Abstract
In vitro propagation greatly boosts the propagation rate and allows for the development of disease-free plants. In the near future, new in vitro propagation methods could make it easier to produce plants true to type on a wide scale and to use genetic engineering to improve genotypes. Various factors, such as genotype, explant type, size of explants, position of explants on the medium, plant growth regulators and certain additives, incubation conditions, and sub-culturing time, all have a significant impact on the in vitro generation of plantlets and bulblets. However, numerous studies on in vitro propagation have been published, but there is very little information on the parameters that affect the in vitro efficiency of tuberose. The efficiency of bulblet production in ornamental tuberose as well as different physical, nutritional, and hormonal aspects are discussed in this manuscript along with several in vitro propagation strategies (direct, indirect, and somatic embryogenesis). Future research opportunities and the use of creative ways to improve ornamental tuberose are also highlighted. As a whole, this review provides an insight toward a complete protocol for in vitro propagation in tuberose, highlighting the factors influencing the in vitro efficiency and future strategies for improving in vitro plantlets and bulblets in ornamental tuberose.
1. Introduction
Polianthes tuberosa L., better known as tuberose, is one of the important bulbous flowering plants that is part of the Amaryllidaceae family. It is now placed under the Agavaceae family. A native of Mexico, it was cultivated before its conquest in 1522. The basic chromosome number of single-stemmed tuberose is 2n = 60, whereas double-flowered species have chromosome numbers ranging from 2n = 50 to 2n = 54, 60, and 120 [1,2]. Tuberose flowers are widely used in perfumes as a source of essential oils and fragrances. Single-flowered plant genotypes (one row of corolla section) are often used to extract essential oils, loose flowers, flower arrangements, etc., while double types of tuberoses (more than two rows of corolla section) are used for cut flowers and garden displays. The flowers of the ‘Single’ type of tuberose plants are more fragrant than those of the ‘Double’ variety and vary in concentration from 0.08 to 0.14 percent [3].
The genus Polianthes contains 15 tuberose species [4,5]. These species have a wide range of colors and vary from white, orange, and red to striped. All species are found in the wild except for Polianthes tuberosa, which have never been found anywhere other than in cultivation. P. tuberosa L. (white), P. palustris (white), P. durangensis (purplish), P. longiflora (white on white), P. montana (white), P. nelsonii (white), P. geminiflora var. graminifolia McVaugh, P. geminiflora var. clivicola McVaugh (bright red), P. platyphylla (white on red), and P. sessiliflora (white) are other notable species [6].
Tuberose is commonly propagated using vegetative bulbs or bulblets rather than the seed. Alternatively, in vitro procedures, in which whole plant components can be employed for the growth and development of new plants, in tuberose are becoming more popular [7]. These approaches should be adopted at the commercial level to fill the demand gap. Micro-plant production, somatic embryogenesis, protoplast culture, haploid generation, somaclonal hybridization, and somaclonal variations all benefit from in vitro culture, which includes growing plant cells or tissues in a synthetic medium. Various in vitro protocols for the regeneration of tuberose plants have been developed using various media and plant sources as explants [8,9,10,11,12,13,14,15].
The micro-propagation effectiveness of plantlets is regulated by a number of factors [16]. Explant kind, genotype, and explant position are frequent characteristics of donor plants that influence the efficacy of the in vitro treatment. Environmental factors, such as culture temperature, gaseous environment, physical condition of the medium, and chemical factors, such as media, hormonal types and concentrations, carbohydrate sources, gelling agents, additives, such as vitamins, salts, and others, have an impact on in vitro plantlet generation and organ efficiency [17]. For a significant reduction in manufacturing costs, both automated environmental control systems and enhanced in vitro culture systems are required [18]. Therefore, in this review, we used the literature from around the world over the last 40 years, with special emphasis on in vitro propagation strategies for the growth and development of in vitro plantlets and bulblets, and factors impacting in vitro plant and bulblet creation. The important topics for future ornamental tuberose research are also covered.
2. In Vitro Propagation
Various methods have been tried to produce regenerative plants (Table 1) by in vitro morphogenesis in tuberose, including direct organogenesis [19,20,21,22,23], indirect organogenesis [10,15], and somatic embryogenesis [7,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65]. The pace of regeneration in vitro, on the other hand, is highly influenced by a variety of factors, including the type of planting materials utilized and the cultural environment established, type and focus of plant growth regulators used, and the genotype of the plant. As illustrated in Figure 1, the entire process of in vitro plantlets, bulblets, acclimatization, and field transfer of plantlets and bulblets for the purpose of obtaining flowers and bulblets can be summarized into the following stages.

Table 1.
In vitro studies based on different propagation conditions in ornamental tuberose (Polianthes tuberosa L.).
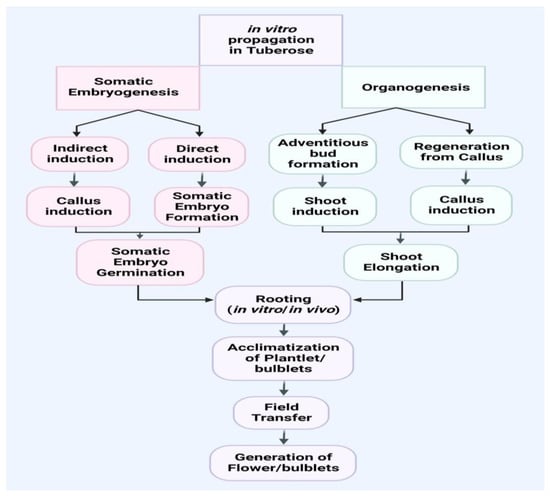
Figure 1.
Schematic chart involving somatic embryogenesis and organogenesis methods for the development of in vitro plantlets and bulblets in tuberose.
2.1. Somatic Embryogenesis in Cell Suspension
Callus growth is induced in the MS medium integrated by 4 μM 2,4-D [7]. However, the 2-week-old calli embedded in the basal MS medium showed nodular shapes resembling embryoid-like structures. In prolonged incubation in a nutritious environment for up to 8 weeks, nodular bodies were enlarged. Separated embryos transferred to the MS liquid medium, supplemented with a double concentration of ammonium nitrate and 15% coconut water (v/v), and stirred at 50–60 RPM at 25 °C showed elongated shoots after three weeks of culture. The regeneration of embryos succeeded in breaking down their dormancy and was also cultured at various hormonal concentrations, but did not produce the desired results. Somatic explants (e.g., leaves, peduncles, nodes, and internodes) with varied amounts of auxin (2,4-D, NAA, IAA) and without BAP were used by [24] to create embryogenic callus cultures of Polianthes tuberosa. The best explant for inducing embryogenic callus was the peduncle. An extremely high embryogenic callus was yielded in the MS medium with 2 mg L−1 IAA and 0.5 mg L−1 BAP. In comparison to peduncles, leaf explants have a reduced rate of embryonic callus.
2.2. Organogenesis
Organogenesis refers to the development of plants from organs, such as soil-born roots, bulbs, and corms, as well as aerial portions, such as shoots and leaves, which can arise directly from the meristem or indirectly by callus induction.
2.2.1. Direct Organogenesis
Direct organogenesis is a very fast way to regenerate micro-plants, but the quality of the in vitro regenerated plants is affected by a variety of things, including the type of explants, the formation of the cultural environment, plant growth regulators and its concentrations, and plant genotype. The flow chart involving different stages is depicted in Figure 1. Direct organogenesis in tuberose was performed by various workers using different explants (Table 1).
2.2.2. Indirect Organogenesis
In indirect organogenesis the selected tissue or plant material is placed in the growth media to produce callus. After callus induction, the callus is moved to the growth media added with plant growth regulators and some additives for future organ development. After a few weeks, the new plant was gradually introduced into the environment. The rate at which plants are regenerated through indirect organogenesis depends largely on a number of factors, including plant type, quantity and age of callus, culture formation, type and concentration of plant growth hormones, and genotype (Table 1). A flowchart involving different stages of in vitro propagation in tuberose is depicted in Figure 1.
3. Stages in the Micro-Propagation Process
An efficient micro-propagation protocol follows a number of steps, each with its own set of prerequisites. These are (i) the implementation of aseptic cultures, (ii) the stage of regeneration of shoots, (iii) the growth of young plants, and (iv) the resilience, field transfer, and survival of growing tissue plants.
