Abstract
Climate change is an inevitable process characterized by an abrupt increase in global temperature and a decrease in precipitations leading to drought incidents. Biostimulants could be a valuable tool for mitigating these harsh conditions. The objective of our study was to test the efficiency of two biostimulants, a silicon-based seaweed and the seaweed Ascophyllum nodosum, to mitigate the drought stress endured by watermelon transplants during the first few weeks after transplanting. In order to achieve this, three water treatments (100%, 75%, and 50% of field capacity) were applied in pots. Important growth parameters (leaf number, fresh weight, and plant area) deteriorated depending on water availability. This was also the case for the root system development displayed by root dry weight, total length, and surface area. It is the first time the OJIP transient has been evaluated after the application of A. nodosum for drought-stressed plants. Chlorophyll fluorescence parameters showed that the photosynthetic apparatus was more stressed when A. nodosum was applied, especially in the harshest conditions (i.e., 50% field capacity). Overall, the silicon-based biostimulant failed to demonstrate drought-mitigating potential compared to the non-treated counterparts. On the other hand, A. nodosum alleviated the negative effects of water deficit, especially in the harshest conditions.
1. Introduction
Climate change is an inevitable human-induced process. According to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, global surface temperature rose by 0.85 °C between 1880 and 2021, and is expected to rise further by 0.3–0.7 °C between 2016 and 2035. In addition, heat waves and heavy rainfall have increased in frequency over large parts of Europe [1]. The effects of global warming are already being observed on a global level and are expected to further worsen in the coming years. In addition to the expected increase in average seasonal temperatures, crops are expected to suffer from another key stressor: extreme summer drought that is largely associated with extreme heat [2]. Such conditions prevailed in Central Europe in 2003 [3] and 2018, in Italy in 2017 [4], and an exceptional heatwave also occurred in Europe in early summer of 2019 [5] and 2021.
Drought is nowadays a common but very important stress factor responsible for declining crop yields. This abiotic stressor leads to morphological and physiological alterations in plants by affecting their metabolism. Estimations of water deficiency effects on plants highlighted drought’s negative impact on crop quality [6] and production, which exhibited 50% losses [7], while the electron transport chain within the photosynthetic apparatus is also severely impaired [8].
Biostimulants could be a valuable tool for improving plant production. Biostimulants consist of many subclasses (humic and fulvic acids, hydrolyzed proteins, pollen grains, mycorrhiza fungi, etc.) with different modes of action showing positive results in the treatment of specific issues such as drought and salinity implications [9,10,11,12,13]. A silicon-based biostimulant mitigated the effects of water stress on lettuce [9]. Furthermore, brown seaweed (Ascophyllum nodosum) is nowadays accepted as an effective agronomic input in crop production to alleviate the effects of drought and salinity [10,11]. For example, in a study with tomatoes, A. nodosum alleviated the negative effects of saline irrigation [12].
Watermelon (Citrullus lanatus) is native to West Africa, cultivated for its fruits and established in the soil mainly through transplanted seedlings. While it is considered a berry, the crop’s fruit is large and contains over 90% water. According to the FAOSTAT database, watermelon is an economically important crop throughout the world, producing over 100 million tons per year (FAOSTAT database), and is grown mainly in East Asian and Mediterranean/South European countries [14,15]. In 2021, the value of watermelon exports worldwide amounted to EUR 2.02 billion, while European countries accounted for 47% (EUR 949 million) of the global export value.
The first few weeks after transplantation are critical for plant development due to the occurrence of transplanting shock. The objective of our study was to test the efficiency of two biostimulants to mitigate the water deficiency conditions endured by watermelon transplants until flower blooming. The biostimulants used were a silicon-based seaweed and the seaweed A. nodosum, which were applied on the foliar, while three water (including the control) treatments were applied. Environmental stress factors can damage the photosynthetic mechanism. This damage can be evaluated by the chlorophyll fluorescence OJIP transients which correspond to the reduction stages of the electron transport chain. The photosynthetic performance of rice using silicon has already been tested with the OJIP transient [16]. However, our study is the first attempt to evaluate the plant photosynthetic apparatus under drought stress using the OJIP transient after the application of the seaweed A. nodosum.
