The Feasibility of Using Autofluorescence to Detect Lignin Deposition Pattern during Defense Response in Apple Roots to Pythium ultimum Infection
Abstract
1. Introduction
2. Results and Discussion
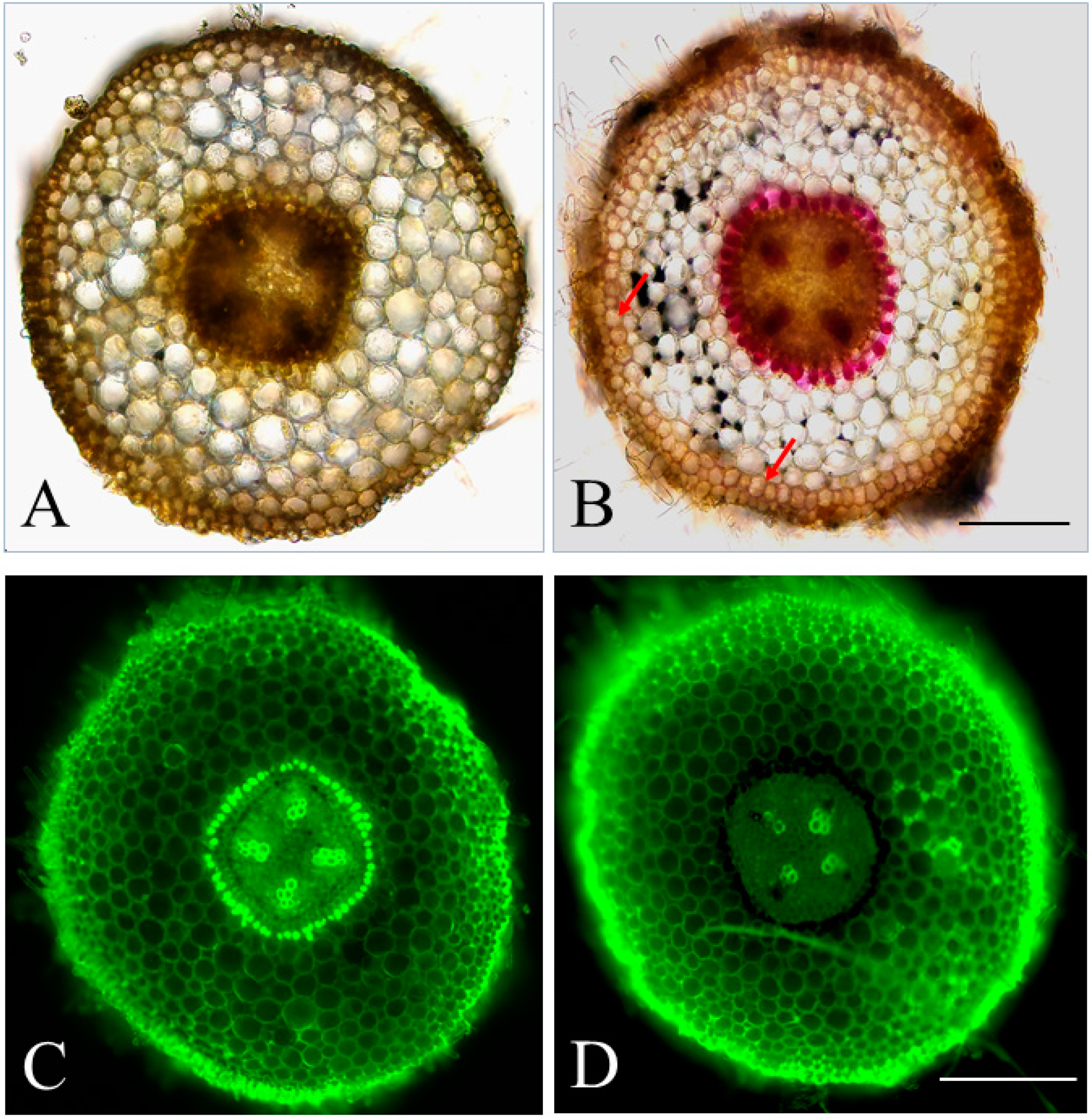
2.1. The Feasibility of Detecting Lignin Deposition Patterns through Autofluorescence
2.2. Upregulated Phenylpropanoid and Flavonoid Biosynthesis Pathways Due to P. ultimum Infection
2.3. Contrasting Regulation Patterns of Apple MATE Gene Expression between Apple Rootstock Genotypes
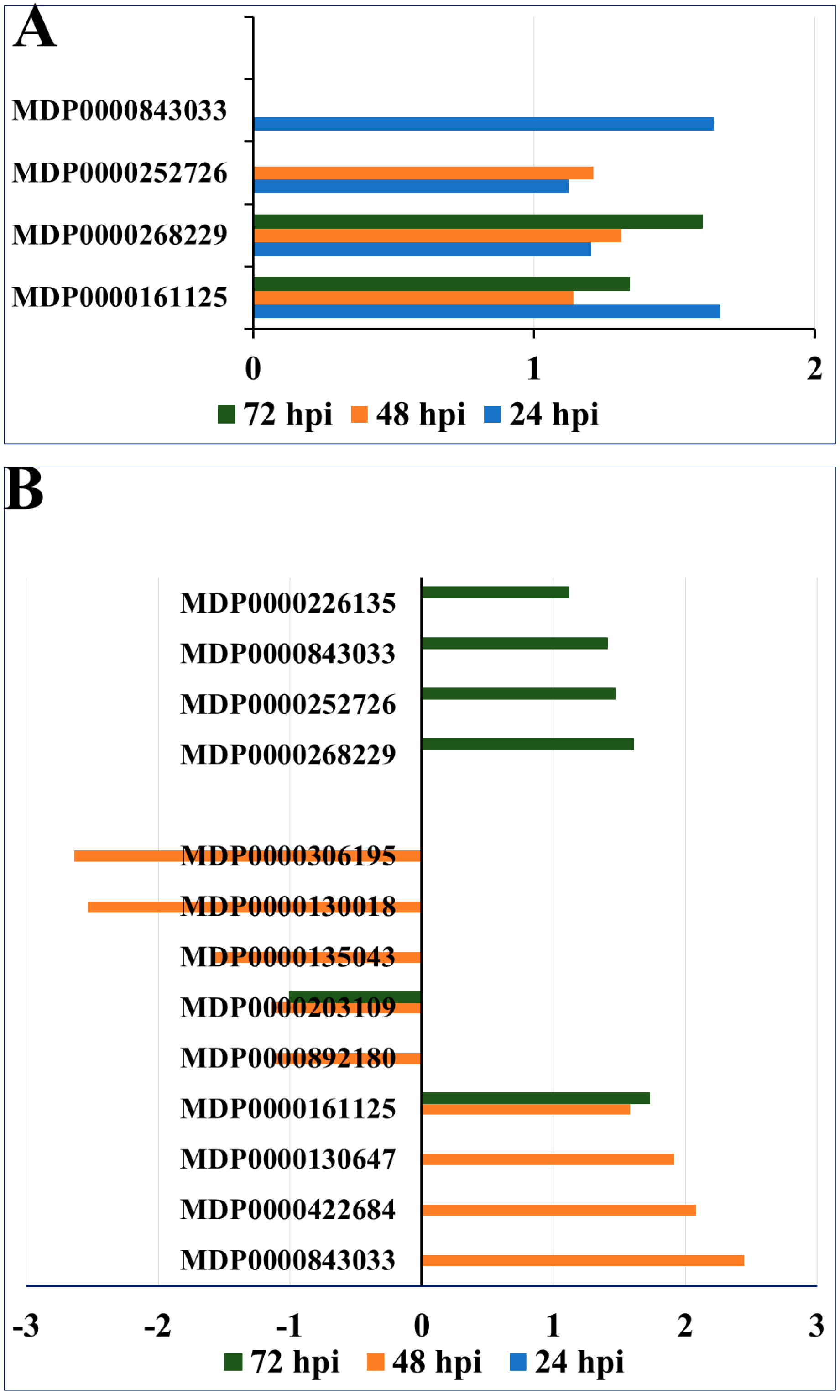
2.4. Epigenetic Regulation of Secondary Metabolism and Lignin Formation in Apple Roots in Response to P. ultimum Infection
3. Materials and Methods
3.1. Preparation of Apple Plants by Tissue Culture
3.2. Wiesner Staining of Lignin and Microscope Images of Sectioned Apple Root Tissue
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Li, G.; Singh, J.; Khan, A.; Fazio, G.; Saltzgiver, M.; Xia, R. Laccase Directed Lignification Is One of the Major Processes Associated with the Defense Response Against Pythium ultimum Infection in Apple Roots. Front. Plant Sci. 2021, 12, 629776. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shao, J.; Zhou, Z.; Davis, R.E. Genotype-specific suppression of multiple defense pathways in apple root during infection by Pythium ultimum. Hortic. Res. 2019, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Zheng, P.; Fazio, G.; Mazzola, M.; Main, D.; Zhu, Y. Transcriptome changes specifically associated with apple (Malus domestica) root defense response during Pythium ultimum infection. Physiol. Mol. Plant Pathol. 2016, 94, 16–26. [Google Scholar] [CrossRef]
- Zhu, Y.; Fazio, G.; Mazzola, M. Elucidating the molecular responses of apple rootstock resistant to ARD pathogens: Challenges and opportunities for development of genomics-assisted breeding tools. Hortic. Res. 2014, 1, 14043. [Google Scholar] [CrossRef]
- Zhu, Y.; Shin, S.; Mazzola, M. Genotype responses of two apple rootstocks to infection by Pythium ultimum causing apple replant disease. Can. J. Plant Pathol. 2016, 38, 483–491. [Google Scholar] [CrossRef]
- Zhou, Z.; Tian, Y.; Cong, P.; Zhu, Y. Functional characterization of an apple (Malus × domestica) LysM domain receptor encoding gene for its role in defense response. Plant Sci. 2018, 269, 56–65. [Google Scholar] [CrossRef]
