Assessing Physicochemical Parameters, Bioactive Profile and Antioxidant Status of Different Fruit Parts of Greek Eggplant Germplasm
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Experimentation
2.3. Sample Preparation
2.4. Evaluated Parameters
2.4.1. Physicochemical Parameters
2.4.2. Sample Extraction
2.4.3. Bioactive Compounds Determination
Total Phenolics Content (TPC)
Total Flavonoids Content (TFC)
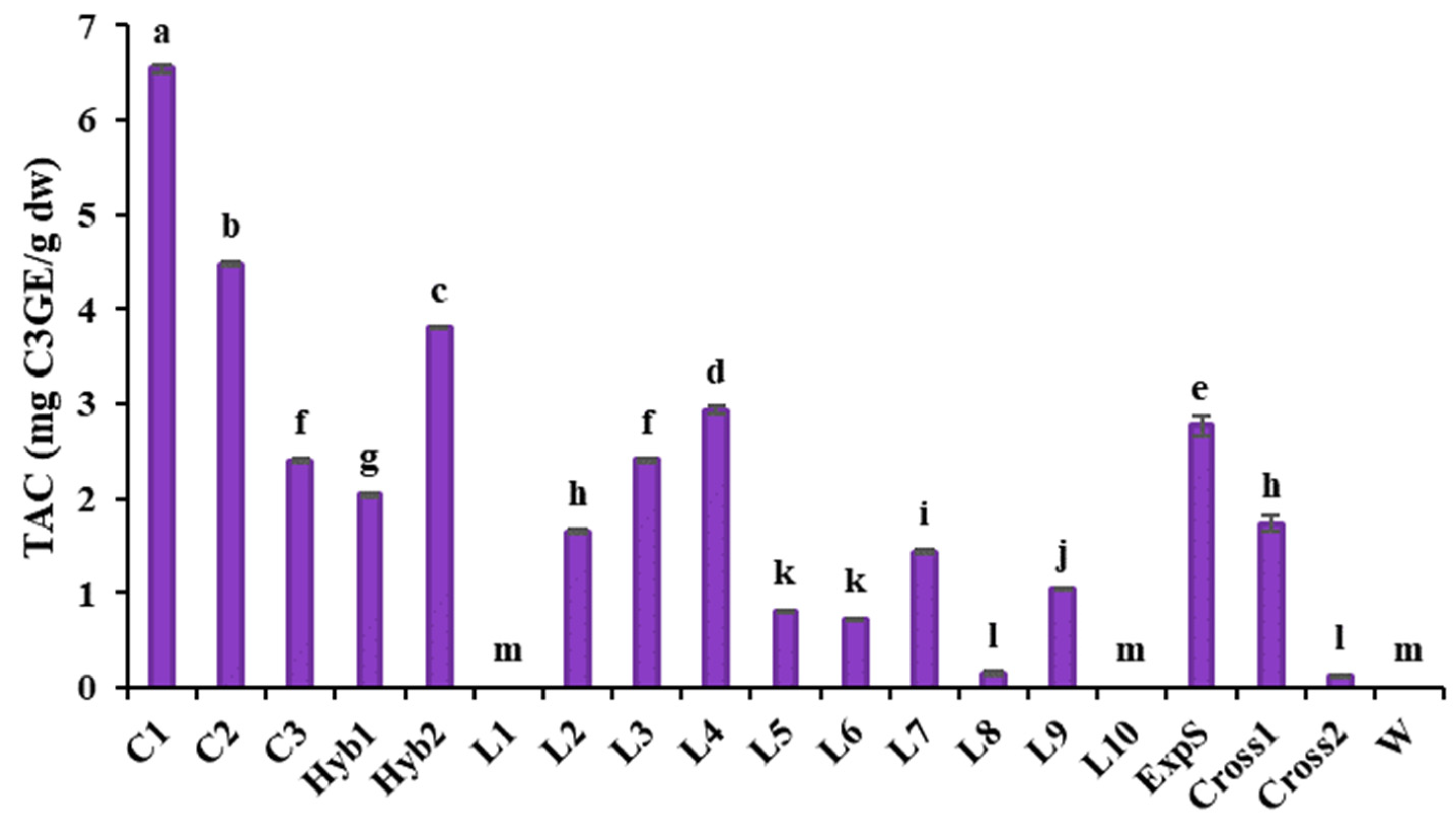
Total Monomeric Anthocyanins Content (TAC)
Chlorogenic Acids (CAs)
2.4.4. Antioxidant Activity Determination
2,2′-Azinobis-(3-ethylbenzothiazoline-6-sulfonate) (ABTS) Radical Scavenging Activity
2,2-Diphenylpicrylhydrazyl (DPPH) Radical Scavenging Activity
Ferric Reducing Antioxidant Power (FRAP) Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical Parameters
3.2. Bioactive Compounds Determination
3.2.1. Total Phenolics Content
3.2.2. Total Flavonoids Content
3.2.3. Total Monomeric Anthocyanins Content
3.2.4. Identification and Quantification of Chlorogenic Acids
3.3. Antioxidant Capacity
3.4. Correlation Coefficients among Physicochemical Traits, Bioactive Compounds, Antioxidant Capacity and CAs
3.5. Principal Component Analysis and Hierarchical Clustering
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A
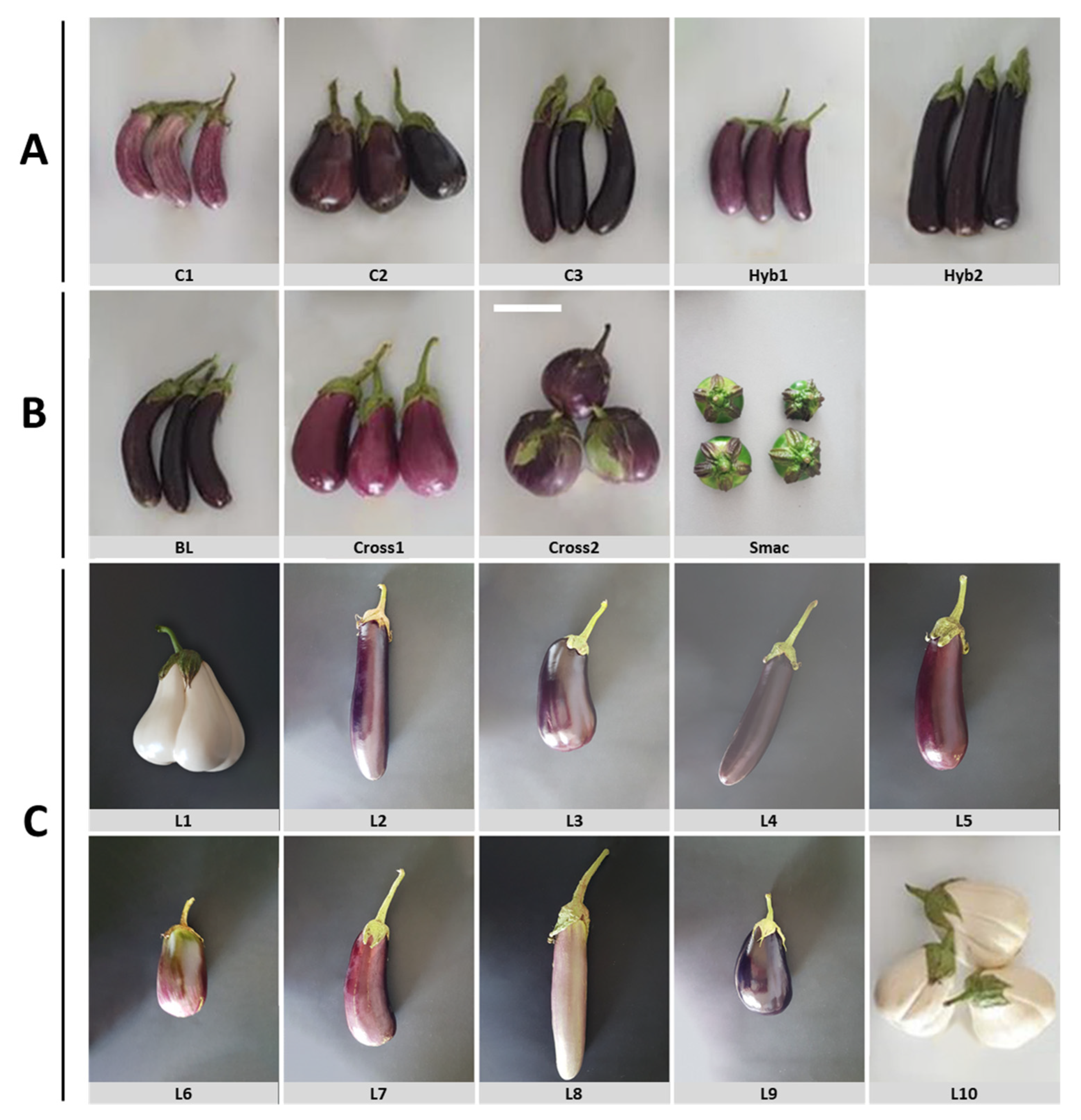
Appendix B
| No | Germplasm | Abbreviation | Type | Source |
| 1 | Tsakoniki | C1 | Greek commercial landrace 1 | ELGO-DIMITRA 2 |
| 2 | Emi | C2 | Greek commercial landrace | ELGO-DIMITRA |
| 3 | Langada | C3 | Greek commercial landrace | ELGO-DIMITRA |
| 4 | Anamika | Hyb1 | Commercial F1 hybrid | Sungro Seeds |
| 5 | Nilo | Hyb2 | Commercial F1 hybrid | Rijk Zwaan |
| 6 | LKK95/07 | L1 | Greek landrace | GGB 3 |
| 7 | KD053/07 | L2 | Greek landrace | GGB |
| 8 | KD054/07 | L3 | Greek landrace | GGB |
| 9 | KD209/07 | L4 | Greek landrace | GGB |
| 10 | KD047/07 | L5 | Greek landrace | GGB |
| 11 | GRC094/05 | L6 | Greek landrace | GGB |