2. Materials and Methods
2.1. Plant Growth
The experiment was performed at the greenhouse of the Laboratory of Vegetable Crops (N 40.536; E 22.995) of the Aristotle University of Thessaloniki, Greece, in May 2021. The grafted seedlings were provided by a professional nursery (Agris S.A., Kleidi, Imathia, Greece) at the stage of 3–4 leaves. Specifically, the plant material consisted of watermelon “Celine F1” scions grafted onto interspecific squash (Cucurbita maxima × C. moschata) “TZ-148” hybrid rootstocks. The root system was completely cut-off during grafting, as suggested by Lee and Oda [17] for rapid root growth.
The seedlings were immediately transplanted in plastic pots (volume of 1L) filled with a 2:1 mixture of peat and perlite. Each pot contained one seedling. The transplanted seedlings were irrigated with plenty of water in order for the substrate to be fully saturated. Following this, the pots were transferred into a plastic greenhouse and placed on a large bench at a randomized complete block (RCBD) with six replicates (pots).
2.2. Water Deficiency and Biostimulant Treatments
The drought experiment was initiated two days after transplanting (DAT). At DAT 2 and every two days onwards, the plants were irrigated with different amounts of nutrient solution (Hoagland pH 6.5; electric conductivity 2.6 mS cm−1). Specifically, 18 pots were irrigated with 200 mL which was designated as 100% of field capacity (Control treatment) after watering, 18 pots were irrigated with 150 mL, which was designated as 75% of field capacity after watering, and 18 pots were irrigated with 100 mL, which was designated as 50% of field capacity after watering. The 100% of field capacity (200 mL) was designated upon irrigating several pots (not involved in this experiment) until a runoff of 15–20% water. In the 75% and 50% treatments, there was no runoff water from the pots. A preliminary experiment was conducted in order to determine the proper field water capacity treatments.
Moreover, at DAT 2, six plants per water deficiency treatment (18 pots in total) were sprayed with a silicon-based biostimulant (30 kg/ha) hereby labeled as “Si”, while six different plants per water deficiency treatment (18 pots in total) were sprayed with an A. nodosum seaweed biostimulant (4 L/ha) hereby labeled as “Asc”. The Si biostimulant is comprised of >85% SiO2 (w/v). The Asc is comprised of seaweed extract of A. nodosum 19.5% (w/v), P2O5 (9.8%), and K2O (13.7%). The amounts of foliar application for each biostimulant were in accordance with the instructions on the label for watermelon crops. Biostimulant applications were only performed once throughout the experiment, at DAT 2.
2.3. Determinations and Analysis
The plants were grown until the blooming of the first few flowers at DAT 20. Upon blooming initiation, we determined the leaf number, the stem diameter (using a digital caliper), the female and male flowers, and the relative chlorophyll content using a CCM-200 plus chlorophyll meter (Opti-Sciences, Hudson, NH, USA) which provides dimensionless values. Plant area was determined from images using WinRHIZO Pro software (Regent Instruments Inc., Québec, QC, Canada).
Chlorophyll fluorescence parameters (after 20 min dark adaptation) were determined using a Pocket PEA chlorophyll fluorometer (Hansatech, King’s Lynn, UK). Specifically, PIabs (performance index), φP0 (maximum quantum yield for primary photochemistry), ψE0 (probability that an electron moves further than QA), RC/ABS (QA reducing reaction centers per PSII antenna), VJ (the relative fluorescence at the J-step), and ΔVIP (relative fluorescence increase between the intersystem carriers and electron end acceptors of PSI [18,19].
Upon plant destruction, we measured the shoot fresh weight, as well as the relative water content (RWC). RWC was measured on three fully developed leaves (4th, 5th, and 6th leaf from the lateral bud) per sample using the equation “RWC [%] = [(FW − DW) / (TW − DW)] × 100” with FW = fresh weight, TW = turgor weight, DW = dry weight. TW was measured after the leaves were moisturized in plastic bags filled with water for 24 h. The root dry weight was measured after drying the rinsed roots in an oven (72 °C for 3 days). In addition, the root architecture parameters, such as total root length and root surface area, were determined using WinRHIZO Pro software (Regent Instruments Inc., Québec, QC, Canada).
