Current Developments on Chemical Compositions, Biosynthesis, Color Properties and Health Benefits of Black Goji Anthocyanins: An Updated Review
Abstract
1. Introduction
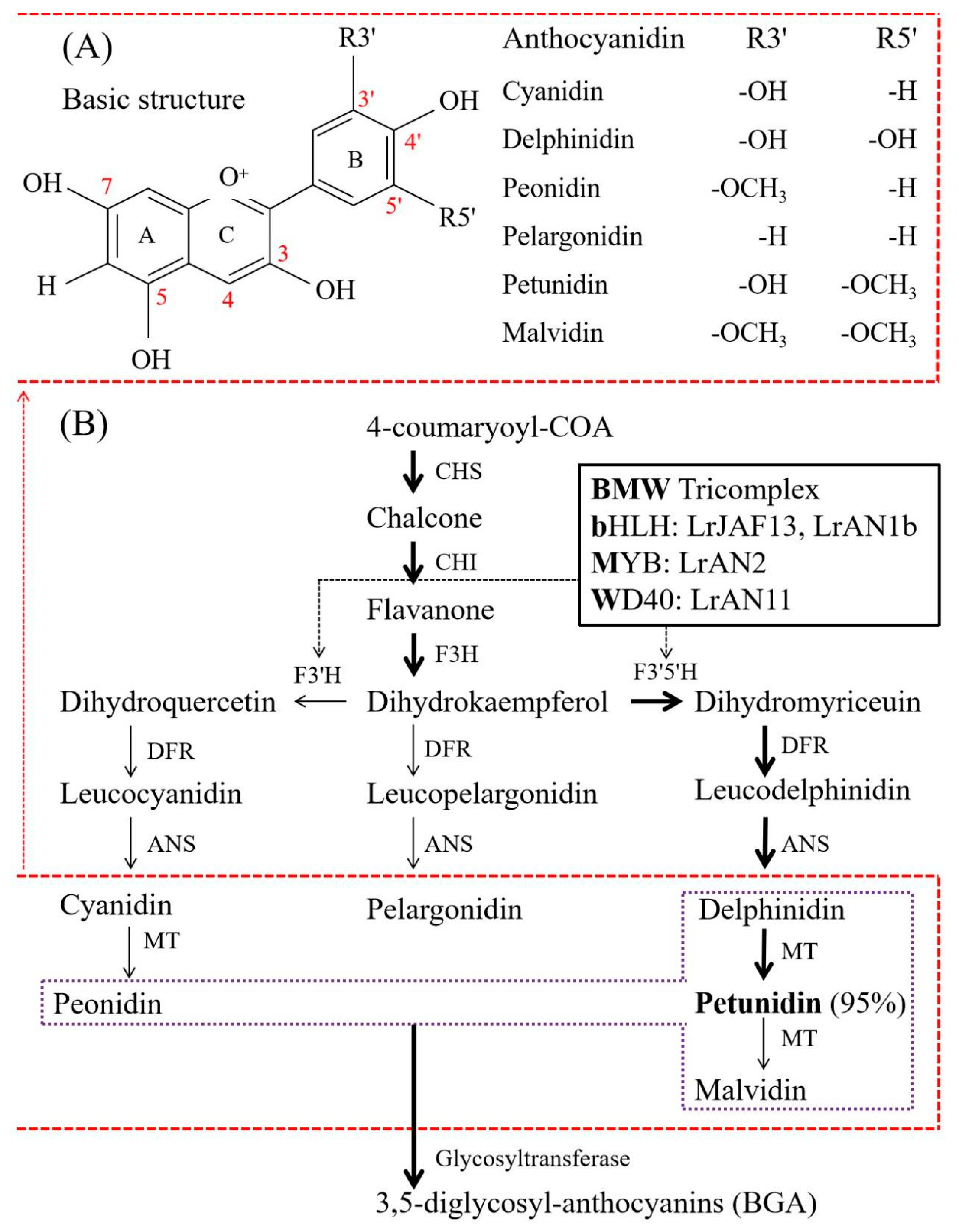
2. Chemical Compositions and Biosynthesis of BGAs
2.1. Extraction and Purification
2.2. Characterization
2.3. Biosynthesis of BGAs
2.4. Factors Influencing the Composition of BGAs
2.4.1. Degree of Ripeness
2.4.2. Variety
2.4.3. Processing Techniques
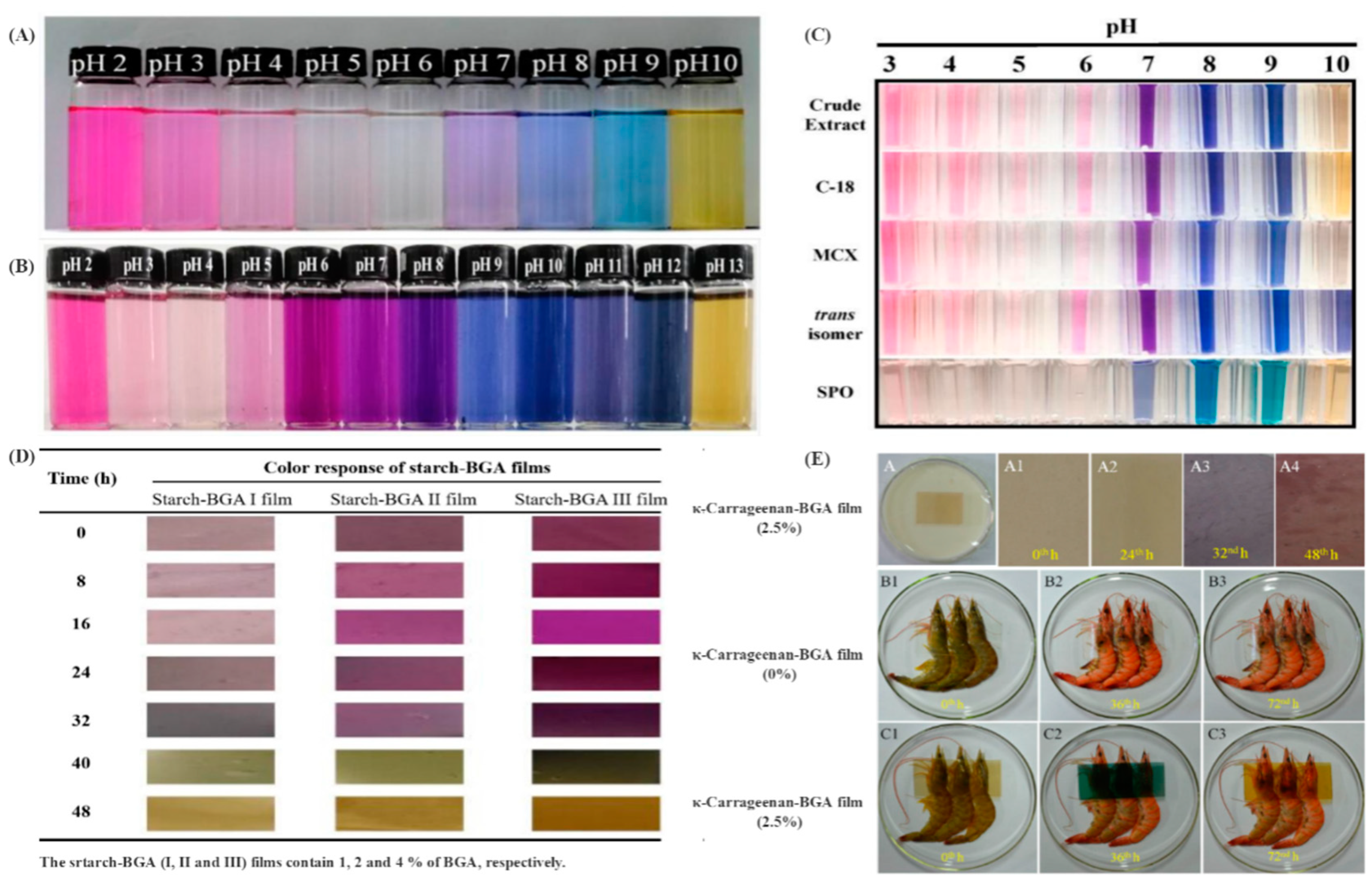
3. Color Properties of BGAs
4. Health Benefits of BGAs
4.1. Antioxidant Activities
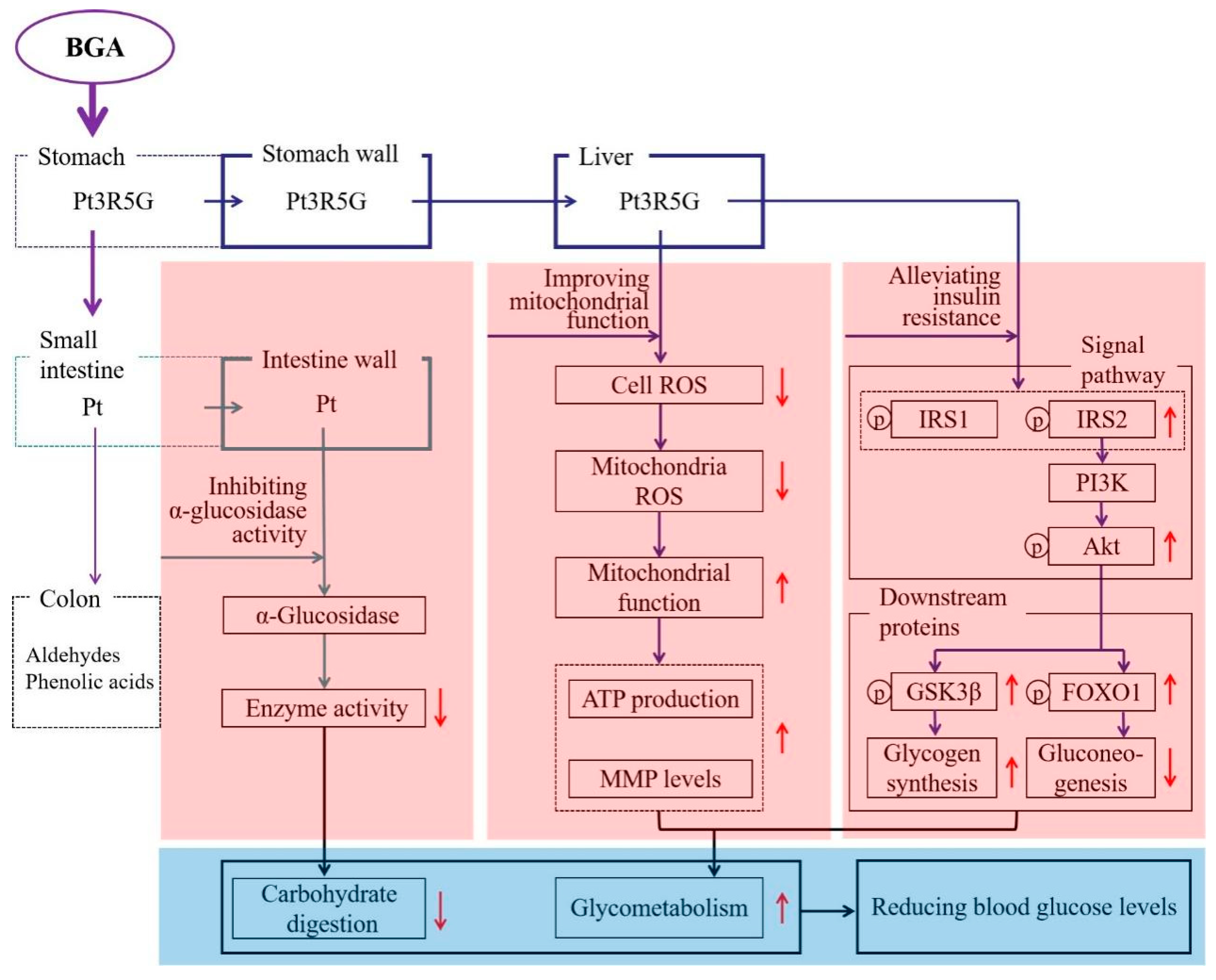
4.2. α-Glucosidase Inhibiting Activity
4.3. Alleviating Insulin Resistance
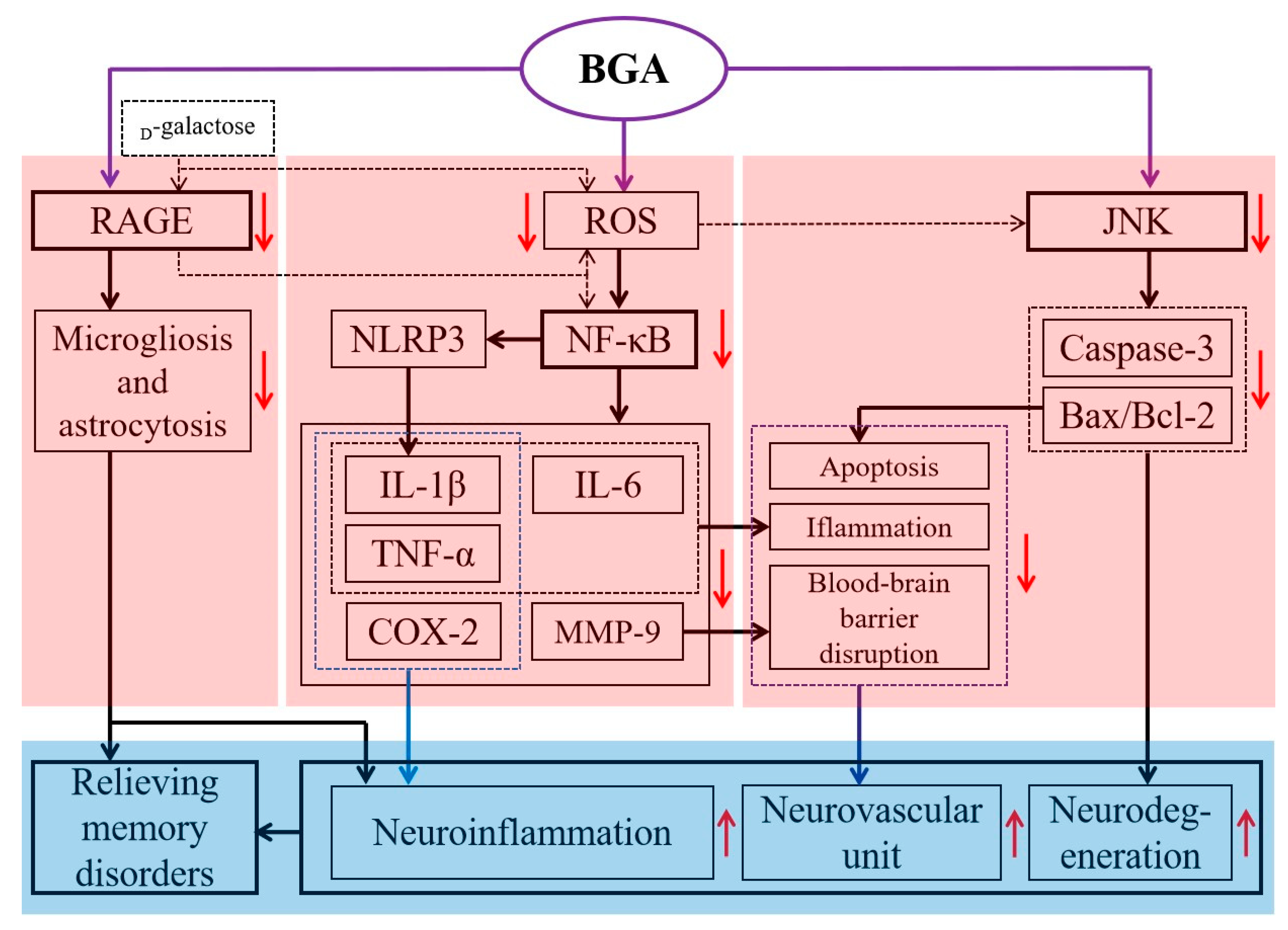
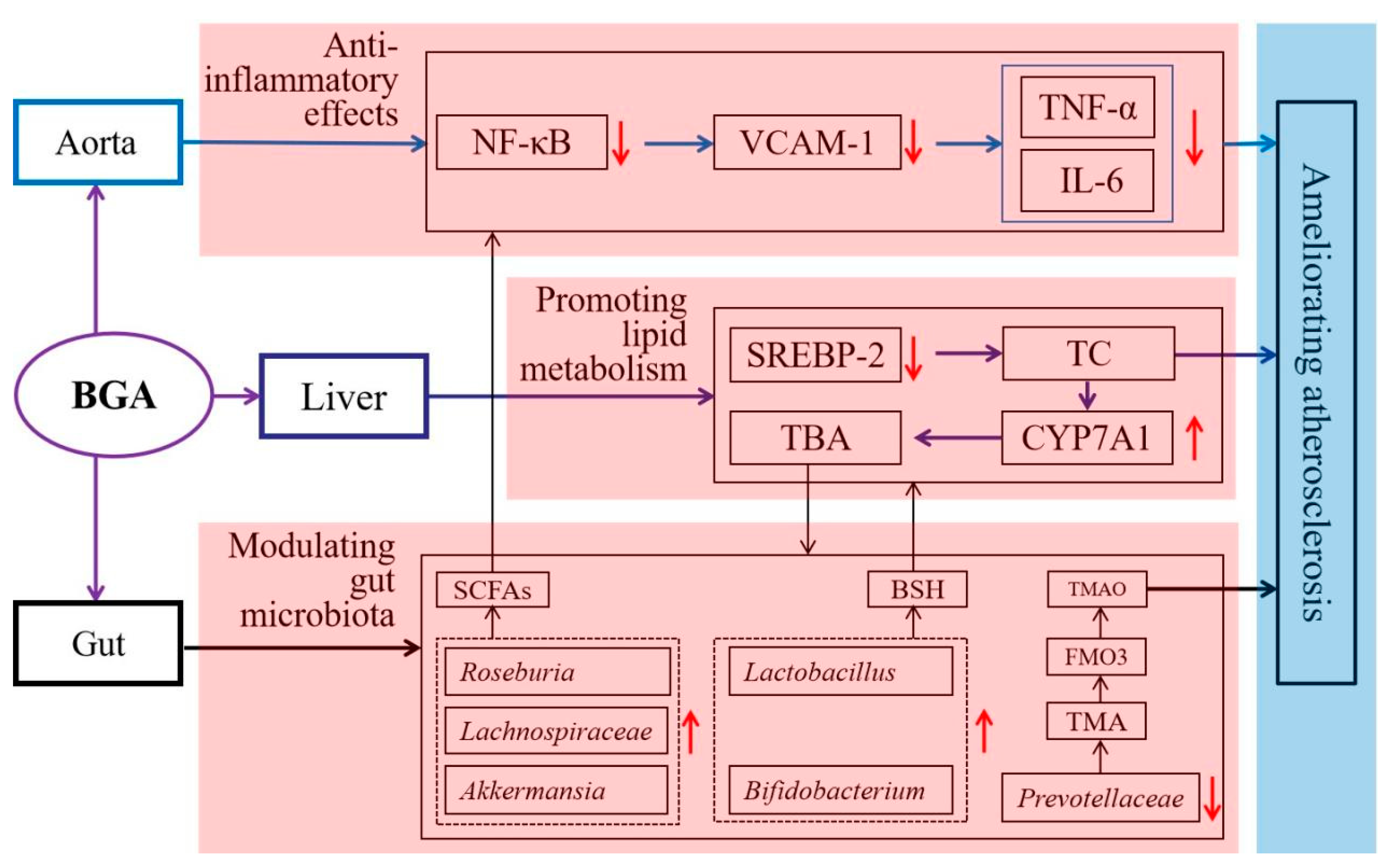
4.4. Anti-Inflammatory Effects
4.5. Promoting Lipid Metabolism
4.6. Modulating Gut Microbiota
4.7. Other Health Benefits
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.; Dong, B.; Liu, C.; Zong, Y.; Shao, Y.; Liu, B.; Yue, H. Variation of anthocyanin content in fruits of wild and cultivated Lycium ruthenicum. Ind. Crop. Prod. 2020, 146, 112208. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Nisar, T.; Zou, L.; Yang, X.; Niu, P.; Sun, L.; Guo, Y. Comparison and multivariate statistical analysis of anthocyanin composition in Lycium ruthenicum Murray from different regions to trace geographical origins: The case of China. Food Chem. 2018, 246, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sun, L.; Dong, Y.; Fang, Z.; Nisar, T.; Zhao, T.; Wang, Z.-C.; Guo, Y. Chemical compositions and alpha-glucosidase inhibitory effects of anthocyanidins from blueberry, blackcurrant and blue honeysuckle fruits. Food Chem. 2019, 299, 125102. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, J.; Zhou, W.; Shen, A.; Yang, F.; Liu, Y.; Guo, Z.; Zhang, X.; Tao, Y.; Peng, X.; et al. Preparative separation of a challenging anthocyanin from Lycium ruthenicum Murr. by two-dimensional reversed-phase liquid chromatography/hydrophilic interaction chromatography. RSC Adv. 2015, 5, 62134–62141. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Xia, Y.; Qiao, Z.; Feng, F.; Bian, J.; Liu, W.; Qu, W. Comparison of the origin and phenolic contents of Lycium ruthenicum Murr. by high-performance liquid chromatography fingerprinting combined with quadrupole time-of-flight mass spectrometry and chemometrics. J. Sep. Sci. 2017, 40, 1234–1243. [Google Scholar] [CrossRef]
