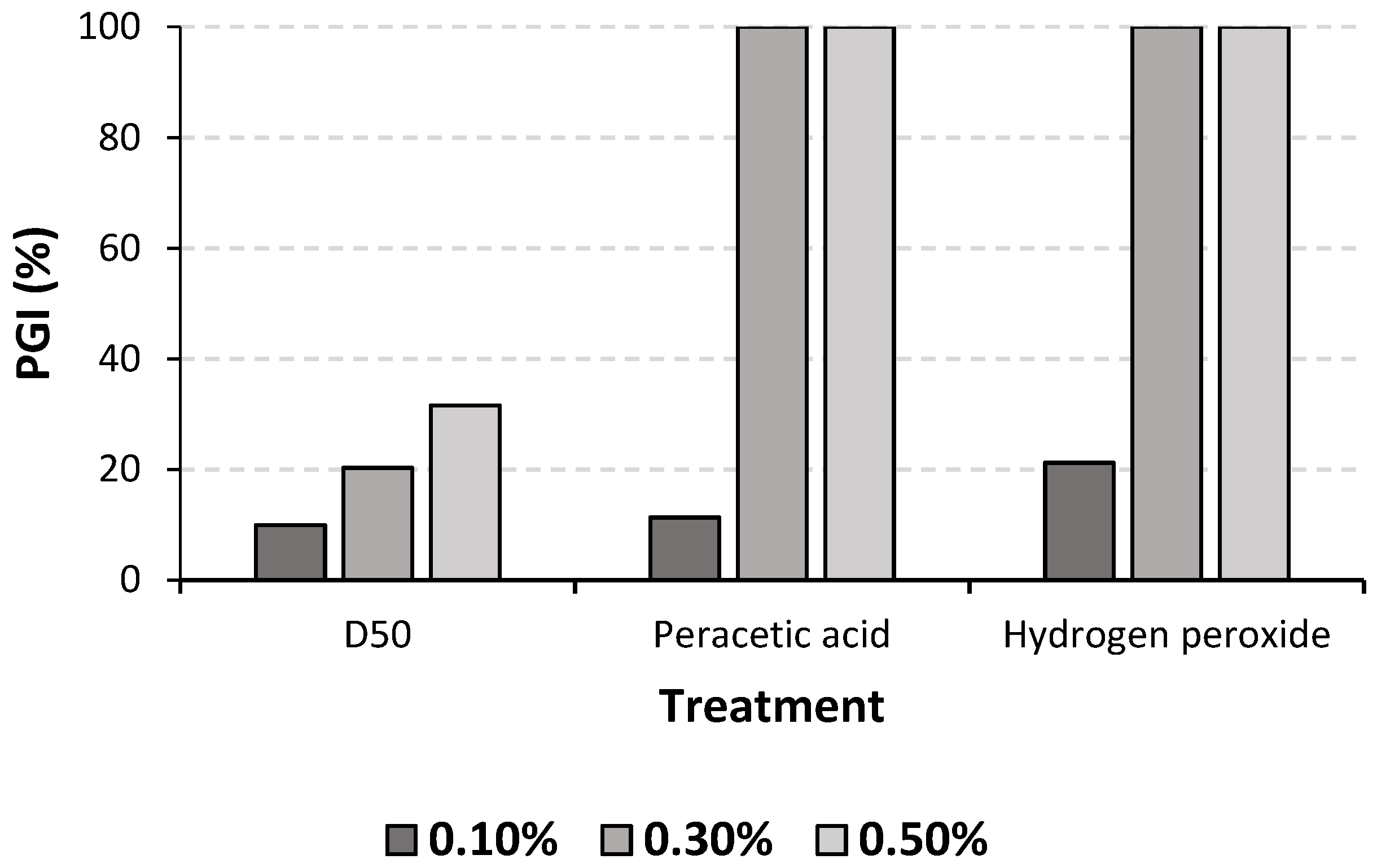1. Introduction
Garlic (
Allium sativum L.) is amongst the oldest horticultural crops cultivated in the world, especially in temperate regions [
1]. In addition to its culinary utility, a lot of beneficial properties have been reported from its consumption, including antioxidant, anti-microbial, anti-diabetic, anti-coagulant, anti-carcinogenic and immunomodulation effects [
2,
3]. The world’s annual garlic production is about 28.49 million tonnes, cultivated on approx. 1.54 million ha. The most important garlic-producing countries include China, India, Bangladesh, Egypt, South Korea and Spain. In Europe, Italy is the 4th largest producer with approx. 29,270 tonnes, and the 7th in terms of cultivation areas (3410 ha) [
4]. While the Italian growing areas are limited, the focus is on high quality garlic and includes products with Denomination of Origin Protected (DOP), controlled quality (QC) characteristics, which are appreciated for their special flavors and aromas. Amongst them, Aglio di Voghiera DOP, from the province of Ferrara, Emilia-Romagna region, and Aglio Bianco Polesano DOP, from Polesine, in Veneto, are particularly noteworthy. In addition, there are garlic varieties from Caraglio (Slow Food Presidium), in the province of Cuneo, Piedmont, and the Aglio Bianco Piacentino, in the Piacenza area, in Emilia-Romagna, which are famous for their aromatic richness and high concentrations of allicin. In Northern Italy, garlic is commonly sown from mid-October to early November and harvested in July. Garlic cloves harvested in the previous cropping season are used as seeds and sown for the following growing season [
5].
