The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review
Abstract
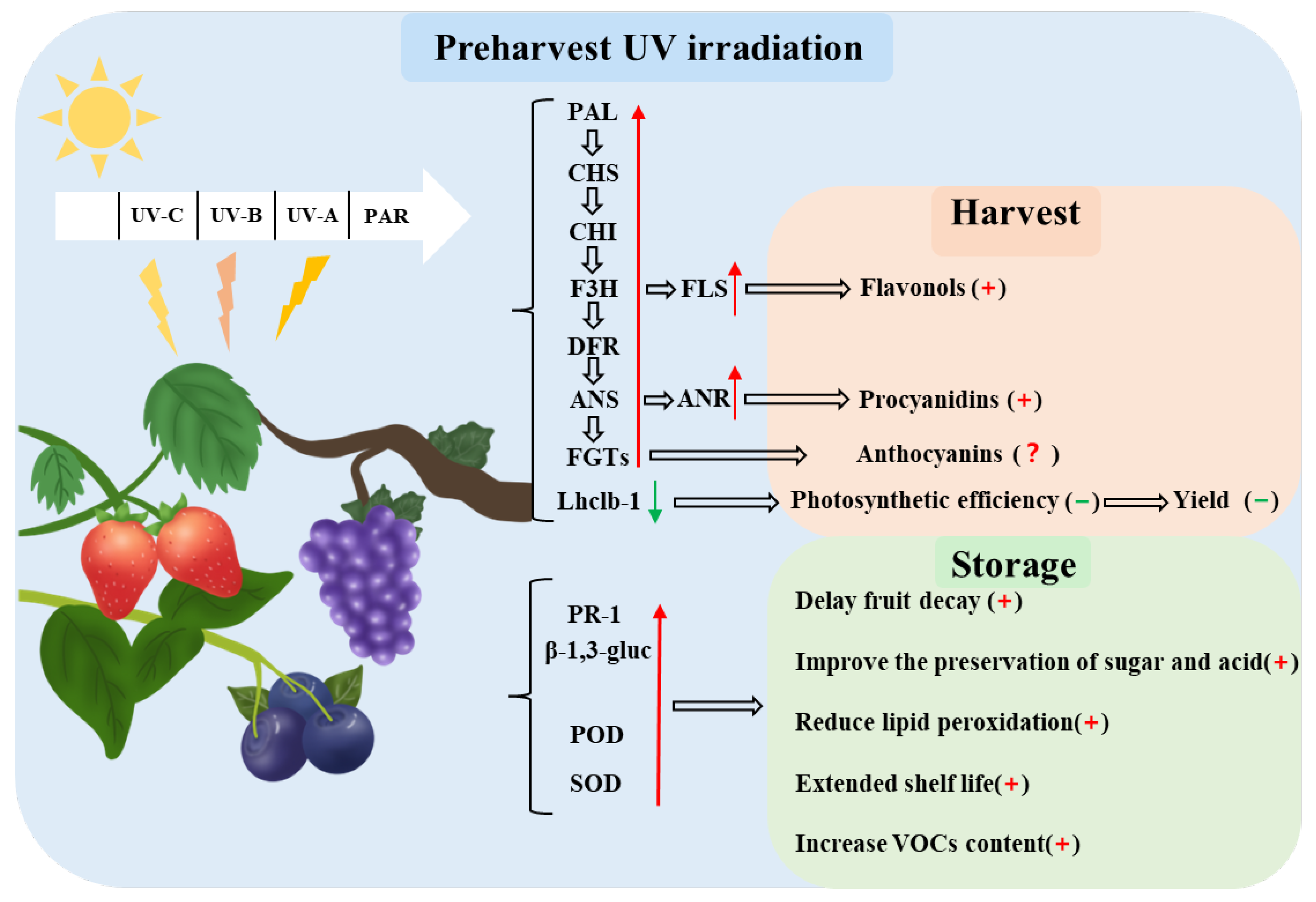
1. Introduction
2. Effect of Preharvest UV Light Treatment on Berries Appearance Quality
2.1. Effect of Preharvest UV Light Treatment on Berries firmness
2.2. Effect of Preharvest UV Light Irradiation on Berries Skin Color
2.3. Effects of Preharvest UV Light Irradiation on Flavonoids and Phenols in Berries
3. Effect of Preharvest UV Light Treatment on Berries Flavor
3.1. Effect of Preharvest UV Light Treatment on Sugar and Acid Content of Berry
3.2. Effect of Preharvest UV Light Irradiation on Volatile Compounds in Berries
4. Effect of Preharvest UV Light Irradiation on Disease Resistance of Berries
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cisneros-Zevallos, L. The Use of Controlled Postharvest Abiotic Stresses as a Tool for Enhancing the Nutraceutical Content and Adding-Value of Fresh Fruits and Vegetables. J. Food Sci. 2003, 68, 1560–1565. [Google Scholar] [CrossRef]
- Barka, E.A. Protective enzymes against reactive oxygen species during ripening of tomato (Lycopersicon esculentum) fruits in response to low amounts of UV-C. Funct. Plant Biol. 2001, 28, 785–791. [Google Scholar] [CrossRef]
- Baka, M.; Mercier, J.; Corcuff, R.; Castaigne, F.; Arul, J. Photochemical Treatment to Improve Storability of Fresh Strawberries. J. Food Sci. 1999, 64, 1068–1072. [Google Scholar] [CrossRef]
- Mercier, J.; Baka, M.; Reddy, B.; Corcuff, R.; Arul, J. Shortwave Ultraviolet Irradiation for Control of Decay Caused by Botrytis cinerea in Bell Pepper: Induced Resistance and Germicidal Effects. J. Am. Soc. Hortic. Sci. 2001, 126, 128–133. [Google Scholar] [CrossRef]
- Mullenders, L.H.F. Solar UV damage to cellular DNA: From mechanisms to biological effects. Photochem. Photobiol. Sci. 2018, 17, 1842–1852. [Google Scholar] [CrossRef]
- Batista, L.F.Z.; Kaina, B.; Meneghini, R.; Menck, C.F.M. How DNA lesions are turned into powerful killing structures: Insights from UV-induced apoptosis. Mutat. Res. 2009, 681, 197–208. [Google Scholar] [CrossRef]
- Stevens, C.; Wilson, C.L.; Lu, J.Y.; Khan, V.A.; Chalutz, E.; Droby, S.; Kabwe, M.K.; Haung, Z.; Adeyeye, O.; Pusey, L.P.; et al. Plant hormesis induced by ultraviolet light-C for controlling postharvest diseases of tree fruits. Crop Prot. 1996, 15, 129–134. [Google Scholar] [CrossRef]
- Shama, G.; Alderson, P. UV hormesis in fruits: A concept ripe for commercialisation. Trends Food Sci. Technol. 2005, 16, 128–136. [Google Scholar] [CrossRef]
- Luckey, T.D. Hormesis with ionizing radiation. Nucl. Sci. Eng. 1982, 82, 5912626. [Google Scholar]
- Giovannoni, J.J. Genetic Regulation of Fruit Development and Ripening. Plant Cell 2004, 16 (Suppl. 1), 170–180. [Google Scholar] [CrossRef]
- Stevens, C.; Khan, V.A.; Lu, J.Y.; Wilson, C.L.; Pusey, P.L.; Igwegbe, E.C.K.; Kabwe, K.; Mafolo, Y.; Liu, J.; Chalutz, E.; et al. Integration of Ultraviolet (UV-C) Light with Yeast Treatment for Control of Postharvest Storage Rots of Fruits and Vegetables. Biol. Control 1997, 10, 98–103. [Google Scholar] [CrossRef]
- Jaleh-Rezaee, H.; Taheri, M.; Noorju, A. Effect of Irrigation, Nutrition and Harvest Time on the Quality and Storage Life of Red Delicious Apple. 2004. Available online: https://agris.fao.org/agris-search/search.do?recordID=IR2006000277 (accessed on 6 December 2022). (In Farsi).
- Rapoport, H.F.; Costagli, G.; Gucci, R. The effect of water deficit during early fruit development on olive fruit morphogenesis. J. Am. Soc. Hortic. Sci. 2004, 129, 121–127. [Google Scholar] [CrossRef]
- Smith, D.L.; Stommel, J.R.; Fung, R.W.M.; Wang, C.Y.; Whitaker, B.D. Influence of cultivar and harvest method on postharvest storage quality of pepper (Capsicum annuum L.) fruit. Postharvest Biol. Technol. 2006, 42, 243–247. [Google Scholar] [CrossRef]