| 12 | IS031/07 | L7 | Greek landrace | GGB |
| 13 | HL050/07 | L8 | Greek landrace | GGB |
| 14 | MFS030/07 | L9 | Greek landrace | GGB |
| 15 | Santorini | L10 | Greek landrace | GGB |
| 16 | Male parent of F1 Meliton | BL | Breeding line | GGB |
| 17 | Santorini × Tsakoniki | Cross1 | Experimental F1 hybrid | GGB |
| 18 | Santorini × BL | Cross2 | Experimental F1 hybrid | GGB |
| 19 | Solanum macrocarpon | Smac | Cultivated eggplant relative | GGB |
Appendix C
| Significance of F-Ratio | ||||||||||||||
| Variation Source | Df 1 | DM | pH | TSS | TA | TPC | TFC | ABTS | DPPH | FRAP | TAC | CA | n-CA | c-CA |
| Genotype (G) | 18 | *** 2 | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Fruit Part (P) | 1 | na 3 | na | na | na | *** | *** | *** | *** | *** | na | *** | *** | *** |
| G x P | 18 | *** | *** | *** | *** | *** | *** | *** | *** | |||||
| CV% | 5.24 | 0.36 | 3.83 | 3.45 | 2.71 | 2.99 | 17.30 | 2.73 | 11.73 | 2.00 | 11.71 | 1.63 | 9.21 | |
References
- Wei, Q.; Wang, J.; Wang, W.; Hu, T.; Hu, H.; Bao, C. A high-quality chromosome-level genome assembly reveals genetics for important traits in eggplant. Hortic. Res. 2020, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Kalloo, G.; Banerjee, M.K.; Singh, S. Genetics of yield and its component characters in brinjal (Solanum melongena L.). Veg. Sci. 2002, 29, 24–26. [Google Scholar]
- Mwinuka, P.R.; Mbilinyi, B.P.; Mbungu, W.B.; Mourice, S.K.; Mahoo, H.F.; Schmitter, P. Optimizing water and nitrogen application for neglected horticultural species in tropical sub-humid climate areas: A case of African eggplant (Solanum aethiopicum L.). Sci. Hortic. 2021, 276, 109756. [Google Scholar] [CrossRef]
- Sharma, M.; Kaushik, P. Biochemical composition of eggplant fruits: A review. Appl. Sci. 2021, 11, 7078. [Google Scholar] [CrossRef]
- FAOSTAT. Statistical Database of the Food and Agriculture Organization of the United Nations. Online Database. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 4 August 2022).