- Wang, Z.-C.; Nisar, T.; Sun, L.; Fang, Z.; Yan, Y.; Li, D.; Xie, H.; Wang, H.; Guo, Y. Effect of in vitro gastrointestinal digestion on the composition and bioactivity of anthocyanins in the fruits of cultivated Lycium ruthenicum Murray. CYTA—J. Food 2019, 17, 552–562. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J.A. Lycium ruthenicum studies: Molecular biology, phytochemistry and pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, H.; Shi, S.; Li, M. Lycium ruthenicum polysaccharide attenuates inflammation through inhibiting TLR4/NF-kappaB signaling pathway. Int. J. Biol. Macromol. 2014, 67, 330–335. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, H.; Lei, H.; Wang, X. Relationship between structure and im-munological activity of an arabinogalactan from Lycium ruthenicum. Food Chem. 2016, 194, 595–600. [Google Scholar] [CrossRef]
- Qi, Y.; Zhu, C.; Chen, J.; Liu, G.; Yang, Z.; Chen, W. Comparative analysis of the quality and health-promoting compounds of two-shaped fruits of wild Lycium ruthenicum Murr. from the Qinghai-Tibet Plateau. Acta Physiol. Plant. 2019, 41, 101. [Google Scholar] [CrossRef]
- Zheng, J.; Ding, C.; Wang, L.; Li, G.; Shi, J.; Li, H.; Wang, H.; Suo, Y. Anthocyanins composition and antioxidant activity of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Food Chem. 2011, 126, 859–865. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical profiles, nutritional constituents and antioxidant activity of black wolfberry (Lycium ruthenicum Murr.). Ind. Crop. Prod. 2020, 154, 112692. [Google Scholar] [CrossRef]
