In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. In Vitro Germination
2.3. Micropropagation
2.3.1. Establishment of Initial Cultures
2.3.2. Multiplication
2.3.3. In Vitro Rooting
2.3.4. In Vitro Culture Conditions and Data Collection
2.3.5. Ex Vitro Acclimatisation
2.4. Statistical Analysis
3. Results
3.1. Seed Germination
3.2. Micropropagation
3.2.1. Establishment of Initial Cultures
3.2.2. Multiplication
3.2.3. In Vitro Rooting and Ex Vitro Acclimatisation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganatsas, P.; Daskalakou, E.; Paitaridou, D. First results on early post-fire succession in an Abies cephalonica forest (Parnitha National Park, Greece). IForest Biogeosci. For. 2012, 5, 6–12. [Google Scholar] [CrossRef]
- Aplada, E.; Georgiadis, T.; Tiniakou, A.; Theocharopoulos, M. Phytogeography and ecological evaluation of the flora and vegetation of Mt Parnitha (Attica, Greece). Edinb. J. Bot. 2007, 64, 185–207. [Google Scholar] [CrossRef]
- Le Roux, J.J.; Hui, C.; Castillo, M.L.; Iriondo, J.M.; Keet, J.H.; Khapugin, A.A.; Médail, F.; Rejmánek, M.; Theron, G.; Yannelli, F.A.; et al. Recent anthropogenic plant extinctions differ in biodiversity hotspots and coldspots. Curr. Biol. 2019, 29, 2912–2918. [Google Scholar] [CrossRef] [PubMed]
- Dragozi, E.; Gitas, I.Z.; Stavrakoudis, D.G.; Theocharis, J.B. Burned area mapping using support vector machines and the FuzCoC feature selection method on VHR IKONOS imagery. Remote Sens. 2014, 6, 12005–12036. [Google Scholar] [CrossRef]
- Karani, P. National Park Parnes: Present and Future. Ph.D. Thesis, Harokopio University, Athens, Greece, 2008. (In Greek). [Google Scholar]
- Chatzimpiros, P.; Roumelioti, N.; Zamba, A.; Hadjibiros, K. Road transport emissions and capacity of forests in the region of Athens for sequestring these emissions: Carbon flow before and after forest fires. Eur. J. Environ. Sci. 2016, 6, 18–24. [Google Scholar] [CrossRef][Green Version]
- Fraga, B.M. Phytochemistry and chemotaxonomy of Sideritis species from the Mediterranean region. Phytochemistry 2012, 76, 7–24. [Google Scholar] [CrossRef] [PubMed]
- Patelou, E.; Chatzopoulou, P.; Polidoros, A.N.; Mylona, P.V. Genetic diversity and structure of Sideritis raeseri Boiss. & Heldr. (Lamiaceae) wild populations from Balkan Peninsula. J. Appl. Res. Med. Arom. Plants 2020, 16, 100241. [Google Scholar] [CrossRef]
- Grigoriadou, K.; Krigas, N.; Sarropoulou, V.; Papanastasi, K.; Tsoktouridis, G.; Maloupa, E. In vitro propagation of medicinal and aromatic plants: The case of selected Greek species with conservation priority. Vitr. Cell Dev. Biol. Plant 2019, 55, 635–646. [Google Scholar] [CrossRef]
- Papanikolaou, K.; Kokkini, S. A taxonomic revision of Sideritis L. section Empedoclia (RAFIN.) BENTHAM (Labiatae) in Greece. In Aromatic Plants; Springer: Dordrecht, The Netherland, 1982; pp. 101–128. [Google Scholar]
- Phoitos, D.; Constantinidis, T.; Kamari, G. The Red Data Book of Rare and Threatened Plants of Greece; Hellenic Botanical Society: Patras, Greece, 2009; Volume II, pp. 295–297. ISBN 978-960-9407-09-0. [Google Scholar]
- Kougioumoutzis, K.; Kokkoris, I.P.; Panitsa, M.; Strid, A.; Dimopoulos, P. Extinction risk assessment of the Greek endemic flora. Biology 2021, 10, 195. [Google Scholar] [CrossRef] [PubMed]
- Constantinidis, T. The flora and vegetation of the mountains Gerania, Pateras and Kitheron (SE Sterea Ellas, Greece). Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 1997. (In Greek with an English summary). [Google Scholar] [CrossRef]