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential health benefits of plant food-derived bioactive components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef]
- Niño-medina, G.; Muy-rangel, D.; Gardea-béjar, A.; González-aguilar, G.; Heredia, B.; Báez-sañudo, M.; Siller-cepeda, J.; Vélez, R.; Rocha, D.E.L.A. Nutritional and Nutraceutical Components of Commercial Eggplant Types Grown in Sinaloa, Mexico. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 538–544. [Google Scholar] [CrossRef]
- Niño-Medina, G.; Urías-Orona, V.; Muy-Rangel, M.D.; Heredia, J.B. Structure and content of phenolics in eggplant (Solanum melongena)—A review. S. Afr. J. Bot. 2017, 111, 161–169. [Google Scholar] [CrossRef]
- Scorsatto, M.; de Castro Pimentel, A.; da Silva, A.J.R.; Sabally, K.; Rosa, G.; de Oliveira, G.M.M. Assessment of Bioactive Compounds, Physicochemical Composition, and In Vitro Antioxidant Activity of Eggplant Flour. Int. J. Cardiovasc. Sci. 2017, 30, 235–242. [Google Scholar] [CrossRef]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing Capacity, Chlorogenic Acid Content and Biological Activity in a Collection of Scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) Eggplants. Int. J. Mol. Sci 2014, 15, 17221–17241. [Google Scholar] [CrossRef]
- Gürbüz, N.; Uluişik, S.; Frary, A.; Frary, A.; Doğanlar, S. Health benefits and bioactive compounds of eggplant. Food Chem. 2018, 268, 602–610. [Google Scholar] [CrossRef]
- Jung, E.J.; Bae, M.S.; Jo, E.K.; Jo, Y.H.; Lee, S.C. Antioxidant activity of different parts of eggplant. J. Med. Plant Res. 2011, 5, 4610–4615. [Google Scholar]
- Sadilova, E.; Stintzing, F.F.C.; Carle, R. Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts. Z. fur Naturforsch. C 2006, 61, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Kashiwada, Y.; Shida, Y.; Ikeshiro, Y.; Kaneyuki, T.; Konishi, T. Nasunin from eggplant consists of cis-trans isomers of delphinidin 3-[4-(p-coumaroyl)-L-rhamnosyl (1→6)glucopyranoside]-5-glucopyranoside. J. Agric. Food Chem. 2005, 53, 9472–9477. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, K.; Kaneyuki, T.; Miyake, T.; Mori, M. Antiangiogenic activity of nasunin, an antioxidant anthocyanin, in eggplant peels. J. Agric. Food Chem. 2005, 53, 6272–6275. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y.; Kaneyuki, T.; Igarashi, K.; Mori, A.; Packer, L. Antioxidant activity of nasunin, an anthocyanin in eggplant peels. Toxicology 2000, 148, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Okmen, B.; Sigva, H.O.; Mutlu, S.; Doganlar, S.; Yemenicioglu, A.; Frary, A. Total antioxidant activity and total phenolic contents in different turkish eggplant (Solanum Melongena L.) cultivars. Int. J. Food Prop. 2009, 12, 616–624. [Google Scholar] [CrossRef]
- Piao, X.; Chung, J.; Lee, G.; Lee, J.; Cho, G.; Lee, H.; Ma, K.; Guo, J.; Kim, H.S.; Lee, S. Variation in antioxidant activity and flavonoid aglycones in eggplant (Solanum melongena L.) germplasm. Plant Breed. Biotechnol. 2014, 2014, 396–403. [Google Scholar] [CrossRef]
- Stommel, J.R.; Whitaker, B.D. Phenolic Acid Content and composition of eggplant fruit in a germplasm core subset. J. Am. Soc. Hort. Sci. 2003, 128, 704–710. [Google Scholar] [CrossRef]
- Plazas, M.; Andújar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Breeding for Chlorogenic Acid Content in Eggplant: Interest and Prospects. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef]
- Prohens, J.; Rodríguez-Burruezo, A.; Raigón, M.D.; Nuez, F. Total Phenolic Concentration and Browning Susceptibility in a Collection of Different Varietal Types and Hybrids of Eggplant: Implications for Breeding for Higher Nutritional Quality and Reduced Browning. J. Am. Soc. Hortic. Sci. 2007, 132, 638–646. [Google Scholar] [CrossRef]
- Hanson, P.M.; Yang, R.-Y.Y.; Tsou, S.C.S.S.; Ledesma, D.; Engle, L.; Lee, T.-C.C. Diversity in eggplant (Solanum melongena) for superoxide scavenging activity, total phenolics, and ascorbic acid. J. Food Compos. Anal. 2006, 19, 594–600. [Google Scholar] [CrossRef]
- Muñoz-Falcón, J.E.; Prohens, J.; Vilanova, S.; Nuez, F. Diversity in commercial varieties and landraces of black eggplants and implications for broadening the breeders’ gene pool. Ann. Appl. Biol. 2009, 154, 453–465. [Google Scholar] [CrossRef]
- Gramazio, P.; Prohens, J.; Plazas, M.; Herraiz, F.J.; Castillo, E.; Knapp, S.; Meyer, R.S.; Vilanova, S. Location of chlorogenic acid biosynthesis pathway and polyphenol oxidase genes in a new interspecific anchored linkage map of eggplant. BMC Plant Biol. 2014, 14, 350. [Google Scholar] [CrossRef] [PubMed]