Garlic dry rot is a major post-harvest issue for garlic production worldwide. Since 2002, it has been reported in several countries [
6,
7,
8,
9,
10,
11,
12,
13,
14,
15], including Italy [
16]. Bulbs, even if apparently firm at harvest, become emptied post-harvest, develop necrotic spots, and can become centrally depressed when observing the bulb sheaths. Sometimes, white mycelium even becomes visible.
Fusarium proliferatum has been identified as the main causal agent of garlic dry rot and it is predominantly isolated from the cloves;
F. oxysporum can co-occur with
F. proliferatum, but it mainly affects the basal plate/roots [
5].
F. proliferatum is a cosmopolitan saprophytic fungal pathogen which can attack different plant species, such as maize [
17], rice [
18], date palm [
19] and ornamental palms [
8,
20]. It is known to produce fumonisins (FBs), mainly fumonsin B
1 and B
2 (FB
1, FB
2), in addition to other toxic metabolites, including moniliformin [
21], beauvericin [
22,
23], fusaric acid (FA) [
24] and fusaproliferin [
25]. FBs are a group of mycotoxins with a similar structure and, among these, FB
1 is the most toxic. It is not only involved in many animal diseases, such as equine leukoencephalomalacia [
26] and pulmonary edema in swine [
27], but it is also possibly carcinogenic to humans [
28]. Therefore, as fresh garlic is consumed worldwide, the production of FBs in infected cloves requires serious consideration, and reducing
F. proliferatum infection and dissemination in garlic bulbs is crucial to reduce losses due to waste and to conserve yield, quality and safety.
Although the symptoms of the disease are mostly recorded post-harvest, seeds play an important role in garlic dry rot. Indeed, Dugan, et al. [
29] observed that up to 77% of visibly healthy bulbs at harvest developed symptoms after 9–16 months, indicating that the cloves used as seeds, even if apparently healthy, can be systemically infected with
Fusarium spp. Similarly, Mondani, et al. [
5,
30] noted that apparently healthy cloves could develop symptoms during the early stages of garlic growth. Thus, with this evidence that garlic dry rot is seed-transmitted, sowing healthy cloves is of critical importance. In the past, seed treatments were carried out mainly by applying fungicides. Today, alternative non-chemical methods are more commonly applied to minimize environmental impacts and the development of pathogen resistance. These include physical techniques such as thermotherapy and ozone (O
3) application, and seed coating using biocontrol agents (BCAs) or plant extracts that contain natural antimicrobial compounds [
31].
Temperature is a crucial factor that can influence pathogen occurrence, including
Fusarium spp., as revealed by studies conducted on chickpeas, oat and corn [
32,
33,
34]. Nevertheless, heat stress can affect seed germination, as shown for
Arabidopsis, wheat and rice [
35,
36,
37]. Therefore, it is necessary to find the right combination of temperature and exposure time to reduce fungal growth without damaging seed germination. Gaseous O
3 application leaves no harmful residue because of its rapid conversion into oxygen (O
2); its efficacy against fungi has already been demonstrated in wheat, barley, maize and pea seeds [
38,
39].
Thus, the objective of this work was to examine different physical approaches, including (a) gaseous O3, (b) heat treatment and (c) chemical disinfectants, to reduce F. proliferatum incidence in garlic cloves while conserving high seed germinability. Furthermore, microscopy techniques were used to identify the location of the fungal mycelium inside garlic cloves.
3. Results
3.1. Gaseous Ozone
- (a)
In vitro studies
O
3 concentration, exposure and incubation time significantly affected spore germination (
p < 0.01), whereas no statistical differences were detected between the fungal species (
Table 1).