- Crupi, P.; Pichierri, A.; Basile, T.; Antonacci, D. Postharvest stilbenes and flavonoids enrichment of table grape cv Redglobe (Vitis vinifera L.) as affected by interactive UV-C exposure and storage conditions. Food Chem. 2013, 141, 802–808. [Google Scholar] [CrossRef]
- Yang, J.; Shi, W.; Li, B.; Bai, Y.; Hou, Z. Preharvest and postharvest UV radiation affected flavonoid metabolism and antioxidant capacity differently in developing blueberries (Vaccinium corymbosum L.). Food Chem. 2019, 301, 125248. [Google Scholar] [CrossRef]
- Charles, M.T.; Arul, J. UV treatment of fresh fruits and vegetables for improved quality: A status report. Stewart Postharvest Rev. 2007, 3, 1–8. [Google Scholar] [CrossRef]
- Obande, M.A.; Tucker, G.A.; Shama, G. Effect of preharvest UV-C treatment of tomatoes (Solanum lycopersicon Mill.) on ripening and pathogen resistance. Postharvest Biol. Technol. 2011, 62, 188–192. [Google Scholar] [CrossRef]
- Topcu, Y.; Dogan, A.; Kasimoglu, Z.; Sahin-Nadeem, H.; Polat, E.; Erkan, M. The effects of UV radiation during the vegetative period on antioxidant compounds and postharvest quality of broccoli (Brassica oleracea L.). Plant Physiol. Biochem. 2015, 93, 56–65. [Google Scholar] [CrossRef]
- Xie, Z.; Charles, M.T.; Fan, J.; Charlebois, D.; Khanizadeh, S.; Rolland, D.; Roussel, D.; Deschênes, M.; Dubé, C. Effects of preharvest ultraviolet-C irradiation on fruit phytochemical profiles and antioxidant capacity in three strawberry (Fragaria × ananassa Duch.) cultivars. J. Sci. Food Agric. 2015, 95, 2996–3002. [Google Scholar] [CrossRef]
- Xie, Z.; Charles, M.T.; Charlebois, D.; Rolland, D.; Roussel, D.; Deschênes, M.; Dubé, C.; Khanizadeh, S.; Fan, J. Preharvest exposure to UV-C radiation: Impact on strawberry fruit quality. Acta Hortic. 2014, 1079, 589–592. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, J.; Charles, M.T.; Charlebois, D.; Khanizadeh, S.; Rolland, D.; Roussel, D.; Zhang, Z. Preharvest ultraviolet-C irradiation: Influence on physicochemical parameters associated with strawberry fruit quality. Plant Physiol. Biochem. 2016, 108, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Janisiewicz, W.J.; Takeda, F.; Nichols, B.; Glenn, D.M.; Jurick, W.M., II; Camp, M.J. Use of low-dose UV-C irradiation to control powdery mildew caused by Podosphaera aphanis on strawberry plants. Can. J. Plant Pathol. 2016, 38, 430–439. [Google Scholar] [CrossRef]
- Van Hemelrijck, W.; Van Laer, S.; Hoekstra, S.; Aiking, A.; Creemers, P. UV-c radiation as an alternative tool to control powdery mildew on apple and strawberry. In Proceedings of the 14th International Conference on Organic Fruit-Growing, Hohenheim, Germany, 22–24 February 2010; pp. 22–24. [Google Scholar]
- Mahdavian, K.; Ghorbanli, M.; Kalantari, K.M. The effects of ultraviolet radiation on the contents of chlorophyll, flavonoid, anthocyanin and proline in Capsicum annuum L. Turk. J. Bot. 2008, 32, 25–33. [Google Scholar]
- Kondo, S.; Fiebig, A.; Okawa, K.; Ohara, H.; Kowitcharoen, L.; Nimitkeatkai, H.; Kittikorn, M.; Kim, M. Jasmonic acid, polyamine, and antioxidant levels in apple seedlings as affected by Ultraviolet-C irradiation. Plant Growth Regul. 2011, 64, 83–89. [Google Scholar] [CrossRef]
- Tang, K.; Zhan, J.-C.; Yang, H.-R.; Huang, W.-D. Changes of resveratrol and antioxidant enzymes during UV-induced plant defense response in peanut seedlings. J. Plant Physiol. 2010, 167, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Park, H.L.; Lee, S.-W.; Jung, K.-H.; Hahn, T.-R.; Cho, M.-H. Transcriptomic analysis of UV-treated rice leaves reveals UV-induced phytoalexin biosynthetic pathways and their regulatory networks in rice. Phytochemistry 2013, 96, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Feliziani, E.; Romanazzi, G. Postharvest decay of strawberry fruit: Etiology, epidemiology, and disease management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Bian, Z.H.; Yang, Q.C.; Liu, W.K. Effects of light quality on the accumulation of phytochemicals in vegetables produced in controlled environments: A review. J. Sci. Food Agric. 2015, 95, 869–877. [Google Scholar] [CrossRef]