Statistical analysis was performed with SPSS software (SPSS 23.0, IBM Corp., Armonk, NY, USA) using analysis of variance (ANOVA). Post-hoc analysis was performed with the Tukey method at α = 0.05.
3. Results and Discussion
Climate change is nowadays associated with elevated heat and less precipitations leading to increased frequency and severity of drought incidents. Among the risks arising from global warming, its impact on crop physiological and developmental processes will be devastating.
In our research, we studied the potential of silicon-based and A. nodosum seaweed biostimulants to alleviate the effects of water deficiency imposed on watermelon transplants. Among the evaluated morphometric parameters, the stem diameter and female flower number did not show significant differences regardless of the water and biostimulant treatments (Table 1). Watermelon is a monoecious plant where male flowers start to bloom a few days earlier than female ones. Since the experiment lasted until the blooming of the first few flowers, female flowers did not manage to exhibit potentially significant differences among the different treatments, which, in any case, was not the aim of the study. In addition, stem diameter was also similar in all treatments, which can be attributed to the transplanting shock which ensues the first few weeks after transplantation. However, male flowers were significantly more at 100% compared to 100% Asc, and 50% Si (Table 1). Following this, A. nodosum foliar application alleviated the effects of water deficiency stress on several important growth parameters. Specifically, the leaf number was significantly greater at 100% Asc compared to 75%, 75% Si, and all the 50% water treatments (Figure 1A). The shoot fresh weight was significantly greater at 100% Asc compared to all the 75% and 50% treatments (Figure 1B). Both in the leaf area and in the shoot fresh weight, the 100% treatments showed greater values compared to 75% and 50%, irrespective of the biostimulants, while 50% showed the lowest values. In addition, the plant area was significantly greater at 100% Si and 100% Asc than 50% (Figure 1C). It is obvious that plants irrigated with greater amounts of nutrient solution responded better compared to the water-deficient ones, an effect which was visible even after a rather short period of 20 days after transplantation.

Table 1.
Developmental and physiological parameters of watermelon plants 20 days after transplantation treated with two biostimulants (Si: silicon-based, and Asc: Ascophyllum nodosum seaweed) and additionally irrigated with different amounts of nutrient solution. Mean values (n = 6; ± SE) within a column followed by different letters are significantly different (a < 0.05). RWC: relative water content; RCC: relative chlorophyll content.
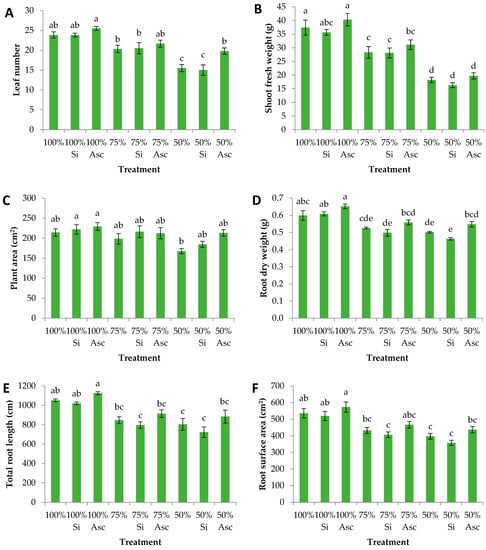
Figure 1.
(A) Leaf number, (B) shoot fresh weight, (C) plant area, (D) root dry weight, (E) total root length, and (F) root surface area of watermelon plants 20 days after transplantation treated with two biostimulants (Si: silicon-based, and Asc: Ascophyllum nodosum seaweed) and additionally irrigated with different amounts of nutrient solution. Mean values (n = 6; ± SE) within a row followed by different letters are significantly different (a < 0.05).
Lettuce plants treated with a silicon-based biostimulant under water deficit showed greater shoot biomass and increased antioxidant content compared to the non-treated counterpart [9], an effect that was not observed in our case. In a study with water-stressed tomatoes, a product including seaweeds (A. nodosum and Laminaria digitata) and yeast resulted in greater shoot fresh weight during flowering (similar to our experiment) compared to non-treated plants [20]. Upon seaweed extract application for plant growth, phytohormones such as auxins and cytokinins or macro- and micronutrients might impose significant positive effects [21,22]. Overall, the plant growth-promoting factors found in A. nodosum induce cell division, subsequently affecting leaf formation and general biomass accumulation [23].
