Enhanced Production of Apocarotenoids by Salicylic Acid Elicitation in Cell Suspension Cultures of Saffron (Crocus sativus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Saffron Suspension-Cultured Cells and SA Elicitation
2.2. Extraction and Separation of Crocin and Carotenoids from Saffron Treated Cells
2.3. HPLC-DAD Analysis of Crocin and Carotenoids
2.4. Isolation of Nucleic Acids and cDNA Synthesis
2.5. Quantitative RT-PCR Analysis
2.6. Statistical Analysis
3. Results
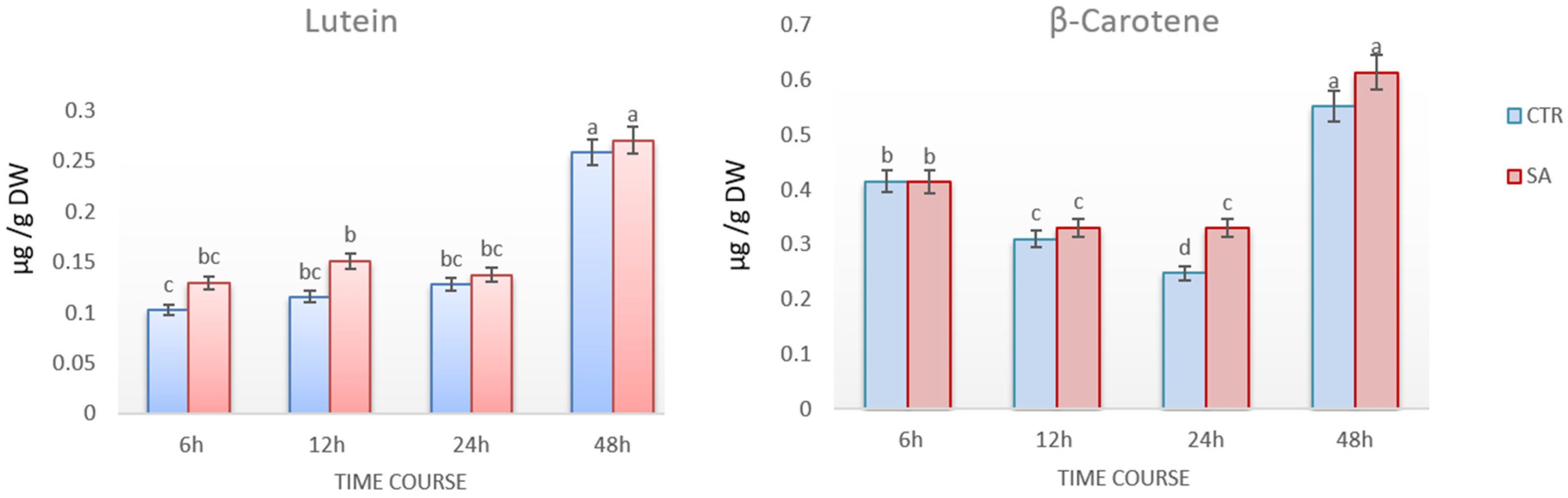
3.1. Carotenoid Contents after 0.5 mM SA Elicitation
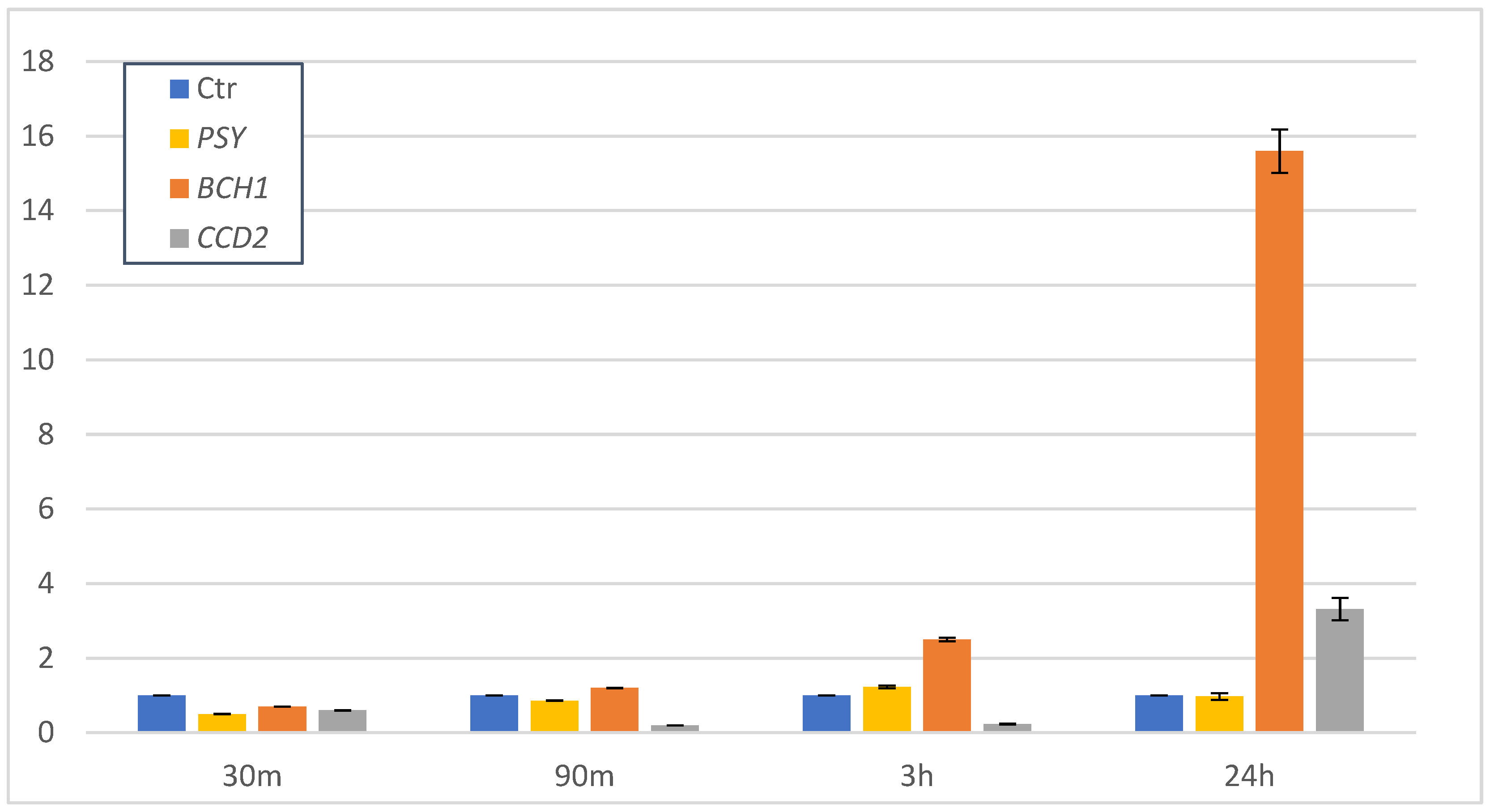
3.2. Gene Expression of Crocin Biosynthetic Pathway after SA Treatment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, R.; Ahmed, H.; Dixit, R.K.; Dharamveer; Saraf, S.A. Crocus sativus L.: A comprehensive review. Pharmacogn. Rev. 2010, 4, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Gohari, A.R.; Saeidnia, S.; Mahmoodabadi, M.K. An overview on saffron, phytochemicals, and medicinal properties. Pharmacogn. Rev. 2013, 7, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.I.; Manzoor, M.; Dhar, M.K. A comprehensive review of the pharmacological potential of Crocus sativus and its bioactive apocarotenoids. Biomed. Pharmacother. 2018, 98, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Rahaiee, S.; Moini, S.; Hashemi, M.; Shojaosadati, S.A. Evaluation of antioxidant activities of bioactive compounds and various extracts obtained from saffron (Crocus sativus L.): A review. J. Food Sci. Technol. 2015, 52, 1881–1888. [Google Scholar] [CrossRef]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z. Evaluation of Crocus sativus L. stigma phenolic and flavonoid compounds and its antioxidant activity. Molecules 2010, 15, 6244–6256. [Google Scholar] [CrossRef]
- Amin, A.; Hamza, A.A.; Bajbouj, K.; Ashraf, S.S.; Daoud, S. Saffron: A potential candidate for a novel anticancer drug against hepatocellular carcinoma. Hepatology 2011, 54, 857–867. [Google Scholar] [CrossRef]
- Seifi, A.; Shayesteh, H. Chapter 15—Molecular biology of Crocus sativus. In Saffron; Koocheki, A., Khajeh-Hosseini, M., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 247–258. [Google Scholar] [CrossRef]