3.1. Initiation of Aseptic Cultures and Factors Affecting In Vitro Establishment
Three main factors are involved in this phase, which include the correct selection of genotypes, the correct selection of explants, and the sterilization process. Among them, the right choice of explants allows for discriminate growth in a defined set of cultural situations and growing media. A flowchart involving some important internal and external factors for the in vitro establishment of ornamental tuberose is depicted in Figure 2.
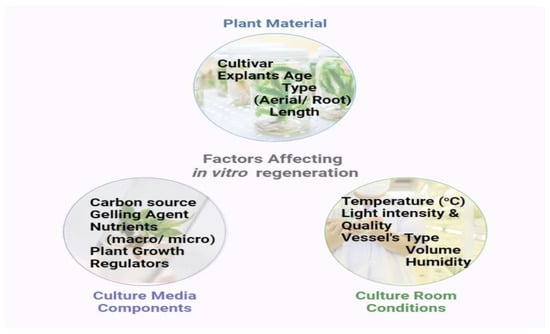
Figure 2.
In vitro regeneration of plantlets and bulblets in ornamental tuberose.
3.1.1. Genotype
One of the most critical elements influencing the success of in vitro tuberose development is genotype (Table 1). In vitro studies [19] reported a higher percentage of establishment, i.e., 26.6 and 30.0, and 23.3 and 30.0 of axenic cultures in the event of axillary and terminal scale segments under ‘Shringar’ and ‘Suvasini’ genotypes, respectively. In the single and double types of cultivars (cvs) [25] we observed high in vitro establishment efficiency in “Single” (85%), followed by “Double” (65%) and lowly in “Swarna Rekha” (45%). Cultivar Prajwal responded better to aseptic culture than cultivar Vaibhav when used for in vitro efficiency [26]. Similarly, [14] found better performance in the cultivar Nagpur Local-1 than in Nagpur Local-2 or Amravati Local-1.
3.1.2. Selection and Type of Explants
The choice of explants depends largely on the method used for in vitro broadcasting. The explant type plays an important role in increasing tissue culture efficiency. In tuberose, in vitro morphogenesis has been used to grow regenerative plants from a variety of explants, including shoot tips [27], bulb scale [9,10,11,12,27], rhizome/bulbul [28,29,30], leaf disc [25], stem disc [13,14], and roots [31]. The inner leaf was found to be suitable explants with respect to initiation and fragile callus formation as compared to the outer leaf and bulb [32]. Similarly, the inner leaf was found to be more suitable in relation to the onset and formation of a weaker calli compared to the outer leaf and bulb [33]. Scale stem segments from the bulbs were found to be appropriate explants for improving the in vitro protocol [34], while portions of the inflorescence containing ripe flower buds were ideal for somatic organogenesis, either straight or callus mediated. In comparison to various explants for callus frequency, Ref. [10] showed a 100% frequency of callus with shoot bud, while bulb fragments had only a 70% frequency. However, Ref. [24] noted that the peduncle was the best explant for the introduction of embryogenic callus compared to leaf, node, and inter-node. The bulb exhibited negative effects, such as explants, because of contamination. When two types of explants, namely axillary bud and bulblets, were used by [22], it was observed that the axillary bud showed the most appropriate results with regard to its response to in vitro regeneration.
Different responses were reported from the same and from different explants collected from various sources (Table 1). Jyoti et al. [12] expended buds and bud scales as an explant for two tuberose plants and the results revealed a low level of results as compared to the shoot scale on both plants. The same explants of two genotypes were examined by [35] and uncovered that those axillary buds showed a high response rate of 95.00% and 92.5% in the Prajwal and Suvasini plants, respectively, and reported that they were the most active explants as compared to others. Variable differences in callus structure were observed, when three different types of explants were used and a compact green color callus was created by the leaves [15]. The calli formed by the stem, scale, and petal produced a soft, clearly distinguishable callus; although the color of the flowering stem callus was dissimilar. The large nodular callus was induced with leaf explants, while the flower produced an unusually soft callus, the roots developed a huge but compact callus, while the tuber generated a soft and tiny nodular callus [36].
3.1.3. Sanitation Conditions of Mother Plant and Explant Sterilization
Tuberose is usually planted in the ground by bulbs or bulbils, and these organs are also used as explants. Due to soil-generated organs, proper sanitation and sterilization must be done before they can be used as explants. Several studies have used a single sterilant, such as HgCl2, followed by a water rinse. Bulb treatment with 0.1 percent HgCl2 followed by cleaning 3–4 times in clear and de-ionized water is recommended in this case [29,36]. Then, they were washed with Teepol (6%) for 10 min before rinsing in clean water and handling the contents for 15 min with 1% HgCl2 before fully cleaning the air flow cabinet with double distilled water as directed [20,25,32,33]. However, [37] used only hot water in the temperature range of 46–57 °C for 1 h to kill the contaminant agents. The lowest contamination is shown on plants grown at 55 °C. Radial cuttings of bulbs are treated with 70% ethanol for 30 s before rinsing 3–4 times with clean water. An additional sterilization was performed with 0.1% mercuric chloride for six minutes and properly washed several times with pure water [9]. Explants were treated with 70% ethyl alcohol for 2 min and 1% HgCl2 for 3–4 min, followed by several washes in sterile water as optimized by [10], and explants were kept in 70% ethanol for a few seconds, followed by a solution containing 0.1% HgCl2 for 4–5 min, and then they were washed several times with disinfected water [38]. Explants were cleaned for 5 min with detergent and 96% ethyl alcohol before being immersed in 0.1 percent mercuric chloride for 2 to 3 min [39]. Following a 15 min surfactant treatment in 20% sodium hypochlorite, the material was thoroughly rinsed 2 to 3 times with purified water [40], followed by cleaning several times with autoclave water. Bulbs, a type of soil-generated explant, are sterilized with 70% ethanol (v/v) for 30 sec followed by 4% sodium hypochlorite (w/v) for 30 min. After that, the bulbs were washed three times each for 10 min in sterilized water [15]. However, in another study [23], bulbs were sterilized for 30 s with ethanol 70% followed by treatment for 20 min with NaClO containing a 2.5% solution, while seeds were sterilized for 20 min with a 1% NaClO solution, and promising results were obtained.
Some researchers have reported the use of detergent and antiseptic agents, such as Savlon etc. The authors of [41] treated initial explants with a detergent (5%) liquid solution for 10 min, then rinsed them with tap water and sterilized them with 70% ethanol for 30 s, then, 0.1 percent HgCl2 for 2 min, and washed them thrice in plain water. Explants were washed with 5% Savlon for 30 min, followed by three times with distilled water. Again, explants were disinfected with 5% sodium hypochloride for 10 min and cleaned three times with distilled water. Finally, the explants were disinfected with 0.1% mercuric chloride for 10 min and rinsed three times with distilled water [42]. Jala and Kachonpadungkitti [43] sterilized explants by immersing them in Clorox 20% (v/v) for 15 min, then in 5% (v/v) for 10 min, and then washed three times with pure clear water for 2 min.
Some research reports indicated the use of pesticides and fungicides with disinfectant agents. Bavistin with other sterilants has been used by various studies, including [19], where the authors treated explants with Bavistin (1000 ppm) + Kavach (1000 ppm) + Cetrimide (500 ppm) overnight followed by HgC12 (0.1%) for 15 min, and finally, cleaned the explants five to six times in sterile water. In a comparison of different disinfectant agents [34], 0.10 percent mercuric chloride for 10 min followed by cleaning in water was found to be sufficient to disinfect explants. Explants were best disinfected by 70% ethanol for 30 s, followed by 1% Bavistin mixed with 0.1% HgCl2 for 10 min [44]. A disinfectant protocol was developed by [45], where they treated explants with sodium hypochlorite 90% (v/v) and ethanol for 30 s, then 1% Bavistin combined with 0.1% HgCl2 for 10 min, and vacuumed to 100 psi before being rinsed 4–5 times with clear, double-filtered water. Explants were submerged in 90% ethanol (v/v) for 30 s, then in an aqueous solution of mercuric chloride (0.1%) and Bavistin (1%) for 10 min, before being rinsed 4–5 times with pure water [14]. Explants were dipped in a solution containing Bavistin (0.4%) for 15 min, followed by treatment with HgCl2 (0.1%) for 5–7 min, and washed 5–7 times with sterile pure water, as suggested by [30]. Explants disinfected with 0.5% Bavistin solution and Teepol for 2 h and 5% NaOCl for 10 min were found to be very effective in disinfecting media [22]. Similarly, Diathane M-45 has been used by [7], where they treated the bulbs as explants with hot water at 58 °C for 30 min, and then immersed them in a 3 g/L solution of Diathane M-45 for 30 min before further experiments.