RWC has been shown to be an important indicator of water stress in Brassica [24]. In our case, the relative water content was unaffected by the different water treatments, but 50% water led to significantly lower values regardless of biostimulant application (Table 1). It seems that watermelon has developed a drought avoidance strategy through its evolution by which it forms a dense root system allowing the roots to exploit more water, while the leaves are structured in a way to avoid water loses through transpiration [25]. Moreover, other strategies include modification of photosynthetic proteins to protect photosystem II from photoinhibition, and additionally, alteration in the proteome and transcriptome to regulate tolerance [26]. Drought stress is known to reduce stomatal conductance leading to lower RWC values [27]. Silicon has been shown to increase RWC in wheat [28] but this was not evident in our case where no differences were observed within each water treatment. The difference could be attributed to the shorter time of our experiment, which was possibly insufficient for the exhibition of variable RWC values. Quite similarly, Salvi et al. [29] did not observe stem water potential differences in Vitis vinifera treated with A. nodosum extract compared to non-treated plants. Moreover, a study with Brassica juncea showed greater RWC and related water parameters (i.e., water and osmotic potential) when plants were foliarly sprayed with silicon-based and A. nodosum biostimulants [22]. Galvao et al. [30] performed an experiment including the application of Bacillus amyloliquefaciens (rhizobacteria) and A. nodosum to alleviate drought stress in common beans, and they reported that it was not possible to correlate the use of biostimulants with the mitigation of water deficit effect. It is possible that the stress imposed by the water deficit is very difficult to strongly mitigate only by applying the known biostimulants, especially in crops already equipped with drought resistance mechanisms such as watermelon. In another study, protein hydrolysates derived from casein and soybean showed a great ability to reduce water stress in Vitis vinifera cv. Corvina [31].
The root system is probably the first plant tissue affected by water deficit. The root dry weight was significantly enhanced at the 100% water treatments compared to the 75% and 50% treatments, irrespective of the biostimulants. Specifically, 100% Asc showed significantly greater root dry mass than all the 75% and 50% treatments (Figure 1D). Regarding root architecture parameters, the total root length and root surface area were significantly enhanced at the 100% water treatments compared to the 75% and 50% treatments, irrespective of the biostimulants. Specifically, 100% Asc showed significantly greater total root length and root surface area than all the 75% and 50% treatments (except for 75% Asc root surface area) (Figure 1E,F). Tobacco plants treated with silicon-based biostimulants were shown to develop mechanisms such as greater root surface area and root biomass in order to absorb water in a more sufficient manner [27]. However, this was not the case for watermelon plants where silicon did not affect their root system development compared to 100%. After Si application, a thin layer of silicon was evenly spread on top of the substrate particles, which possibly reduced water transpiration, allowing the plants to absorb more water and limiting the need to develop a substantial root system. On the other hand, A. nodosum showed a strong tendency for increased root system development displayed by root dry biomass, total root length and surface area, which nonetheless was not significant compared to the other treatments. In an experiment with sorghum, the authors reported that silicon treatments mitigated the effects of reduced water on the root system attributes leading to higher drought tolerance, while silicon treatments did not increase the relative expression of aquaporin genes [32]. In addition, humic and fulvic acids in combination with maleic hydrazide showed a clear desmutagenic action in the root tips of Vicia faba [33].
The OJIP transient is a means to display the efficiency of primary photochemistry as well as the structure of the photosystem II (PSII) photosynthetic structure [34]. Even though chlorophyll fluorescence measurements revealed significant differences between certain treatments, all plants developed well, which was also evident by the absolute values of each chlorophyll fluorescence parameter. In our case, PIabs, a parameter that sums up the effects of φP0, ψE0, and RC/ABS, was greater at the non-biostimulant and Si treatments compared to the Asc treatments. Specifically, 75% had greater values than 100%, 75% Asc and 50% Asc (Figure 2A). Similarly, light trapping (i.e., RC/ABS) was significantly greater at 100% Asc, 75%, 75% Si, 50%, and 50% Si compared to 50% Asc (Figure 2B). The latter followed a similar trend with PIabs showing that differences in PIabs can mostly be attributed to the RC/ABS component. In a study with rice, Wang et al. [16] found that the PIabs of drought-stressed plants diminished, while the addition of silicon enhanced the activity of the electron transfer chain and improved their adaptability. The authors concluded that silicon alters the components of the thylakoid membrane protein, ultimately playing an important part in the transfer of light energy. However, drought-imposed watermelon transplants did not significantly alter their photosynthetic reactions after silicon application.
