Establishment of a Mutant Library of Fragaria nilgerrensis Schlechtendal ex J. Gay via EMS Mutagenesis
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. EMS Dose Trials for Apical Meristems
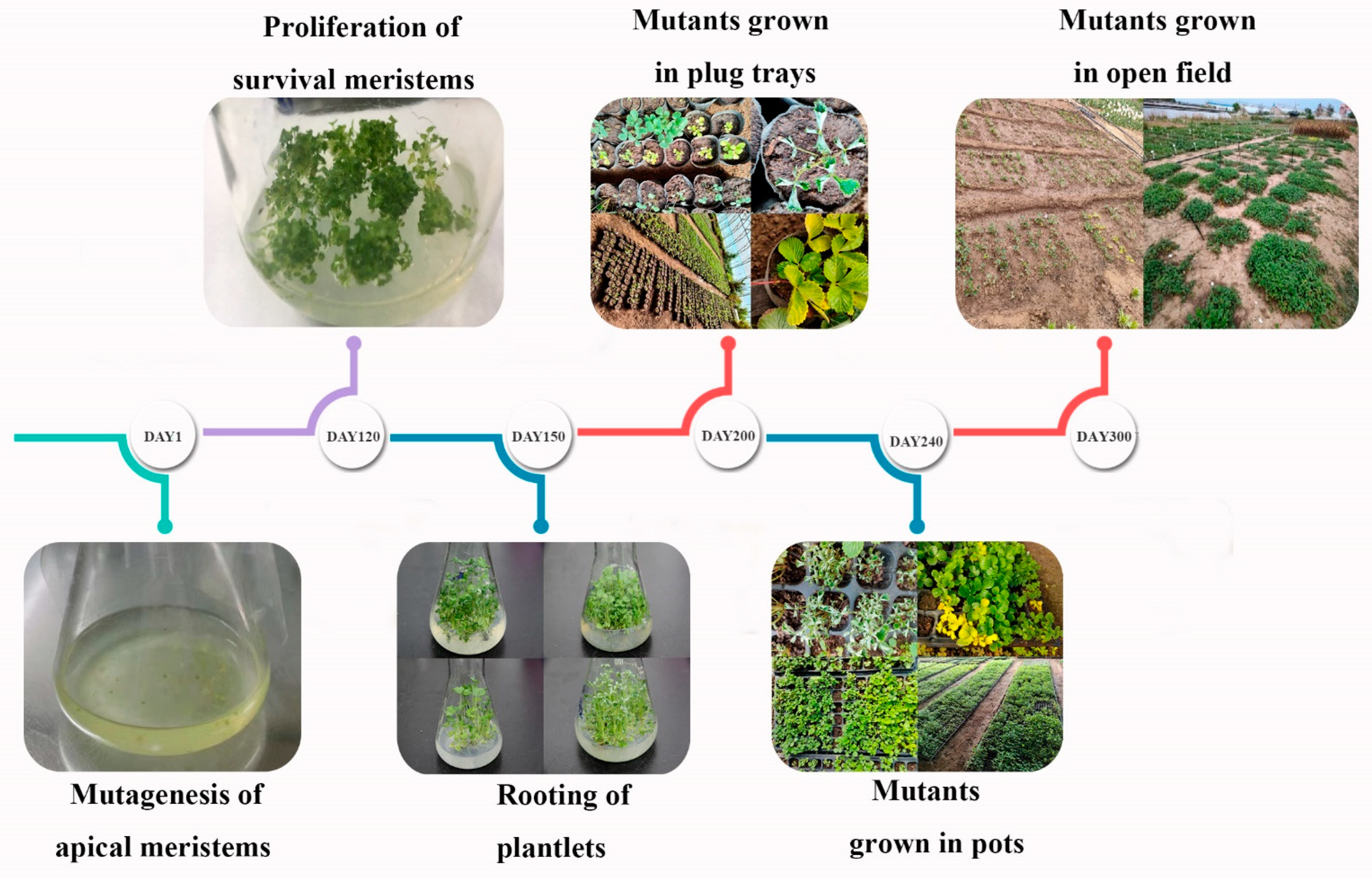
2.3. Large-Scale EMS Treatment and Mutant Library Construction
2.4. Measurement of Some Physiological Indexes of Mutants
2.5. Cytological Observation of Mutants
2.6. Data Analysis
3. Results
3.1. Identification of Optimal EMS Concentrations for Mutagenesis in F. nilgerrensis
3.2. Large-Scale EMS Treatment and Mutant Library Construction of F. nilgerrensis
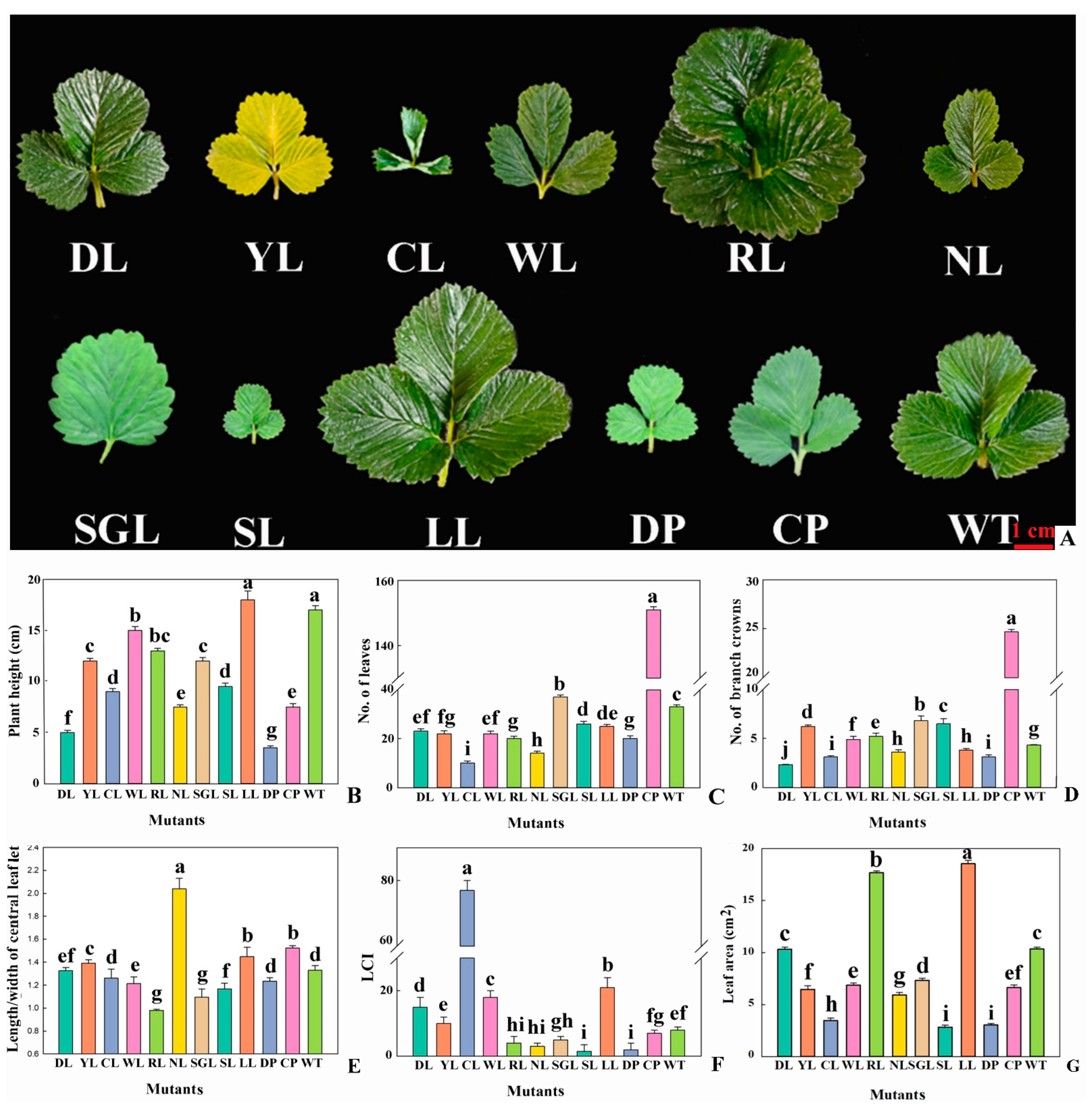
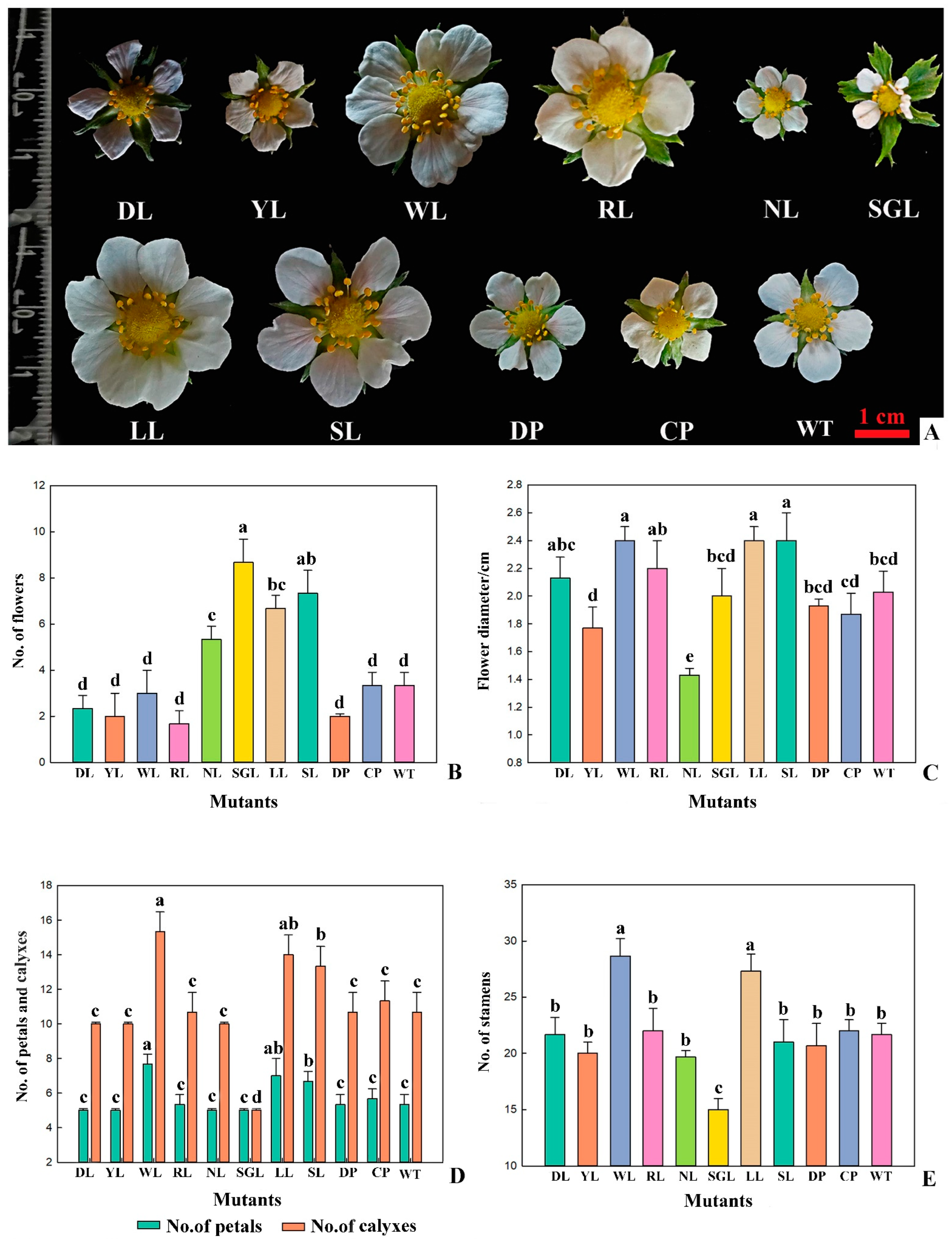
3.3. Botanical Traits Analysis of Mutants in F. nilgerrensis
3.3.1. Plant and Leaf
3.3.2. Flower
3.3.3. Fruit
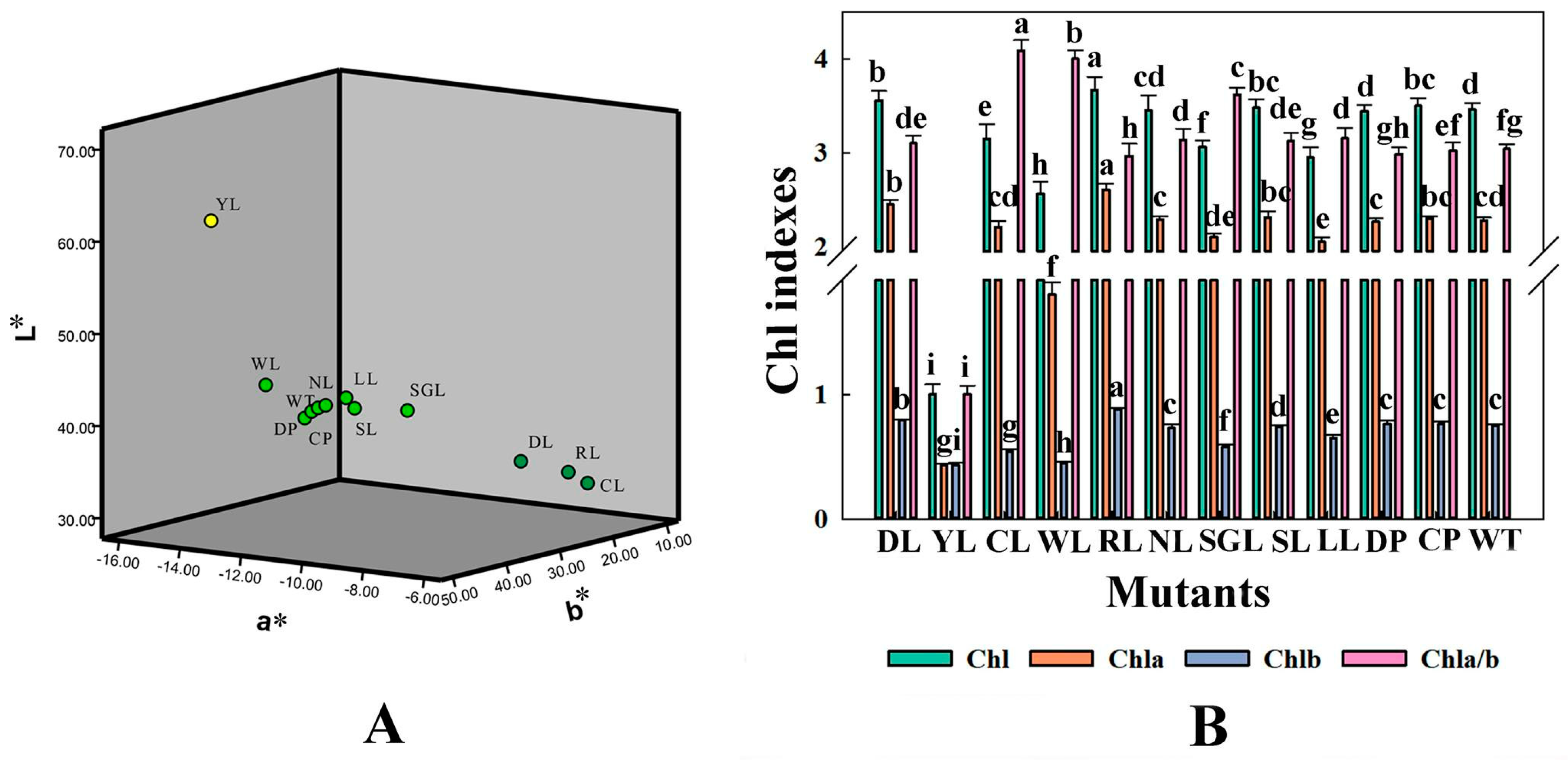
3.4. Measurement of Some Physiological Indexes of Mutants in F. nilgerrensis
3.4.1. The Values of L*, a*, and b*
3.4.2. The Contents of Chl, Chla, and Chlb
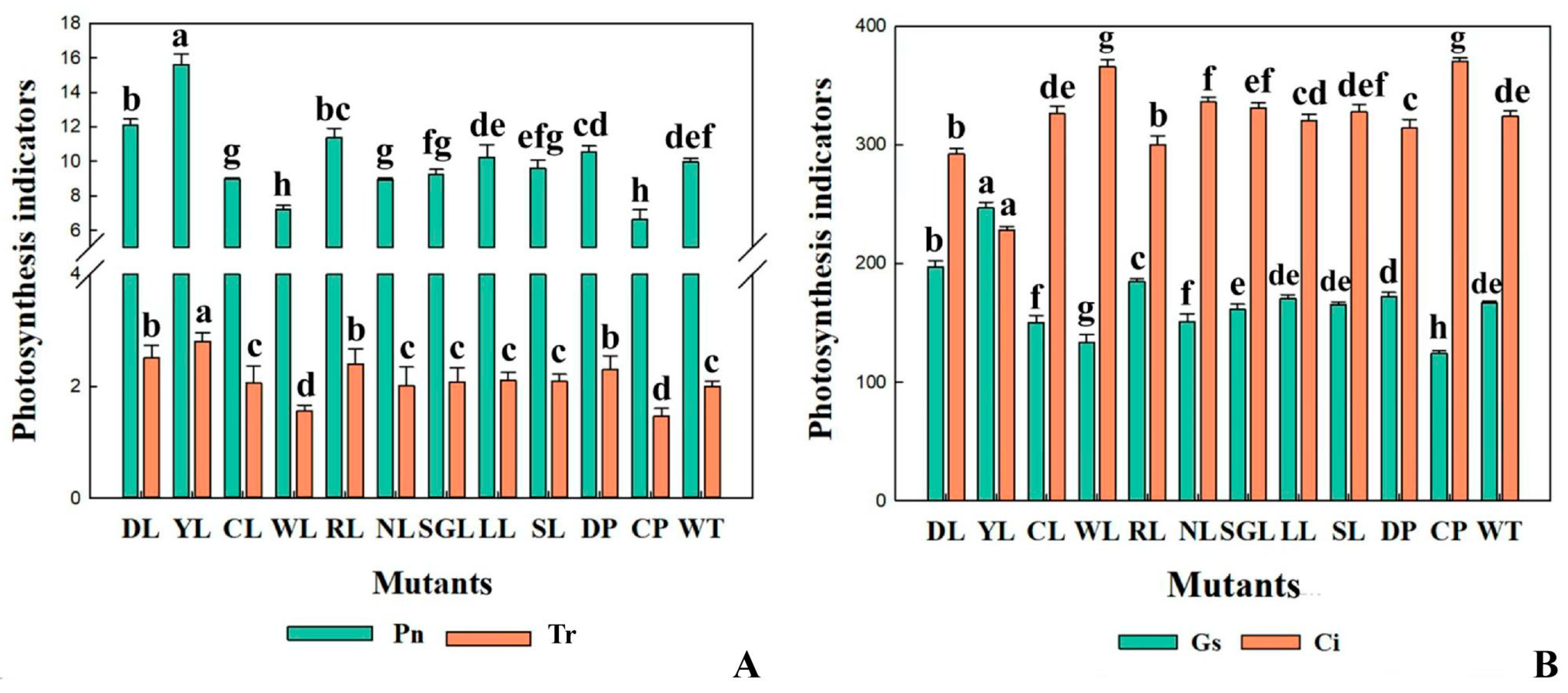
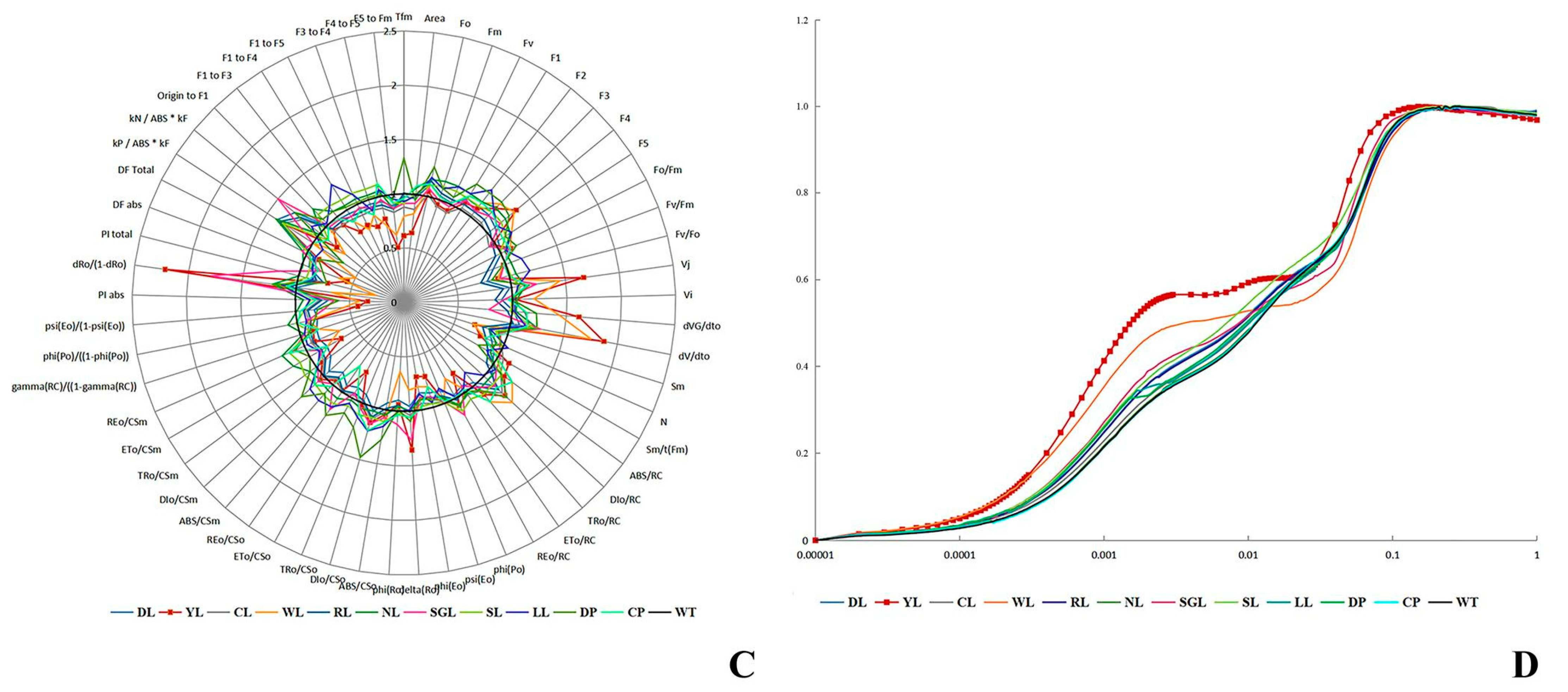
3.4.3. The Indexes of Photosynthesis and Fluorescence
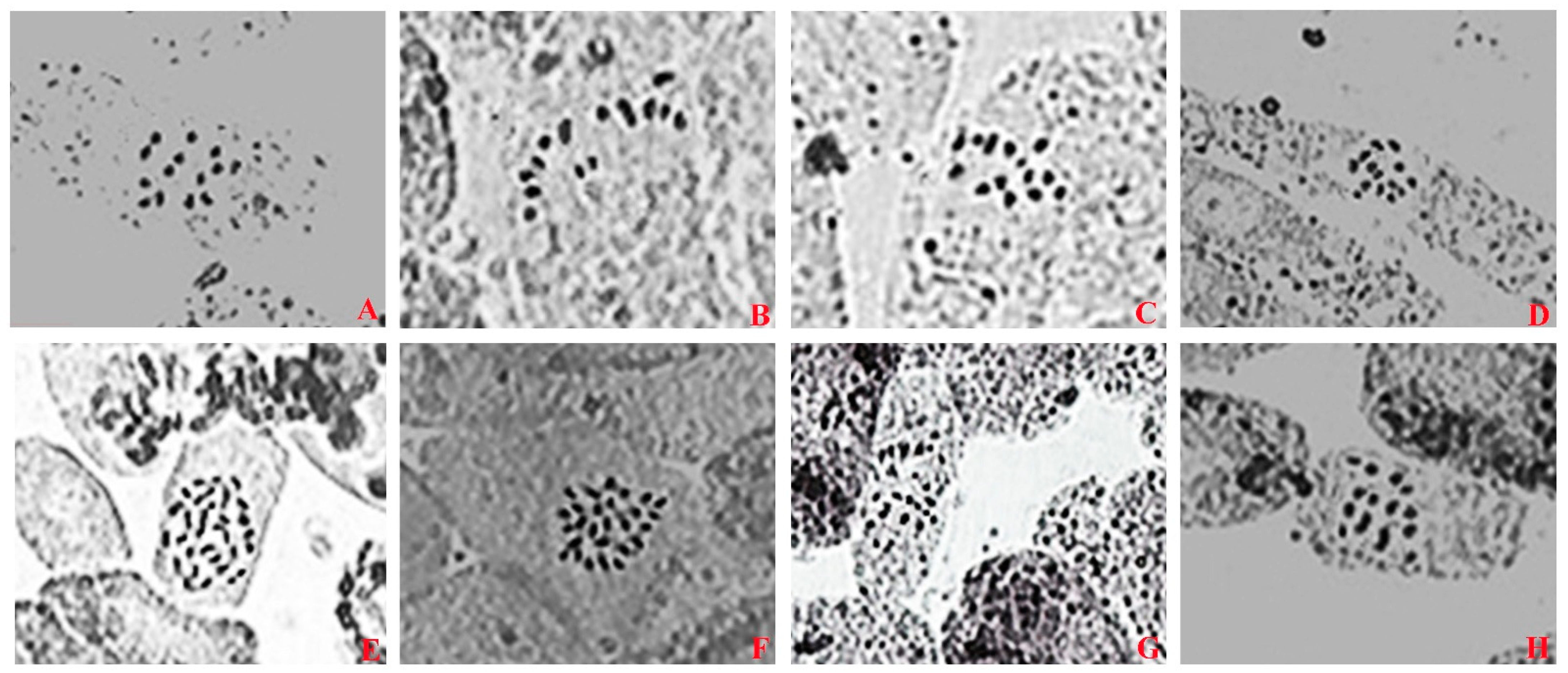
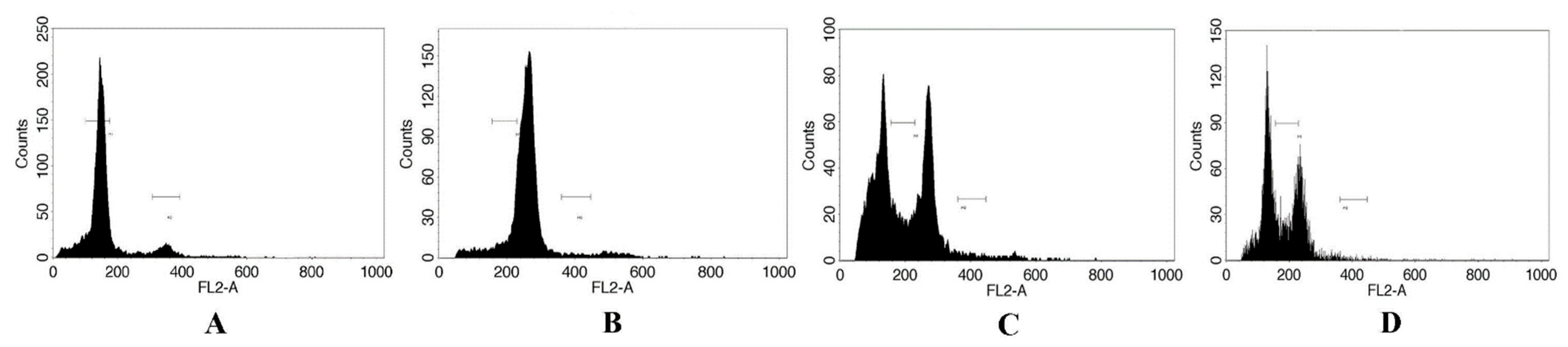
3.5. Cytological Observation of Mutants in F. nilgerrensis
4. Discussion
4.1. Effects of EMS Concentration and Treatment Time on Apical Meristem Mutagenesis in F. nilgerrensis
4.2. Prospect and Utilization of F. nilgerrensis Mutants
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staudt, G. The species of Fragaria, their taxonomy and geographical distribution. Acta Hortic. 1989, 265, 23–34. [Google Scholar] [CrossRef]
- Folta, K.M.; Davis, T.M. Strawberry genes and genomics. CRC Crit. Rev. Plant Sci. 2006, 25, 399–415. [Google Scholar] [CrossRef]
- Lei, J.J.; Xue, L.; Guo, R.X.; Dai, H.P. The Fragaria species native to China and their geographical distribution. Acta Hortic. 2017, 1156, 37–46. [Google Scholar] [CrossRef]