- Gonzalez-Burgos, E.; Carretero, M.E.; Gomez-Serranillos, M.P. Sideritis spp.: Uses, chemical composition and pharmacological activities-a review. J. Ethnopharmacol. 2011, 135, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Lall, N.; Chrysargyris, A.; Lambrechts, I.; Fibrich, B.; Blom Van Staden, B.; Twilley, D.; Nuno de Canha, M.; Oosthuizen, C.B.; Bodiba, D.; Tzortzakis, N. Sideritis perfoliate (subsp. perfoliata) nutritive value and its potential medicinal properties. Antioxidants 2019, 8, 521. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Kulbat-Warycha, K.; Oracz, J.; Żyżelewicz, K. Polyphenols and other bioactive compounds of Sideritis plants and their potential biological activity. Molecules 2020, 25, 3763. [Google Scholar] [CrossRef] [PubMed]
- Kassi, E.; Papoutsi, Z.; Fokialakis, N.; Messari, I.; Mitakou, S.; Moutsatsou, P. Greek plant extracts exhibit selective estrogen receptor modulator (SERM)-like properties. J. Agric. Food Chem. 2004, 52, 6956–6961. [Google Scholar] [CrossRef] [PubMed]
- Tsaknis, J.; Lalas, S. Extraction and identification of natural antioxidant from Sideritis euboea (mountain tea). J. Agric. Food Chem. 2005, 53, 6375–6381. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized Phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef] [PubMed]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Kloukina, C.; Tomou, E.M.; Krigas, N.; Sarropoulou, V.; Madesis, P.; Maloupa, E.; Skaltsa, H. Non-polar secondary metabolites and essential oil of ex situ propagated and cultivated Sideritis syriaca L. subsp. syriaca (Lamiaceae) with consolidated identity (DNA Barcoding): Towards a potential new industrial crop. Ind. Crops Prod. 2020, 158, 112957. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Maloupa, E. Effect of the NO donor “sodium nitroprusside” (SNP), the ethylene inhibitor “cobalt chloride” (CoCl2) and the antioxidant vitamin E “α-tocopherol” on In Vitro shoot proliferation of Sideritis raeseri Boiss. & Heldr. subsp. raeseri. PCTOC 2017, 128, 619–629. [Google Scholar] [CrossRef]
- Krishnan, P.N.; Decruse, S.W.; Radha, R.K. Conservation of medicinal plants of Western Ghats, India and its sustainable utilization through in vitro technology. Vitr. Cell Dev. Biol.-Plant 2011, 47, 110–122. [Google Scholar] [CrossRef]
- Reed, B.M.; Sarasan, V.; Kane, M.; Bunn, E.; Pence, V.C. Biodiversity conservation and conservation biotechnology tools. Vitr. Cell Dev. Biol.-Plant 2011, 47, 1–4. [Google Scholar] [CrossRef]
- Galus, A.; Chenari Bouket, A.; Belbahri, L. In vitro propagation and acclimatization of dragon tree (Dracaena draco). Horticulturae 2019, 5, 64. [Google Scholar] [CrossRef]
- Rafiq, S.; Wagay, N.A.; Bhat, I.A.; Kaloo, Z.A.; Rashid, S.; Lin, F.; El-Abedin, T.K.Z.; Wani, S.H.; Mahmoud, E.A.; Almutairi, K.F.; et al. In vitro propagation of Aconitum chasmanthum Stapf Ex Holmes: An endemic and critically endangered plant species of the western Himalaya. Horticulturae 2021, 7, 586. [Google Scholar] [CrossRef]
- Sarasan, V.; Kite, G.C.; Sileshi, G.W.; Stevenson, P.C. Applications of phytochemical and in vitro techniques for reducing overharvesting of medicinal and pesticidal plants and generating income for the rural poor. Plant Cell Rep. 2011, 30, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.F.; de Souza, D.C.; Resente, L.V.; Gonçalves, W.M. Manejo de recursos genéticos vegetais. An da Acad. Pernambucana de Ciência Agronômica 2018, 15, 109–126. Available online: http://www.journals.ufrpe.br/index.php/apca/article/view/1824 (accessed on 4 March 2022).