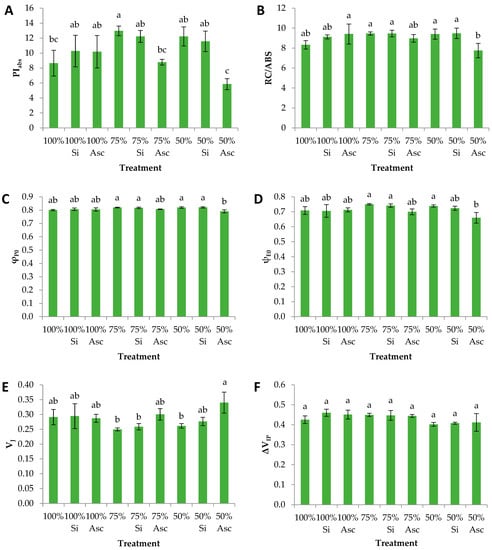
Figure 2.
(A) PIabs, (B) RC/ABS, (C) φP0, (D) ψE0, (E) VJ, and (F) ΔVIP of watermelon plants 20 days after transplantation treated with two biostimulants (Si: silicon-based, and Asc: Ascophyllum nodosum seaweed) and additionally irrigated with different amounts of nutrient solution. Mean values (n = 6; ± SE) within a row followed by different letters are significantly different (a < 0.05).
Both the quantum efficiency of QA reduction (φP0) and the probability of electron transport from QA to the intersystem carriers of the photosynthetic apparatus (ψE0) showed greater values at 75%, 75% Si, 50%, and 50% Si compared to 50% Asc (Figure 2C,D). φP0 was greater at the Si than the Asc treatments, while ψΕ0 was greater at the non-biostimulant than the Asc treatments, irrespective of the water amount. Nonetheless, both parameters showed high values in all treatments. In particular, φP0 (also known as Fv/Fm) has been reported to reach values of 0.78–0.86 in non-stressed plants [35]. In drought-stressed lettuce plants irrigated at 75% field capacity, PIabs and φP0 (stated as Fv/Fm) were greater after the input of a silicon-based biostimulant [27]. Nonetheless, PIabs is considered a more sensitive parameter for the detection of abiotic stress factors compared to φP0 [36,37,38]. The relative fluorescence at the J-step of OJIP transient (VJ) was significantly greater at 50% Asc than at 50%, 75%, and 75% Si (Figure 2E). In the same parameter, the Si and Asc water treatments showed greater values compared to the non-biostimulant treatments, irrespective of the water amount. The increased VJ values in the Asc treatments mentioned above indicate inhibition of the photosystem II through diminished electron flow to QA. This could be due to damage caused by water deficit or by controlled silencing to protect the photosynthetic apparatus from over-reduction.
Watermelon is a very sturdy species with great ability to withstand prolonged drought which is displayed by the vast root system and thick leaves. In our study, the artificial reduction of field water capacity did not manage to considerably deteriorate the crops’ photosynthetic performance. However, foliar application of A. nodosum seaweed during the first few days after transplanting led to (statistically significant) diminished photosynthetic activity, as displayed by several OJIP parameters. This discomfort might have been perceived by the plants as a threat; an alarm provoking them to orientate their efforts to form an extensive root system, to accumulate biomass, and to form plenty of leaves, hence the relatively reduced number of flowers (c.f. Table 1). The above leads to the conclusion that a positive response, a eustress, ensues with the inclusion of the seaweed A. nodosum during a strong water deficit imposed on watermelon transplants.