- Qiao, Q.; Edger, P.P.; Xue, L.; Qiong, L.; Lu, J.; Zhang, Y.C.; Cao, Q.; Yocca, A.E.; Platts, A.E.; Zhang, T.C.; et al. Evolutionary history and pan-genome dynamics of strawberry (Fragaria spp.). Proc. Natl. Acad. Sci. USA 2021, 118, e2105431118. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.X.; Xue, L.; Luo, G.; Zhang, T.; Lei, J.J. Investigation and taxonomy of wild Fragaria resources in Tibet, China. Genet. Resour. Crop Evol. 2017, 65, 405–415. [Google Scholar] [CrossRef]
- Luo, G.J.; Xue, L.; Guo, R.X.; Ding, Y.; Xu, W.J.; Lei, J.J. Creating interspecific hybrids with improved cold resistance in Fragaria. Sci. Hortic. 2018, 234, 1–9. [Google Scholar] [CrossRef]
- Li, C.L.; Hiroshi, I.; Hideaki, O. Fragaria Linnaeus, Sp. Pl. 1: 494. 1753. Flora China 2003, 9, 335–338. [Google Scholar]
- Noguchi, Y.; Morishita, M.; Muro, T. ‘Tokun’: A new aromatic decaploid interspecific hybrid strawberry. Bull. Natl. Inst. Veg. Tea Sci. 2011, 10, 59–67. [Google Scholar]
- Zhang, J.X.; Lei, Y.Y.; Wang, B.T.; Li, S.; Yu, S.; Wang, Y.; Li, H.; Liu, Y.X.; Ma, Y.; Dai, H.Y.; et al. The high-quality genome of diploid strawberry (Fragaria nilgerrensis) provides new insights into anthocyanin accumulation. Plant Biotechnol. J. 2020, 18, 1908–1924. [Google Scholar] [CrossRef]
- Wang, L.N.; Cao, C.X.; Zheng, S.S.; Zhang, H.Y.; Liu, P.J.; Ge, Q.; Li, J.R.; Ren, Z.H. Transcriptomic analysis of short-fruit 1 (sf1) reveals new insights into the variation of fruit-related traits in Cucumis sativus. Sci. Rep. 2017, 7, 2950. [Google Scholar] [CrossRef]
- Xu, T.; Bian, N.F.; Wen, M.X.; Xiao, J.; Yuan, C.X.; Cao, A.Z.; Zhang, S.Z.; Wang, X.E.; Wang, H.Y. Characterization of a common wheat (Triticum aestivum L.) high-tillering dwarf mutant. Theor. Appl. Genet. 2017, 130, 483–494. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Wang, P.P.; Li, H.J.; Zhao, Y.W.; Lu, Y.; Dai, P.; Ren, T.Q.; Wang, X.F.; Li, X.Z.; Shao, Q.; et al. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J. Integr. Plant Biol. 2018, 60, 591–607. [Google Scholar] [CrossRef] [PubMed]