A significant decrease of spore germination was observed, from 97.0% to 32.3%, when comparing the untreated samples with the highest gas concentration (200 mg·kg−1). The 30- and 60-min exposure periods had a greater effect on spore germination than shorter (15 min) exposure where a decrease of only 26% was observed. There was an increase of the germination observed after 48 h incubation suggesting that the spores had recovered some germinative capacity between 24 and 48 h.
Regarding fungal growth,
F. proliferatum reached a significantly larger diameter compared to
F. oxysporum (3.4 cm vs. 3.1 cm;
p < 0.01) (
Table 1). This was probably related to a better adaptability to the GM medium. However, both 100 and 200 mg·kg
−1 O
3 significantly reduced the mycelial growth of the two species when compared to 0 and 50 mg·kg
−1 (
p < 0.01).
- (b)
In vivo studies
Gaseous O
3 treatment of garlic cloves did not significantly affect the CFUs·g
−1 depending on clove categories. However, there were differences amongst the O
3 treatments (
p < 0.01;
Table 2).
Treatments in which the garlic cloves were exposed to the maximum gas concentration for a longer period were more effective. In particular, the samples exposed for 20 min or those with 30/40 min exposure showed reductions of 91.9%, 92.6% and 96.1%, respectively. The interaction between treatments and clove quality categories were also significant (p < 0.05).
However, there was little effect on seed germination. Indeed, no statistical differences were detected between treatments and their untreated controls. Furthermore, more of the first-category seeds germinated than did second-category seeds (85.9% vs. 73.1%) (p < 0.05).
3.2. High Temperatures
In the first experiment, steam was found to be more effective than dry heat in reducing the fungal CFUs·g
−1 (mean reduction of 77.2% vs. 65.8%, compared to the untreated control) (
Table 3). The most effective treatments were those with steam at 49 °C for 30 min, 49 °C for 20 min and 46 °C for 60 min. They caused reductions of 92.0%, 86.5% and 76.7%, in CFUs·g
−1, respectively. However, steam also significantly reduced seed germination (average reduction of 36.9%;
p < 0.01).
In the second experiment done with dry heat, the fungal CFUs·g
−1 were not reduced compared to the untreated control. Again, treatments significantly affected seed germination (average reduction of 11.6%;
p < 0.05) (
Table 4). Regarding the two qualities of garlic cloves used, the first category germinated better than the second one (75.6% vs. 65.2%, respectively) (
p < 0.01) (