- Gunness, P.; Kravchuk, O.; Nottingham, S.M.; D’Arcy, B.R.; Gidley, M.J. Sensory analysis of individual strawberry fruit and comparison with instrumental analysis. Postharvest Biol. Technol. 2009, 52, 164–172. [Google Scholar] [CrossRef]
- Tsormpatsidis, E.; Ordidge, M.; Henbest, R.G.C.; Wagstaffe, A.; Battey, N.H.; Hadley, P. Harvesting fruit of equivalent chronological age and fruit position shows individual effects of UV radiation on aspects of the strawberry ripening process. Environ. Exp. Bot. 2011, 74, 178–185. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E.; Puertas, B.; Richard, T. Daily preharvest UV-C light maintains the high stilbenoid concentration in grapes. J. Agric. Food Chem. 2016, 64, 5139–5147. [Google Scholar] [CrossRef] [PubMed]
- Alessandrini, M.; Gaiotti, F.; Belfiore, N.; Matarese, F.; D’Onofrio, C.; Tomasi, D. Influence of vineyard altitude on Glera grape ripening (Vitis vinifera L.): Effects on aroma evolution and wine sensory profile. J. Sci. Food Agric. 2017, 97, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Rosenthal, A. Food texture and structure. In Modifying Food Texture; Chen, J., Rosenthal, A., Eds.; Novel Ingredients and Processing Techniques; Woodhead Publishing: Cambridge, UK, 2015; Volume 1, pp. 3–24. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Cantos-Villar, E.; Fernández-Marín, M.I.; Puertas, B.; Serrano-Albarrán, M.J. Optimising UV-C preharvest light for stilbene synthesis stimulation in table grape: Applications. Innov. Food Sci. Emerg. Technol. 2015, 29, 222–229. [Google Scholar] [CrossRef]
- Li, T.; Yamane, H.; Tao, R. Preharvest long-term exposure to UV-B radiation promotes fruit ripening and modifies stage-specific anthocyanin metabolism in highbush blueberry. Hortic. Res. 2021, 8, 67. [Google Scholar] [CrossRef]
- Castagna, A.; Chiavaro, E.; Dall’Asta, C.; Rinaldi, M.; Galaverna, G.; Ranieri, A. Effect of postharvest UV-B irradiation on nutraceutical quality and physical properties of tomato fruits. Food Chem. 2013, 137, 151–158. [Google Scholar] [CrossRef]
- Dong, Y.H.; Mitra, D.; Kootstra, A.; Lister, C.; Lancaster, J. Postharvest Stimulation of Skin Color in Royal Gala Apple. J. Am. Soc. Hortic. Sci. 1995, 120, 95–100. [Google Scholar] [CrossRef]
- Zhang, D.; Qian, M.; Yu, B.; Teng, Y. Effect of fruit maturity on UV-B-induced post-harvest anthocyanin accumulation in red Chinese sand pear. Acta Physiol. Plant. 2013, 35, 2857–2866. [Google Scholar] [CrossRef]
- Yang, J.; Li, B.; Shi, W.; Gong, Z.; Chen, L.; Hou, Z. Transcriptional Activation of Anthocyanin Biosynthesis in Developing Fruit of Blueberries (Vaccinium corymbosum L.) by Preharvest and Postharvest UV Irradiation. J. Agric. Food Chem. 2018, 66, 10931–10942. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Li, X.-X.; Chu, Y.-N.; Zhang, M.-X.; Wen, Y.-Q.; Duan, C.-Q.; Pan, Q.-H. Three types of ultraviolet irradiation differentially promote expression of shikimate pathway genes and production of anthocyanins in grape berries. Plant Physiol. Biochem. 2012, 57, 74–83. [Google Scholar] [CrossRef]
- Willson, M.F.; Whelan, C.J. The Evolution of Fruit Color in Fleshy-Fruited Plants. Am. Nat. 1990, 136, 790–809. [Google Scholar] [CrossRef]
