 e–g (+46) | 0.456 E | 21.89 k–m | 24.90 g–l (+14) | 30.01 b–h (+37) | 25.60 D |
| Shahrekord | 0.3506 ml | 0.4246 h–m (+20) | 0.5010 f–h (+42) | 0.425 E | 17.72 m | 20.73 l–m (+16) | 24.28 i–l (+37) | 20.91 E |
| Mashhad | 0.4895 f–i | 0.7468 ab (+52) | 0.8276 a (+67) | 0.688 A | 22.41 j–m | 29.50 d–i (+32) | 31.81 b–f (+41) | 27.91 CD |
| Avg. | 0.3968 C | 0.5302 B | 0.6399 A | 23.85 C | 29.32 B | 33.29 A | ||
| Sources of Changes | DF | Plant Height |
|---|---|---|
| I | 2 | 3241.38 ** |
| Y | 1 | 307.91 ns |
| I × Y | 2 | 27.33 ns |
| Error | 12 | 11.63 |
| G | 9 | 2296.89 ** |
| G× Y | 9 | 36.97 ns |
| G × I | 18 | 148.08 ** |
| I × Y × G | 18 | 45. 36 ns |
| Total error | 108 | 867.99 |
| Plant Height (cm) | ||||
|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. |
| Afghanistan | 36.69 e–h † | 34.56 g–i (−6) ‡ | 31.47 hi (−15) | 34.90 G § |
| Pakistan | 36.35 e–h | 28.00 j–m (−23) | 27.15 l (−26) | 30.50 EF |
| Syria | 35.38 e–i | 31.55 g–i (−5) | 29.11 hi (−18) | 32.01 FG |
| India | 33.31 e–i | 30.25 hi (−10) | 27.22 i (−19) | 30.25 D |
| Arak | 61.75 a | 42.35 de (−32) | 33.27 f–i (−46) | 45.80 A |
| Isfahan | 59.33 a | 34.67 e–i (−42) | 32.13 g–k (−46) | 41.37 E |
| Semirom | 57.18 ab | 49.03 d (−15) | 31.30 d–g (−45) | 45.80 CD |
| Shahreza | 62.93 a | 48.74 b–d (−22) | 41.79 d–f (−34) | 59.90 B |
| Shahrekord | 55.88 a | 47.51 cd (−15) | 40.54 d–g (−28) | 48.00 BC |
| Mashhad | 58.68 a | 48.75 d (−17) | 30.29 hi (−48) | 45.90 A |
| Avg. | 49.74 A | 39.70 B | 32.20 C | |
| Seeds per Capsule | Capsules Number per Plant | |||||||
|---|---|---|---|---|---|---|---|---|
| Genotypes | I1 * | I2 | I3 | Avg. | I1 | I2 | I3 | Avg. |
| Afghanistan | 14.08 g–l | 11.32 i–n (−20) | 10.23 c (−28) | 10.43 F § | 57.33 g–i † | 47.50 k–m (−18) ‡ | 35.00 o (−38) | 46.27 C |
| Pakistan | 19.31 c–f | 9.32 n–p (−52) | 6.66 o–p (−60) | 11.76 E–F | 72.16 c | 49.16 j–m (−32) | 38.0 no (−48) | 53.22 D |
| Syria | 19.25 c–f | 10.76 j–o (−44) | 6.97 o–p (−63) | 12.33 D–F | 61.00 e–g | 46.25 lm (−25) | 35.00 o (−43) | 47.50 C |
| India | 21.14 cd | 10.45 n–p (−51) | 8.50 n–p (−60) | 13.36 C–E | 76.33 c | 46.04 lm (−40) | 38.92 no (−49) | 53.90 B |
| Arak | 26.06 ab | 22.06 b–d (−16) | 10.61 j–o (−60) | 19.58 A | 95.0 a | 59.00 fh (−38) | 37.82 no (−60) | 64.93 A |
| Isfahan | 18.33 d–g | 14.01 h–l (−24) | 11.61 k–n (−36) | 14.65 CD | 70.50 cd | 51.16 i–l (−28) | 35.50 o (−32) | 52.55 B |
| Semirom | 16.66 e–h | 14.40 g–k (−13) | 13.85 h–l (−17) | 14.97 BC | 70.83 cd | 60.16 e–g (−16) | 55.20 g–i (−22) | 62.11 A |
| Shahreza | 23.46 abc | 20.57 cde (−13) | 13.54 k–m (−40) | 19.17 A | 85.33 b | 65.33 de (−24) | 43.83 mn (−48) | 64.83 A |
| Shahrekord | 15.17 f–i | 14.63 g–j (−4) | 12.10 k–n (−20) | 13.96 C–E | 71.66 e–f | 64.33 ef (−10) | 52.00 i–k (−27) | 62.94 A |
| Mashhad | 26.76 a | 14.79 g–j (−44) | 10.07 l–p (−65) | 17.21 AB | 90.16 ab | 54.83 h–j–f (−38) | 43.40 m–n (−53) | 62.61 A |
| Avg. | 19.96 A | 14.21 B | 10.39 C | 75.03 A | 54.43 B | 41.51 C | ||
| Stress Susceptibility Index under I3 (SSI3) | Stress Susceptibility Index under I2 (SSI2) | |||||
|---|---|---|---|---|---|---|
| Genotypes | SSI2 | Ranking | Group | SSI3 | Ranking | Group |
| Afghanistan | 0.63 | 2 | Tolerant | 0.989 | 4 | Moderate |
| Pakistan | 1.562 | 10 | Susceptible | 1.184 | 10 | Susceptible |
| Syria | 1.252 | 8 | Susceptible | 1.118 | 8 | Susceptible |
| India | 1.379 | 9 | Susceptible | 1.1 | 6 | Susceptible |
| Arak | 0.746 | 5 | Moderate | 1.118 | 7 | Susceptible |
| Isfahan | 0.748 | 6 | Moderate | 0.748 | 3 | Tolerant |
| Semirom | 0.723 | 4 | Tolerant | 0.586 | 1 | Tolerant |
| Shahreza | 0.713 | 3 | Tolerant | 1 | 5 | Moderate |
| Shahrekord | 0.572 | 1 | Tolerant | 0.621 | 2 | Tolerant |
| Mashhad | 1.247 | 7 | Susceptible | 1.137 | 9 | Susceptible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayati, P.; Karimmojeni, H.; Razmjoo, J.; Pucci, M.; Abate, G.; Baldwin, T.C.; Mastinu, A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae 2022, 8, 193. https://doi.org/10.3390/horticulturae8030193
Bayati P, Karimmojeni H, Razmjoo J, Pucci M, Abate G, Baldwin TC, Mastinu A. Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae. 2022; 8(3):193. https://doi.org/10.3390/horticulturae8030193
Chicago/Turabian StyleBayati, Pedram, Hassan Karimmojeni, Jamshid Razmjoo, Mariachiara Pucci, Giulia Abate, Timothy C. Baldwin, and Andrea Mastinu. 2022. "Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress" Horticulturae 8, no. 3: 193. https://doi.org/10.3390/horticulturae8030193
APA StyleBayati, P., Karimmojeni, H., Razmjoo, J., Pucci, M., Abate, G., Baldwin, T. C., & Mastinu, A. (2022). Physiological, Biochemical, and Agronomic Trait Responses of Nigella sativa Genotypes to Water Stress. Horticulturae, 8(3), 193. https://doi.org/10.3390/horticulturae8030193