- Laia, C.P.; Victor, S.; Virginie, P.; Aurelie, C.; Aurelie, H.; Alexia, G.; Daniel, V.; Laurence, C.; Mathilde, G.; Raphael, M. The HEM lines: A new library of homozygous Arabidopsis thaliana EMS mutants and its potential to detect meiotic phenotypes. Front. Plant Sci. 2018, 9, 1339. [Google Scholar] [CrossRef]
- Yugandhar, P.; Sun, Y.F.; Liu, L.; Neqi, M.; Nallamothu, V.; Sun, S.B.; Neelamraju, S.; Rai, V.; Jain, A. Characterization of the loss-of-function mutant NH101 for yield under phosphate deficiency from EMS induced mutants of rice variety Nagina22. Plant Physiol. Biochem. 2018, 130, 1–13. [Google Scholar] [CrossRef]
- Rime, J.; Dinesh, M.R.; Sankaran, M.; Shivashankara, K.S.; Rekha, A.; Ravishankar, K.V. Evaluation and characterization of EMS derived mutant populations in mango. Sci. Hortic. 2019, 254, 55–60. [Google Scholar] [CrossRef]
- Lian, X.; Liu, Y.; Guo, H.H.; Fan, Y.J.; Wu, J.F.; Guo, H.X.; Jiao, C.Z.; Tang, Z.M.; Zhang, L.; Fan, Y.P.; et al. Ethyl methane sulfonate mutant library construction in Gossypium hirsutum L. for allotetraploid functional genomics and germplasm innovation. Plant J. 2020, 103, 858–868. [Google Scholar] [CrossRef]
- Sun, X.X.; Li, X.; Lu, Y.; Wang, S.; Zhang, X.; Zhang, K.; Su, X.; Liu, M.; Feng, D.; Luo, S.; et al. Construction of a high-density mutant population of Chinese cabbage facilitates the genetic dissection of agronomic traits. Mol. Plant. 2022, 15, 913–924. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Z.Y.; Yun, Z.; Li, C.Y.; Feng, H. Identification and fine mapping of a stay-green gene (Brnye1) in pakchoi (Brassica campestris L. ssp. chinensis). Theor. Appl. Genet. 2018, 131, 673–684. [Google Scholar] [CrossRef]