- Nguyen, C.T.T.; Kim, J.; Yoo, K.S.; Lim, S.; Lee, E.J. Effect of prestorage UV-A,-B, and-C radiation on fruit quality and anthocyanin of ‘Duke’ blueberries during cold storage. J. Agric. Food Chem. 2014, 62, 12144–12151. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Chen, C.-T.; Wang, S.Y. Changes of flavonoid content and antioxidant capacity in blueberries after illumination with UV-C. Food Chem. 2009, 117, 426–431. [Google Scholar] [CrossRef]
- Carbonell-Bejerano, P.; Diago, M.-P.; Martínez-Abaigar, J.; Martínez-Zapater, J.M.; Tardáguila, J.; Núñez-Olivera, E. Solar ultraviolet radiation is necessary to enhance grapevine fruit ripening transcriptional and phenolic responses. BMC Plant Biol. 2014, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Tuan, P.A.; Saito, T.; Honda, C.; Hatsuyama, Y.; Ito, A.; Moriguchi, T. Epigenetic regulation of MdMYB1 is associated with paper bagging-induced red pigmentation of apples. Planta 2016, 244, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Tao, R.; Yin, L.; Ni, J.; Yang, Q.; Yan, X.; Yang, F.; Guo, X.; Li, H.; Teng, Y. Two B-box proteins, PpBBX18 and PpBBX21, antagonistically regulate anthocyanin biosynthesis via competitive association with Pyrus pyrifolia ELONGATED HYPOCOTYL 5 in the peel of pear fruit. Plant J. 2019, 100, 1208–1223. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishimaru, M.; Ding, C.K.; Yakushiji, H.; Goto, N. Comparison of UDP-glucose: Flavonoid 3-O-glucosyltransferase (UFGT) gene sequences between white grapes (Vitis vinifera) and their sports with red skin. Plant Sci. 2001, 160, 543–550. [Google Scholar] [CrossRef]
- Henry-Kirk, R.A.; Plunkett, B.; Hall, M.; McGhie, T.; Allan, A.C.; Wargent, J.J.; Espley, R.V. Solar UV light regulates flavonoid metabolism in apple (Malus × domestica). Plant Cell Environ. 2018, 41, 675–688. [Google Scholar] [CrossRef]
- Loyola, R.; Herrera, D.; Mas, A.; Wong, D.C.J.; Höll, J.; Cavallini, E.; Amato, A.; Azuma, A.; Ziegler, T.; Aquea, F.; et al. The photomorphogenic factors UV-B RECEPTOR 1, ELONGATED HYPOCOTYL 5, and HY5 HOMOLOGUE are part of the UV-B signalling pathway in grapevine and mediate flavonol accumulation in response to the environment. J. Exp. Bot. 2016, 67, 5429–5445. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.-Y.; Rolland, D.; Roussel, D. Preharvest ultraviolet C irradiation increased the level of polyphenol accumulation and flavonoid pathway gene expression in strawberry fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Roussel, D.; Rolland, D. Potential link between fruit yield, quality parameters and phytohormonal changes in preharvest UV-C treated strawberry. Plant Physiol. Biochem. 2017, 116, 80–90. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Che, X.-N.; Pan, Q.-H.; Li, X.-X.; Duan, C.-Q. Transcriptional activation of flavan-3-ols biosynthesis in grape berries by UV irradiation depending on developmental stage. Plant Sci. 2013, 208, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Das, P.K.; Geul, B.; Choi, S.-B.; Yoo, S.-D.; Park, Y.-I. Photosynthesis-dependent anthocyanin pigmentation in Arabidopsis. Plant Signal. Behav. 2011, 6, 23–25. [Google Scholar] [CrossRef] [PubMed]
- Azodanlou, R.; Darbellay, C.; Luisier, J.-L.; Villettaz, J.-C.; Amadò, R. Changes in flavour and texture during the ripening of strawberries. Eur. Food Res. Technol. 2004, 218, 167–172. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Vérronneau, P.-Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Treatment Affected Senescence of Stored Strawberry Fruit with a Potential Role of MicroRNAs in the Activation of the Antioxidant System. J. Agric. Food Chem. 2018, 66, 12188–12197. [Google Scholar] [CrossRef]
- Vicente, A.R.; Martínez, G.A.; Chaves, A.R.; Civello, P.M. Effect of heat treatment on strawberry fruit damage and oxidative metabolism during storage. Postharvest Biol. Technol. 2006, 40, 116–122. [Google Scholar] [CrossRef]