- Zhu, Y.; Saltzgiver, M.; Zhao, J. A phenotyping protocol for detailed evaluation of apple root resistance responses utilizing tissue culture micropropagated apple plants. Am. J. Plant Sci. 2018, 9, 2183. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, J.; Zhou, Z. Identifying an elite panel of apple rootstock germplasm with contrasting root resistance to Pythium ultimum. J. Plant Pathol. Microbiol. 2018, 9, 11. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, Y.; Tian, Y.; Yao, J.-L.; Bian, S.; Zhang, H.; Zhang, R.; Gao, Q.; Yan, Z. MdPR4, a pathogenesis-related protein in apple, is involved in chitin recognition and resistance response to apple replant disease pathogens. J. Plant Physiol. 2021, 260, 153390. [Google Scholar] [CrossRef]
- Zhu, Y.; Shao, J.; Zhou, Z.; Davis, R.E. Comparative transcriptome analysis reveals a preformed defense system in apple root of a resistant genotype of G.935 in the absence of pathogen. Int. J. Plant Genom. 2017, 2017, 8950746. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Kokubun, T. Plant-fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Geilfus, C.-M. Plant secondary compounds. In Controlled Environment Horticulture: Improving Quality of Vegetables and Medicinal Plants; Springer International Publishing: Cham, Switzerland, 2019; pp. 19–33. [Google Scholar]
- Meyer, J.; Murray, S.L.; Berger, D.K. Signals that stop the rot: Regulation of secondary metabolite defences in cereals. Physiol. Mol. Plant Pathol. 2016, 94, 156–166. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, G.; Norris, A.; Chen, X.; Chen, F. Studying plant secondary metabolism in the age of genomics. Crit. Rev. Plant Sci. 2013, 32, 369–382. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Phytoalexins: What have we learned after 60 years? Annu. Rev. Phytopathol. 1999, 37, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Grayer, R.J.; Harborne, J.B. A survey of antifungal compounds from higher plants, 1982–1993. Phytochemistry 1994, 37, 19–42. [Google Scholar] [CrossRef]
- Nicholson, R.L.; Hammerschmidt, R. Phenolic compounds and their role in disease resistance. Annu. Rev. Phytopathol. 1992, 30, 369–389. [Google Scholar] [CrossRef]
- Vance, C.; Kirk, T.; Sherwood, R. Lignification as a mechanism of disease resistance. Annu. Rev. Phytopathol. 1980, 18, 259–288. [Google Scholar] [CrossRef]
- Miedes, E.; Vanholme, R.; Boerjan, W.; Molina, A. The role of the secondary cell wall in plant resistance to pathogens. Front. Plant Sci. 2014, 5, 358. [Google Scholar] [CrossRef]