- Li, C.; Tang, J.; Hu, Z.Y.; Wang, J.W.; Yu, T.; Yi, H.Y.; Cao, M.A. Novel maize dwarf mutant generated by Ty1-copia LTR-retrotransposon insertion in Brachytic2 after spaceflight. Plant Cell Rep. 2020, 39, 393–408. [Google Scholar] [CrossRef]
- Sagan, M.; Morandi, D.; Tarenghi, E.; Duc, G. Selection of nodulation and mycorrhizal mutants in the model plant Medicago truncatula (Gaertn.) after γ-ray mutagenesis. Plant Sci. 1995, 111, 63–71. [Google Scholar] [CrossRef]
- Staudt, G. Strawberry biogeography, genetics, and systematics. Acta Hortic. 2009, 842, 71–84. [Google Scholar] [CrossRef]
- Greene, E.A.; Codomo, C.A.; Taylor, N.E.; Henikoff, J.G.; Till, B.J.; Reynolds, S.H.; Enns, L.C.; Burtner, C.; Johnson, J.E.; Odden, A.R.; et al. Spectrum of chemically induced mutations from a large-scale reverse-genetic screen in Arabidopsis. Genetics 2003, 164, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Caruana, J.C.; Sittmann, J.W.; Wang, W.; Liu, Z. Suppressor of runnerless encodes a DELLA protein that controls runner formation for asexual reproduction in strawberry. Mol Plant. 2018, 11, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Dai, C.; Li, Y.; Feng, J.; Liu, Z.; Kang, C. Reduced anthocyanins in petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J. Exp. Bot. 2018, 69, 2595–2608. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.F.; Xie, Z.B.; Zhang, Y.; Wang, S.M. The FvCYP714C2 gene plays an important role in gibberellin synthesis in the woodland strawberry. Genes Genom. 2021, 43, 11–16. [Google Scholar] [CrossRef]
- Zheng, G.H.; Wei, W.; Li, Y.P.; Kan, L.J.; Wang, F.X.; Zhang, X.; Li, F.; Liu, Z.C.; Kang, C.Y. Conserved and novel roles of miR164-CUC2 regulatory module in specifying leaf and floral organ morphology in strawberry. New Phytol. 2019, 224, 480–492. [Google Scholar] [CrossRef]