- Wang, X.; Fu, X.; Chen, M.; Huan, L.; Liu, W.; Qi, Y.; Gao, Y.; Xiao, W.; Chen, X.; Li, L.; et al. Ultraviolet B irradiation influences the fruit quality and sucrose metabolism of peach (Prunus persica L.). Environ. Exp. Bot. 2018, 153, 286–301. [Google Scholar] [CrossRef]
- Naeem ul Hassan, M.; Zainal, Z.; Ismail, I. Green leaf volatiles: Biosynthesis, biological functions and their applications in biotechnology. Plant Biotechnol. J. 2015, 13, 727–739. [Google Scholar] [CrossRef]
- Neri, F.; Cappellin, L.; Spadoni, A.; Cameldi, I.; Algarra Alarcon, A.; Aprea, E.; Romano, A.; Gasperi, F.; Biasioli, F. Role of strawberry volatile organic compounds in the development of Botrytis cinerea infection. Plant Pathol. 2015, 64, 709–717. [Google Scholar] [CrossRef]
- Myung, K.; Hamilton-Kemp, T.R.; Archbold, D.D. Interaction with and effects on the profile of proteins of Botrytis cinerea by C6 aldehydes. J. Agric. Food Chem. 2007, 55, 2182–2188. [Google Scholar] [CrossRef]
- Croft, K.P.C.; Juttner, F.; Slusarenko, A.J. Volatile Products of the Lipoxygenase Pathway Evolved from Phaseolus vulgaris (L.) Leaves Inoculated with Pseudomonas syringae pv phaseolicola. Plant Physiol. 1993, 101, 13–24. [Google Scholar] [CrossRef]
- Vandendriessche, T.; Keulemans, J.; Geeraerd, A.; Nicolai, B.M.; Hertog, M.L.A.T.M. Evaluation of fast volatile analysis for detection of Botrytis cinerea infections in strawberry. Food Microbiol. 2012, 32, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Piesik, D.; Wenda-Piesik, A.; Weaver, D.K.; Macedo, T.B.; Morrill, W.L. Influence of Fusarium and wheat stem sawfly infestation on volatile compounds production by wheat plants. J. Plant Prot. Res. 2009, 49, 167–174. [Google Scholar] [CrossRef]
- Taniguchi, S.; Hosokawa-Shinonaga, Y.; Tamaoki, D.; Yamada, S.; Akimitsu, K.; Gomi, K. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant Cell Environ. 2013, 37, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Pinar, A.L.; Rauhut, D.; Ruehl, E.; Buettner, A. Effects of Botrytis cinerea and Erysiphe necator fungi on the aroma character of grape must: A comparative approach. Food Chem. 2016, 207, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Roussel, D.; Rolland, D.; Véronneau, P.-Y. Preharvest UV-C treatment affected postharvest senescence and phytochemicals alternation of strawberry fruit with the possible involvement of abscisic acid regulation. Food Chem. 2019, 299, 125138. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.; Bottini, R.; Berli, F.; Pontin, M.; Silva, M.F.; Piccoli, P. Volatile organic compounds characterized from grapevine (Vitis vinifera L. cv. Malbec) berries increase at pre-harvest and in response to UV-B radiation. Phytochemistry 2013, 96, 148–157. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, Z.; Charles, M.T.; Rolland, D.; Roussel, D. Pre-harvest UV-C irradiation triggers VOCs accumulation with alteration of antioxidant enzymes and phytohormones in strawberry leaves. J. Plant Physiol. 2017, 218, 265–274. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Charles, M.T.; Arul, J. UV-C hormesis: A means of controlling diseases and delaying senescence in fresh fruits and vegetables during storage. In Postharvest Pathology of Fresh Horticultural Produce, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019; pp. 539–594. [Google Scholar]
- Charles, M.T.; Goulet, A.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit: IV. Biochemical modification of structural barriers. Postharvest Biol. Technol. 2008, 47, 41–53. [Google Scholar] [CrossRef]
- Charles, M.T.; Makhlouf, J.; Arul, J. Physiological basis of UV-C induced resistance to Botrytis cinerea in tomato fruit: II. Modification of fruit surface and changes in fungal colonization. Postharvest Biol. Technol. 2008, 47, 21–26. [Google Scholar] [CrossRef]