- Frusciante, S.; Diretto, G.; Bruno, M.; Ferrante, P.; Pietrella, M.; Prado-Cabrero, A.; Rubio-Moraga, A.; Beyer, P.; Gomez-Gomez, L.; Al-Babili, S.; et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 12246–12251. [Google Scholar] [CrossRef]
- Baba, S.A.; Mohiuddin, T.; Basu, S.; Swarnkar, M.K.; Malik, A.H.; Wani, Z.A.; Abbas, N.; Singh, A.K.; Ashraf, N. Comprehensive transcriptome analysis of Crocus sativus for discovery and expression of genes involved in apocarotenoid biosynthesis. BMC Genom. 2015, 16, 698. [Google Scholar] [CrossRef]
- Yue, J.; Wang, R.; Ma, X.; Liu, J.; Lu, X.; Balaso Thakar, S.; An, N.; Liu, J.; Xia, E.; Liu, Y. Full-length transcriptome sequencing provides insights into the evolution of apocarotenoid biosynthesis in Crocus sativus. Comput. Struct. Biotechnol. J. 2020, 18, 774–783. [Google Scholar] [CrossRef]
- Cazzonelli, C.I.; Pogson, B.J. Source to sink: Regulation of carotenoid biosynthesis in plants. Trends Plant Sci. 2010, 15, 266–274. [Google Scholar] [CrossRef]
- Castillo, R.; Fernandez, J.A.; Gomez-Gomez, L. Implications of carotenoid biosynthetic genes in apocarotenoid formation during the stigma development of Crocus sativus and its closer relatives. Plant Physiol. 2005, 139, 674–689. [Google Scholar] [CrossRef] [PubMed]
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Huhn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Paolis, A.; Frugis, G.; Giannino, D.; Iannelli, M.A.; Mele, G.; Rugini, E.; Silvestri, C.; Sparvoli, F.; Testone, G.; Mauro, M.L.; et al. Plant Cellular and Molecular Biotechnology: Following Mariotti’s Steps. Plants 2019, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Ee, S.F.; Oh, J.M.; Mohd Noor, N.; Kwon, T.R.; Mohamed-Hussein, Z.A.; Ismail, I.; Zainal, Z. Transcriptome profiling of genes induced by salicylic acid and methyl jasmonate in Polygonum minus. Mol. Biol. Rep. 2013, 40, 2231–2241. [Google Scholar] [CrossRef]
- Gadzovska, S.; Maury, S.; Delaunay, A.; Spasenoski, M.; Hagège, D.; Courtois, D.; Joseph, C. The influence of salicylic acid elicitation of shoots, callus, and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. (PCTOC) 2013, 113, 25–39. [Google Scholar] [CrossRef]