- Pi, M.T.; Hu, S.Q.; Cheng, L.C.; Zhong, R.H.; Cai, Z.Y.; Liu, Z.C.; Yao, J.L.; Kang, C.Y. The MADS box gene FveSEP3 plays essential roles in flower organogenesis and fruit development in woodland strawberry. Hort. Res. 2020, 8, 3533–3547. [Google Scholar] [CrossRef]
- Feng, J.; Cheng, L.C.; Zhu, Z.Y.; Yu, F.Q.; Dai, C.; Liu, Z.C.; Guo, W.W.; Wu, X.M.; Kang, C.Y. GRAS transcription factor loss of axillary meristems is essential for stamen and runner formation in wild strawberry. Plant Physiol. 2021, 186, 1970–1984. [Google Scholar] [CrossRef]
- Zhao, M.Z.; Wang, G.X.; Qian, Y.M. Descriptors and Data Standard for Strawberry (Fragaria spp.); China Agricultural Press: Beijing, China, 2006. [Google Scholar]
- Zhang, Q.; Folta, K.M.; Davis, T.M. Somatic embryogenesis, tetraploidy, and variant leaf morphology in transgenic diploid strawberry (Fragaria vesca subspecies vesca ‘Hawaii 4’). BMC Plant Biol. 2014, 14, 23. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, J.; Wang, R. Change law of hyperspectral data in related with chlorophyll and carotenoid in rice at different developmental stages. Rice Sci. 2004, 11, 274–282. Available online: http://www.ricesci.cn/CN/Y2004/V11/I5-6/274 (accessed on 20 November 2004).
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, E., Eds.; Taylor & Francis: London, UK, 2000; pp. 445–483. [Google Scholar]
- Lei, J.J.; Li, Y.H.; Du, G.D.; Dai, H.P.; Deng, M.Q. A natural pentaploid strawberry genotype from the Changbai Mountains in Northeast China. HortScience 2005, 40, 1194–1195. [Google Scholar] [CrossRef]
- Dolezel, J.; Greilhuber, J.; Suda, J. Estimation of nuclear DNA content in plants using flow cytometry. Nat. Protoc. 2007, 2, 2233. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Liu, Z.Z.; Chang, S.F.; Zhao, Y.X. An EMS mutant library for carrot and genetic analysis of some mutants. Breed. Sci. Preview 2020, 70, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cui, Q.Z.; Huang, S.W.; Wang, S.H.; Liu, X.H.; Lu, X.Y.; Chen, H.M.; Tian, Y. An EMS mutant library for cucumber. J. Integr. Agric. 2018, 17, 1612–1619. [Google Scholar] [CrossRef]