Moreover, ΔVIP, which is the relative fluorescence increase between the intersystem carriers and the PSI electron end acceptors, exhibited insignificant differences among the water deficiency treatments (Figure 2F). This was also the case for the relative chlorophyll content, which showed similar values in all treatments, even though 50% Asc showed a strong tendency for reduced values (Table 1). Nonetheless, studies [39] showed that drought stress causes chlorophyll decomposition in plants, an effect that can be alleviated in rice by the addition of silicon [16]. On the other hand, Xu and Leskovar [40] reported that under 100% field capacity the seaweed A. nodosum imposed no effects on the chlorophyll content and chlorophyll fluorescence of spinach. Chlorophyll decomposition is a protective mechanism of the photosynthetic apparatus during extreme conditions [41]. The insignificant differences in relative chlorophyll content enhance the scenario by which watermelon crops have the capacity to withstand extreme water deficit without considerably damaging their photosynthetic apparatus and ultimately diminishing their development. Chlorophyll degradation is also known to be a result of reduced nitrogen content, which is rather used by plants for active growth [42]. Moreover, the photosynthetic capacity is often positively correlated with the nitrogen concentration in the leaves, thus, chlorophyll and nitrogen content can be used to determine the plant’s photosynthetic activity [43].
4. Conclusions
Two biostimulants, a silicon-based seaweed and the seaweed A. nodosum, were tested as means to mitigate the water deficit effects on watermelon transplants. As expected, 100% field water capacity led to greater aboveground and underground development compared to water-deficient counterparts of 75% and 50% field capacity. Silicon neither deteriorated nor improved the situation. However, A. nodosum significantly enhanced important parameters such as leaf number and plant area, and showed a strong tendency for extensive root system development. Moreover, our study is the first attempt to evaluate the plant photosynthetic apparatus under drought stress using the OJIP transient after the application of the seaweed A. nodosum. Watermelon is a very sturdy species with great ability to withstand prolonged drought, thus photosynthetic parameters were not considerably depleted when water was deficient. In addition, silicon application did not significantly attenuate the impact of reduced water on the photosynthetic apparatus. However, A. nodosum application triggered a slight stress which provoked a positive response during a strong water deficit. Therefore, watermelon transplants concentrated their efforts to form a vast root system, to accumulate biomass, and to form plenty of leaves. Overall, water reduction depleted watermelon growth attributes, but supplementation with A. nodosum mitigated the impact of stress.
Author Contributions
Conceptualization, methodology, and data analysis, F.B. and A.K.; experimental measurements, F.B.; writing—original draft preparation, F.B.; writing—review and editing, F.B. and A.K.; supervision and project administration, A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEARCH—CREATE—INNOVATE (project code: T1EDK-00960, LEDWAR.gr).
Acknowledgments
The authors would like to express their gratitude to Christodoulos Dangitsis and Agris S.A. for their valuable suggestions regarding the seedlings, as well as Eleni Papoui, Emmanouil Kokolakis, and Anna Gkotzamani, Agronomists, for their assistance with the cultivation practices.
Conflicts of Interest
The authors declare no conflict of interest.
References
- IPCC. Climate Change 2021: The Physical Science Basis. In Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; 2391p. [Google Scholar]
- Carnicer, J.; Coll, M.; Ninyerola, M.; Pons, X.; Sanchez, G.; Penuelas, J. Widespread crown condition decline, food web disruption, and amplified tree mortality with increased climate change-type drought. Proc. Natl. Acad. Sci. USA 2011, 108, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Breda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Puletti, N.; Mattioli, W.; Bussotti, F.; Pollastrini, M. Monitoring the effects of extreme drought events on forest health by Sentinel-2 imagery. J. Appl. Remote Sens. 2019, 13, 020501. [Google Scholar] [CrossRef]