A comparative study of various surface sterilants for the survival of tuberose explants was conducted by [11], and they observed that subsequent use of ethanol 70%, HgCl2 (0.1%), and NaOCl (1%) was found to be a better solution for tuberose explant sterilants compared to a single sterilant, either HgCl2 or NaOCl. In order to achieve the greatest outcomes, [26] administered HgCl2 and NaOCl at 1.0 percent for 8 min as a surface sterilant to all the explants used. Explants pretreated with 0.1% each of carbendazim, mancozeb, and 200 mg L−1 8-HQC for 4 h [12] observed high cultural survival (89.20% and 82.5%), while explants with HgCl2 (0.1%) and NaOCl (1%) resulted in higher cultural survival, i.e., 88.30% and 87.45% of the bud scales and bud explants, respectively. A single sterilant, such as Ca(OCl)2 or HgCl2, was ineffective in establishing an aseptic culture, and increasing the time spent exposed to sterilants resulted in explant death [44]. Peña et al. [46] found that axenic tissue had a higher percentage efficiency of 65% for 20 min than other treatments. In a bacterial contamination environment, [47] examined the frequency of rescue of the culture product with different chemicals. The effect of rescue treatments on contaminated cultures of Prajwal and Suvasini showed survival of 76.84% and 78.09%, respectively. Wrapping up cultural waste parts with 70% ethanol-based sterile paper provides 75.47% and 75.00% rescue for both plants. A combination of 0.3% Bavistin and 0.4% 8-HQC for 3 h followed by 70% ethanol for 30 s, 0.25% NaOCl, and 0.5% HgCl2 for 10 min and 2 min, respectively, resulted in a higher percentage of survival for the two genotypes [35].
3.2. Shoot Multiplication Stage
The pace and mechanism of shoot multiplication determine the success of any micro-propagation procedure. The following are some important elements that influence in vitro shoot multiplication in ornamental tuberose (Figure 1).
3.2.1. Factors Affecting the Shoot Multiplication Stage
Genotypes/Cultivars/Species
In vitro propagation studies with single genotypes resulted in 50% of shoots within seven days, whereas double-flowered tuberose resulted in only 40% of shoots [32]. However, another cultural medium, the single and double variants, have produced 80% and 70% shooting, respectively. Similarly, [19] found that the cultivar Suvasini has a higher reproductive capacity than the Shringar genotype. The Suvasini, a double-type cultivar, produces a higher number of shoots than the Shringar, which is a single-type of tuberose. The best responses to multi-shoot production (70%, 50%, and 20%) in cvs are “Single”, “Double”, and “Swarna Rekha”, respectively, as noted by [20]. In another study, Prajwal produced superior results under in vitro experiment compared to the Vaibhav genotype [12]. On the other hand, the Mexican Single was found to have the best shoot multiplication, followed by Prajwal, Phule Rajni, and Shringar [44]. In their in vitro studies, [48] used two tuberose varieties, namely Prajwal and Sringar, as explants. The frequency of shooting was slightly higher in Prajwal compared to Shringar. Surendranath et al. [35] observed a higher frequency of shootings in Suvasini compared to the cultivar Prajwal; however, mean shoot length was higher in Prajwal than in Suvasini. In vitro shoot performance was better in Nagpur Local-1 as compared to Nagpur Local-2 and Amravati Local-1 [14], and the largest number of shoots was in the Calcutta Single, followed by the Calcutta Double and the smaller shoot produced by Srinagar [36].
Media
The MS basal medium was employed by a variety of researchers (Table 1) in all phases of micro-propagation [7,9,10,11,12,14,15,25,29,30,34,35,36,39,43]. However, other media were also used by other laboratories, including [49,50], where they used three standard media: white, Gamborg B5, and the Linsmair and Skook (LS) medium. Among the media that appeared, LS showed high production of extracellular polysaccharides. Various media, including MS [51], WH [52], and B5 [53], were examined by [13,44] to find the best response under in vitro conditions. During the initial investigation, the WH basal medium was found to be more acceptable than the MS and B5 media and was eventually used for in vitro studies on tuberose. WH media is also superior for in vitro enlargement and elongation shooting in the three tuberose genotypes [14].
Size and Position of Explants
Most in vitro regenerated studies in tuberose uniform growth stage/size of explants have been used (Table 1). However, some research reports have demonstrated differences in explant size. The variable explant size of tuberose under in vitro conditions has been optimized, including 3–5 mm by [32,33], 4–5 mm by [20], 5–10 mm by [13], 1–3 cm by [42], and 2–3 cm by [39]. In vitro cultures of tuberose [54] used basic segment (1 × 1 cm2) scales with lateral buds.
Growth Regulators
The formation of media containing a strong role for PGR is crucial to the proliferation and multiplication of in vitro shoots. In vitro regeneration and proliferation of tuberose shoots have been observed using cytokinins and auxins in media (Table 1). Various researchers have stated that indole acetic acid (IAA), benzyl aminopurine (BAP), or benzyladenine (BA) has a beneficial effect over other cytokinins [11,19,28]. Jyothi et al. [12] reproduced micro-plantlets from two tuberose cvs with 4 mg/L BAP. BAP combined with IAA was reported by various studies, including [19], which reported high BAP concentrations of 2.0 mg L−1 BAP and low 0.1 mg L−1 IAA concentrations for favorable results. Media supplemented with 4.0 mg L−1 BAP and 0.2 mg L−1 IBA produced the highest number of shoots, whereas media supplemented with only BAP (4.0 mg L−1) decreased the quantity and number of shoots [11]. The best response for multiple shoot production was observed with media consisting of 0.5 mg L−1 IAA and 3 mg L−1 BAP, as observed by [29]. Higher IAA and lower BAP concentrations are recommended in various reports, including [24], who received the best callus in media containing 2 mg L−1 IAA and 0.5 mg L−1 BAP, while media containing IAA 3.0 mg L−1 and 0.5 mg L−1 BAP, induced higher callus frequencies [28], and direct micro-plantlets were obtained using 1.5 mg L−1 BA and 0.5 mg L−1 IAA [23]. Tuberose propagation in vitro has been successful using NAA alone and in conjunction with other PGRs. In one report [25], hired NAA alone and a 0.5 g/L concentration of NAA was the most effective treatment for callus induction. Similarly, high concentrations of BAP and low concentrations of NAA were more efficient in vitro tuberose. Hernández-Mendoza et al. [55] obtained high-quality micro-plants in the media containing 2.0 mg L−1 BAP and 0.5 mg L−1 NAA. The medium containing 0.5 mg L−1 BAP and 2.0 mg L−1 NAA yielded the best results in terms of complete regeneration of the plants [30], and media consisting of BAP 2 mg L−1 and NAA 0.5 mg L−1 demonstrated excellent in vitro reproduction [22]. Liquid culture containing 1 mg/L benzyladenine and 0.2 mg L−1 naphthalene acetic acid (NAA) yielded more micro-plantlets [45]. However, Ref. [46] developed adventitious shoots through direct organogenesis with a frequency of up to four shoots per treatment at 7.0 uM BA in the absence of NAA. Some researchers have also reported Kinetin singles and combinations with other growth regulators for in vitro multiplication of tuberose. High shoot formation and shoot length with media containing 3.0 mg L−1 of Kinetin showed that the increase in Kinetin inhibited positive results [9]. Ali et al. [38] reported initiation in shoots under the medium fortified with 1.0 mg L−1 KIN, while media containing 60 mg L−1 Kinetin are needed to release the axillary bud of tuberose under in vitro conditions [57]. Media consisting of 0.2 mg L−1 each of BAP and Kinetin caused the maximum number of shoots in the Calcutta Single cultivar [58] and high-frequency recurrent shoots were observed in media containing 0.5 mg L−1 BAP, Kinetin 0.5 mg L−1, and IAA 0.8 mg L−1 [42]. Well-developed shoots with leaves were recorded under media consisting of 0.5 mg L−1 IAA and 1.0 mg L−1 KIN, while the maximum number of leaves and leaf length was found with 0.5 mg L−1 IAA and 1.0 mg L−1 KIN [21]. Instead, [34] optimized very high concentrations of NAA and adenine sulfate for callus induction and found that treatment containing NAA of 15.0–20.0 mg L−1 and adenine sulfate of 10.0 mg L−1 showed the best results.