- Siddique, M.I.; Seungki, B.; Lee, J.H.; Jo, J.K.; Jang, S.Y.; Han, K.; Venkatesh, J.; Kwon, J.K.; Jo, Y.D.; Kang, B.C. Development and characterization of an ethyl methane sulfonate (EMS) induced mutant population in Capsicum annuum L. Plants 2020, 9, 396. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, G.Y.; Huang, S.N.; Liu, Z.Y.; Zhang, M.D.; Fu, W.; Ren, J.; Feng, H. Comparison between germinated seed and isolated microspore EMS mutagenesis in Chinese cabbage (Brassica rapa L. ssp. pekinensis). Horticulturae 2022, 8, 232. [Google Scholar] [CrossRef]
- Huang, S.N.; Liu, Z.Y.; Li, D.Y.; Yao, R.P.; Feng, H. A new method for generation and screening of Chinese cabbage mutants using isolated microspore culturing and EMS mutagenesis. Euphytica 2016, 207, 23–33. [Google Scholar] [CrossRef]
- Khan, A.; Jalil, S.; Cao, H.; Sunusi, M.; Tsago, Y.; Chen, J.; Shi, C.H.; Jin, X.L. Identification and fine mapping of candidate gene for yellow leaf mutant (ygl54) exhibiting yellow leaf colour in rice. Russ. J. Plant Physiol. 2021, 68, 1069–1078. [Google Scholar] [CrossRef]
- Jillian, A.P.; Trevor, L.; Wang, T.J.; Welham, S.G.; Jodie, M.P.; Satoko, Y.; Martin, P. A TILLING reverse genetics tool and a web-accessible collection of mutants of the legume Lotus japonicus. Plant Physiol. 2003, 131, 866–871. [Google Scholar] [CrossRef]
- Yang, M.; He, J.B.; Wan, S.B.; Li, W.Y.; Chen, W.J.; Wang, Y.M.; Jiang, X.M.; Cheng, P.F.; Chu, P.; Shen, W.B.; et al. Fine mapping of the BnaC04.BIL1 gene controlling plant height in Brassica napus L. BMC Plant Biol. 2021, 21, 359. [Google Scholar] [CrossRef]