- Moradi, A.; Zarinkamar, F.; De Domenico, S.; Mita, G.; Di Sansebastiano, G.P.; Caretto, S. Salycilic Acid Induces Exudation of Crocin and Phenolics in Saffron Suspension-Cultured Cells. Plants 2020, 9, 949. [Google Scholar] [CrossRef]
- Moradi, A.; Zarinkamar, F.; Caretto, S.; Azadi, P. Influence of thidiazuron on callus induction and crocin production in corm and style explants of Crocus sativus L. Acta Physiol. Plant. 2018, 40, 185. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Sadler, G.; Davis, J.; Dezman, D. Rapid Extraction of Lycopene and β-Carotene from Reconstituted Tomato Paste and Pink Grapefruit Homogenates. J. Food Sci. 1990, 55, 1460–1461. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Rizzello, F.; De Paolis, A.; Durante, M.; Blando, F.; Mita, G.; Caretto, S. Enhanced Production of Bioactive Isoprenoid Compounds from Cell Suspension Cultures of Artemisia annua L. Using β-Cyclodextrins. Int. J. Mol. Sci. 2014, 15, 19092–19105. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Srivastava, P.L.; Verma, M.; Ghangal, R.; Garg, R. De novo transcriptome assembly and comprehensive expression profiling in Crocus sativus to gain insights into apocarotenoid biosynthesis. Sci. Rep. 2016, 6, 22456. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M. Chemical and Medicinal Properties of Saffron. Bull. Environ. Pharmacol. Life Sci. 2015, 4, 69–81. [Google Scholar]
- Veisi, A.; Akbari, G.; Mard, S.A.; Badfar, G.; Zarezade, V.; Mirshekar, M.A. Role of crocin in several cancer cell lines: An updated review. Iran. J. Basic Med. Sci. 2020, 23, 3–12. [Google Scholar] [CrossRef]
- Gorelick, J.; Bernstein, N. Chapter Five—Elicitation: An Underutilized Tool in the Development of Medicinal Plants as a Source of Therapeutic Secondary Metabolites. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 124, pp. 201–230. [Google Scholar]
- Bhat, Z.Y.; Mohiuddin, T.; Kumar, A.; Lopez-Jimenez, A.J.; Ashraf, N. Crocus transcription factors CstMYB1 and CstMYB1R2 modulate apocarotenoid metabolism by regulating carotenogenic genes. Plant Mol. Biol. 2021, 107, 49–62. [Google Scholar] [CrossRef]