- Bantis, F.; Graap, J.; Fruchtenicht, E.; Bussotti, F.; Radoglou, K.; Bruggemann, W. Field Performances of Mediterranean Oaks in Replicate Common Gardens for Future Reforestation under Climate Change in Central and Southern Europe: First Results from a Four-Year Study. Forests 2021, 12, 678. [Google Scholar] [CrossRef]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of plants to water stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P.; Machado, R.M.A.; Serralheiro, R.P. Soil Salinity: Effect on Vegetable Crop Growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Hayat, S.; Hasan, S.A.; Fariduddin, Q.; Ahmad, A. Growth of tomato (Lycopersicon esculentum) in response to salicylic acid under water stress. J. Plant Interact. 2008, 3, 297–304. [Google Scholar] [CrossRef]
- Hidalgo-Santiago, L.; Navarro-León, E.; López-Moreno, F.J.; Arjó, G.; González, L.M.; Ruiz, J.M.; Blasco, B. The application of the silicon-based biostimulant Codasil® offset water deficit of lettuce plants. Sci. Hortic. 2021, 285, 110177. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Miras Moreno, M.B.; Reynaud, H.; Canaguier, R.; Trtílek, M.; et al. A combined phenotypic and metabolomic approach for elucidating the biostimulant action of a plant-derived protein hydrolysate on tomato grown under limited water availability. Front. Plant Sci. 2019, 10, 493. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro-and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef]
- Ikuyinminu, E.; Goñi, O.; O’Connell, S. Enhancing Irrigation Salinity Stress Tolerance and Increasing Yield in Tomato Using a Precision Engineered Protein Hydrolysate and Ascophyllum nodosum-Derived Biostimulant. Agronomy 2022, 12, 809. [Google Scholar] [CrossRef]
- Papoui, E.; Bantis, F.; Kapoulas, N.; Ipsilantis, I.; Koukounaras, A. A Sustainable Intercropping System for Organically Produced Lettuce and Green Onion with the Use of Arbuscular Mycorrhizal Inocula. Horticulturae 2022, 8, 466. [Google Scholar] [CrossRef]
- Lee, J.M.; Kubota, C.; Tsao, S.J.; Bie, Z.; Hoyos Echevarria, P.; Morra, L.; Oda, M. Current status of vegetable grafting: Diffusion, grafting techniques, automation. Sci. Hortic. 2010, 127, 93–105. [Google Scholar] [CrossRef]
- Bantis, F.; Panteris, E.; Dangitsis, C.; Carrera, E.; Koukounaras, A. Blue light promotes hormonal induced vascular reconnection, while red light boosts the physiological response and quality of grafted watermelon seedlings. Sci. Rep. 2021, 11, 21754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, B.; Jiang, D.; Chen, G. Silicon improves photosynthetic performance by optimizing thylakoid membrane protein components in rice under drought stress. Environ. Exp. Bot. 2019, 158, 117–124. [Google Scholar] [CrossRef]
- Lee, J.M.; Oda, M. Grafting of Herbaceous Vegetable and Ornamental Crops. In Horticultural Review; Janick, J., Ed.; John Wiley & Sons: New York, NY, USA, 2003; pp. 61–124. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Bantis, F.; Fruchtenicht, E.; Graap, J.; Stroll, S.; Reininger, N.; Schafer, L.; Pollastrini, M.; Holland, V.; Bussotti, F.; Radoglou, K.; et al. The JIP-test as a tool for forestry in times of climate change. Photosynthetica 2020, 58, 224–236. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Mansori, M.; Chernane, H.; Latique, S.; Benaliat, A.; Hsissou, D.; El Kaoua, M. Seaweed extract effect on water deficit and antioxidative mechanisms in bean plants (Phaseolus vulgaris L.). J. Appl. Phycol. 2015, 27, 1689–1698. [Google Scholar] [CrossRef]
- Goyal, V.; Baliyan, V.; Avtar, R.; Mehrotra, S. Alleviating Drought Stress in Brassica juncea (L.) Czern & Coss. by Foliar Application of Biostimulants—Orthosilicic Acid and Seaweed Extract. Appl. Biochem. Biotechnol. 2022, 1–29. [Google Scholar]
- Jacomassi, L.M.; Viveiros, J.O.; Oliveira, M.P.; Momesso, L.; de Siqueira, G.F.; Crusciol, C.A.C. A seaweed extract-based biostimulant mitigates drought stress in sugarcane. Front. Plant Sci. 2022, 13, 865291. [Google Scholar] [CrossRef]