- Abdul-Awal, S.M.; Chen, J.P.; Xin, Z.G.; Harmon, F.G. A Sorghum gigantea mutant attenuates florigen gene expression and delays flowering time. Plant Direct. 2020, 4, e00281. [Google Scholar] [CrossRef] [PubMed]
- Hossen, Q.M.M.; Rahman, M.B.; Rahman, M.N.; Sarker, M.D.H.; Moniruzzaman, M.; Tareq, M.Z.; Sadat, M.A.; Arafat, K.M.Y.; Jahan, M.S.; Haque, M.S. Development of early flowering, short life-spanned jute (Corchorus spp.) mutant via ethyl methane sulfonate mutagenesis. J. Crop Sci. Biotechnol. 2022, 25, 489–500. [Google Scholar] [CrossRef]
- Naama, M.; Yaniv, S.; Dror, P.; Yuval, E.; Dani, Z. In silico screening of a saturated mutation library of tomato. Plant J. 2004, 38, 861–872. [Google Scholar] [CrossRef]
- Gao, L.Y.; Yang, G.H.; Li, Y.F.; Fan, N.N.; Li, H.J.; Zhang, M.; Xu, R.B.; Zhang, M.Y.; Zhao, A.J.; Ni, Z.F.; et al. Fine mapping and candidate gene analysis of a QTL associated with leaf rolling index on chromosome 4 of maize (Zea mays L.). Theor. Appl. Genet. 2019, 132, 3047–3062. [Google Scholar] [CrossRef]
- Lei, J.J.; Wu, L.P.; Dai, H.P.; Hu, W.Y.; Ge, H.B. Study on chromosome doubling of apical meristem in strawberry. Acta Hortic. Sin. 1999, 26, 13–18. [Google Scholar]


| Treatment | CK | 0.30% | 0.60% | 0.90% | 1.20% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | 3 h | 6 h | 9 h | ||
| Survival rate (%) | 100.00 ± 0.00 a | 86.67 ± 4.73 b | 78.33 ± 5.86 c | 57.67 ± 2.52 e | 73.33 ± 4.04 d | 47.00 ± 2.00 f | 31.33 ± 2.08 g | 32.33 ± 2.08 g | 10.00 ± 1.00 h | 4.67 ± 1.53 ij | 6.00 ± 1.00 hi | 0.60 ± 1.15 j | 0.00 ± 0.00 j |
| Mutant Categories | Mutant Types | No. of Mutants | Percentage of Mutation (%) |
|---|---|---|---|
| Plant posture | 28 | 2.33 | |
| Dwarf plant | 26 | 2.17 | |
| Clumped plant | 2 | 0.17 | |
| Leaf color and shape | 48 | 4.00 | |
| Dark green leaf | 2 | 0.17 | |
| Yellow-green leaf | 7 | 0.58 | |
| Mottled yellow leaf | 5 | 0.42 | |
| Albino leaf | 1 | 0.08 | |
| Curled leaf | 2 | 0.17 | |
| Wrinkled leaf | 1 | 0.08 | |