- Balasubramanian, V.K.; Rai, K.M.; Thu, S.W.; Hii, M.M.; Mendu, V. Genome-wide identification of multifunctional laccase gene family in cotton (Gossypium spp.); expression and biochemical analysis during fiber development. Sci. Rep. 2016, 6, 34309. [Google Scholar] [CrossRef]
- Yang, C.; Liang, Y.; Qiu, D.; Zeng, H.; Yuan, J.; Yang, X. Lignin metabolism involves Botrytis cinerea BcGs1-induced defense response in tomato. BMC Plant Biol. 2018, 18, 103. [Google Scholar] [CrossRef] [PubMed]
- Arcuri, M.L.; Fialho, L.C.; Nunes-Laitz, A.V.; Fuchs-Ferraz, M.C.P.; Wolf, I.R.; Valente, G.T.; Marino, C.L.; Maia, I.G. Genome-wide identification of multifunctional laccase gene family in Eucalyptus grandis: Potential targets for lignin engineering and stress tolerance. Trees 2020, 34, 745–758. [Google Scholar] [CrossRef]
- Lee, M.H.; Jeon, H.S.; Kim, S.H.; Chung, J.H.; Roppolo, D.; Lee, H.; Cho, H.J.; Tobimatsu, Y.; Ralph, J.; Park, O.K. Lignin-based barrier restricts pathogens to the infection site and confers resistance in plants. EMBO J. 2019, 38, e101948. [Google Scholar] [CrossRef] [PubMed]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin biosynthesis and structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, N.H.; Selvaraj, G.; Wei, Y.; King, J. Role of lignification in plant defense. Plant Signal. Behav. 2009, 4, 158–159. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, L.; Tu, L.; Liu, L.; Yuan, D.; Jin, L.; Long, L.; Zhang, X. Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J. Exp. Bot. 2011, 62, 5607–5621. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Z. The genotype-specific laccase gene expression and lignin deposition patterns in apple root during Pythium ultimum infection. Fruit Res. 2021, 1, 12. [Google Scholar] [CrossRef]
- Boudet, A.; Lapierre, C.; Grima-Pettenati, J. Tansley review No. 80. Biochemistry and molecular biology of lignification. New Phytol. 1995, 129, 203–236. [Google Scholar] [CrossRef]
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546. [Google Scholar] [CrossRef]
- Zhao, Q.; Dixon, R.A. Transcriptional networks for lignin biosynthesis: More complex than we thought? Trends Plant Sci. 2011, 16, 227–233. [Google Scholar] [CrossRef]
- Chaurasia, P.K.; Yadav, R.S.S.; Yadava, S. A review on mechanism of laccase action. Res. Rev. BioSci. 2013, 7, 66–71. [Google Scholar]