Table 4), whereas in the second experiment, the second category of garlic cloves germinated better than the first category (95.3% vs. 80.0%, respectively;
p < 0.01) (
Table 4).
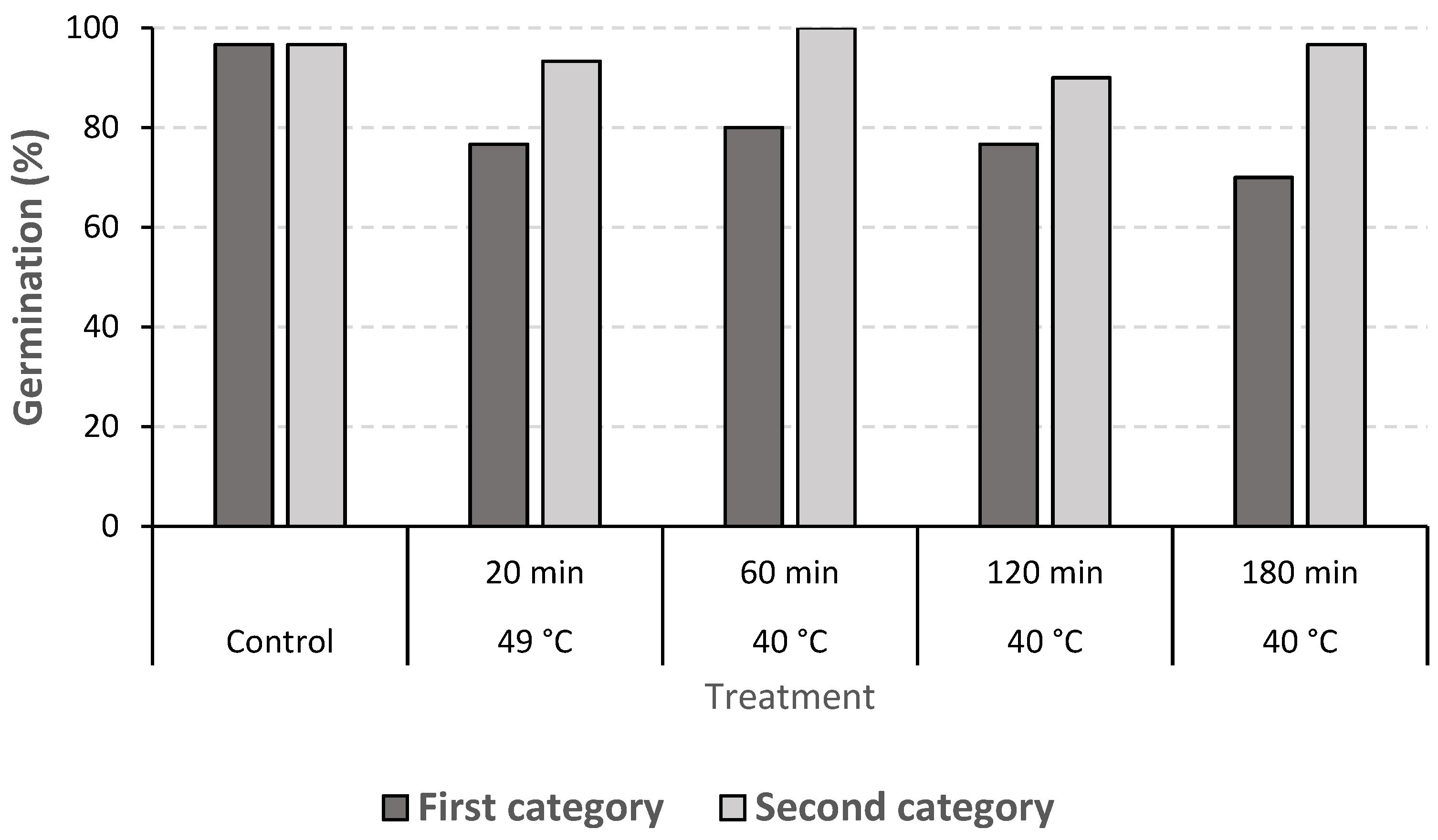
In the second experiment, the interaction between treatments and clove quality categories was significant (
p < 0.01), confirming that, in almost all the treatments, second-category cloves germinated better than first-category ones (
Figure 4).
3.3. Chemical Disinfectants
- (a)
In vitro trial
In this study, C
2H
4O
3 and H
2O
2 were the most effective compounds in reducing pathogen growth (PGI = 70.4, 73.8%, respectively;
Table 5).
Regarding concentrations, a significant increase of reducing pathogen development was observed ranging from 0.1% (PGI = 14.2%) to 0.3% (PGI = 73.4%) and 0.5% (PGI = 77.2%; p < 0.01).
Figure 5 shows the interaction between treatments and concentrations. 0.3% of C
2H
4O
3 and 0.3% of H
2O
2 completely inhibited the pathogen growth.
- (b)
In vivo trial
A statistical difference was detected among treatments in terms of fungal CFUs·g
−1 (
p < 0.01) (
Table 6). Immersion in 0.3% of C
2H
4O
3 for 10 min resulted in the best performance with a decrease of 83.3% of fungal CFUs·g
−1, when compared to the untreated control. However, it significantly affected seed germination (reduction of approx. 70%). Generally, all the treatments reduced seed germination compared to the untreated control; the average germination reduction caused by H
2O
2 was 34.0%, whereas that caused by C
2H
4O
3 was 46.3% (
p < 0.01).
3.4. Fusarium Occurrence in Garlic Cloves
The study aimed to identify the areas where fungi were present in the cloves. This showed that the outer tunics were always infected (100% incidence), and other inner parts of the cloves were also colonized, although with a minor incidence (
Table 7). Sometimes, fungal mycelium was found in the inner germ (incidence 30%).
F. proliferatum was the main detected species; however,
F. oxysporum,
Penicillium spp. and
Alternaria spp. were also detected.
In the second trial, observation through the stereo microscope did not show traces of fungal contamination in the inner portion of the cloves. Nevertheless, the analysis carried out with the other half of the cloves showed that the germ was also infected, although with a lower incidence compared to the base and the remaining part of the cloves (17% vs. 67% and 83%, respectively), thus confirming the results obtained from the first trial. Additionally in this trial, F. proliferatum was the main detected species.