- Charles, M.T.; Mercier, J.; Makhlouf, J.; Arul, J. Physiological basis of UV-C-induced resistance to Botrytis cinerea in tomato fruit: I. Role of pre- and post-challenge accumulation of the phytoalexin-rishitin. Postharvest Biol. Technol. 2008, 47, 10–20. [Google Scholar] [CrossRef]
- Ouhibi, C.; Attia, H.; Rebah, F.; Msilini, N.; Chebbi, M.; Aarrouf, J.; Urban, L.; Lachaal, M. Salt stress mitigation by seed priming with UV-C in lettuce plants: Growth, antioxidant activity and phenolic compounds. Plant Physiol. Biochem. 2014, 83, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Pombo, M.A.; Rosli, H.G.; Martínez, G.A.; Civello, P.M. UV-C treatment affects the expression and activity of defense genes in strawberry fruit (Fragaria × ananassa, Duch.). Postharvest Biol. Technol. 2011, 59, 94–102. [Google Scholar] [CrossRef]
- El Ghaouth, A.; Wilson, C.L.; Callahan, A.M. Induction of Chitinase, β-1,3-Glucanase, and Phenylalanine Ammonia Lyase in Peach Fruit by UV-C Treatment. Phytopathology 2003, 93, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Erkan, M.; Wang, S.Y.; Wang, C.Y. Effect of UV treatment on antioxidant capacity, antioxidant enzyme activity and decay in strawberry fruit. Postharvest Biol. Technol. 2008, 48, 163–171. [Google Scholar] [CrossRef]
- González-Aguilar, G.A.; Zavaleta-Gatica, R.; Tiznado-Hernández, M.E. Improving postharvest quality of mango ‘Haden’ by UV-C treatment. Postharvest Biol. Technol. 2007, 45, 108–116. [Google Scholar] [CrossRef]
- Zhao, Y.; Zuo, J.; Yuan, S.; Shi, W.; Shi, J.; Feng, B.; Wang, Q. UV-C Treatment Maintains the Sensory Quality, Antioxidant Activity and Flavor of Pepino Fruit during Postharvest Storage. Foods 2021, 10, 2964. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, S.; Su, X.; Jiang, Y. Respiratory activity and mitochondrial membrane associated with fruit senescence in postharvest peaches in response to UV-C treatment. Food Chem. 2014, 161, 16–21. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Takeda, F.; Glenn, D.M.; Camp, M.J.; Jurick, W.M., II. Dark Period Following UV-C Treatment Enhances Killing of Botrytis cinerea Conidia and Controls Gray Mold of Strawberries. Phytopathology 2016, 106, 386–394. [Google Scholar] [CrossRef]
- Darras, A.I.; Demopoulos, V.; Tiniakou, C. UV-C irradiation induces defence responses and improves vase-life of cut gerbera flowers. Postharvest Biol. Technol. 2012, 64, 168–174. [Google Scholar] [CrossRef]
- Yao, Y.; Danna, C.H.; Zemp, F.J.; Titov, V.; Ciftci, O.N.; Przybylski, R.; Ausubel, F.M.; Kovalchuk, I. UV-C–irradiated Arabidopsis and tobacco emit volatiles that trigger genomic instability in neighboring plants. Plant Cell 2011, 23, 3842–3852. [Google Scholar] [CrossRef] [PubMed][Green Version]
- de Oliveira, I.R.; Crizel, G.R.; Severo, J.; Renard, C.M.; Chaves, F.C.; Rombaldi, C.V. Preharvest UV-C radiation influences physiological, biochemical, and transcriptional changes in strawberry cv. Camarosa. Plant Physiol. Biochem. 2016, 108, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Bonomelli, A.; Mercier, L.; Franchel, J.; Baillieul, F.; Benizri, E.; Mauro, M.-C. Response of Grapevine Defenses to UV—C Exposure. Am. J. Enol. Vitic. 2004, 55, 51–59. [Google Scholar] [CrossRef]
- Severo, J.; de Oliveira, I.R.; Bott, R.; Le Bourvellec, C.; Renard, C.M.G.C.; Page, D.; Chaves, F.C.; Rombaldi, C.V. Preharvest UV-C radiation impacts strawberry metabolite content and volatile organic compound production. LWT 2017, 85, 390–393. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Morales, F.; Delrot, S.; Sánchez-Díaz, M.; Gomés, E.; Aguirreolea, J.; Pascual, I. Short- and long-term physiological responses of grapevine leaves to UV-B radiation. Plant Sci. 2013, 213, 114–122. [Google Scholar] [CrossRef]