- Prohens, J.; Whitaker, B.D.; Plazas, M.; Vilanova, S.; Hurtado, M.; Blasco, M.; Gramazio, P.; Stommel, J.R. Genetic diversity in morphological characters and phenolic acids content resulting from an interspecific cross between eggplant, Solanum melongena, and its wild ancestor (S. incanum). Ann. Appl. Biol. 2013, 162, 242–257. [Google Scholar] [CrossRef]
- Oboh, G.; Ekperigin, M.M.; Kazeem, M.I. Nutritional and haemolytic properties of eggplants (Solanum macrocarpon) leaves. J. Food Compos. Anal. 2005, 18, 153–160. [Google Scholar] [CrossRef]
- Kaushik, P.; Gramazio, P.; Vilanova, S.; Raigón, M.D.; Prohens, J.; Plazas, M. Phenolics content, fruit flesh colour and browning in cultivated eggplant, wild relatives and interspecific hybrids and implications for fruit quality breeding. Food Res. Int. 2017, 102, 392–401. [Google Scholar] [CrossRef]
- Yadav, V.K.; Singh, R.; Jha, R.K.; Kaushik, P. Biochemical variability of eggplant peel among Indian cultivars. Indian J. Biochem. Biophys. 2020, 57, 634–637. [Google Scholar] [CrossRef]
- Zaro, M.J.; Keunchkarian, S.; Chaves, A.R.; Vicente, A.R.; Concellón, A. Changes in bioactive compounds and response to postharvest storage conditions in purple eggplants as affected by fruit developmental stage. Postharvest Biol. Technol. 2014, 96, 110–117. [Google Scholar] [CrossRef]
- Kadoglidou, K.; Chatzopoulou, P.; Maloupa, E.; Kalaitzidis, A.; Ghoghoberidze, S.; Katsantonis, D. Mentha and Oregano Soil Amendment Induces Enhancement of Tomato Tolerance against Soilborne Diseases, Yield and Quality. Agronomy 2020, 10, 406. [Google Scholar] [CrossRef]
- Leogrande, R.; Lopedota, O.; Vitti, C.; Ventrella, D.; Montemurro, F. Effects of irrigation volumes and organic fertilizers on eggplant grown in Mediterranean environment. Acta Agric. Scand. Sect. B Soil Plant Sci. 2014, 64, 518–528. [Google Scholar] [CrossRef]
- Sadler, G.D.; Murphy, P.A. pH and Titratable Acidity. In Food Analysis; Nielsen, S.S., Ed.; Springer: Boston, MA, USA, 2010; pp. 219–238. ISBN 978-1-4419-1478-1. [Google Scholar]
- Ntinas, G.K.; Kadoglidou, K.; Tsivelika, N.; Krommydas, K.; Kalivas, A.; Ralli, P.; Irakli, M. Performance and Hydroponic Tomato Crop Quality Characteristics in a Novel Greenhouse Using Dye-Sensitized Solar Cell Technology for Covering Material. Horticulturae 2019, 5, 42. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Irakli, M.N.; Samanidou, V.F.; Biliaderis, C.G.; Papadoyannis, I.N. Development and validation of an HPLC-method for determination of free and bound phenolic acids in cereals after solid-phase extraction. Food Chem. 2012, 134, 1624–1632. [Google Scholar] [CrossRef]
- Bao, J.; Cai, Y.; Sun, M.; Wang, G.; Corke, H. Anthocyanins, flavonols, and free radical scavenging activity of Chinese Bayberry (Myrica rubra) extracts and their color properties and stability. J. Agric. Food Chem. 2005, 53, 2327–2332. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Skendi, A.; Irakli, M.; Chatzopoulou, P. Analysis of phenolic compounds in Greek plants of Lamiaceae family by HPLC. J. Appl. Res. Med. Aromat. Plants 2017, 6, 62–69. [Google Scholar] [CrossRef]
- Frankel, E.N.; Meyer, A.S. The problems of using one-dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Metsalu, T.; Vilo, J. ClustVis: A web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 2015, 43, W566–W570. [Google Scholar] [CrossRef] [PubMed]
- San José, R.; Sánchez, M.C.; Cámara, M.M.; Prohens, J. Composition of eggplant cultivars of the Occidental type and implications for the improvement of nutritional and functional quality. Int. J. Food Sci. Technol. 2013, 48, 2490–2499. [Google Scholar] [CrossRef]
- Todaro, A.; Cavallaro, R.; Argento, S.; Branca, F.; Spagna, G. Study and characterization of polyphenol oxidase from eggplant (Solanum melongena L.). J. Agric. Food Chem. 2011, 59, 11244–11248. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Carvalho, J.; Orlanda, J.F.F. Heat stability and effect of pH on enzyme activity of polyphenol oxidase in buriti (Mauritia flexuosa Linnaeus f.) fruit extract. Food Chem. 2017, 233, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.M. Polyphenol oxidases in plants and fungi: Going places? A review. Phytochemistry 2006, 67, 2318–2331. [Google Scholar] [CrossRef]
- Selinheimo, E.; NiEidhin, D.; Steffensen, C.; Nielsen, J.; Lomascolo, A.; Halaouli, S.; Record, E.; O’Beirne, D.; Buchert, J.; Kruus, K. Comparison of the characteristics of fungal and plant tyrosinases. J. Biotechnol. 2007, 130, 471–480. [Google Scholar] [CrossRef]
- Roudsari, M.H.; Signoret, A.; Crouzet, J. Eggplant polyphenol oxidase: Purification, characterisation and properties. Food Chem. 1981, 7, 227–235. [Google Scholar] [CrossRef]