- Chen, S.; Zeng, Z.; Hu, N.; Bai, B.; Wang, H.; Suo, Y. Simultaneous optimization of the ultrasound-assisted extraction for phenolic compounds content and antioxidant activity of Lycium ruthenicum Murr. fruit using response surface methodology. Food Chem. 2018, 242, 1–8. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium ruthenicum Murr. ameliorated d-galactose-Induced memory impairment, oxidative stress, and neuroinflammation in adult rats. J. Agr. Food Chem. 2019, 67, 3140–3149. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Fang, J.-L.; Yuan, K.; Jin, S.-H.; Guo, Y. Ameliorative effect of purified anthocyanin from Lycium ruthenicum on atherosclerosis in rats through synergistic modulation of the gut microbiota and NF-κB/SREBP-2 pathways. J. Funct. Foods 2019, 59, 223–233. [Google Scholar] [CrossRef]
- Yan, Y.; Peng, Y.; Tang, J.; Mi, J.; Lu, L.; Li, X.; Ran, L.; Zeng, X.; Cao, Y. Effects of anthocyanins from the fruit of Lycium ruthenicum Murray on intestinal microbiota. J. Funct. Foods 2018, 48, 533–541. [Google Scholar] [CrossRef]
- Zeng, S.; Wu, M.; Zou, C.; Liu, X.; Shen, X.; Hayward, A.; Liu, C.; Wang, Y. Comparative analysis of anthocyanin biosynthesis during fruit development in two Lycium species. Physiol. Plantarum. 2014, 150, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Zheng, J.; Li, W.; Suo, Y. Isolation, stability, and antioxidant activity of anthocyanins from Lycium ruthenicum Murray and Nitraria Tangutorum Bobr of Qinghai-Tibetan Plateau. Sep. Sci. Technol. 2014, 49, 2897–2906. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Yang, F.; Wang, J.; Fu, D.; Zhang, X.; Peng, X.; Liang, X. Characterization of anthocyanins in wild Lycium ruthenicum Murray by HPLC-DAD/QTOF-MS/MS. Anal. Methods 2015, 7, 4947–4956. [Google Scholar] [CrossRef]
- Pan, Z.; Cui, M.; Dai, G.; Yuan, T.; Li, Y.; Ji, T.; Pan, Y. Protective effect of anthocyanin on neurovascular unit in cerebral ischemia/reperfusion injury in rats. Front. Neurosci. 2018, 12, 947. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, L.; Fang, Z.; Nisar, T.; Zou, L.; Li, D.; Guo, Y. Lycium ruthenicum Murray anthocyanins effectively inhibit α-glucosidase activity and alleviate insulin resistance. Food Biosci. 2021, 41, 100949. [Google Scholar] [CrossRef]
- Yang, X.; Lin, S.; Jia, Y.; Rehman, F.; Zeng, S.; Wang, Y. Anthocyanin and spermidine derivative hexoses coordinately increase in the ripening fruit of Lycium ruthenicum. Food Chem. 2020, 311, 125874. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Liu, Y.; Wu, M.; Liu, X.; Shen, X.; Liu, C.; Wang, Y. Identification and validation of reference genes for quantitative real-time PCR normalization and its applications in Lycium. PLoS ONE 2014, 9, e97039. [Google Scholar] [CrossRef] [PubMed]
- Gamage, G.C.V.; Lim, Y.Y.; Choo, W.S. Black goji berry anthocyanins: Extraction, stability, health benefits, and applications. ACS Food Sci. Technol. 2021, 1, 1360–1370. [Google Scholar] [CrossRef]
- Tian, Z.; Aierken, A.; Pang, H.; Du, S.; Feng, M.; Ma, K.; Gao, S.; Bai, G.; Ma, C. Constituent analysis and quality control of anthocyanin constituents of dried Lycium ruthenicum Murray fruits by HPLC-MS and HPLC-DAD. J. Liq. Chromatogr. R. T. 2016, 39, 453–458. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, F.; Ji, T.; Li, J. A new spermidine from the fruits of Lycium ruthenicum. Chem. Nat. Compd. 2014, 50, 880–883. [Google Scholar] [CrossRef]
- Samec, D.; Valek-Zulj, L.; Martinez, S.; Grúzc, J.; Piljac, A.; Piljac-Zegarac, J. Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Ind. Crop. Prod. 2016, 84, 104–107. [Google Scholar] [CrossRef]
- Chen, C.; Shao, Y.; Tao, Y.; Mei, L.; Shu, Q.; Wang, L. Main anthocyanins compositions and corresponding H-ORAC assay for wild Lycium ruthenicum Murr. fruits from the Qaidam Basin. J. Pharm. Technol. Drug Res. 2013, 2, 1. [Google Scholar] [CrossRef]