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- International Seed Testing Association. International rules for seed testing. Seed Sci. Technol. 1999, 27, 333. [Google Scholar]
- Soltani, A.; Galeshi, S.; Zeinali, E.; Latifi, N. Genetic variation for and interrelationships among seed vigor traits in wheat from the Caspian Sea coasts of Iran. Seed Sci. Technol. 2001, 29, 653–662. [Google Scholar]
- Maguire, J.D. Speed of germination-aid in selection and evaluation for seedling emergence and vigor. Crop. Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Lloyd, G.; McCown, B. Commercially feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Int. Plant Prop. Soc. Proc. 1980, 30, 421–427. [Google Scholar]
- Estrelles, E.; Güemes, J.; Riera, J.; Boscai, U.; Ibars, A.; Costa, M. Seed germination behavior in Sideritis from different Iberian habitats. Not. Bot. Horti Agrobot. 2010, 38, 9–13. [Google Scholar] [CrossRef]
- Yankova-Tsvetkova, E.; Yurukova-Grancharova, P.; Vitkova, A. Reproductive biology of the Balkan endemic Sideritis scardica (Lamiaceae). Bot. Serb. 2013, 37, 83–87. [Google Scholar]
- Uçar, E.; Turgut, K. In Vitro Propagation of Some Mountain Tea (Sideritis) Species. Ziraat Fakültesi Derg. Akdeniz Üniversitesi 2009, 22, 51–57. [Google Scholar]
- Papafotiou, M.; Kalantzis, A. Seed germination and in vitro propagation of Sideritis athoa. Acta Hortic. 2009, 813, 471–476. [Google Scholar] [CrossRef]
- Shtereva, L.A.; Vassilevska-Ivanova, R.D.; Kraptchev, B.V. In vitro cultures for micropropagation, mass multiplication and preservation of an endangered medicinal plant Sideritis scardica Griseb. Bot. Serbica 2015, 39, 11–120. Available online: https://botanicaserbica.bio.bg.ac.rs/arhiva/pdf/2015_39_2_633_full.pdf (accessed on 10 October 2022).
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Thanos, C.A.; Doussi, M.A. Ecophysiology of seed germination in endemic labiates of Crete. Isr. J. Plant Sci. 1995, 43, 227–237. [Google Scholar] [CrossRef]
- Kadis, C.; Georghiou, K. Seed dispersal and germination behaviour of three threatened endemic labiates of Cyprus. Plant Spec. Biol. 2010, 25, 77–84. [Google Scholar] [CrossRef]
- Picciau, R.; Pritchard., H.W.; Mattana, E.; Bacchetta, G. Thermal thresholds for seed germination in Mediterranean species are higher in mountain compared with lowland areas. Seed Sci. Res. 2019, 29, 44–54. [Google Scholar] [CrossRef]
- Donohue, K. Completing the cycle: Maternal effects as the missing link in plant life histories. Philos. Trans. R. Soc. B. Biol. Sci. 2009, 364, 1059–1074. [Google Scholar] [CrossRef] [PubMed]
- Donohue, K.; De Casas, R.R.; Burghardt, L.T.; Kovach, K.; Willis, C.G. Germination, postgermination adaptation, and species ecological ranges. Annu. Rev. Ecol. Evol. Syst. 2010, 41, 293–319. Available online: https://www.annualreviews.org/doi/10.1146/annurev-ecolsys-102209-144715 (accessed on 10 October 2022). [CrossRef]
- Finch-savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Baskin, C.C. Plant population differences in dormancy and germination characteristics of seeds: Heredity or environment? Am. Midl. Nat. 1973, 90, 493–498. [Google Scholar] [CrossRef]
- Copete, M.A.; Herranz, J.M.; Ferrandis, P.; Copete, E. Annual dormancy cycles in buried seeds of shrub species: Germination ecology of Sideritis serrata (Labiatae). Plant Biol. 2015, 17, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Crocker, W.M. Mechanics of dormancy in seeds. Am. J. Bot. 1916, 3, 99–120. [Google Scholar] [CrossRef]
- Baskin, J.M.; Baskin, C.C. The seed bank in a population of an endemic plant species and its ecological significance. Biol. Consev. 1978, 14, 125–130. [Google Scholar] [CrossRef]
- Cristaudo, A.; Catara, S.; Mingo, A.; Restuccia, A.; Onofri, A. Temperature and storage time strongly affect the germination success of perennial Euphorbia species in Mediterranean regions. Ecol. Evol. 2019, 9, 10984–10999. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Maloupa, E. Effect of polyamine type and concentration on in vitro propagation and ex situ conservation of Sideritis raeseri Boiss & Heldr. Subsp. raeseri. Res. J. Life Sci. Bioinform. Pharmaceut. Chem. Sci. 2019, 5, 583–598. [Google Scholar] [CrossRef]
- Sahini, R.; Gupta, S.C. In vitro plantlet regeneration from seedling nodal explants of Acacia catechu. Indian J. Exp. Biol. 2002, 40, 1050–1055. Available online: https://pubmed.ncbi.nlm.nih.gov/12587736/ (accessed on 10 October 2022).