- Vijayanand, S.; Nagasathya, S.; Peter, B. Extraction, Characterization and Inhibition of Polyphenol Oxidase in Solanum Melongena. Artic. Int. J. Pharm. Sci. Res. 2021, 12, 4292. [Google Scholar] [CrossRef]
- De Pascale, S.; Maggio, A.; Fogliano, V.; Ambrosino, P.; Ritieni, A. Irrigation with saline water improves carotenoids content and antioxidant activity of tomato. J. Hortic. Sci. Biotechnol. 2001, 76, 447–453. [Google Scholar] [CrossRef]
- Hanifah, A.; Maharijaya, A.; Putri, S.P.; Laviña, W.A. Sobir Untargeted Metabolomics Analysis of Eggplant (Solanum melongena L.) Fruit and Its Correlation to Fruit Morphologies. Metabolites 2018, 8, 49. [Google Scholar] [CrossRef]
- Ayaz, F.A.; Colak, N.; Topuz, M.; Tarkowski, P.; Jaworek, P.; Seiler, G.; Inceer, H. Comparison of Nutrient Content in Fruit of Commercial Cultivars of Eggplant (Solanum melongena L.). Polish J. Food Nutr. Sci. 2015, 65, 251–259. [Google Scholar] [CrossRef]
- Bidaramali, V.; Akhtar, S.; Das, A. Proximate Composition and Bioactive Compounds in Diverse Eggplant Genotypes. Curr. J. Appl. Sci. Technol. 2020, 39, 113–121. [Google Scholar] [CrossRef][Green Version]
- Johnson, R.W.; Dixon, M.A.; Lee, D.R. Water relations of the tomato during fruit growth. Plant. Cell Environ. 1992, 15, 947–953. [Google Scholar] [CrossRef]
- Serrano, R. Salt tolerance in plants and microorganisms: Toxicity targets and defense responses. Int. Rev. Cytol. 1996, 165, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, M.; Katarzyna, K.; Bajer, M. The influence of postharvest storage on quality characteristics of fruit of eggplant cultivars. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 200–205. [Google Scholar]
- Arkoub-Djermoune, L.; Boulekbache-Makhlouf, L.; Zeghichi-Hamri, S.; Bellili, S.; Boukhalfa, F.; Madani, K. Influence of the thermal processing on the physico-chemical properties and the antioxidant activity of A solanaceae vegetable: Eggplant. J. Food Qual. 2016, 39, 181–191. [Google Scholar] [CrossRef]
- Luthria, D.; Singh, A.P.; Wilson, T.; Vorsa, N.; Banuelos, G.S.; Vinyard, B.T. Influence of conventional and organic agricultural practices on the phenolic content in eggplant pulp: Plant-to-plant variation. Food Chem. 2010, 121, 406–411. [Google Scholar] [CrossRef]
- Apak, R.; Gorinstein, S.; Böhm, V.; Schaich, K.M.; Özyürek, M.; Güçlü, K. Methods of measurement and evaluation of natural antioxidant capacity/activity (IUPAC Technical Report). Pure Appl. Chem. 2013, 85, 957–998. [Google Scholar] [CrossRef]
- Koley, T.K.; Tiwari, S.K.; Sarkar, A.; Nishad, J.; Goswami, A.; Singh, B. Antioxidant Potential of Indian Eggplant: Comparison Among White, Purple and Green Genotypes Using Chemometrics. Agric. Res. 2019, 8, 9–20. [Google Scholar] [CrossRef]
- Raigon, M.D.; Prohens, J.; Munoz-Falcon, J.E.; Nuez, F. Comparison of eggplant landraces and commercial varieties for fruit content of phenolics, minerals, dry matter and protein. J. Food Compos. Anal. 2008, 21, 370–376. [Google Scholar] [CrossRef]
- Chioti, V.; Zeliou, K.; Bakogianni, A.; Papaioannou, C.; Biskinis, A.; Petropoulos, C.; Lamari, F.N.; Papasotiropoulos, V. Nutritional Value of Eggplant Cultivars and Association with Sequence Variation in Genes Coding for Major Phenolics. Plants 2022, 11, 2267. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, A.; Wu, X.; Zhu, Z.; Yang, Z.; Zhu, Y.; Zha, D. Transcriptome analysis revealed expression of genes related to anthocyanin biosynthesis in eggplant (Solanum melongena L.) under high-temperature stress. BMC Plant Biol. 2019, 19. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, B.D.; Stommel, J.R. Distribution of hydroxycinnamic acid conjugates in fruit of commercial eggplant (Solanum melongena L.) cultivars. J. Agric. Food Chem. 2003, 51, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Flores, M.I.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Systematic study of the content of phytochemicals in fresh and fresh-cut vegetables. Antioxidants 2015, 4, 345–358. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence and dietary burden. J. Sci. Food Agric. 1999, 79, 362–372. [Google Scholar] [CrossRef]
- Clifford, M.N. Chlorogenic acids and other cinnamates—Nature, occurrence, dietary burden, absorption and metabolism. J. Sci. Food Agric. 2000, 80, 1033–1043. [Google Scholar] [CrossRef]
- Luthria, D.L. A simplified UV spectral scan method for the estimation of phenolic acids and antioxidant capacity in eggplant pulp extracts. J. Funct. Foods 2012, 4, 238–242. [Google Scholar] [CrossRef]
