Web Architecture Affects the Functional Response of the Space Web-Builder Kochiura aulica against Trioza erytreae in the Laboratory
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling of Canopy Spiders
2.2. Characterization of Spider Webs in the Field
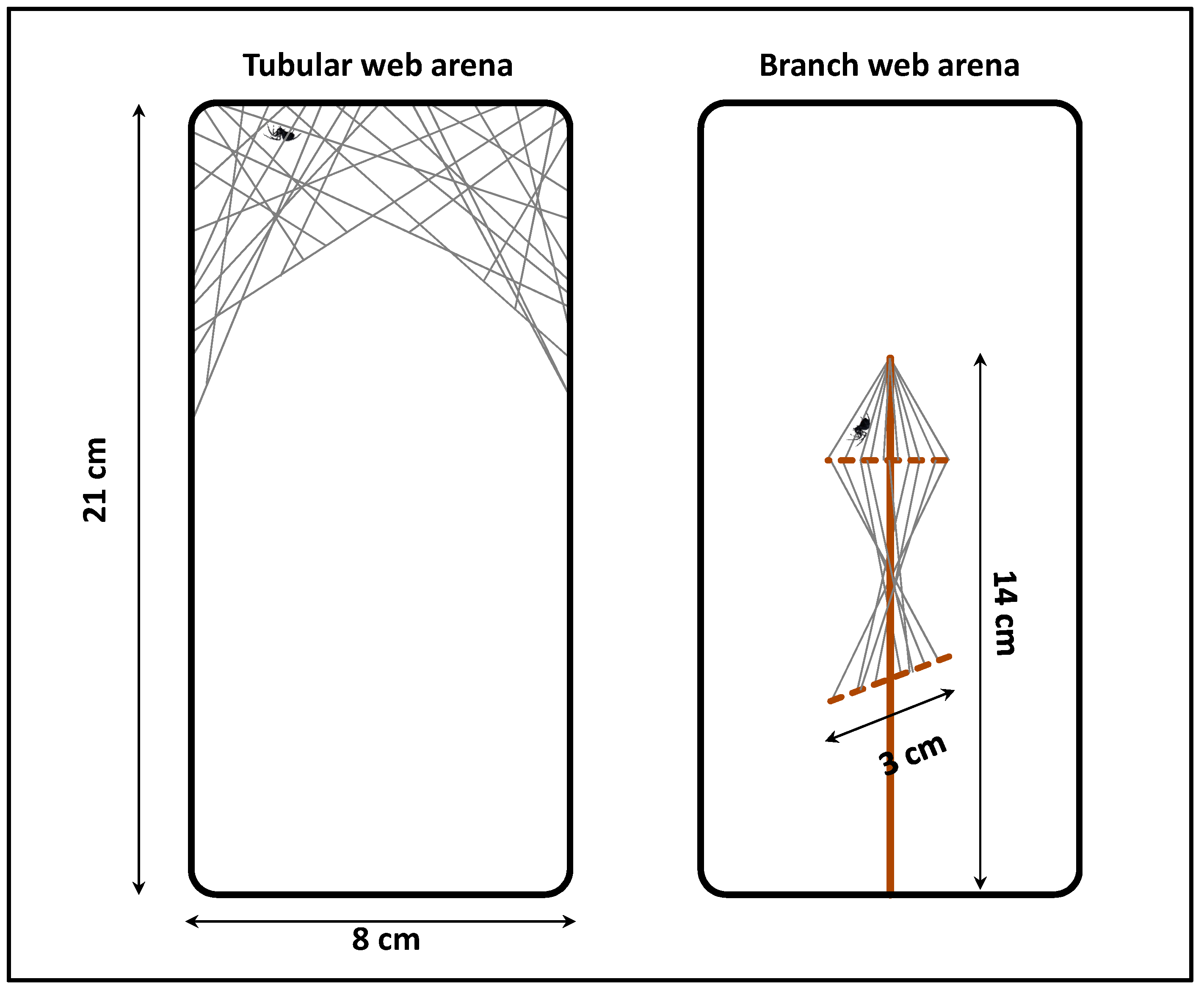
2.3. Selection of Model Species and Functional Response Trials
2.4. Functional Response Assays
2.5. Data Analysis
3. Results
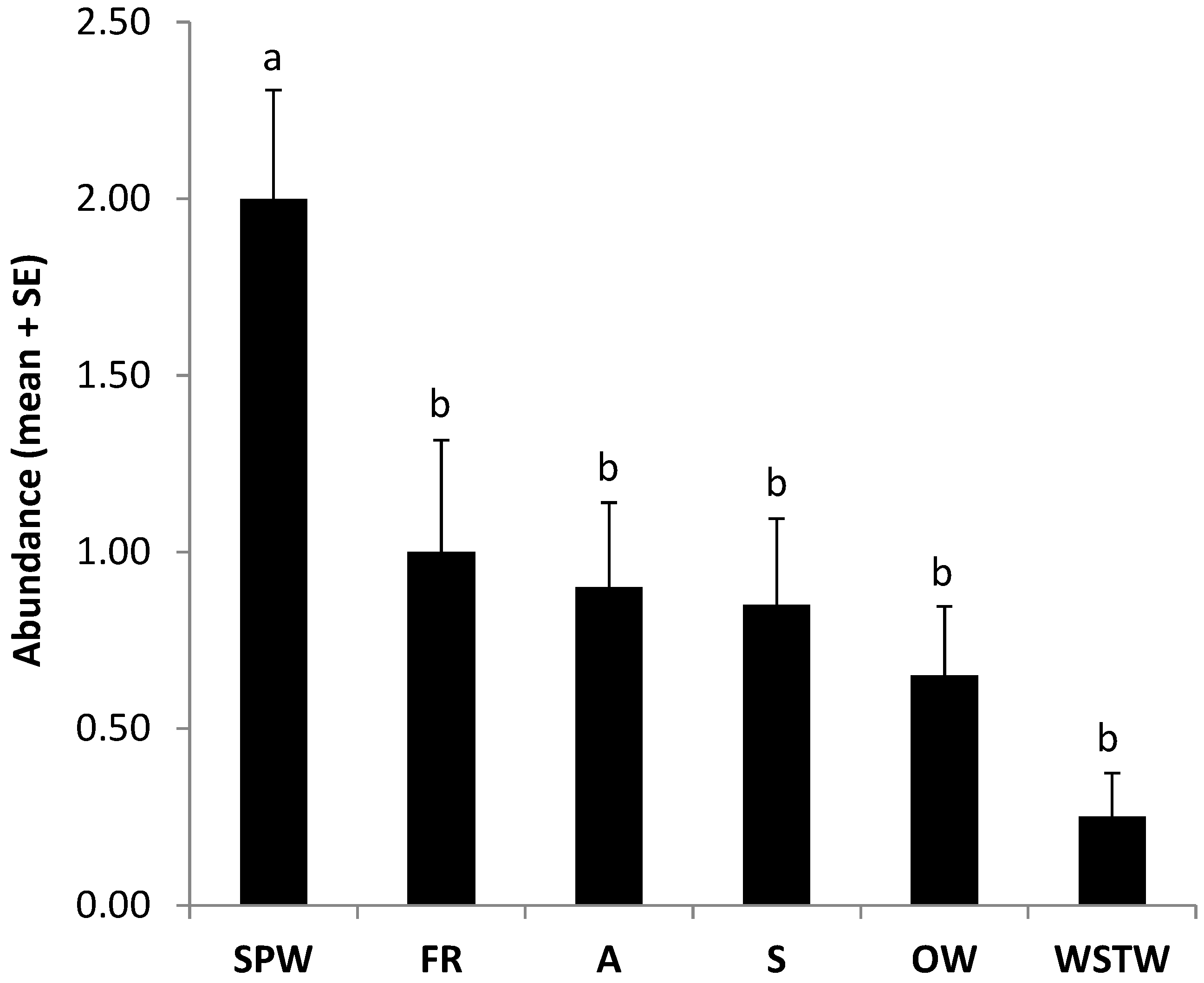
3.1. Sampling of Spiders and Webs in the Field
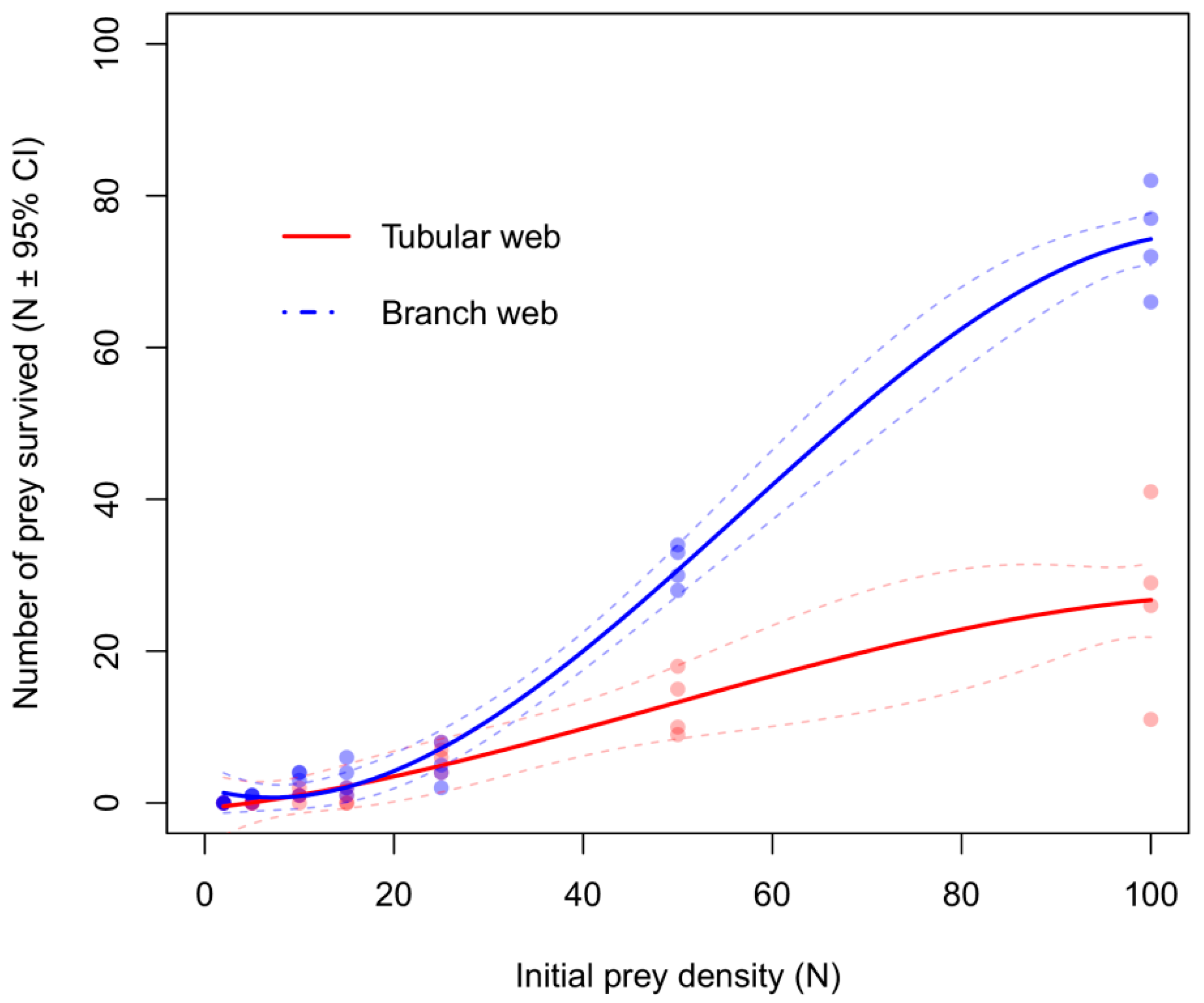