- Shukla, S.; Shukla, S.K.; Mishra, S.K. In vitro plant regeneration from seedling explants of Stereospermum personatum DC: A medicinal tree. Trees 2009, 23, 409–413. [Google Scholar] [CrossRef]
- Aki, C.; Cördük, N. Inhibition of browning problem during micropropagation of Sideritis trojana Bornm., an endemic medicinal herb of Turkey, Rom. Biotechnol. Lett. 2011, 16, 6760–6765. [Google Scholar]
- Rout, G.R.; Samantaray, S.; Das, P. Manipulation and propagation of medicinal plants. Biotechnol. Adv. 2000, 18, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.D. The role of activated charcoal in plant tissue culture. Biotechnol. Adv. 2008, 26, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Aicha, N.; Abdelmalek, E.M. Rapid in vitro regeneration and clonal multiplication of Thymus bleicherianus pomel, a rare and threatened medicinal and aromatic plant in Morocco. Med. Aromat. Plants 2014, 3, 145. [Google Scholar]
- Liao, Z.; Chen, M.; Tan, F.; Sun, X.; Tang, K. Microprogagation of endangered Chinese aloe. PCTOC 2004, 76, 83–86. [Google Scholar] [CrossRef]
- Hamdeni, I.; Slim, S.; Sanaa, A.; Louhaichi, M.; Boulila, A.; Bettaieb, T. Rosemary essential oil enhances culture establishment and inhibits contamination and enzymatic browning: Applications for in vitro propagation of Aloe vera L. S. Afr. J. Bot. 2022, 147, 1199–1205. [Google Scholar] [CrossRef]
- Carelli, B.P.; Echeverrigaray, S. An improved system for the In Vitro propagation of rose cultivars. Sci. Hortic. 2002, 92, 69–74. [Google Scholar] [CrossRef]