A broadleaf weed management herbicide called 2,4-D was employed as a growth medium with several PGRs for plant micro-propagation. Media consisting of 4 mg L−1, 2,4-D and 0.5 mg L−1 BAP initiated more shooting and the maximum number of shoots [20]. Multiple shoots were intensified in media enriched with 6.0 mg L−1 BAP + 0.5 mg L−1 NAA + 0.7 mg L−1 2,4-D + 0.5 mg L−1 TDZ [59].
When cultured in a single or a combination of plant growth regulators, the results of direct and indirect organogenesis with different genotypes varied (Table 1). Media consisting of 4 mg/l,2,4-D and 0.5 mg L−1 BA showed 50% shooting in single-type tuberose, while similar media had 40% shooting in double-type tuberose [19], and shoot bud explants cultured with 4 mg L−1 2,4-D and 0.5 mg L−1 BA resulted in 80% shooting in the single type and 70% detection in the double type [32]. In another study, a significant increase in shoots of the Hyderabad cultivar was obtained in comparison to Double Navsari Local when media was integrated by 0.2 mg L−1 for BAP and 0.2 mg L−1 Kn [60]. However, significant spread of shoots through media consisting of BAP 1.0 mg L−1 and Kn 1.0 mg L−1 was noted in the Phule Rajni cultivar, while 2.5 mg each of L−1 BAP and Kn exhibited superior results in Calcutta Double [61]. The highest number of axillary buds was recorded in media intensified by 4.0 mg L−1 BAP and 0.5 mg L−1 NAA, but combined culture media intensified with 4 mg L−1 BAP, 0.5 mg L−1 NAA, and 0.5 mg L−1 TDZ produced longer micro-shoots in the Prajwal and Suvasini genotypes [35]. Another genotype, Nagpur Local-1, performed better in media comprising 2.5 mg L−1 BAP and 0.5 mg L−1 NAA, followed by Nagpur Local-2 in the same media [14].
Direct and indirect processes of micro-plants have been achieved using two or more types of growth regulators and multiple types of explants (Table 1). The medium containing 2 Tm of Kin showed a very high percentage of shoots per plant. Shoots of the greatest length were discovered in media containing 3 TM NAA + 30 TM BA [37]. Gajbhiye et al. [44] reported that media consisting of 0.3 mg L−1 TDZ and 0.5 mg L−1 NAA were recorded to be more responsive to their ability to produce shoots, and media containing 3.0 mg L−1 NAA and 0.5 mg L−1 TDZ has been shown to improve shooting duration. When explants were enlarged by 3.0 mg L−1 BAP in the media [39], 93% of the shoots were formed. TDZ (18 M) as a single growth regulator was also found to be suitable for the shoot formation. However, regenerated shoots transferred to the media intensified with 2.5 mg L−1 BAP, 0.5 mg L−1 NAA, and 0.1 mg L−1 kinetin were found to have increased shooting levels. A good quality callus was induced in the media containing 4.0 mg L−1 2,4-D [13], while callus induced from media comprising 1.0 mg L−1 TDZ and 0.5 mg L−1 1NAA showed effective efficacy for shoot growth. A maximum number of shoots was obtained using 2.0 mg L−1 TDZ and 0.5 mg L−1 1NAA. A nutrient medium consisting of 0.5 mg L−1 BA and 0.5 mg L−1 NAA produced longer shoots. Jala and Kachonpadungkitti [43] concluded that callus weight depends on BA concentration, varying from 0.5 to 2 mg L−1. Axillary bud explants grown without growth regulators in the media provided a small amount of callus. When the media was boosted by 3.0 mg L−1 BAP, the most shoots were identified, whereas the media enhanced by 0.5 mg L−1 BA and 2.0 mg/L NAA was ideal for shot duplication. A high-quality callus was induced in media supplemented with 1.95 M of 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T) [15], but the highest frequency of regeneration of shoots was found in the enriched medium with 2.26 M thidiazuron (TDZ).
Carbon Source
For in vitro tuberose plant multiplication, sucrose is the most frequent carbon source, and the ideal concentration at all phases of micro-propagation is between 2 and 3 percent (Table 1). Among the carbon sources, sucrose is cheaper, readily available, and stable in autoclaving and easily absorbed by micro-plants [9,28,39,41]. Various researchers have recommended 3% sucrose in the growing media as a carbon source for the in vitro propagation of tuberose [11,15,23,28,39,46]. Changes in the value of the carbon source, such as increased sucrose content (up to 15%), could not achieve the desired results [7]. When employing pure and commercial grade sucrose in an in vitro culture of tuberose, the authors of [13] observed no discernible differences in the reaction between pure and commercially generated sucrose. In one report, the authors of [14] applied 2% sucrose to media with a combination of different growth regulators in tuberose for micro-shoot growth.
Gelling Agents
Tuberose in vitro development is commonly gelled by agar and Phytagel, which are commonly used as gelling agents during tuberose in vitro development (Table 1). Concerning appropriate agar concentrations in the cultural medium, minor differences were observed by various research workers. In vitro media containing 0.8% agar in culture are recommended by [9,25,29,30,36]. Several researchers have optimized agar ranges, including 6.4 g/L [56], 0.7 percent [15,23], 7.5 g/L [44], 0.9% [10], and 1% [14]. Phytogel has also been optimized in several ranges, including 1.5 g/L [39], 0.25% [19,42], and 0.3% [7]. In contrast, [46] used 0.25% Gelrite in cultural media. The role of Bacto agar in pure or commercial grade was examined by [13] and found no significant variation in the response of media sources integrated with Bacto agar for pure or commercial grade.
Inorganic Salts, Organic Compounds, Antibiotics, and Vitamins
Reducing the number of nitrogen sources in media, such as casein hydrolyzate (CH) and coconut water (CW) by 15% (v/v) in accordance with the growth regulators resulted in favorable results [7]. When excessive salt and vitamins were used in the cultural media, [13] noted the best results. Jala and Kachonpadungkitti [43] found that the highest survival rate of callus was observed at low Oryzalin concentrations (0, 0.1, and 0.5 mg L−1) with the shortest time interval. In a recent study, [15] used various concentrations of proline (0, 50, 100, 150, 200, and 250 mg L−1). In vitro, a 250 mg L−1 concentration of proline in the media demonstrated a significantly higher frequency of regenerated shoots than in vitro tuberose.
Incubation Conditions and Environmental Factors
Incubation conditions and environmental factors are more important and play a critical role in tuberose in vitro reproduction [61]. There is a lack of information available about the cultural conditions of in vitro multiplication of tuberose, and most authors use the same photoperiod, light intensity, and temperature in all stages of micro-propagation (Table 1). Most of the studies reported that media cultures were exposed to temperatures of 25 ± 2 °C and the photoperiod of 16 h light/8 h dark period was better for direct and indirect organogenesis [9,13,14,25,36,41,43,44,58], and some authors optimized 25 ± 1 °C temperature and 16 h light/ 8 h for the best in vitro conditions of tuberose [10,11,32,54]. Light intensity at 2400 lux with a 27 ± 2 °C and 16 h light/8 h photoperiod per day exhibited direct and indirect organogenesis [33]. An adjusted pH of 5.8 and a flow chamber and incubation room temperature at 27 ± 2 °C and 40 mmol·m−2·s−1 light had the best results (46). Ahmad et al. [7] discovered that when cultures were kept at a light intensity of 2000 lux and a photoperiod of 16 h, but callus cultures were kept in darkness, exposure to extreme light and persistent low temperature at 4 °C before incubation was ineffective in helping embryos differentiate normally without rhizogenesis.
Variation in day and night temperatures for in vitro tuberose was reported by [28] where they maintained long day conditions (16 h of light at 360 nmol m−2·s−1, 3500-lux with fluorescent tube and temperature was adjusted at 27 °C during the day and 21 °C at night). The highest shooting rates per plant were obtained with pH values between 5.5 and 6.0.
Some authors optimized minor lower temperatures for culture media, including [39], where they kept the culture at 22 ± 2 °C with day and night light of 16 h/8 h while 16/8-h day and night light cycle and temperature 23 ± 1 °C was the best for in vitro tuberose distribution [23]. Nalousi et al. [15] induced callus and regenerated shoots at 24 ± 2 °C with a relative humidity of 60–65%.
4. In Vitro Rooting of Shoots and Ex Vitro Rooting
One of the most crucial features of any micro-propagation strategy is the in vitro rooting of micro-shoots and their long-term viability in the soil. Very little work has been done to improve root growth efficiency in different types of tuberose genotypes. Root initiation in micro-plants can be carried out both in vitro and ex vitro. The rooting capacity in vitro is determined by the interaction of internal and extrinsic factors (Figure 1).