- Singh, A.P.; Luthria, D.; Wilson, T.; Vorsa, N.; Singh, V.; Banuelos, G.S.; Pasakdee, S. Polyphenols content and antioxidant capacity of eggplant pulp. Food Chem. 2009, 114, 955–961. [Google Scholar] [CrossRef]
- Šilarová, P.; Boulekbache-Makhlouf, L.; Pellati, F.; Česlová, L. Monitoring of chlorogenic acid and antioxidant capacity of Solanum melongena L. (eggplant) under different heat and storage treatments. Antioxidants 2019, 8, 234. [Google Scholar] [CrossRef]
- Lo Scalzo, R.; Fibiani, M.; Francese, G.; D’Alessandro, A.; Rotino, G.L.; Conte, P.; Mennella, G. Cooking influence on physico-chemical fruit characteristics of eggplant (Solanum melongena L.). Food Chem. 2016, 194, 835–842. [Google Scholar] [CrossRef]
- Irakli, M.; Skendi, A.; Bouloumpasi, E.; Chatzopoulou, P.; Biliaderis, C.G. Lc-ms identification and quantification of phenolic compounds in solid residues from the essential oil industry. Antioxidants 2021, 10, 2016. [Google Scholar] [CrossRef] [PubMed]
- Baderschneider, B.; Winterhalter, P. Isolation and characterization of novel benzoates, cinnamates, flavonoids, and lignans from Riesling wine and screening for antioxidant activity. J. Agric. Food Chem. 2001, 49, 2788–2798. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Dueñas, M.; Estrella, I.; Hernández, T.; Benitez, V.; Esteban, R.M.; Martín-Cabrejas, M.A. Evaluation of phenolic profile and antioxidant properties of Pardina lentil As affected by industrial dehydration. J. Agric. Food Chem. 2010, 58, 10101–10108. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.G.; Hu, Q.P.; Liu, Y. Antioxidant and DNA-protective activities of chlorogenic acid isomers. J. Agric. Food Chem. 2012, 60, 11625–11630. [Google Scholar] [CrossRef]

| Genotype | Dry Matter g /100 g fw 1 | pH | Total Soluble Solids (°Brix) | Total Acidity (%) |
|---|---|---|---|---|
| C1 | 6.19 ± 0.34 def | 5.13 ± 0.02 n | 1.25 ± 0.05 k | 0.19 ± 0.006 c |
| C2 | 9.45 ± 0.45 a | 5.30 ± 0.01 g | 2.25 ± 0.05 ef | 0.12 ± 0.003 i |
| C3 | 5.38 ± 0.51 f | 5.12 ± 0.01 o | 3.05 ± 0.05 c | 0.29 ± 0.010 a |
| Hyb1 | 5.21 ± 0.45 fg | 5.05 ± 0.01 q | 1.60 ± 0.02 j | 0.16 ± 0.003 f |
| Hyb2 | 9.08 ± 0.71 a | 4.99 ± 0.03 s | 3.15 ± 0.05 c | 0.12 ± 0.006 h |
| L1 | 8.61 ± 0.40 ab | 5.30 ± 0.01 f | 2.67 ± 0.06 d | 0.09 ± 0.001 m |
| L2 | 6.72 ± 0.07 cde | 5.53 ± 0.01 b | 1.63 ± 0.15 ij | 0.11 ± 0.001 j |
| L3 | 5.57 ± 0.41 cde | 5.57 ± 0.01 a | 2.47 ± 0.22 de | 0.09 ± 0.001 l |
| L4 | 6.99 ± 0.40 cd | 5.43 ± 0.01 e | 1.63 ± 0.06 ij | 0.08 ± 0.001 n |
| L5 | 8.70 ± 0.11 ab | 5.29 ± 0.01 i | 2.40 ± 0.17 def | 0.10 ± 0.004 k |
| L6 | 9.20 ± 0.10 a | 5.27 ± 0.02 j | 1.53 ± 0.06 j | 0.12 ± 0.001 i |
| L7 | 6.28 ± 0.28 def | 5.29 ± 0.02 h | 1.90 ± 0.02 ghi | 0.09 ± 0.005 l |
| L8 | 7.67 ± 0.40 bc | 5.44 ± 0.01 d | 1.73 ± 0.11 hij | 0.12 ± 0.005 h |
| L9 | 7.21 ± 0.20 cd | 5.19 ± 0.01 m | 1.77 ± 0.06 hij | 0.15 ± 0.010 g |
| L10 | 4.19 ± 0.32 gh | 5.24 ± 0.06 k | 4.20 ± 0.01 b | 0.18 ± 0.003 d |
| BL | 3.43 ± 0.12 h | 5.46 ± 0.02 c | 1.90 ± 0.02 ghi | 0.26 ± 0.001 b |
| Cross1 | 9.14 ± 0.39 a | 5.22 ± 0.01 l | 4.65 ± 0.15 a | 0.11 ± 0.003 k |
| Cross2 | 6.58 ± 0.43 cde | 5.03 ± 0.01 r | 1.95 ± 0.05 gh | 0.16 ± 0.010 fg |
| Smac | 5.83 ± 0.27 ef | 5.06 ± 0.01 p | 2.15 ± 0.05 fg | 0.17 ± 0.001 e |
| CV 2 % | 5.24 | 0.36 | 3.83 | 3.45 |
| HSD 3 | 0.2113 | 0.0006 | 0.0516 | 0.0006 |
| Genotype | Total Phenolics Content (mg GAE/g dw) | Total Flavonoids Content (mg CATE /g dw) | ||
|---|---|---|---|---|
| Pulp | Peel | Pulp | Peel | |
| C1 | 7.06 ± 0.20 a | 6.63 ± 0.18 jk | 7.46 ± 0.09 c | 5.99 ± 0.10 j |
| C2 | 3.81 ± 0.13 d | 8.09 ± 0.13 h | 3.24 ± 0. 04 f | 5.75 ± 0.21 j |
| C3 | 4.63 ± 0.02 c | 10.82 ± 0.13 c | 4.37 ± 0.06 e | 11.07 ± 0.32 e |
| Hyb1 | 3.64 ± 0.03 de | 10.64 ± 0.46 c | 2.59 ± 0.02 gh | 11.66 ± 0.32 d |
| Hyb2 | 3.25 ± 0.09 ef | 12.74 ± 0.06 a | 2.43 ± 0.17 h | 14.24 ± 0.32 a |
| L1 | 3.85 ± 0.10 d | 7.27 ± 0.14 i | 2.91 ± 0.18 fg | 6.87 ± 0.16 i |
| L2 | 3.09 ± 0.05 fg | 8.66 ± 0.17 fg | 2.23 ± 0.23 hi | 7.32 ± 0.05 hi |
| L3 | 7.43 ± 0.46 a | 9.82 ± 0.27 d | 8.12 ± 0.30 b | 8.90 ± 0.16 f |
| L4 | 2.67 ± 0.08 g | 9.31 ± 0.44 de | 1.74 ± 0.01 j | 7.59 ± 0.09 h |
| L5 | 2.66 ± 0.18 g | 7.01 ± 0.15 ij | 1.94 ± 0.12 ij | 7.17 ± 0.05 hi |
| L6 | 7.05 ± 0.19 a | 10.47 ± 0.11 c | 7.54 ± 0.13 c | 12.52 ± 0.06 c |
| L7 | 2.06 ± 0.14 h | 7.27 ± 0.27 i | 1.49 ± 0.12 j | 7.40 ± 0.25 h |
| L8 | 4.82 ± 0.16 c | 8.14 ± 0.53 gh | 4.48 ± 0.27 e | 8.37 ± 0.20 g |
| L9 | 2.73 ± 0.18 fg | 6.52 ± 0.20 jk | 1.63 ± 0.18 j | 4.71 ± 0.25 k |
| L10 | 5.63 ± 0.17 b | 9.21 ± 0.14 ef | 5.75 ± 0.01 d | 10.68 ± 0.19 e |