- Papafotiou, M.; Martini, A.N.; Vlachou, G. In Vitro propagation as a tool to enhance the use of native ornamentals in archaeological sites of Greece. Acta Hortic. 2017, 1155, 301–308. [Google Scholar] [CrossRef]
- Faria, J.L.C.; Kostenyuk, I.; Segura, J. Isolation, culture and plant regeneration from proto-plasts of Sideritis angustifolia. J. Plant Physiol. 1998, 153, 251–254. [Google Scholar] [CrossRef]
- Yavuz, D.Ö. Optimization of regeneration conditions and in vitro propagation of Sideritis stricta Boiss & Heldr. Int. J. Biol. Macromol. 2016, 90, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Siddique, I.; Anis, M. An improved plant regeneration system and ex vitro acclimatization of Ocimum basilicum L. Acta Physiol. Plant. 2008, 30, 493–499. [Google Scholar] [CrossRef]
- Iyer, P.V.; Pai, J.S. Micropropagation of sweet marjoram (Majorana hortensis Moench). J. Spices Aromat. Crops 1998, 7, 47–49. [Google Scholar]
- Chen, G.; Sun, W. The role of botanical gardens in scientific research, conservation, and citizen science. Plant Divers 2018, 40, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Maunder, M. Plant reintroduction: An overview. Biodivers. Conserv. 1992, 1, 51–61. [Google Scholar] [CrossRef]
- Chokheli, V.A.; Dmitriev, P.A.; Rajput, V.D.; Bakulin, S.D.; Azarov, A.S.; Varduni, T.V.; Stepanenko, V.V.; Tarigholizadeh, S.; Singh, R.K.; Verma, K.K.; et al. Recent development in micropropagation techniques for rare plant species. Plants 2020, 9, 1733. [Google Scholar] [CrossRef] [PubMed]

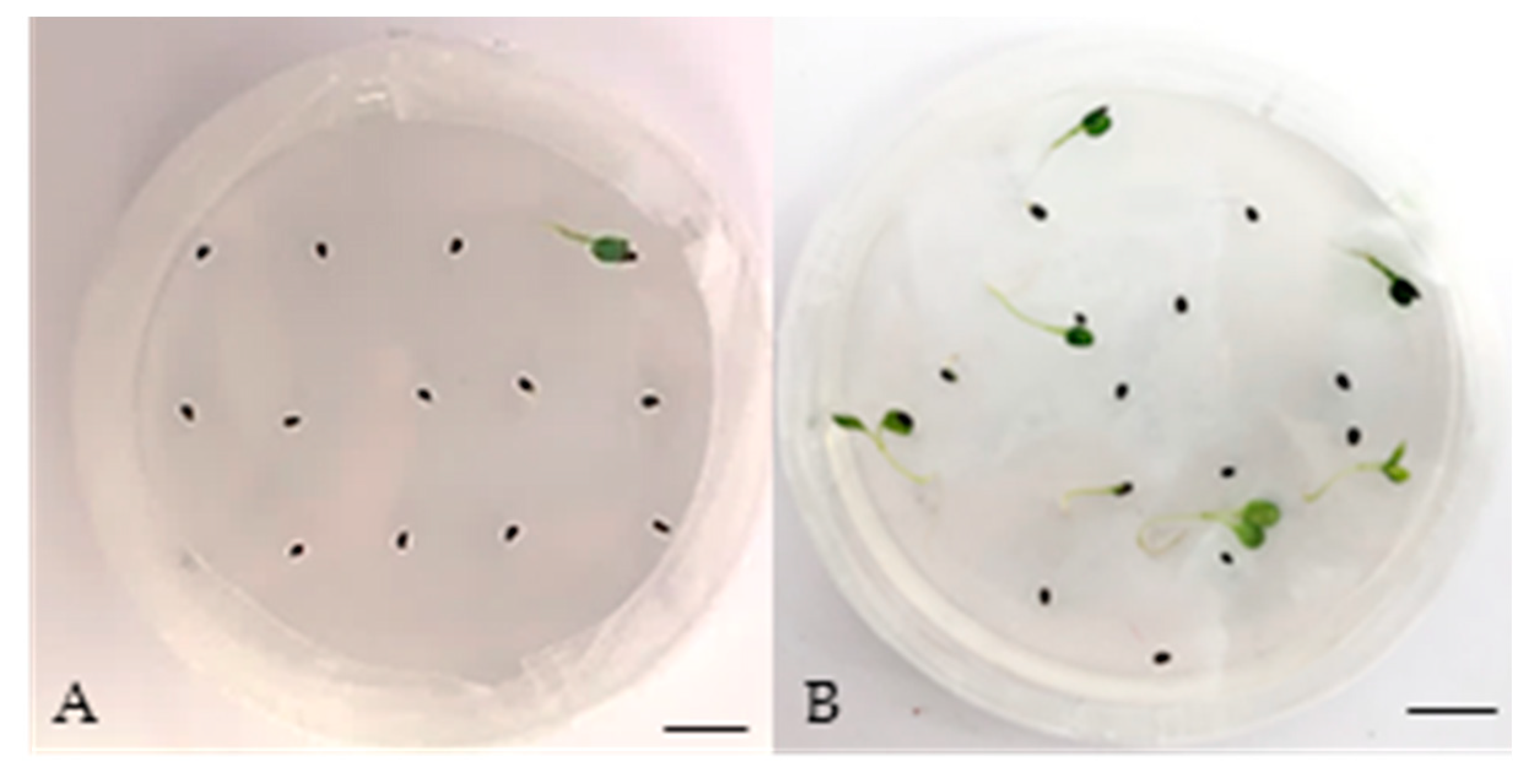
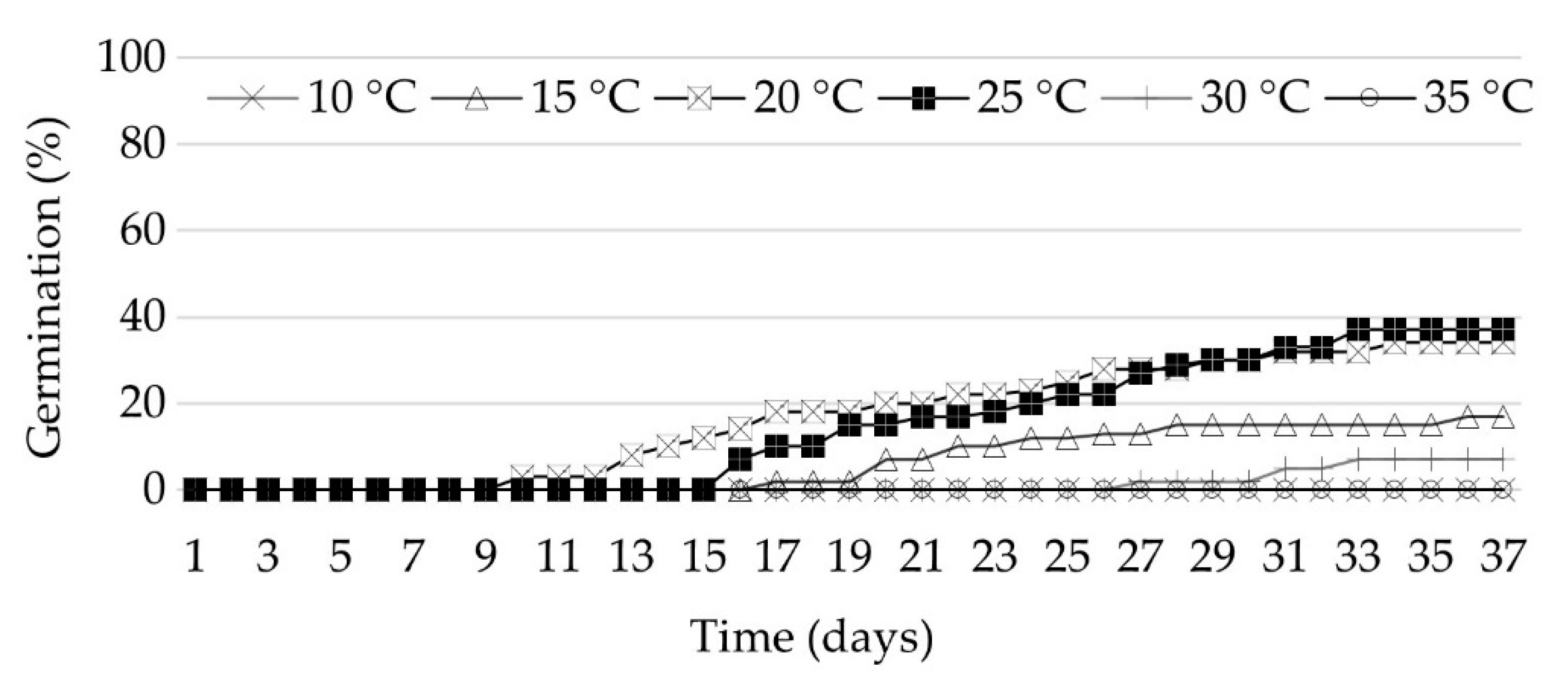

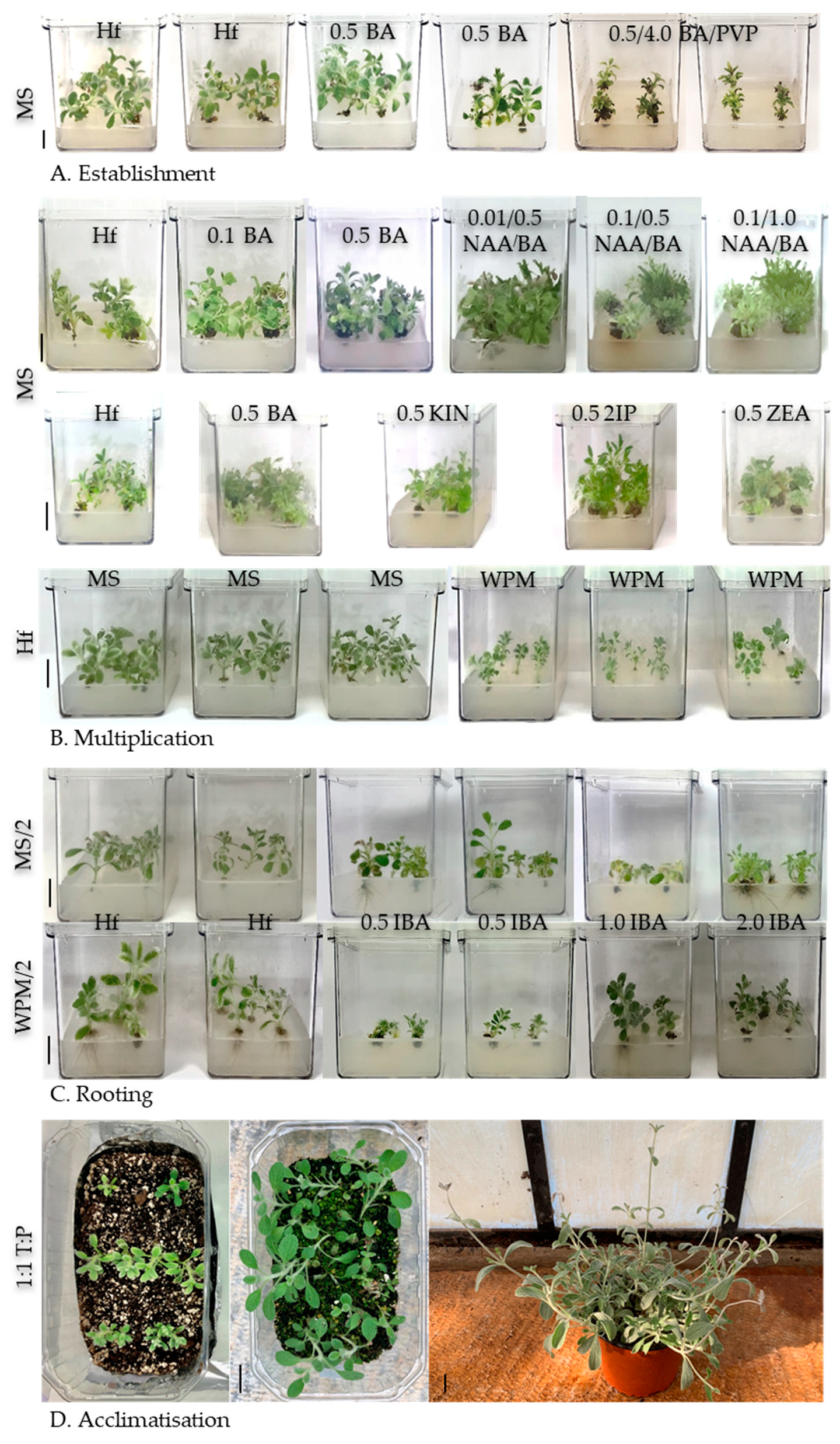
| Temperature (°C) | Germination (%) ± SE † | T50 †† (Days) | Time for Full Germination (Days) | GSI |