| Round leaf | 4 | 0.33 | |
| Narrow leaf | 1 | 0.08 | |
| Leaf vein bulge | 1 | 0.08 | |
| Single leaflet | 1 | 0.08 | |
| Large leaf | 3 | 0.25 | |
| Small leaf | 20 | 1.67 | |
| Flower characteristics | 10 | 0.83 | |
| Large flower | 2 | 0.17 | |
| Small flower | 3 | 0.25 | |
| Increased petal number | 5 | 0.42 | |
| Total | 86 | 7.17 |
| Mutant Lines | The Transect of the Two Lateral Leaflets/Width of the Central Leaflet |
|---|---|
| DL | 1.72 ± 0.05 d |
| YL | 1.75 ± 0.04 d |
| CL | 3.68 ± 0.07 a |
| WL | 1.53 ± 0.05 e |
| RL | 1.13 ± 0.06 f |
| NL | 2.14 ± 0.06 b |
| SGL | 1.00 ± 0.01 g |
| SL | 1.78 ± 0.03 cd |
| LL | 1.84 ± 0.04 c |
| DP | 1.72 ± 0.03 d |
| CP | 1.78 ± 0.02 cd |
| WT | 1.77 ± 0.04 cd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, S.; Wang, M.; Zhao, C.; Cui, Y.; Cai, Z.; Zhao, J.; Zheng, Y.; Xue, L.; Lei, J. Establishment of a Mutant Library of Fragaria nilgerrensis Schlechtendal ex J. Gay via EMS Mutagenesis. Horticulturae 2022, 8, 1061. https://doi.org/10.3390/horticulturae8111061
Jiang S, Wang M, Zhao C, Cui Y, Cai Z, Zhao J, Zheng Y, Xue L, Lei J. Establishment of a Mutant Library of Fragaria nilgerrensis Schlechtendal ex J. Gay via EMS Mutagenesis. Horticulturae. 2022; 8(11):1061. https://doi.org/10.3390/horticulturae8111061
Chicago/Turabian StyleJiang, Shu, Mingqian Wang, Can Zhao, Yuchen Cui, Zhi Cai, Jun Zhao, Yang Zheng, Li Xue, and Jiajun Lei. 2022. "Establishment of a Mutant Library of Fragaria nilgerrensis Schlechtendal ex J. Gay via EMS Mutagenesis" Horticulturae 8, no. 11: 1061. https://doi.org/10.3390/horticulturae8111061
APA StyleJiang, S., Wang, M., Zhao, C., Cui, Y., Cai, Z., Zhao, J., Zheng, Y., Xue, L., & Lei, J. (2022). Establishment of a Mutant Library of Fragaria nilgerrensis Schlechtendal ex J. Gay via EMS Mutagenesis. Horticulturae, 8(11), 1061. https://doi.org/10.3390/horticulturae8111061