| BL | 4.07 ± 0.03 d | 10.43 ± 0.24 c | 3.24 ± 0.07 f | 11.09 ± 0.49 e |
| Cross1 | 5.55 ± 0.28 b | 11.84 ± 0.27 b | 5.55 ± 0.04 d | 13.75 ± 0.26 b |
| Cross2 | 4.66 ± 0.25 c | 8.24 ± 0.15 gh | 4.56 ± 0.01 e | 8.86 ± 0.19 f |
| Smac | 7.37 ± 0.26 a | 6.33 ± 0.18 k | 8.92 ± 0.06 a | 7.27 ± 0.12 hi |
| CV 1 % | 6.33 | 2.05 | 3.44 | 1.69 |
| HSD 2 | 0.0983 | 0.1049 | 0.0837 | 0.0876 |
| Genotype | n-CA (mg/g dw) | CA (mg/g dw) | c-CA (mg/g dw) | |||
|---|---|---|---|---|---|---|
| Pulp | Peel | Pulp | Peel | Pulp | Peel | |
| C1 | 0.104 ± 0.005 b | 0.174 ± 0.015 bc | 6.338 ± 0.019 d | 1.963 ± 0.058 n | 0.046 ± 0.003 b | 0.174 ± 0.014 h |
| C2 | 0.024 ± 0.004 f | 0.059 ± 0.005 bc | 3.079 ± 0.051 h | 3.624 ± 0.024 l | 0.014 ± 0.006 b | 0.136 ± 0.010 l |
| C3 | 0.017 ± 0.001 g | 0.110 ± 0.010 bc | 4.240 ± 0.070 g | 8.137 ± 0.058 b | 0.007 ± 0.007 b | 0.155 ± 0.015 j |
| Hyb1 | 0.019 ± 0.001 g | 0.356 ± 0.015 abc | 1.865 ± 0.035 jk | 4.065 ± 0.058 k | 0.034 ± 0.011 b | 0.471 ± 0.021 a |
| Hyb2 | 0.019 ± 0.004 g | 0.145 ± 0.015 bc | 2.022 ± 0.022 j | 7.090 ± 0.020 d | 0.022 ± 0.008 b | 0.225 ± 0.025 g |
| L1 | nd, 1,i | 0.079 ± 0.058 bc | 2.348 ± 0.049 i | 2.990 ± 0.090 m | 0.084 ± 0.014 ab | 0.106 ± 0.014 m |
| L2 | 0.002 ± 0.004 i | 0.548 ± 0.031 a | 1.574 ± 0.025 kl | 4.062 ± 0.062 k | 0.015 ± 0.005 b | 0.240 ± 0.025 e |
| L3 | 0.102 ± 0.016 b | 0.411 ± 0.011 ab | 10.424 ± 0.302 a | 6.559 ± 0.144 e | 0.036 ± 0.006 b | 0.147 ± 0.007 k |
| L4 | nd i | 0.284 ± 0.010 abc | 0.993 ± 0.003 mn | 5.200 ± 0.080 g | 0.014 ± 0.004 b | 0.166 ± 0.016 i |
| L5 | nd i | 0.131 ± 0.021 bc | 0.960 ± 0.059 mn | 4.511 ± 0.061 i | 0.015 ± 0.006 b | 0.087 ± 0.008 n |
| L6 | 0.014 ± 0.012 h | 0.067 ± 0.007 bc | 9.939 ± 0.060 b | 9.774 ± 0.075 a | 0.062 ± 0.013 b | 0.081 ± 0.011 o |
| L7 | nd i | 0.175 ± 0.015 bc | 0.877 ± 0.037 n | 5.250 ± 0.100 g | 0.014 ± 0.004 b | 0.080 ± 0.015 o |
| L8 | nd i | 0.097 ± 0.012 bc | 4.402 ± 0.002 fg | 4.266 ± 0.101 j | 0.013 ± 0.002 b | 0.057 ± 0.008 p |
| L9 | 0.001 ± 0.001 i | 0.381 ± 0.020 abc | 1.271 ± 0.129 lm | 1.855 ± 0.055 o | 0.013 ± 0.002 b | 0.035 ± 0.005 r |
| L10 | 0.034 ± 0.001 e | 0.110 ± 0.010 bc | 5.659 ± 0.180 e | 6.110 ± 0.011 f | 0.023 ± 0.005 b | 0.235 ± 0.005 f |
| BL | 0.025 ± 0.004 f | 0.205 ± 0.020 abc | 2.514 ± 0.015 i | 4.721 ± 0.019 h | 0.038 ± 0.007 b | 0.296 ± 0.026 c |
| Cross1 | 0.057 ± 0.004 d | 0.354 ± 0.010 abc | 5.500 ± 0.090 e | 7.669 ± 0.070 c | 0.022 ± 0.008 b | 0.293 ± 0.013 d |
| Cross2 | 0.066 ± 0.006 c | 0.131 ± 0.011 bc | 4.624 ± 0.086 f | 4.564 ± 0.082 i | 0.023 ± 0.002 b | 0.385 ± 0.015 b |
| Smac | 0.528 ± 0.031 a | 0.017 ± 0.005 c | 7.274 ± 0.095 c | 1.942 ± 0.042 no | 0.210 ± 0.030 a | 0.050 ± 0.010 q |
| CV 2 % | 16.35 | 9.54 | 2.45 | 0.78 | 12.17 | 5.09 |
| HSD 3 | 0.00058 | 0.06583 | 0.05774 | 0.01826 | 0.02582 | 0.00058 |
| Genotype | ABTS (mg TE/g dw) | DPPH (mg TE/g dw) | FRAP (mg TE/g dw) | |||
|---|---|---|---|---|---|---|
| Pulp | Peel | Pulp | Peel | Pulp | Peel | |
| C1 | 8.98 ± 0.14 cd | 10.17 ± 0.60 hi | 7.11 ± 0.09 c | 8.01 ± 0.21 k | 8.74 ± 0.17 b | 8.06 ± 1.45 h |
| C2 | 4.85 ± 0.11 hij | 15.54 ± 2.61 cdefg | 3.20 ± 0.06 g | 10.99 ± 0.35 ef | 4.16 ± 0.15 fg | 15.73 ± 3.01 defg |
| C3 | 6.27 ± 0.22 fg | 20.47 ± 3.08 ab | 4.25 ± 0.07 f | 15.01 ± 0.17 c | 5.24 ± 0.12 e | 23.90 ± 1.37 a |
| Hyb1 | 4.29 ± 0.14 ijk | 16.79 ± 2.73 bcde | 2.71 ± 0.05 h | 14.87 ± 0.23 c | 3.95 ± 0.16 fg | 18.02 ± 1.76 cde |
| Hyb2 | 3.65 ± 0.12 jkl | 24.87 ± 4.70 a | 2.63 ± 0.02 h | 18.74 ± 0.36 a | 3.21 ± 0.16 h | 23.61 ± 2.01 ab |
| L1 | 5.41 ± 0.53 gh | 10.84 ± 1.87 ghi | 3.19 ± 0.04 g | 9.33 ± 0.37 hi | 3.73 ± 0.14 g | 10.82 ± 0.43 gh |
| L2 | 3.66 ± 0.06 jkl | 13.92 ± 2.34 defghi | 2.42 ± 0.20 hi | 11.71 ± 0.29 ef | 2.86 ± 0.16 hi | 17.32 ± 0.56 cde |
| L3 | 10.03 ± 0.22 ab | 16.18 ± 1.36 bcdef | 8.07 ± 0.14 b | 13.69 ± 0.43 d | 9.47 ± 0.29 a | 16.37 ± 1.81 cdef |
| L4 | 3.66 ± 0.72 jkl | 17.58 ± 3.99 bcd | 1.81 ± 0.16 jk | 13.67 ± 0.10 d | 2.29 ± 0.17 jk | 19.06 ± 4.32 abcd |
| L5 | 3.26 ± 0.19 l | 10.07 ± 1.94 hi | 2.05 ± 0.14 ij | 8.82 ± 0.12 ijk | 2.68 ± 0.11 ij | 11.09 ± 0.87 gh |
| L6 | 9.55 ± 0.13 bc | 16.70 ± 1.74 bcde | 7.43 ± 0.14 c | 14.44 ± 0.57 cd | 8.96 ± 0.27 b | 21.05 ± 0.01 abc |
| L7 | 2.97 ± 0.08 l | 11.54 ± 2.21 fghi | 1.58 ± 0.08 k | 9.92 ± 0.29 gh | 2.23 ± 0.06 k | 10.99 ± 1.03 gh |
| L8 | 6.15 ± 0.71 fg | 10.32 ± 0.98 hi | 4.29 ± 0.14 f | 9.62 ± 0.53 hi | 5.16 ± 0.07 e | 10.25 ± 0.57 h |
| L9 | 3.39 ± 0.13 kl | 11.04 ± 1.77 ghi | 2.22 ± 0.14 i | 8.22 ± 0.20 jk | 2.38 ± 0.07 jk | 9.21 ± 0.25 h |
| L10 | 7.50 ± 0.18 e | 12.58 ± 1.65 efghi | 5.47 ± 0.10 d | 10.66 ± 0.12 fg | 6.15 ± 0.08 d | 13.03 ± 1.91 efgh |