- Liu, L.; Gregan, S.M.; Winefield, C.; Jordan, B. Comparisons of controlled environment and vineyard experiments in Sauvignon blanc grapes reveal similar UV-B signal transduction pathways for flavonol biosynthesis. Plant Sci. 2018, 276, 44–53. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Martínez-Abaigar, J.; Núñez-Olivera, E. Phenolic characteristics acquired by berry skins of Vitis vinifera cv. Tempranillo in response to close-to-ambient solar ultraviolet radiation are mostly reflected in the resulting wines. J. Sci. Food Agric. 2020, 100, 401–409. [Google Scholar] [CrossRef]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Ranieri, A.; Castagna, A.; Martínez-Abaigar, J.; Núñez-Olivera, E. Secondary metabolites and related genes in Vitis vinifera L. cv. Tempranillo grapes as influenced by ultraviolet radiation and berry development. Physiol. Plant. 2021, 173, 709–724. [Google Scholar] [CrossRef]
- Dzakovich, M.P.; Ferruzzi, M.G.; Mitchell, C.A. Manipulating Sensory and Phytochemical Profiles of Greenhouse Tomatoes Using Environmentally Relevant Doses of Ultraviolet Radiation. J. Agric. Food Chem. 2016, 64, 6801–6808. [Google Scholar] [CrossRef]
- Sullivan, J.H.; Muhammad, D.; Warpeha, K.M. Phenylalanine Is Required to Promote Specific Developmental Responses and Prevents Cellular Damage in Response to Ultraviolet Light in Soybean (Glycine max) during the Seed-to-Seedling Transition. PLoS ONE 2014, 9, e112301. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.F.; Ballaré, C.L.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Natarajan, S.; Sullivan, J.H. Impact of solar ultraviolet-B radiation on the antioxidant defense system in soybean lines differing in flavonoid contents. Environ. Exp. Bot. 2008, 63, 39–48. [Google Scholar] [CrossRef]
- Petit, A.-N.; Baillieul, F.; Vaillant-Gaveau, N.; Jacquens, L.; Conreux, A.; Jeandet, P.; Clément, C.; Fontaine, F. Low responsiveness of grapevine flowers and berries at fruit set to UV-C irradiation. J. Exp. Bot. 2009, 60, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Ferreira de Oliveira, J.M.P.; Melo, P.; Santos, C. Tomato plants use non-enzymatic antioxidant pathways to cope with moderate UV-A/B irradiation: A contribution to the use of UV-A/B in horticulture. J. Plant Physiol. 2018, 221, 32–42. [Google Scholar] [CrossRef]
- Maharaj, R.; Arul, J.; Nadeau, P. Effect of photochemical treatment in the preservation of fresh tomato (Lycopersicon esculentum cv. Capello) by delaying senescence. Postharvest Biol. Technol. 1999, 15, 13–23. [Google Scholar] [CrossRef]
- Ben-Yehoshua, S.; Rodov, V.; Kim, J.J.; Carmeli, S. Preformed and induced antifungal materials of citrus fruits in relation to the enhancement of decay resistance by heat and ultraviolet treatments. J. Agric. Food Chem. 1992, 40, 1217–1221. [Google Scholar] [CrossRef]
- Cia, P.; Pascholati, S.F.; Benato, E.A.; Camili, E.C.; Santos, C.A. Effects of gamma and UV-C irradiation on the postharvest control of papaya anthracnose. Postharvest Biol. Technol. 2007, 43, 366–373. [Google Scholar] [CrossRef]
| Crops | UV | Dose Rate/Total Dose (kJ/m2/d)/(kJ/m2) | Treatment Time | Response | Reference |
|---|---|---|---|---|---|
| Grape | UV-C | 9.33/9.33, 18.66, 27.99 | 1, 2, 3 days | Promote fruit ripening and the accumulation of resveratrol and other stilbene compounds, and improves disease resistance | [33,36] |
| Grape | UV-C | 1.92/1.92 | 1 day | Induce phenol accumulation | [87] |
| Strawberry | UV-C | 0.5/22.5, 42.5 | 45 days, 85 days | Improve the content of phenols, anthocyanins, and ascorbic acid, and delay the decay of postharvest fruits | [86] |
| Strawberry | UV-C | 0.01236/0.17304 | twice/week × 7 | Reduce the incidence rate of B. cinerea | [83] |