- Loc, N.H.; Giang, N.T.; Huy, N.D. Effect of salicylic acid on expression level of genes related with isoprenoid pathway in centella (Centella asiatica (L.) Urban) cells. 3 Biotech 2016, 6, 86. [Google Scholar] [CrossRef]
- Yamamoto, R.; Ma, G.; Zhang, L.; Hirai, M.; Yahata, M.; Yamawaki, K.; Shimada, T.; Fujii, H.; Endo, T.; Kato, M. Effects of Salicylic Acid and Methyl Jasmonate Treatments on Flavonoid and Carotenoid Accumulation in the Juice Sacs of Satsuma Mandarin In Vitro. Appl. Sci. 2020, 10, 8916. [Google Scholar] [CrossRef]
- Ahrazem, O.; Rubio-Moraga, A.; Lopez, R.C.; Gomez-Gomez, L. The expression of a chromoplast-specific lycopene beta cyclase gene is involved in the high production of saffron’s apocarotenoid precursors. J. Exp. Bot. 2010, 61, 105–119. [Google Scholar] [CrossRef]
- Moraga, Á.R.; Rambla, J.L.; Ahrazem, O.; Granell, A.; Gómez-Gómez, L. Metabolite and target transcript analyses during Crocus sativus stigma development. Phytochemistry 2009, 70, 1009–1016. [Google Scholar] [CrossRef]
- Ashraf, N.; Jain, D.; Vishwakarma, R.A. Identification, cloning and characterization of an ultrapetala transcription factor CsULT1 from Crocus: A novel regulator of apocarotenoid biosynthesis. BMC Plant Biol. 2015, 15, 25. [Google Scholar] [CrossRef] [PubMed]
- Ahrazem, O.; Rubio-Moraga, A.; Berman, J.; Capell, T.; Christou, P.; Zhu, C.; Gomez-Gomez, L. The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 2016, 209, 650–663. [Google Scholar] [CrossRef]
- Balestrini, R.; Brunetti, C.; Cammareri, M.; Caretto, S.; Cavallaro, V.; Cominelli, E.; De Palma, M.; Docimo, T.; Giovinazzo, G.; Grandillo, S.; et al. Strategies to Modulate Specialized Metabolism in Mediterranean Crops: From Molecular Aspects to Field. Int. J. Mol. Sci. 2021, 22, 2887. [Google Scholar] [CrossRef]
- Dong, J.; Wan, G.; Liang, Z. Accumulation of salicylic acid-induced phenolic compounds and raised activities of secondary metabolic and antioxidative enzymes in Salvia miltiorrhiza cell culture. J. Biotechnol. 2010, 148, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Kawano, T. Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 2003, 21, 829–837. [Google Scholar] [CrossRef]