| BL | 4.58 ± 0.16 hij | 16.86 ± 2.66 bcde | 3.10 ± 0.04 g | 15.05 ± 0.03 c | 3.99 ± 0.21 fg | 20.14 ± 0.91 abcd |
| Cross1 | 8.09 ± 0.03 de | 19.86 ± 2.68 bc | 5.08 ± 0.24 e | 16.83 ± 0.22 b | 6.59 ± 0.06 c | 18.63 ± 2.15 bcd |
| Cross2 | 6.42 ± 0.02 f | 14.48 ± 2.28 defgh | 4.27 ± 0.01 f | 9.13 ± 0.33 hij | 5.15 ± 0.03 e | 11.68 ± 0.14 fgh |
| Smac | 10.57 ± 0.20 a | 9.13 ± 1.76 i | 9.04 ± 0.18 a | 9.99 ± 0.33 gh | 9.42 ± 0.05 a | 10.34 ± 0.78 h |
| CV 1 % | 5.06 | 10.48 | 2.94 | 2.63 | 2.70 | 10.68 |
| HSD 2 | 0.1742 | 0.8884 | 0.0707 | 0.1826 | 0.0796 | 0.9390 |
| Parameters | DM 1,2 | pH | TSS | TA | TPC-Pulp | TPC-Peel | TFC-Pulp | TFC-Peel | TAC-Peel | ABTS-Pulp | ABTS-Peel | DPPH-Pulp | DPPH-Peel | FRAP-Pulp | FRAP-Peel | CA-Pulp | n-CA-Pulp | c-CA-Pulp | CA-Peel | n-CA-Peel | c-CA-Peel |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DM | 1 | −0.007 | 0.106 | −0.676 ** | −0.083 | 0.045 | −0.084 | −0.013 | 0.033 | −0.041 | 0.140 | −0.073 | 0.063 | −0.051 | 0.032 | 0.028 | −0.187 | −0.120 | 0.185 | −0.103 | −0.345 |
| pH | 1 | −0.166 | −0.319 | −0.066 | −0.068 | −0.088 | −0.248 | −0.043 | −0.080 | −0.161 | −0.091 | −0.055 | −0.085 | 0.019 | 0.035 | −0.305 | −0.229 | 0.055 | 0.387 | −0.252 | |
| TSS | 1 | 0.050 | 0.117 | 0.449 | 0.0121 | 0.509 * 3 | −0.184 | 0.171 | 0.369 | 0.091 | 0.378 | 0.093 | 0.250 | 0.123 | −0.001 | −0.091 | 0.436 | −0.78 | 0.143 | ||
| TA | 1 | 0.213 | 0.155 | 0.191 | 0.208 | 0.165 | 0.156 | 0.145 | 0.171 | 0.121 | 0.176 | 0.228 | 0.086 | 0.153 | 0.094 | 0.023 | −0.219 | 0.254 | |||
| TPC-pulp | 1 | 0.047 | 0.991 ** | 0.194 | 0.029 | 0.987 ** | −0.082 | 0.985 ** | 0.032 | 0.993 ** | −0.051 | 0.954 ** | 0.567 * | 0.553 * | 0.174 | −0.221 | −0.076 | ||||
| TPC-peel | 1 | −0.003 | 0.915 ** | 0.180 | 0.014 | 0.930 ** | −0.037 | 0.958 ** | 0.033 | 0.897 ** | 0.128 | −0.293 | −0.303 | 0.778 ** | 0.196 | 0.524 * | |||||
| TFC-pulp | 1 | 0.161 | −0.007 | 0.991 ** | −0.119 | 0.995 ** | −0.004 | 0.993 ** | −0.087 | 0.949 ** | 0.637 ** | 0.597 ** | 0.162 | −0.255 | −0.126 | ||||||
| TFC-peel | 1 | −0.037 | 0.173 | 0.764 ** | 0.125 | 0.856 ** | 0.184 | 0.742 ** | 0.244 | −0.117 | −0.101 | 0.782 ** | −0.009 | 0.497 * | |||||||
| TAC-pulp | 1 | −0.052 | 0.346 | −0.027 | 0.239 | 0.043 | 0.243 | −0.045 | −0.138 | −0.230 | −0.067 | 0.129 | 0.143 | ||||||||
| ABTS-pulp | 1 | −0.104 | 0.985 ** | 0.008 | 0.986 ** | −0.080 | 0.952 ** | 0.606 ** | 0.586 ** | 0.188 | −0.241 | −0.118 | |||||||||
| ABTS-peel | 1 | −0.143 | −0.022 | 0.989 ** | −0.104 | 0.944 ** | 0.668 ** | 0.641 ** | 0.127 | −0.244 | −0.170 | ||||||||||
| DPPH-pulp | 1 | 0.928 ** | −0.084 | 0.919 ** | 0.005 | −0.280 | −0.333 | 0.694 ** | 0.176 | 0.459 * | |||||||||||
| DPPH-peel | 1 | 0.033 | 0.925 ** | 0.096 | −0.141 | −0.141 | 0.706 ** | 0.210 | 0.424 | ||||||||||||
| FRAP-pulp | 1 | −0.054 | 0.960 ** | 0.589 ** | 0.563 * | 0.189 | −0.219 | −0.103 | |||||||||||||
| FRAP-peel | 1 | 0.042 | −0.253 | −0.229 | 0.744 ** | 0.159 | 0.364 | ||||||||||||||
| CA-pulp | 1 | 0.432 | 0.420 | 0.359 | −0.175 | −0.129 | |||||||||||||||
| n-CA-pulp | 1 | 0.899 ** | −0.313 | −0.262 | −0.75 | ||||||||||||||||
| c-CA-pulp | 1 | −0.304 | −0.336 | −0.240 | |||||||||||||||||
| CA-peel | 1 | −0.035 | 0.127 | ||||||||||||||||||
| n-CA-peel | 1 | 0.334 | |||||||||||||||||||
| c-CA-peel | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadoglidou, K.I.; Krommydas, K.; Ralli, P.; Mellidou, I.; Kalyvas, A.; Irakli, M. Assessing Physicochemical Parameters, Bioactive Profile and Antioxidant Status of Different Fruit Parts of Greek Eggplant Germplasm. Horticulturae 2022, 8, 1113. https://doi.org/10.3390/horticulturae8121113
Kadoglidou KI, Krommydas K, Ralli P, Mellidou I, Kalyvas A, Irakli M. Assessing Physicochemical Parameters, Bioactive Profile and Antioxidant Status of Different Fruit Parts of Greek Eggplant Germplasm. Horticulturae. 2022; 8(12):1113. https://doi.org/10.3390/horticulturae8121113
Chicago/Turabian StyleKadoglidou, Kalliopi I., Konstantinos Krommydas, Parthenopi Ralli, Ifigeneia Mellidou, Apostolos Kalyvas, and Maria Irakli. 2022. "Assessing Physicochemical Parameters, Bioactive Profile and Antioxidant Status of Different Fruit Parts of Greek Eggplant Germplasm" Horticulturae 8, no. 12: 1113. https://doi.org/10.3390/horticulturae8121113
APA StyleKadoglidou, K. I., Krommydas, K., Ralli, P., Mellidou, I., Kalyvas, A., & Irakli, M. (2022). Assessing Physicochemical Parameters, Bioactive Profile and Antioxidant Status of Different Fruit Parts of Greek Eggplant Germplasm. Horticulturae, 8(12), 1113. https://doi.org/10.3390/horticulturae8121113