3.2. Functional Response Experiments in the Laboratory
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Graça, J.V. Citrus greening disease. Annu. Rev. Phytopathol. 1991, 29, 109–136. [Google Scholar] [CrossRef]
- Carvalho, J.P.; Aguiar, A.M.F. Espécies da entomofauna nociva. In Pragas Dos Citrinos na Ilha da Madeira; Secretaria Regional de Agricultura Florestas e Pescas, Instituto Nacional de Investigaçao Agrária, Direçao Regional de Agricultura da Regiao Autónoma da Madeira: Funchal, Portugal, 1997; pp. 83–91. [Google Scholar]
- González-Hernández, A. Trioza erytreae (Del Guercio 1918): Nueva plaga de los cítricos en Canarias. Phytoma España 2003, 153, 12–117. [Google Scholar]
- Pérez-Otero, R.; Mansilla, J.P.; del Estal, P. Detección de la psila africana de los cítricos, Trioza erytreae (Del Guercio, 1918) (Hemiptera: Psylloidea: Triozidae), en la Península Ibérica. Arq. Entomolóxicos 2015, 13, 119–122. [Google Scholar]
- DGAV (Direção Geral de Alimentação e Veterinária). Available online: https://www.dgav.pt/plantas/conteudo/sanidade-vegetal/inspecao-fitossanitaria/informacao-fitossanitaria/trioza-erytreae/ (accessed on 6 December 2021).