- Voxeur, A.; Wang, Y.; Sibout, R. Lignification: Different mechanisms for a versatile polymer. Curr. Opin. Plant Biol. 2015, 23, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Donaldson, L. Autofluorescence in plants. Molecules 2020, 25, 2393. [Google Scholar] [CrossRef]
- Pegg, T.J.; Gladish, D.K.; Baker, R.L. Algae to angiosperms: Autofluorescence for rapid visualization of plant anatomy among diverse taxa. Appl. Plant Sci. 2021, 9, e11437. [Google Scholar] [CrossRef]
- Lichtman, J.W.; Conchello, J.-A. Fluorescence microscopy. Nat. Method. 2005, 2, 910–919. [Google Scholar] [CrossRef]
- Metzker, M.L. Sequencing technologies—The next generation. Nat. Rev. Genet. 2010, 11, 31. [Google Scholar] [CrossRef]
- Zhu, Y.; Saltzgiver, M. A systematic analysis of apple root resistance traits to Pythium ultimum infection and the underpinned molecular regulations of defense activation. Hortic. Res. 2020, 7, 62. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Ma, Q.H.; Zhu, H.H.; Qiao, M.Y. Contribution of both lignin content and sinapyl monomer to disease resistance in tobacco. Plant Pathol. 2018, 67, 642–650. [Google Scholar] [CrossRef]
- Eckardt, N.A. Move It on Out with MATEs. Plant Cell 2001, 13, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, K.; Shitan, N.; Yazaki, K. The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol. 2014, 31, 417–430. [Google Scholar] [CrossRef]
- Upadhyay, N.; Kar, D.; Deepak Mahajan, B.; Nanda, S.; Rahiman, R.; Panchakshari, N.; Bhagavatula, L.; Datta, S. The multitasking abilities of MATE transporters in plants. J. Exp. Bot. 2019, 70, 4643–4656. [Google Scholar] [CrossRef] [PubMed]
- Bednarek, P.; Schulze-Lefert, P. Role of Plant Secondary Metabolites at the Host-Pathogen Interface. In Annual Plant Reviews, Molecular Aspects of Plant Disease Resistance; Wiley Blackwell: New York, NY, USA, 2009; Volume 34, pp. 220–260. [Google Scholar]
- Ko, J.H.; Jeon, H.W.; Kim, W.C.; Kim, J.Y.; Han, K.H. The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann. Bot. 2014, 114, 1099–1107. [Google Scholar] [CrossRef]
- Yang, B.; Li, Y.; Song, Y.; Wang, X.; Guo, Q.; Zhou, L.; Xue, X.; Zhang, C. The R2R3-MYB transcription factor VcMYB4a inhibits lignin biosynthesis in blueberry (Vaccinium corymbosum). Tree Genet. Genomes 2022, 18, 27. [Google Scholar] [CrossRef]
- McCully, M.E. Roots in soil: Unearthing the complexities of roots and their rhizospheres. Annu. Rev. Plant Biol. 1999, 50, 695–718. [Google Scholar] [CrossRef]