|---|---|---|---|---|
| 10.0 | 0.0 ± 0.0 c | - | - | - |
| 15.0 | 17.0 ± 4.6 b | 21.0 | 35.0 | 9.0 b |
| 20.0 | 34.0 ± 5.3 a | 16.0 | 33.0 | 24.3 a |
| 25.0 | 37.0 ± 7.5 a | 20.0 | 32.0 | 20.3 a |
| 30.0 | 7.0 ± 6.5 b | 30.0 | 32.0 | 1.7 b |
| 35.0 | 0.0 ± 0.0 c | - | - | - |
| Fone-way ANOVA | * | * |
| BA (mg L−1) | PVP (g L−1) | Shooting (%) | Shoot Number | Shoot Length (cm) | Node Number | HyperhyDricity | MI † | Lateral Shoot Number | Length of Lateral Shoots (cm) | Node Number of Lateral Shoots | Rooting (%) | Root Number | Root Length (cm) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| - | - | 89.0 a | 2.2 a | 1.1 a | 4.2 a | - | 3.6 ab | 0.6 a | 0.1 a | 0.4 a | 12.5 a | 15.0 a | 2.6 a |
| 0.5 | - | 84.0 a | 2.1 a | 1.4 a | 4.5 a | 19.0 a | 4.1 a | 0.2 a | 0.1 a | 0.1 b | 13.0 a | 18.0 a | 1.5 a |
| 0.5 | 4.0 | 95.0 a | 2.0 a | 0.6 b | 3.3 b | 12.0 a | 1.9 b | 0.5 a | 0.1 a | 0.1 b | - | - | - |
| Fone-way ANOVA | ns | ns | ** | ** | ns | ** | ns | ns | * | ns | ns | ns | |
| Hormone (mg L−1) | Shooting (%) | Shoot Number | Shoot Length | Node Number | HyperhyDricity | Callus Formation | MI † | Lateral Shoot Number | Length of Lateral Shoots (cm) | Node Number of Lateral Shoots | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BA | NAA | ||||||||||
| - | - | 81.0 a | 2.1 b | 1.1 a | 3.9 a | 6.0 b | 23.0 c | 3.1 b | 0.3 b | 0.2 a | 0.5 a |
| 0.1 | - | 78.0 a | 2.4 b | 1.2 a | 3.2 a | 32.0 ab | 50.0 b | 3.7 b | 0.2 b | 0.1 a | 0.3 a |
| 0.5 | - | 87.0 a | 2.8 ab | 0.9 a | 3.2 a | 23.0 ab | 55.0 b | 3.7 b | 0.2 b | 0.1 a | 0.2 a |