| Strawberry | UV-C | 0.5/14 | every 4 days × 28 | Improve the content of phenols (especially procyanidins and anthocyanins) and volatile esters | [88] |
| Strawberry | UV-C | 0.6/3.6 | twice/week × 3 | Increase hardness and ellagic acid content | [20,21,22] |
| Strawberry | UV-C | 0.6/9.6, 15, 29.4 0.6/6.6 | Every 3/2/1 days × 16/25/49 Every 2 day × 11 | Promote the accumulation of anthocyanins, and polyphenols, facilitate the preservation of sugars and acids Enhance the activity of antioxidant enzymes, promote the accumulation of VOCs, inhibit lipid peroxidation of fruits, and enhance disease resistance | [52,53,57,68,70] |
| Tomato | UV-C | 8/8 | 1 day | Increase hardness, improve storage, and inhibit the growth of P. digitatum | [18] |
| Tomato | UV-C | -/3.7 | 1 day | Delay fruit decay and inhibit the growth of B. cinerea | [72,73,74] |
| Strawberry/Apple | UV-C | 0.3/4.8 | 16 days | Effectively control the growth of Sphaerotheca aphanis | [24] |
| Blueberry | UV-C/B | 4.14/12.42 | Once/week × 3 | Promote the synthesis of anthocyanins and increase the content of sugar, flavonols, and procyanidins. | [16,41] |
| Blueberry | UV-B | 3.528, 4.788/ 24.696~148.176, 33.516~153.216 | 7~42/32 days | Promote fruit growth, coloring, ripening, and sugar accumulation | [37] |
| Grape | UV-B | 4.75/669.75 | 141 days | Induce grape berries to produce VOCs (such as aldehydes, alcohols, and ketones, mainly monoterpenes) that protect the tissues from UV-B itself and other abiotic and biotic stresses | [69] |
| Grape | UV-B | 5.98, 9.66/119.6193.2 | 20 days | Enhance the activity of antioxidant enzymes, preserving leaves from oxidative stress | [89] |
| Grape | UV-B | 86.4/86.4, 172.8, 259.2, 345.6, 432, 604.8 | 1, 2, 3, 4, 5, 7 days | Increase Flavonols content (particularly quercetin/kaempferol 3-O-glycosides) | [90] |
| Peach | UV-B | 1.44/30.24, 50.4, 70.56, 90.72, 110.88 | 21, 35, 49, 63, 77 days | Promote sugar accumulation, increase the anthocyanin contents in peach sarcocarp and pericarp, enhance the sucrose transport to the UV-B-treated fruit | [59] |
| Strawberry | UV | UV transparent/opaque film | 45 days | Promote fruit coloring, increase fruit firmness, anthocyanin, flavonoid, and phenolic contents | [32] |
| Tomato | UV-B | 6.08/60.8, 109.44 | 10 d,18 days | Promote fruit coloring, increased the concentration of ascorbic acid and carotenoids | [38] |
| Apple | UV-A/B | UV transparent/opaque film | 39, 59, 104, 126, 146 days | Increase anthocyanin and flavonol content | [50] |
| Grape | UV-A/B/C | 1.8/1.8 | 1 day | Increase the content of flavan-3-ol in grape during verasion period and anthocyanin in mature period | [42] |
| Grape | UV-A/B | UV transparent/opaque film | Every day | Increase flavonols contents (particularly quercetins and kaempferols), and grape weight and size. | [91,92] |
| Tomato | UV-A | 11.29/- | Every day | Increase VOCs content and acidity | [93] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, H.; Pang, Y.; Liao, Q.; Wang, F.; Qian, C. The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review. Horticulturae 2022, 8, 1171. https://doi.org/10.3390/horticulturae8121171
Peng H, Pang Y, Liao Q, Wang F, Qian C. The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review. Horticulturae. 2022; 8(12):1171. https://doi.org/10.3390/horticulturae8121171
Chicago/Turabian StylePeng, Honggui, Yadan Pang, Qiuhong Liao, Fang Wang, and Chun Qian. 2022. "The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review" Horticulturae 8, no. 12: 1171. https://doi.org/10.3390/horticulturae8121171
APA StylePeng, H., Pang, Y., Liao, Q., Wang, F., & Qian, C. (2022). The Effect of Preharvest UV Light Irradiation on Berries Quality: A Review. Horticulturae, 8(12), 1171. https://doi.org/10.3390/horticulturae8121171