- Benhadi-Marín, J.; Fereres, A.; Pereira, J.A. Potential areas of spread of Trioza erytreae over mainland Portugal and Spain. J. Pest. Sci. 2022, 95, 67–78. [Google Scholar] [CrossRef]
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Cocuzza, G.E.M.; Urbaneja, A.; Hernández-Suárez, E.; Siverio, F.; Di Silvestro, S.; Tena, A.; Carmelo, R. A review on Trioza erytreae (African citrus psyllid), now in mainland Europe, and its potential risk as vector of huanglongbing (HLB) in citrus. J. Pest. Sci. 2017, 90, 1–17. [Google Scholar] [CrossRef]
- Turnbull, A.L. Ecology of true spiders (Araneomorphae). Annu. Rev. Entomol. 1973, 18, 305–348. [Google Scholar] [CrossRef]
- Uetz, G.W.; Halaj, J.; Cady, A. Guild structure of spiders in major crops. J. Arachnol. 1999, 27, 270–280. [Google Scholar]
- Cardoso, P.; Pekár, S.; Jocqué, R.; Coddington, J.A. Global patterns of guild composition and functional diversity of spiders. PLoS ONE 2011, 6, e21710. [Google Scholar] [CrossRef] [Green Version]
- Hassanzadeh-Avval, M.; Sadeghi-Namaghi, H.; Fekrat, L. Prey preference and prey switching in Anthocoris minki Dohrn (Hemiptera: Anthocoridae). J. Asia Pac. Entomol. 2018, 21, 1116–1121. [Google Scholar] [CrossRef]
- Murdoch, W.W. Switching in general predators: Experiments on predator specificity and stability of prey populations. Ecol. Monogr. 1969, 39, 335–354. [Google Scholar] [CrossRef]
- Chan, K.; Boutin, S.; Hossie, T.J.; Krebs, C.J.; O’Donoghue, M.; Murray, D.L. Improving the assessment of predator functional responses by considering alternate prey and predator interactions. Ecology 2017, 98, 1787–1796. [Google Scholar] [CrossRef]
- Holling, C. Some characteristics of simple types of predation and parasitism. Can. Entomol. 1959, 91, 385–398. [Google Scholar] [CrossRef]
- Bolker, B. Ecological Models and Data In R; Princeton University Press: Princeton, NJ, USA, 2008; p. 408. [Google Scholar]
- Coblentz, K.E.; DeLong, J.P. Estimating predator functional responses using the times between prey captures. Ecology 2021, 102, e03307. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why intraspecific trait variation matters in community ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeLong, J.P.; Uiterwaal, S.F.; Dell, A.I. Trait-Based variation in the foraging performance of individuals. Front. Ecol. Evol. 2021, 9, 649542. [Google Scholar] [CrossRef]
- Rossi, M.N.; Reigada, C.; Godoy, W.A.C. The effect of hunger level on predation dynamics in the spider Nesticodes rufipes: A functional response study. Ecol. Res. 2006, 21, 617–623. [Google Scholar] [CrossRef]
- Fathipour, Y.; Karimi, M.; Farazmand, A.; Talebi, A.A. Age-specific functional response and predation capacity of Phytoseiulus persimilis (Phytoseiidae) on the two-spotted spider mite. Acarologia 2018, 58, 31–40. [Google Scholar] [CrossRef]
- Boswell, M.E.; DeLong, J.P. Gravid tetragnathid spiders show an increased functional response. Food Webs 2019, 21, e00122. [Google Scholar] [CrossRef]