4.1. Factors Affecting In Vitro Rooting of Shoots
4.1.1. Species/Cultivars/Genotype/Varieties
In single-flowered tuberose, [32] showed 62.5% root emergence, while the double-flowered tuberose had 50% rooting efficiency. Similarly, in another study, double cvs had higher roots compared to other plants, such as single-flowered [34]. Additionally, Ref. [12] noted that the cultivar Prajwal produces numerous roots compared to other genotypes, such as Vaibhav, under in vitro conditions. Shringar achieved 100% root emergence, premature root development, and a higher number of roots than Suvasini [19]. When rooting frequencies in three tuberose cvs were tested, the highest root growth rate was observed in “Single” (95%), followed by “Double” (90%), and the lowest root growth rate was observed in “Swarna Rekha” (30%) [20]. Furthermore, Ref. [60] noted a higher percentage of roots in Hyderabad Single compared to Double Navsari Local. Similarly, in another study, Ref. [61] noted earlier roots in Phule Rajni compared to Calcutta Double. However, both types of plants have similar roots that work well in vitro. The highest percentage and longer roots were observed in micro-plants of Shringar, while lower values were shown by Prajwal. Similarly, [14] recorded that the highest root growth, early root formation, and high root length were observed in Amaravati Local-1. However, in root formation comparison among three cvs, a higher number of roots were observed in Arka Nirantara and the least in Calcutta Double and Vaibhav [22].
4.1.2. Type of Explants
Khanchana et al. [22] observed the emergence of earlier roots with axillary buds while bulblets as explants indicate lateral root formation in micro-plants.
4.1.3. Media
In vitro and in vivo rooting of tuberose is commonly used with growth regulators (Table 1). Various researchers have reported the suitability of MS media for in vitro rooting, including [11,36], while 1/3 MS media was used for the in vitro rooting of tuberose [35]. In some research reports, the WH basal medium was found superior for in vitro rooting [13,14,44].
4.1.4. Inorganic Salts/Vitamins/Activated Charcoal
There has been no comprehensive research on the effects of inorganic salts, vitamins, or activated charcoal. Some authors, on the other hand, optimized the number of inorganic salts, vitamins, and activated charcoal (Table 1). Krishnan et al. [34] used charcoal in a range varying from 0.1 to 0.4% and noted that elongated shoots when cultured with 0.2% charcoal emerged earlier roots compared to the other concentrations. The same treatment was also found to be superior in terms of the number of roots. Some macro and micro salts and vitamins in the WH media were found suitable for in vitro rooting [42,44,62]. Nalousi et al. [15] employed 1/2 macro and micro salts and 1/2 vitamins with different rooting media for the in vitro rooting of tuberose.
4.1.5. Carbohydrate/Carbon Source
In vitro tuberose rooting with 3% sucrose was demonstrated in [15,39,42,62]. However, in a comparison of different concentrations of sucrose, Ref. [32] found 3% sucrose was superior in comparison with 1% sucrose. Alternatively, Ref. [14] recommended 2% sucrose as a carbon source in rooting media.
4.1.6. Gelling Agent
There are no comparable results available for gelling agents under in vitro rooting in tuberose. However, different concentrations of gelling agents were recommended by various studies, including [44], which found 7.5 g/L agar was very effective for tuberose in vitro rooting. In their in vitro rooting media, Ref. [14] used 1% agar as a gelling agent, while Ref. [11] used 0.6% Bactoagar for in vitro rooting of micro-plantlets of tuberose. Naz et al. [39] used Phytagel (1.5 g/L) and this was found to be the most effective for the rooting of micro-shoots and 2.5 g/L Phytogel for the induction of rooting in micro-plantlets, as reported by [42].
4.1.7. Growth Regulators
In vitro, auxins such as IBA, NAA, and IAA, alone and in combination, were found to be effective in generating in vitro rooting. IBAs have been used for in vitro rooting of tuberose by several studies, including [9,11,19,59] and NAA [10,11] as PGRs. Various concentrations of NAA have been reported by various studies, including [63], which optimized the media containing 2 mg L−1 NAA for in vitro rooting, while Ref. [39] advocated 1 mg L−1 NAA for in vitro rooting in tuberose, while in another study, the best rooting in shoots was achieved by employing 0.5 mg L−1 NAA [41]. Similarly, higher and lower concentrations of IBA have been recommended by various studies. Lower concentrations of IBA for in vitro rooting were optimized by [20,32,36], where they achieved optimal rooting with 0.5 mg L−1 IBA. However, some research reports suggested minute higher concentrations of IBA, such as [12], where media consisting of 1.0 mg L−1 IBA gave the maximum number of roots with the highest percentage, while increasing the concentrations of auxin decreased the root length. In vitro rooting was found to be most efficient when plantlets were fortified by 2 mg L−1 IAA [14,44], while in another study, plantlets treated with a higher concentration of 4.0 mg L−1 IBA produced positive effects on rooting [34]. Some researchers have employed IAA instead of IBA or NAA for tuberose in vitro rooting. The use of 0.5 mg L−1 IAA has been found to be optimal for the in vitro rooting of tuberose [10,42,62].
Some comparative studies containing IBA, NAA, and IAA in various combinations have been suggested by various studies. Shoots treated with 2.0 mg L−1 IBA had 90% root formation, whereas NAA had only 85% root formation at 3.0 mg L−1 [9]. For the in vitro rooting of tuberose, Ref. [11] used IBA and NAA alone. Plantlets treated with 1.0 mg L−1 IBA induced the maximum number of roots. Raghuvanshi et al. [13] employed IBA and NAA alone, and IBA along with BAP and Kn. Media enriched with 1.0 mg L−1 IBA was found to be the most effective in inducing in vitro root proliferating ability, number of roots, and mean root length. Early root induction and the maximum number of roots were recorded on the medium containing IAA 0.25 mg L−1 and IBA 0.25 mg L−1 in Shringar and Suvasini cvs, respectively. However, both cvs showed maximum root length with 0.5 mg L−1 IBA [19]. In a series of studies, Panigrahi and Saiyad [58] observed maximum rooting parameters with media consisting of 0.5 mg L−1 IAA and 0.5 mg L−1 BAP in the genotype Calcutta Single. Plantlets were kept in a medium containing 0.5 mg L−1 BAP, 2 mg L−1 IAA, and 0.5 mg L−1 BAP, 3 mg L−1 IAA at 5 °C to induce rooting of Hyderabad Single and Local Double Navsari, respectively [60]. Plantlets treated with 0.5 mg L−1 NAA and 0.5 mg L−1 IAA induced maximum rooting in Phule Rajni, while 3.5 mg L−1 NAA and 0.5 mg L−1 IAA were found suitable for the cultivar Calcutta Double [61], and immersing plantlets in solutions containing 0.5 mg L−1 IBA, 2.0 mg L−1 IAA, and 0.5 mg L−1 IBA and 2.5 mg L−1 IAA induced rooting in Prajwal and Shringar varieties, respectively [48]. Plantlets treated with 0.5 mg L−1 IAA and 1.0 mg L−1 KIN emerged with earlier roots, while the maximum number and length of roots were recorded with media containing 0.5 mg L−1 IAA and 1.0 mg L−1 KIN [21]. Kumari and Pal [30] obtained a greater number of roots in regenerated shoots with 0.5 mg L−1 BAP and 1.5 mg L−1 NAA, while micro-plants treated with 3 mg L−1 IBA and 1 mg L−1 NAA [35] initiated the highest number of roots. Plantlets treated with 1 mg/L of IBA and 1 mg L−1 of NAA resulted in efficient rooting in four varieties of tuberose [22].
4.1.8. Ex Vitro Rooting
There is very little information on the parameters that influence tuberose in vitro rooting efficiency. In one research report, [12] used a peat and Soilrite-filled glass bottle (1:1) and reported it as the most effective approach for ex vitro root induction in tuberose.