- Janick, J.; Cummins, J.; Brown, S.; Hemmat, M. Apples. Fruit Breed. 1996, 1, 1–77. [Google Scholar] [CrossRef]
- Velasco, R.; Zharkikh, A.; Affourtit, J.; Dhingra, A.; Cestaro, A.; Kalyanaraman, A.; Fontana, P.; Bhatnagar, S.K.; Troggio, M.; Pruss, D.; et al. The genome of the domesticated apple (Malus × domestica Borkh.). Nat. Genet. 2010, 42, 833. [Google Scholar] [CrossRef]
- Lloyd, S.R.; Schoonbeek, H.-J.; Trick, M.; Zipfel, C.; Ridout, C.J. Methods to study PAMP-triggered immunity in Brassica species. Mol. Plant Microbe Interact. 2014, 27, 286–295. [Google Scholar] [CrossRef]
| Pathways with Mostly Enriched DEGs | 24 hpi | 48 hpi | 72 hpi | 96 hpi | |
|---|---|---|---|---|---|
| 1. | Biosynthesis of amino acids | 18 | 56 | 40 | 30 |
| 2. | Carbon metabolism | 0 | 44 | 34 | 21 |
| 3. | Glycolysis/Gluconeogenesis | 0 | 38 | 25 | 0 |
| 4. | Phenylpropanoid biosynthesis | 0 | 25 | 21 | 19 |
| 5. | Flavonoid biosynthesis | 8 | 17 | 17 | 14 |
| 6. | Methane metabolism | 5 | 19 | 15 | 10 |
| 7. | Cyanoamino acid metabolism | 6 | 14 | 14 | 12 |
| 8. | Pyruvate metabolism | 0 | 26 | 20 | 0 |
| 9. | Phenylalanine, tyrosine and tryptophan biosynthesis | 6 | 15 | 13 | 8 |
| 10. | Phenylalanine metabolism | 0 | 15 | 12 | 15 |
| Gene ID | KEGG | Annotated Function | FC at 48 hpi |
|---|---|---|---|
| Phenylpropanoid Biosynthesis (ko00940) | |||
| MDP0000668828 | K10775 | phenylalanine ammonia-lyase [EC:4.3.1.24] | 3.1 |
| MDP0000175949 | K01188 | beta-glucosidase [EC:3.2.1.21] | 85.8 |
| MDP0000315857 | 99.1 | ||
| MDP0000293578 | K01904 | 4-coumarate—CoA ligase [EC:6.2.1.12] | 2.6 |
| MDP0000225698 | K00487 | trans-cinnamate 4-monooxygenase [EC:1.14.13.11] | 2.6 |
| MDP0000576346 | 4.6 | ||
| MDP0000376347 | K12355 | coniferyl-aldehyde dehydrogenase [EC:1.2.1.68] | 7.6 |
| MDP0000438458 | 5.1 | ||
| MDP0000123993 | K00083 | cinnamyl-alcohol dehydrogenase [EC:1.1.1.195] | 5.2 |
| MDP0000233961 | 5.1 | ||
| MDP0000488361 | 2.3 | ||
| MDP0000509183 | K00430 | peroxidase [EC:1.11.1.7] | 5.4 |
| MDP0000215414 | 2.7 | ||
| MDP0000233961 | 4.2 | ||
| MDP0000818140 | K12356 | coniferyl-alcohol glucosyltransferase [EC:2.4.1.111] | 4.3 |
| MDP0000160216 | K13065 | shikimate O-hydroxycinnamoyl transferase [EC:2.3.1.133] | 4.6 |
| MDP0000630030 | K09755 | ferulate-5-hydroxylase [EC:1.14.-.-] | 7.5 |
| Flavonoid biosynthesis (ko00941) | |||
| MDP0000686666 | K00660 | chalcone synthase [EC:2.3.1.74] | 3.4 |
| MDP0000686661 | 2.9 | ||