4. Discussion
Garlic dry rot is an emerging disease particularly noticeable during the post-harvest phase in garlic cloves, mainly attributed to
F. proliferatum, which has caused significant economic losses. The role of garlic seeds as a source of inoculum was confirmed and sowing healthy seeds is considered fundamental in ensuring that the product has both a high yield and quality as well as good preservability [
44]. Few fungicides are allowed for garlic seed treatments, but they are not very effective. It was recently confirmed the poor efficacy of chemical and biological fungicides applied at sowing as spray seed treatments [
45]. Therefore, this study was carried out to reduce
F. proliferatum incidence in garlic cloves with low impact interventions, while facilitating the maintenance of high levels of seed germination. To achieve this goal, three strategies were tested: heat, O
3 and chemical disinfectants.
The growth and multiplication rate of
Fusarium spp., as well as the development of diseases, are affected by temperature. Several studies have shown that 25 °C is a suitable temperature for
Fusarium mycelial growth [
46], while exposure to both low (15–20 °C) and high (30–35 °C) temperature ranges were found to inhibit mycelial development [
47]. Mogensen, et al. [
48] reported that no mycelial growth was observable at temperatures ≥40 °C for five strains of
Fusarium species, including
F. proliferatum,
F. verticillioides and
F. oxysporum. Thus, heat, applied mainly via water, air or vapor, can be an interesting zero-impact technique to control fungal diseases of garlic cloves [
32,
49,
50]. However, temperature might influence the percentage and rate of seed germination. Seed germination is, in fact, a complex process involving many individual reactions and phases, each of which is affected by this parameter. Once seeds start to germinate, high temperatures stimulate faster germination up to an optimal point, after which, the speed of germination declines rapidly [
51]. Indeed, in oilseed rape,
Arabidopsis, wheat and rice, heat stress was found to affect seed performance, dormancy and seedling growth [
35,
36,
37,
52]. Similar results were also obtained by studies conducted on
Lolium rigidum and sunflower seeds [
53,
54]. Therefore, for successful application of heat treatments, pre-tests using germination assays are needed to determine the optimal temperature-time combination for a given batch of seeds that does not affect its germination. The present study has thus investigated both steam and dry heat on garlic
Fusarium strains and on seed germination. Steam provided the best control of the pathogen, but it also affected seed germination. In contrast, dry heat did not impact negatively on germination, but it had no significant effect on the reduction of
Fusarium incidence. Thus, none of the treatments tested fully achieved the goal of the study. Nevertheless, to have a more complete overview of the effect of high temperatures on garlic seeds, further combinations of temperature and time should be considered.
Similar results were obtained with H
2O
2 and C
2H
4O
3. H
2O
2 produces free radicals, which cause oxidative damage to proteins and membrane lipids of pathogens. Similarly, C
2H
4O
3 also denatures proteins and disrupt cell wall permeability. Thus, these two chemical disinfectants may be effective against a wide range of microorganisms [
55,
56,
57,
58]. In the present study, both H
2O
2 and C
2H
4O
3 were applied to
Fusarium-infected garlic seeds at a concentration of 0.3% by two different application systems, spraying and immersion. This choice was based on the results of preliminary in vitro assays. Some treatments were successful in reducing
Fusarium growth immersion in C
2H
4O
3 for 10 min. On the other hand, they strongly affected seed germination. Therefore, these products are not fully suitable for garlic seed treatments.
Amongst all the physical treatments examined, the most promising approach was found to be the use of gaseous O
3, a powerful antimicrobial agent, which is currently used as a disinfectant for microorganisms. Its antimicrobial activity is based on its oxidizing effect, which causes damage to the fatty acids in the cell membrane and to proteins and DNA [
59]. The application of gaseous O
3 to
Fusarium-infected garlic cloves in the present study reduced the pathogen growth and conserved high levels of seed germination, similar to the untreated samples. Treatments in which cloves were exposed to the maximum gas concentration for a longer period performed the best. Therefore, gaseous O
3 is a promising approach for the disinfection of garlic seeds, which can be used as a safe and green technology for the control of the disease. O
3 was previously effectively used to control
Fusarium infections in germinated barley and whole wheat grains [
60,
61,
62]. Furthermore, it reduced the growth of many other fungal species in wheat, barley and pea seeds [
38], and mycotoxin contamination in different crops, such as peanuts, figs and Brazil nuts [
63,
64,
65,
66,
67,
68]. One previous study on the efficacy of O
3 on garlic post-harvest found a notable reduction of garlic decay caused by
F. proliferatum, while at the same time, conserving the sensory profile [
69]. Its potential and the possible combination with other actions should be explored in more depth.