5. Acclimatization and Field Transfer
The effectiveness of an effective in vitro protocol depends on various factors, including explant type, a combination of hormones, and organogenesis mode that maintains the success of environmentally sustainable plants (Table 1). Plantlets that have become hardened on vermiculite should be transplanted into pots containing garden soil and sand [9]. However, Ref. [19] found a survival rate of 100% using a closed sachet procedure. In this application, the presence of Sandrite may improve the survival rate because of good ventilation conditions, water retention capacity, and nutrients present in this medium. Rooted micro-plants in pots in a greenhouse, initially followed under the conditions of the open field, were found to be effective for in vitro-raised plants [41]. When micro-plants were grown in an open field, all micro-plants died completely within three weeks, while the plants grown under coco-peat showed a survival rate of 65% in the single type, followed by double and Suvasini, each with 60% survival, and the cultivar Shringar survived by 55% [34]. The length of time and exposure time were increased daily to adapt to the environment and grow in the field. A total of 50% of the plants were successfully propagated and formed healthy bulbs [32]. Nazneen et al. [10] obtained impressive plantlets in an area consisting of sandy loam (1:2) and covered them with light polythene bags. After two weeks, the polythene bags were removed and the plants were firmly planted in the ground. Root-bearing micro-plants were placed in plastic containers containing sterile sand and stored inside the culture room for 10 days until firm, and then placed in other plastic pots containing compost soil and stored outside the culture room to adapt to the external environment [20]. After the plants settled, they were transferred to clay pots containing garden soil. When the rooted plantlet leaves were treated with 1.0% glycerol for 15 min before planting, the fertile clay combination combining 1 part sand + 1 part soil + 1-part FYM, [11] achieved the maximum survival rate (86.6%). Jyothi et al. [12] used two adaptation techniques for both types of plants, namely, glass with a peat-filled glass and Sandrite (1:1) covered with a polyproline cap, and other techniques for mixing plastic pots covered with polythene. A glass bottle filled with peat and Groundrite (1:1) worked very well on both types of plants. Pohare et al. [42] transferred in vitro mutagenesis plantlets to trays containing coco peat and sub-soil, followed by keeping them in dark polythene bags containing soil. Ali et al. [38] kept in vitro plantlets in compound-containing cow dung, sand, and garden soil (1:2:1). Plants can grow up to 5–8 cm tall after being washed in tap water and planted in a pot containing sterile clay mixture, followed by plants covered with wet polythene bags to prevent rot, and kept in the growing room for 7–15 days in controlled areas and sprinkled with distilled water every 24 h to keep the plants at a high humidity level. After a period of 10–15 days, the polythene bags were removed when the plant material seemed to be self-sufficient and placed in a natural place. Misra and Saema [64] planted them initially under glasshouse conditions and later planted them in a field where they flourished as a true variety. In the hardening of micro-plants produced from three varieties of tuberose, Ref. [14] achieved the highest survival rate (53.84%) in the cultivar Nagpur Local-1 followed by Nagpur Local-2 (40.90%), and Amravati Local-1 had the lowest survival rate (36.36%). In sand, 81% of tuberose micro-plants survived and produced strong growth [39]. Singh et al. [36] washed the roots of micro-plants gently with plain water and removed agar gel residues before planting them in a soil mixture in jam bottles. At the start of the week, jam bottles were sealed with lids to keep enough moisture within. The lid was progressively removed after one week, and the healthy plants were transplanted into 4-inch pots loaded with regular garden soil. In another study, all four micro-plantlets (B, S4, S6, and S13) planted in two particular areas (CREA and CREAM) produced bulbs, and these bulbs were acclimatized. When these bulbs were grown in open fields, the survival rates were 99.3%, 99.4%, 100.0%, and 98.9% in B, S4, S6, and S13, respectively [23].
To better survive as micro-plantlets, some appropriate environmental conditions are required. In this context, Ref. [7] cleaned the roots of micro-plants with a solution (3 g/L Dithane M-45) to protect them from soil-borne diseases before planting them in a 1:1:1 ratio of loamy soil, sand, and peat moss, a measure paired with Hogland’s solution to meet the nutritional needs of plants. The pots were covered with light polythene bags and kept in a growth room at 25 °C for 4–6 weeks, until the plants had hardened in the pots and had grown to the appropriate size for planting in the garden. Micro-plants planted into plastic pots containing soil, sand, and compost after they had been adequately cleaned (1:1:1) followed by plants covered with light polythene bags for a week to keep them wet, then transported to the greenhouse on a 28 °C Day, 20 °C night, 16-h day-length, and 70% relative humidity [39]. Rooted micro-plantlets were transported to the growth room for 10–25 days at 30 °C and 60% relative humidity followed by plantlets moved to a net house for 30–35 days to acclimatize, and finally, ordinary plants were eventually transplanted to the field [13]. Gajbhiye et al. [44] kept micro-plants at 30 ± 2 °C with relative humidity of 60 ± 5% for 10–25 days in an environmental growth chamber for stabilization, followed by the net house for 30–35 days to adapt, and then transferred to open space. Kumari and Pal [30] transferred rooted micro-plants to empty plug trays with wet coil dust. After four weeks of practice, the plants grew rapidly and survived in a pot filled with garden soil and sand. After a few weeks, the healthy plants were moved to the field and had a 100 percent survival rate. Micro-plants kept in media consisting of 1:1 media of sterile peat and perlite were irrigated every two days and fed with MS basal nutrient solution biweekly while being stored in a 16-hour light and dark photoperiod at 25 °C and 18 °C, respectively, with 80% relative humidity and 100% survival rates without morphological abnormalities recorded in the greenhouse [15].
6. In vitro Bulb/Rhizome Formation
There is a scarcity of data on the parameters that influence tuberose bulb/rhizome efficiency in vitro. Micro-plants treated with IBA at a concentration of 2.0 mg L−1 were found to be effective in hastening early rhizogenesis compared to the other auxins tried, while increasing IBA beyond 2.0 mg L−1 delayed rhizogenesis in in vitro plants [9]. However, acclimatized micro-plantlets transplanted into the field produce smaller bulbs compared to the mother plant [10].
7. Conclusions and Future Prospects
In this review, the results of multiple studies employing various in vitro approaches to produce micro-plantlets and in vitro bulblets in ornamental tuberose have been evaluated. In vitro plant and bulblet development are influenced by a variety of physical and chemical factors, which are extensively discussed in the literature. Plant regeneration by somatic embryogenesis is an appropriate technology for the genetic modification of plants because it comes from a single cell. The ability of cell suspension cultures to create somatic embryos may reduce the time it takes to harvest bulbs, particularly in the case of tuberose. In another bulbous flowering crop, such as gladiolus, it was reported that a 2-year-old callus obtained through somatic embryogenesis retained its regeneration capacity. It is also possible that embryogenic callus derived from mature tuberose plants can be stored for a long time under the right conditions, and that the stability of the stored embryogenic callus may be a potential source of tuberose mass multiplication in tuberose. Because there has been very little research on somatic embryogenesis, additional research is needed. Ex vitro rooting, in vitro bulblet production, and factors affecting the success of these in vitro protocols are all areas where there is a lack of information. As a result, we believe that more research into these parameters is necessary. New procedures for high rates of shoot multiplication and the creation of cost-effective solutions are becoming increasingly important these days. As a result, more research into low cost in vitro protocols are required. Although the use of a “bioreactor” in plant propagation can speed up the growth of cultures and multiplication, it also saves space, energy, and labor costs. There is no information on the use of a “bioreactor” in tuberose plantlet creation. As a result, we recommend that a bioreactor study be conducted for speed multiplication.
Author Contributions
Conceptualization, U.S., A.K., V.C. and S.P.; resources, S.M., S.K. and G.K.A.; writing—original draft preparation, V.P.; writing—review and editing, J.S., M.K.Y. and M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Authors are agreed with data availability rules governed by the journal.
Acknowledgments
The authors are thankful to anonymous reviewers and the Central Library, Sardar Vallabhbhai Patel University of Agriculture and Technology, Meerut, UP, India for providing access to online articles and the e-resources.
Conflicts of Interest
The authors declare no competing interest.
Abbreviations
°C—degree Celsius; µg/mL—microgram per milliliter; µM—micromole; 2,4-D—2,4-dichlorophenoxyacetic acid; ABA—abscisic acid; AC—activated charcoal; AgNO3—silver nitrate; BA/BAP/6—BA-6-Benzylaminopurine/benzyladenine; C—callus; BF—bulblet formation; CI—callus induction; CM—callus multiplication; dm−3—cubic decimeter; FYM—farm yard manure; g/L—gram per liter; GA3—gibberellic acid; Gy—gamma rays; HgCl2—mercuric chloride; h—hour; IAA—indole-3-acetic acid; IBA—indole-3-butyric acid; IC—initial culture; Kin—Kinetin; kPa—kilopascal; LB medium—Luria broth medium; lx/lux—luminous flux per unit area; mg L−1—milligram per liter; mmol/L—millimoles per liter; Min—minutes; MS—Murashige and Skoog; NAA—naphthalene acetic acid; NaOCI—sodium hypochlorite; PGR—plant growth regulator; pH—potential of Hydrogen; ppm—part per million; psi—pound per square inch; R.H—relative humidity; RI—root induction, RL—root length; SE—somatic embryogenesis; SEL—shoot elongation; SM—shoot multiplication; TDZ—thidiazuron; temp.—temperature; v/v—volume/volume; w/v—weight/volume.