| MDP0000274127 | K01859 | chalcone isomerase [EC:5.5.1.6] | 2.7 |
| MDP0000759336 | 2.2 | ||
| MDP0000239947 | K00475 | naringenin 3-dioxygenase [EC:1.14.11.9] | 2.2 |
| MDP0000166375 | 2.4 | ||
| MDP0000225698 | K00487 | trans-cinnamate 4-monooxygenase [EC:1.14.13.11] | 2.6 |
| MDP0000576346 | 4.6 | ||
| MDP0000127185 | K05280 | flavonoid 3′-monooxygenase [EC:1.14.13.21] | 2.4 |
| MDP0000286933 | 2.3 | ||
| MDP0000788934 | K05277 | leucoanthocyanidin dioxygenase [EC:1.14.11.19] | 2.3 |
| MDP0000225491 | K13081 | leucoanthocyanidin reductase [EC:1.17.1.3] | 4.5 |
| Transcription Factors and Lignin Formation being Targeted by microRNA Degradation | Example of Target Genes | Involved miRNA Family Members |
|---|---|---|
| NAC domain-containing protein | HF09293 | |
| HF24823 | miR164 (a, d, h) | |
| HF22809 | ||
| Transcription factor GAMYB | HF16566 | miR319 (a, c, f) |
| Transcription factor MYB101 | HF03499 | |
| Transcription repressor MYB4 | HF00466 | |
| Transcription factor MYB26 | HF08482 | |
| Transcription factor MYB3 | HF13279 | |
| Transcription factor MYB15 | HF16086 | |
| Transcription factor MYB44 | HF21717 | miR858 |
| Transcription factor MYB1 | HF24028 | |
| Transcription factor MYB102 | HF29485 | |
| Transcription factor MYB7 | HF05712 | |
| Transcription factor GAMYB | HF17403 | miR159 (a–c) |
| Laccase-3 | HF40034 | |
| Laccase-5 | HF23917 | |
| Laccase-7 | HF26400 | miR397 (b) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Y. The Feasibility of Using Autofluorescence to Detect Lignin Deposition Pattern during Defense Response in Apple Roots to Pythium ultimum Infection. Horticulturae 2022, 8, 1085. https://doi.org/10.3390/horticulturae8111085
Zhu Y. The Feasibility of Using Autofluorescence to Detect Lignin Deposition Pattern during Defense Response in Apple Roots to Pythium ultimum Infection. Horticulturae. 2022; 8(11):1085. https://doi.org/10.3390/horticulturae8111085
Chicago/Turabian StyleZhu, Yanmin. 2022. "The Feasibility of Using Autofluorescence to Detect Lignin Deposition Pattern during Defense Response in Apple Roots to Pythium ultimum Infection" Horticulturae 8, no. 11: 1085. https://doi.org/10.3390/horticulturae8111085
APA StyleZhu, Y. (2022). The Feasibility of Using Autofluorescence to Detect Lignin Deposition Pattern during Defense Response in Apple Roots to Pythium ultimum Infection. Horticulturae, 8(11), 1085. https://doi.org/10.3390/horticulturae8111085