- Usha Rani, P.; Jyothsna, Y. Biochemical and enzymatic changes in rice plants as a mechanism of defense. Acta Physiol. Plant. 2010, 32, 695–701. [Google Scholar] [CrossRef]
- Hao, W.; Guo, H.; Zhang, J.; Hu, G.; Yao, Y.; Dong, J. Hydrogen peroxide is involved in salicylic acid-elicited rosmarinic acid production in Salvia miltiorrhiza cell cultures. Sci. World J. 2014, 2014, 843764. [Google Scholar] [CrossRef]


| Gene | Accession Number | Primer | Sequence (5’------>3’) |
|---|---|---|---|
| Cs18s | Fw | GGCGCCAAGGAACACTTCT | |
| AJ489273 | Rv | CTCCCTATCGTGGGACAGACA | |
| Probe | CGTCGCGGCCCTCTCCACCT | ||
| CsPSY | Fw | GGCCGCCCATATGACATG | |
| AJ888514.1 | Rv | AAGGGCTGAATGTCAACTGGAA | |
| Probe | TCGATGCTGCCTTGTCTGATACCGTCTC | ||
| CsCCD2 | Fw | TGAGTTGGGACCTAGAAGATATGGT | |
| KJ541749.1 | Rv | CCGTCATCCTCATCAGATTTGA | |
| Probe | AGGCAATATTTGTGCCATGCCAACCTG | ||
| CsBCH1 | Fw | CGACGTCTTCGCCATAATCAA | |
| AJ416711.2 | Rv | CTGTGGAAGAAGCCGAAGTTG | |
| Probe | TCCCCGCCATCGCCCTCC | ||
| Cs(g) BCH1 | AJ416711.2 | Fw | CCGACGTGCCCTACTTC |
| Rv | AATCCTCCTGCTCACCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moradi, A.; Zarinkamar, F.; Mita, G.; Caretto, S.; De Paolis, A. Enhanced Production of Apocarotenoids by Salicylic Acid Elicitation in Cell Suspension Cultures of Saffron (Crocus sativus L.). Horticulturae 2022, 8, 1176. https://doi.org/10.3390/horticulturae8121176
Moradi A, Zarinkamar F, Mita G, Caretto S, De Paolis A. Enhanced Production of Apocarotenoids by Salicylic Acid Elicitation in Cell Suspension Cultures of Saffron (Crocus sativus L.). Horticulturae. 2022; 8(12):1176. https://doi.org/10.3390/horticulturae8121176
Chicago/Turabian StyleMoradi, Azar, Fatemeh Zarinkamar, Giovanni Mita, Sofia Caretto, and Angelo De Paolis. 2022. "Enhanced Production of Apocarotenoids by Salicylic Acid Elicitation in Cell Suspension Cultures of Saffron (Crocus sativus L.)" Horticulturae 8, no. 12: 1176. https://doi.org/10.3390/horticulturae8121176
APA StyleMoradi, A., Zarinkamar, F., Mita, G., Caretto, S., & De Paolis, A. (2022). Enhanced Production of Apocarotenoids by Salicylic Acid Elicitation in Cell Suspension Cultures of Saffron (Crocus sativus L.). Horticulturae, 8(12), 1176. https://doi.org/10.3390/horticulturae8121176