References
- Lin, T.S.; Shen, T.M. Study on karyotype of Polianthes tuberosa L. J. Agric. For. NCYU 2004, 1, 1–12. [Google Scholar]
- Karihaloo, J.L. Cytological and hybridization studies in three cultivars of tuberose (Polianthes tuberosa L.). Cytologia 2019, 84, 47–52. [Google Scholar] [CrossRef]
- Singh, K.P.; Uma, S. Studies on ratoon crop in tuberose cv. Single and Double. Indian Perfum. 1995, 39, 158–160. [Google Scholar]
- Espejo, S.A. Las Monocotiledoneas Mexicanas: Una Synopsis Floristica [Enlinea]; Serna, A.E.S., López-Ferrari, A.R., Eds.; UAM, Consejo Nacional de la Flora de México, A.C.: Iztapalapa, México, 1993; p. 112. [Google Scholar]
- Datta, S.K. Breeding of ornamentals: Tuberose (Polianthes tuberosa L.). Curr. Sci. 2017, 113, 1255–1263. [Google Scholar] [CrossRef]
- Misra, R.L.; Mahesh, K.S. Bulbous ornamental breeding. Adv. Hortic.-Ornam. Plants 1995, 12, 475–494. [Google Scholar]
- Ahmad, M.S.; Ahmad, T.; Zaidi, N.; Nasir, I.A. High frequency in vitro propagation of Polianthes tuberosa. Pak. J. Sci. Ind. Res. 2006, 49, 344–348. [Google Scholar]
- Khan, N.H.; Zaidi, N.; Jabeen, S.; Javaid, I. Micropropagation potential of Polianthes tuberosa L. bulb scales and leaves. Pak. J. Sci. Ind. Res. 2000, 43, 118–122. [Google Scholar]
- Rajasekharan, V.; Haripriya, K.; Arumugam, S.; Shakila, A. In vitro propagation of tuberose (Polianthes tuberosa L.). In Proceedings of the Centennial Conference on Spices and Aromatic Plants: Challenges and Opportunities in the New Century, Calicut, India, 20–23 September 2000; pp. 86–88. [Google Scholar]
- Nazneen, S.; Jabeen, M.; Ilahi, I. Micropropagation of Polianthus tuberosa (tuberose) through callus formation. Pak. J. Bot. 2003, 35, 17–25. [Google Scholar]
- Mishra, A.; Pandey, R.K.; Gupta, R.K. Micropropagation of tuberose (Polianthus tuberosa L.) cv Calcattia Double. Progress. Hortic. 2005, 37, 226–236. [Google Scholar]
- Jyothi, R.; Singh, A.K.; Singh, K.P. In vitro propagation studies in tuberose (Polianthes tuberosa L.). J. Ornam. Hortic. 2008, 11, 196–201. [Google Scholar]
- Raghuvanshi, S.; Tripathi, M.K.; Vidhya-Sankar, M.; Singh, O.P. Establishment of low-cost effective protocol for massive in vitro propagation in Polianthes tuberosa L. Plant Cell Biotechnol. Mol. Biol. 2013, 14, 49–59. [Google Scholar]
- Taksande, P.N.; Patil, S.R.; Rathod, A.D.; Karad, G.W.; Sayyad, R.A.; Jayade, V.S. Direct shoot organogenesis from stem disc explants of tuberose (Polianthes tuberose L.). J. Soils Crops 2018, 28, 157–164. [Google Scholar]
- Nalousi, A.M.; Hatamzadeh, A.; Azadi, P.; Mohsenpour, M.; Lahiji, H.S. A procedure for indirect shoot organogenesis of Polianthes tuberosa L. and analysis of genetic stability using ISSR markers in regenerated plants. Sci. Hortic. 2019, 244, 315–321. [Google Scholar] [CrossRef]
- Cheesman, L.; Finnie, J.F.; Van Staden, J. Eucomiszambesiaca baker: Factors affecting in vitro bulblet induction. South Afr. J. Bot. 2010, 76, 543–549. [Google Scholar] [CrossRef]
- Ascough, G.D.; Erwin, J.E.; Van Staden, J. Reduced temperature, elevated sucrose, continuous light and gibberellic acid promote corm formation in Watsonia vanderspuyiae. PCTOC 2008, 95, 275–283. [Google Scholar] [CrossRef]
- Aitken-Christie, J.; Kozai, T.; Smith, M.A.L. Automation and Environmental Control in Plant Tissue Culture; Springer Science + Business Media: Dordrecht, The Netherlands, 1995; p. 500. [Google Scholar]
- Krishnamurthy, K.B.; Mythili, J.B.; Srinivas, M. Micro-propagation studies in ‘single’ vs. ‘double’ types of tuberoses (Polianthes tuberosa L.). J. Appl. Hortic. 2001, 3, 82–84. [Google Scholar] [CrossRef]
- Bindhani, B.K.; Dalai, A.K.; Behera, B. In vitro multiple shoot induction in Polianthes tuberosa L. using shoot bud explants. Plant Sci. J. 2004, 26, 24–27. [Google Scholar]
- Ali, M.R.; Akand, M.H.; Hoque, M.E.; Homayra, H.; Mehraj, H.; Jamal Uddin, A.F.M. In vitro regeneration and rapid multiplication of tuberose. Int. J. Bus. Soc. Sci. Res. 2015, 3, 35–38. [Google Scholar]
- Khanchana, K.; Kannan, M.; Hemaprabha, K.; Ganga, M. Standardization of protocol for sterilization and in vitro regeneration in tuberose (Polianthes tuberose L.). Int. J. Chem. Stud. 2019, 7, 236–241. [Google Scholar]
- Copetta, A.; Marchioni, I.; Mascarello, C.; Pistelli, L.; Cambournac, L.; Dimita, R.; Ruffoni, B. Polianthes tuberosa as edible flower: In vitro propagation and nutritional properties. Int. J. Food Eng. 2020, 6, 57–62. [Google Scholar] [CrossRef]
- Kahrizi, D.; Barzegar, B.; Azadi, P. Study on somatic embryogenesis in Polianthes tuberosa. J. Biotechnol. 2008, 136, S150. [Google Scholar] [CrossRef]
- Bindhani, B.K.; Dalal, A.K.; Behara, B. Role of auxins for callus induction and chromosomal variation in Polianthes tuberosa L. ‘Single’. Indian J. Genet. Plant Breed. 2004, 64, 173–174. [Google Scholar]
- Kadam, G.B.; Singh, K.P.; Jyoti, R. Role of sterilants in establishment of aseptic culture using different explants in tuberose (Polianthes tuberosa L.). Progress. Hortic. 2011, 43, 105–109. [Google Scholar]
- Hutchinson, M.J.; Onamu, R.; Obukosia, S. Effect of thidiazurone, benzylaminopurine and naphthalene acetic acid on in vitro propagation of tuberose (Polianthes tuberosa L.) from shoot tip explants. J. Agric. Sci. Technol. 2004, 6, 48–59. [Google Scholar]
- Sangavai, C.; Chellapandi, P. In vitro propagation of tuberose plant (Polianthes tuberosa L.). Electron. J. Biol. 2008, 4, 98–101. [Google Scholar]
- Samanta, A.; Maity, T.R.; Jana, D.; Saha, B.; Datta, S. Standardization of in vitro propagation of Polianthes tuberosa L. (calcutta double). J. Plant Dev. Sci. 2015, 7, 889–891. [Google Scholar]
- Kumari, N.; Pal, A. Multiple shoot regenera tiple shoot regenera tiple shoot regeneration of tuberose (Polianthes tuberosa L.) cv Prajwal by using bulb as explant. Bioscan 2016, 11, 1407–1410. [Google Scholar]
- Narayanaswamy, S.; Prabhudesai, V.R. Somatic psedoembryogeny in tissue cultures of tuberose (Polianthes tuberose L.). Indian J. Exp. Biol. 1979, 17, 873–875. [Google Scholar]
- Upadhyay, G.K.; Bindhani, B.K.; Behera, B. In vitro micropropagation of two varieties of Polyanthes tuberosa L. Plant Sci. J. 2001, 23, 25–28. [Google Scholar]
- Bindhani, B.K.; Dalai, A.K.; Behera, B. Photomorphogenetic effect on callus initiation in tuberose (Polianthes tuberosa L.). Plant Sci. J. 2002, 24, 43–45. [Google Scholar]
- Krishnan, A.G. In vitro Multiplication and Genetic Improvement of Tuberose (Polianthes tuberosa L.). Ph.D. Thesis, Kerala Agricultural University, Kerala, India, 2003. [Google Scholar]
- Surendranath, R.; Ganga, M.; Jawaharlal, M. In vitro propagation of tuberose. Ecol. Environ. 2016, 34, 2556–2560. [Google Scholar]
- Singh, K.B.M.; Madhavan, J.; Sadhukhan, R.; Chandra, S.; Rao, U.; Mandal, P.K. Production of nematode free plantlets in Polianthes tuberosa using in vitro culture techniques. Hortic. Environ. Biotechnol. 2020, 61, 929–937. [Google Scholar] [CrossRef]
- Beyrami, Z.E.; Azadi, P.; Safari, A.; Shafii, M.R.; Sadeqi, S. Study on Somoclonal Variation in Polianthes Tuberosa In vitro Culture; The National Ornamental Plant Research Station: Mahallat, Iran, 2008; p. 102. Available online: http://agris.fao.org/agris-search/search.do?recordID=IR2010000115 (accessed on 12 April 2022).