| 0.5 | 0.01 | 87.0 a | 3.6 a | 1.2 a | 3.4 a | 31.0 ab | 54.0 b | 6.3 a | 0.6 a | 0.1 a | 0.3 a |
| 0.5 | 0.1 | 92.0 a | 2.6 ab | 1.0 a | 3.0 a | 50.0 a | 85.0 a | 4.0 b | 0.7 a | 0.2 a | 0.2 a |
| 1.0 | 0.1 | 78.0 a | 3.3 ab | 0.8 a | 4.5 a | 20.0 ab | 94.0 a | 3.4 b | 0.0 b | 0.0 a | 0.0 a |
| Fone-way ANOVA | ns | * | ns | ns | * | * | * | * | ns | ns | |
| Cytokinin (mg L−1) | Shooting (%) | Shoot Number | Shoot Length | Node Number | HyperhyDricity | Callus Formation | MI † | Lateral Shoot Number | Length of Lateral Shoots (cm) | Node Number of Lateral Shoots |
|---|---|---|---|---|---|---|---|---|---|---|
| - | 86.5 a | 2.8 b | 1.8 a | 4.7 a | 8.0 b | - | 7.3 a | 0.5 a | 0.2 a | 0.7 a |
| BA | 78.5 a | 4.3 a | 1.1 b | 4.2 ab | 39.0 ab | 62.0 ab | 6.2 a | 0.1 b | 0.1 a | 0.1 b |
| KIN | 89.5 a | 2.4 b | 1.8 a | 4.9 a | 16.0 b | 48.0 b | 6.4 a | 0.1 b | 0.1 a | 0.1 b |
| 2iP | 73.5 a | 3.6 ab | 1.2 b | 4.0 b | 52.0 a | 63.0 a | 5.3 ab | 0.1 b | 0.1 a | 0.3 b |
| ZEA | 81.5 a | 3.4 ab | 1.0 b | 3.9 b | 34.0 ab | 88.0 a | 4.6 b | 0.1 b | 0.1 a | 0.3 b |
| Fone-way ANOVA | ns | *** | *** | *** | * | * | * | ** | ns | * |
| Medium | Shooting (%) | Shoot Number | Shoot Length (cm) | Node Number | HyperhyDricity | Callus Formation | MI † | Lateral Shoot Number | Length of Lateral Shoots (cm) | Node Number of Lateral Shoots |
|---|---|---|---|---|---|---|---|---|---|---|
| MS | 100 a | 1.9 a | 1.7 a | 4.3 a | - | 84.0 | 5.4 a | 0.1 a | 0.0 a | 0.2 a |
| WPM | 100 a | 2.0 a | 1.5 a | 4.0 a | - | - | 5.0 a | 0.1 a | 0.0 a | 0.1 a |
| Fone-way ANOVA | ns | ns | ns | ns | - | - | ns | ns | ns | ns |