- Chaghakaboodi, Z.; Kakaei, M.; Zebarjadi, A. Study of relationship between some agrophysiological traits with drought tolerance in rapeseed (Brassica napus L.) genotypes. Cent. Asian J. Plant Sci. Innov. 2021, 1, 1–9. [Google Scholar]
- Yoshimura, K.; Masuda, A.; Kuwano, M.; Yokota, A.; Akashi, K. Programmed Proteome Response for Drought Avoidance/Tolerance in the Root of a C3 Xerophyte (Wild Watermelon) Under Water Deficit. Plant Cell Physiol. 2008, 49, 226–241. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Mo, Y.; Cui, Q.; Yang, X.; Guo, Y.; Wei, C.; Yang, J.; Zhang, Y.; Ma, Y.; Zhang, X. Transcriptomic and physiological analyses reveal drought adaptation strategies in drought-tolerant and -susceptible watermelon genotypes. Plant Sci. 2019, 278, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Hajiboland, R.; Cheraghvareh, L.; Poschenrieder, C. Improvement of drought tolerance in tobacco (Nicotiana rustica L.) plants by silicon. J. Plant Nutr. 2017, 40, 1661–1676. [Google Scholar] [CrossRef]
- Gong, H.; Chen, K. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Galvao, I.M.; dos Santos, O.F.; de Souza, M.L.C.; Guimaraes, J.J.; Kuhn, I.E.; Broetto, F. Biostimulants action in common bean crop submitted to water deficit. Agric. Water Manag. 2019, 225, 105762. [Google Scholar] [CrossRef]
- Boselli, M.; Bahouaoui, M.A.; Lachhab, N.; Sanzani, S.M.; Ferrara, G.; Ippolito, A. Protein hydrolysates effects on grapevine (Vitis vinifera L., cv. Corvina) performance and water stress tolerance. Sci. Hortic. 2019, 258, 108784. [Google Scholar] [CrossRef]
- Avila, R.G.; Magalhães, P.C.; da Silva, E.M.; Júnior, C.C.G.; Lana, U.G.D.P.; de Alvarenga, A.A.; de Souza, T.C. Silicon Supplementation Improves Tolerance to Water Deficiency in Sorghum Plants by Increasing Root System Growth and Improving Photosynthesis. Silicon 2020, 12, 2545–2554. [Google Scholar] [CrossRef]
- Ferrara, G.; Loffredo, E.; Simeone, R.; Senesi, N. Evaluation of antimutagenic and desmutagenic effects of humic and fulvic acids on root tips of Vicia faba. Environ. Toxicol. 2000, 15, 513–517. [Google Scholar] [CrossRef]
- Lazár, D. The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. Funct. Plant Biol. 2006, 33, 9–30. [Google Scholar] [CrossRef] [PubMed]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origin. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Strasser, R.; Srivastava, A.; Tsimilli-Michael, M. The Fluorescence Transient as a Tool to Characterize and Screen Photosynthetic Samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis Publishers: London, UK, 2000; pp. 445–483. [Google Scholar]
- Christen, D.; Schönmann, S.; Jermini, M.; Strasser, R.J.; Dèfago, G. Characterization and early detection of grapevine (Vitis vinifera) stress responses to esca disease by in situ chlorophyll fluorescence and comparison with drought stress. Environ. Exp. Bot. 2007, 60, 504–514. [Google Scholar] [CrossRef]
- Bantis, F.; Fotelli, M.; Ilic, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with LEDs and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Wu, F.Z.; Bao, W.K.; Li, F.L.; Wu, N. Effects of drought stress and N supply on the growth, biomass partitioning and water-use efficiency of Sophora davidii seedlings. Environ. Exp. Bot. 2008, 63, 248–255. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of A. nodosum seaweed extracts on spinach growth, physiology and nutrition value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Bantis, F.; Radoglou, K.; Brüggemann, W. Differential ecophysiological responses to seasonal drought of three co-existing oak species in northern Greece. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2018, 153, 378–384. [Google Scholar] [CrossRef]
- Furlani Júnior, E.; Nakagawa, J.; Bulhões, L.J.; Moreira, J.A.A.; Grassi Filho, H. Correlation between chlorophyll readings and levels of nitrogen applied in bean. Bragantia 1996, 55, 171–175. [Google Scholar]
- Pal, P.K.; Prasad, R.; Singh, R.D. Evaluating the non-destructive method for determining the chlorophyll and nitrogen content in Stevia rebaudiana (Bertoni) leaf. Plant Biosyst. 2015, 149, 131–135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).