- Ali, M.R.; Mehraj, H.; Jamal Uddin, A.F.M. Kinetin (KIN) and Indole-3-acetic acid (IAA) on in vitro shoot and root initiation of tuberose. Int. J. Sustain. Agric. Res. 2014, 10, 1–4. [Google Scholar]
- Naz, S.; Aslam, F.; Ilyas, S.; Shahzadi, K.; Tariq, A. In vitro propagation of tuberose (Polianthes tuberose L.). J. Med. Plant Res. 2012, 6, 4107–4112. [Google Scholar]
- Lindsey, K. Plant Tissue Culture Manual, 1st ed.; Kluwer Academic Publishing: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Datta, S.K.; Misra, P.; Mandal, A.K.; Chakrabarty, D. Direct shoot organogenesis from different explants of chrysanthemum, marigold, and tuberose. Isr. J. Plant Sci. 2002, 50, 287–291. [Google Scholar] [CrossRef]
- Pohare, M.; Rathod, H.P.; Shahakar, S.B.; Kelatkar, S.K.; Suryawanshi, P. Effects of UV radiations on morphological characters in in vitro regenerated Polianthes tuberosa. Res. J. Agric. Sci. 2012, 3, 1307–1308. [Google Scholar]
- Jala, A.; Kachonpadungkitti, Y. Tuberose (Polianthes tuberose L.) shoots multiplying and callus induction by benzyladenine, naphthaline acetic acid and oryzaline. Thammasat Int. J. Sci. Technol. 2014, 19, 15–20. [Google Scholar]
- Gajbhiye, S.S.; Tripathi, M.K.; Vidya, M.; Singh, M.S.; Baghel, B.S.; Tiwari, S. Direct shoot organogenesis from cultured stem disc explants of tuberose (Polianthes tuberosa L.). J. Agric. Sci. Technol. 2011, 7, 695–709. [Google Scholar]
- Estrada-Basaldua, J.; Pedraza-Santos, M.; De La Cruz Torres, E.; Martínez-Palacios, A.; Saenz-Romero, C.; Morales-García, J. Effect of 60Co gamma rays in tuberose (Polianthes tuberosa L.). Rev. Mex. Cienc. Agric. 2011, 2, 445–458. [Google Scholar]
- Peña, A.Y.T.; López, L.L.P.; Couoh, E.V.; Ramírez, A.R.; López, D.R. Axenic establishment and in vitro formation of adventitious shoots in nardo (Polianthes tuberose L.). In Proceedings of the Biotechnology Summit, Mérida, Mexico, 12–21 March 2012; pp. 170–173. [Google Scholar]
- Surendranath, R.; Ganga, M.; Ranjitha, G. Can in vitro contaminated culture be revived—A case study with contaminated cultures of tuberose (Polianthes tuberosa). Curr. Biot. 2015, 9, 285–288. [Google Scholar]
- Panigrahi, J.; Rana, V.D.; Patel, I. Multiple shoot regeneration of Polianthes tuberosa cultivars Phulerajni and Calcutta double. Paripex-Indian J. Sci. Res. 2013, 3, 12–14. [Google Scholar]
- Otsuji, K.; Honda, Y.; Sugumira, Y.; Takei, A. Production of polysaccharides in liquid cultures of Polianthes tuberosa cells. Biotechnol. Lett. 1994, 16, 943–948. [Google Scholar] [CrossRef]
- Honda, Y.; Inaoka, H.; Takei, A.; Sugimura, Y.; Otsuji, K. Extracellular polysaccharides produced by tuberose callus. Phytochemistry 1996, 41, 1517–1521. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- White, P.R. The Cultivation of Animal and Plant Cells, 2nd ed.; Ronald Press: New York, NY, USA, 1963. [Google Scholar]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 1968, 50, 151–158. [Google Scholar] [CrossRef]
- Dehdezi, A.A.; Mousavi, S.D.; Azadi, P. Evaluation of different growth regulators on proliferation of (Polianthes tuberosa). Bull. Environ. Pharmacol. Life Sci. 2014, 3, 172–174. [Google Scholar]
- Hernández-Mendoza, F.; Carrillo-Castañeda, G.; Pedraza-Santos, M.E.; de la Cruz-Torres, E.; del Carmen Mendoza-Castillo, M. Regeneration in vitro of shoots Polianthes tuberosa L. through vegetative buds of the inflorescence and from tissue corm. Nova Sci. 2014, 7, 32–47. [Google Scholar] [CrossRef]
- Hernández-Mendoza, F.; Carrillo-Castañeda, G.; García-Gaytán, V.; Pedraza-Santos, M.E.; de la Cruz-Torres, E.; del Carmen Mendoza-Castillo, M. In vitro plant regeneration of Polianthes tuberosa L. from leaf and flower buds tissue. Trop. Subtrop. Agroecosyst. 2021, 24, 55. [Google Scholar]
- Wang, P.J.; Hu, C.Y. Regeneration of virus free plants through in vitro culture. In: Fiechter, A. (ed.). Adv. Biochem. Eng. 1980, 18, 61–99. [Google Scholar]
- Panigrahi, J.; Saiyad, M.S.L. In vitro propagation of Polianthes tuberosa L. cultivars (Calcutta Single). Int. J. Plant Anim. Env. Sci. 2013, 3, 76–79. [Google Scholar]
- Kadam, G.B.; Singh, K.P.; Singh, A.K.; Jyothi, R. In vitro regeneration of tuberose through petals and immature flower buds. Indian J. Hortic. 2010, 67, 76–80. [Google Scholar]
- Panigrahi, J.; Chaudhury, A.A. In vitro propagation of hybrid cultivars (Hyderabad Single & Local Double Navsari) of ornamental plant Polianthes tuberosa L. GRA—Glob. Res. Anal. 2013, 2, 3–4. [Google Scholar]
- Kozai, T.; Kubota, C.; Jeong, B.R. Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tissue Organ Cult. 1997, 51, 49–56. [Google Scholar] [CrossRef]
- Pohare, M.; Batule, B.; Bhor, S.; Shahakar, S.B.; Kelatkar, S.K.; Varandani, S. Effect of gamma radiations on the morphological characters in in vitro regenerated Polianthes tuberosa. Indian J. Hort. 2013, 3, 95–97. [Google Scholar]
- Bose, T.K.; Jana, B.K.; Moulik, S. A note on micropropagation of tuberose from stem scale section. Indian J. Hortic. 1987, 42, 100–101. [Google Scholar]
- Misra, P.; Saema, S. Plant tissue culture for in vitro mutagenesis, large-scale propagation, and genetic transformation. In Plant Tissue Culture: Propagation, Conservation and Crop Improvement; Anis, M., Ahmad, N., Eds.; Springer: Singapore, 2016; pp. 309–342. [Google Scholar]
- Stefaniak, B. Somatic embryogenesis and plant regeneration of Gladiolus (Gladiolus hort.). Plant Cell Rep. 1994, 13, 386–389. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).