| Treatments | Rooting (%) | Root Number | Root Length (cm) | |
|---|---|---|---|---|
| IBA (mg L−1) | ||||
| 0.0 (control) | - † | - | - | |
| 0.5 | - | - | - | |
| 1.0 | - | - | - | |
| 2.0 | - | - | - | |
| Medium | ||||
| MS/2 | - | - | - | |
| WPM/2 | - | - | - | |
| Interaction (IBA × Medium) | ||||
| 0.0 mg L−1 (control) × | MS/2 | 51.0 a | 2.3 ab | 0.9 a |
| WPM/2 | 32.0 ab | 0.4 b | 0.4 ab | |
| 0.5 mg L−1 × | MS/2 | 23.0 ab | 2.6 ab | 0.3 b |
| WPM/2 | 20.0 ab | 2.7 ab | 0.4 ab | |
| 1.0 mg L−1 × | MS/2 | 20.0 ab | 2.4 ab | 0.3 ab |
| WPM/2 | 20.0 ab | 3.7 ab | 0.3 ab | |
| 2.0 mg L−1 × | MS/2 | 14.0 b | 2.1 ab | 0.2 b |
| WPM/2 | 28.0 ab | 6.6 a | 0.4 ab | |
| FIBA | ns | ns | ns | |
| Fmedium | ns | ns | ns | |
| F IBA × med | * | * | * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertsouklis, K.; Theodorou, P.; Aretaki, P.-E. In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica. Horticulturae 2022, 8, 1114. https://doi.org/10.3390/horticulturae8121114
Bertsouklis K, Theodorou P, Aretaki P-E. In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica. Horticulturae. 2022; 8(12):1114. https://doi.org/10.3390/horticulturae8121114
Chicago/Turabian StyleBertsouklis, Konstantinos, Panagiota Theodorou, and Paraskevi-Evangelia Aretaki. 2022. "In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica" Horticulturae 8, no. 12: 1114. https://doi.org/10.3390/horticulturae8121114
APA StyleBertsouklis, K., Theodorou, P., & Aretaki, P.-E. (2022). In Vitro Propagation of the Mount Parnitha Endangered Species Sideritis raeseri subsp. Attica. Horticulturae, 8(12), 1114. https://doi.org/10.3390/horticulturae8121114