- Herberstein, M.; Tso, I. Spider webs: Evolution, diversity and plasticity. In Spider Behaviour: Flexibility and Versatility; Herberstein, M., Ed.; Cambridge University Press: Cambridge, UK, 2011; pp. 57–98. [Google Scholar]
- Jocqué, R.; Dippenaar-Schoeman, A.S. Spider Families of the World; Royal Museum for Central Africa: Tervuren, Belgium, 2007; p. 336. [Google Scholar]
- Blamires, S.J. Plasticity in extended phenotypes: Orb web architectural responses to variations in prey parameters. J. Exp. Biol. 2010, 213, 3207–3212. [Google Scholar] [CrossRef] [Green Version]
- Hesselberg, T. The mechanism behind plasticity of web-building behavior in an orb spider facing spatial constraints. J. Arachnol. 2014, 42, 311–314. [Google Scholar] [CrossRef]
- Cardoso, P. Standardization and optimization of arthropod inventories—The case of Iberian spiders. Biodivers. Conserv. 2009, 18, 3949–3962. [Google Scholar] [CrossRef]
- Nentwig, W.; Blick, T.; Bosmans, R.; Gloor, D.; Hänggi, A.; Kropf, C. Spiders of Europe. Version 1. Available online: https://www.araneae.nmbe.ch (accessed on 20 January 2022).
- De Biurrun, G.; Prieto, C.; Baquero, E. Iberian Spider Catalog. Actualización del Mapa Web y Sus Funciones. Arachnomap 2019. Available online: http://sea-entomologia.org/gia/map (accessed on 1 February 2022).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org (accessed on 15 August 2019).
- Pritchard, D. Frair: Tools for Functional Response Analysis. R Package. Version 0.5.100. 2017. Available online: https://CRAN.R-project.org/package=frair (accessed on 15 August 2018).
- Juliano, S.A. Nonlinear curve fitting: Predation and functional response curve. In Design and Analysis of Ecological Experiments; Scheiner, S.M., Gurevitch, J., Eds.; Oxford University Press: New York, NY, USA, 2001; pp. 178–196. [Google Scholar]
- Van den Berg, M.A.; Deacon, V.E.; Fourie, C.J.; Anderson, S.H. Predators of the citrus psylla, Trioza erytreae (Hemiptera: Triozidae), in the Lowveld and Rustenburg areas of Transvaal. Phytophylactica 1987, 19, 285–289. [Google Scholar]
- Van den Berg, M.A.; Dippenaar-Shoeman, A.S.; Deacon, V.E.; Anderson, S.H. Interaction between citrus psylla, Trioza erytreae (Hem. Triozidae), and spiders in an unsprayed citrus orchard in the Transvaal Lowveld. Entomophaga 1992, 37, 599–608. [Google Scholar] [CrossRef]
- Foelix, R.F. Biology of Spiders; Oxford University Press: New York, NY, USA, 2011; p. 419. [Google Scholar]
- Holling, C.S. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 1959, 91, 293–320. [Google Scholar] [CrossRef]
- Ayoub, N.A.; Friend, K.; Clarke, T.; Baker, R.; Correa-Garhwal, S.M.; Crean, A.; Dendev, E.; Foster, D.; Hoff, L.; Kelly, S.D.; et al. Protein composition and associated material properties of cobweb spiders’ gumfoot glue droplets. Integr. Comp. Biol. 2021, 61, 1459–1480. [Google Scholar] [CrossRef] [PubMed]


| Guild | Family | N |
|---|---|---|
| Space web builders | Dictynidae | 26 |
| Theridiidae | 14 | |
| Foliage runners | Anyphaenidae | 12 |
| Cheiracanthiidae | 8 | |
| Ambushers | Philodromidae | 10 |
| Thomisidae | 8 | |
| Stalkers | Salticidae | 15 |
| Oxyopidae | 2 | |
| Orb-weavers | Araneidae | 13 |
| Wandering sheet/tangle weavers | Linyphiidae | 5 |
| Immatures not identified | - | 9 |
| Total | 122 |
| Functional Group | Family | Height > 1.5 m | Height < 1.5 m | ||
|---|---|---|---|---|---|
| Webs (N) | Adults of T. erytreae (N) | Webs (N) | Adults of T. erytreae (N) | ||
| A | Thomisidae | 1 | 0 | 0 | 0 |
| FR | Anyphaenidae | 1 | 0 | 0 | 0 |
| OW | Araneidae | 11 | 4 | 16 | 2 |
| SPW | Theridiidae | 32 | 68 | 0 | 0 |
| S | Salticidae | 5 | 3 | 0 | 0 |
| WSTW | Linyphiidae | 0 | 0 | 34 | 28 |
| Total | 50 | 75 | 50 | 30 | |
| Response | Type of Web | Linear Coefficient | SE | Z-Value | p | FR Type |
|---|---|---|---|---|---|---|
| Prey captured | Tubular | −0.009 | 0.002 | −3.581 | <0.001 | Type-II |
| Branch | −0.026 | 0.002 | −11.542 | <0.001 | Type-II | |
| Prey killed | Tubular | −0.018 | 0.002 | −8.370 | <0.001 | Type-II |
| Branch | −0.014 | 0.002 | −6.332 | <0.001 | Type-II |
| Response | Type of Web | Parameter | Estimate | SE | Z-Value | p | 95% CI |
|---|---|---|---|---|---|---|---|
| Prey captured | Tubular | a | 0.334 a | 0.031 | 10.905 | <0.001 | [0.256, 0.419] |
| Th | 0.031 A | 0.007 | 4.275 | <0.001 | [0, 0.052] | ||
| Branch | a | 0.423 a | 0.055 | 7.622 | <0.001 | [0.297, 0.633] | |
| Th | 0.209 B | 0.017 | 12.423 | <0.001 | [0.161, 0.251] | ||
| Prey killed | Tubular | a | 0.285 a | 0.028 | 10.079 | <0.001 | [0.222, 0.381] |
| Th | 0.095 C | 0.010 | 9.225 | <0.001 | [0.052, 0.155] | ||
| Branch | a | 0.137 b | 0.023 | 5.939 | <0.001 | [0.090, 0.202] | |
| Th | 0.253 B | 0.040 | 6.303 | <0.001 | [0.172, 0.375] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Félix-Oliveira, D.; Pereira, J.A.; Benhadi-Marín, J. Web Architecture Affects the Functional Response of the Space Web-Builder Kochiura aulica against Trioza erytreae in the Laboratory. Horticulturae 2022, 8, 192. https://doi.org/10.3390/horticulturae8030192
Félix-Oliveira D, Pereira JA, Benhadi-Marín J. Web Architecture Affects the Functional Response of the Space Web-Builder Kochiura aulica against Trioza erytreae in the Laboratory. Horticulturae. 2022; 8(3):192. https://doi.org/10.3390/horticulturae8030192
Chicago/Turabian StyleFélix-Oliveira, Diogo, José Alberto Pereira, and Jacinto Benhadi-Marín. 2022. "Web Architecture Affects the Functional Response of the Space Web-Builder Kochiura aulica against Trioza erytreae in the Laboratory" Horticulturae 8, no. 3: 192. https://doi.org/10.3390/horticulturae8030192
APA StyleFélix-Oliveira, D., Pereira, J. A., & Benhadi-Marín, J. (2022). Web Architecture Affects the Functional Response of the Space Web-Builder Kochiura aulica against Trioza erytreae in the Laboratory. Horticulturae, 8(3), 192. https://doi.org/10.3390/horticulturae8030192