- Jin, H.; Liu, Y.; Guo, Z.; Yang, F.; Wang, J.; Li, X.; Peng, X.; Liang, X. High-performance liquid chromatography separation of cis-trans anthocyanin isomers from wild Lycium ruthenicum Murr. employing a mixed-mode reversed-phase/strong anion-exchange stationary phase. J. Agric. Food Chem. 2015, 63, 500–508. [Google Scholar] [CrossRef]
- Peng, Y.; Yan, Y.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.; Mi, J.; Lu, L.; Zhang, Z.; Li, X.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radical Bio. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Yan, Y.; Ran, L.; Mi, J.; Sun, Y.; Lu, L.; Gao, Y.; Zeng, X.; Cao, Y. Isolation, antioxidant property and protective effect on PC12 cell of the main anthocyanin in fruit of Lycium ruthenicum Murray. J. Funct. Foods 2017, 30, 97–107. [Google Scholar] [CrossRef]
- Tang, P.; Giusti, M.M. Black goji as a potential source of natural color in a wide pH range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef]
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of polyphenols from Lycium ruthenicum fruit by UPLC-Q-TOF/MS(E) and their antioxidant activity in Caco-2 Cells. J. Agr. Food Chem. 2016, 64, 2280–2288. [Google Scholar] [CrossRef] [PubMed]
- Celli, G.B.; Ghanem, A.; Brooks, M.S. Optimization of ultrasound-assisted extraction of anthocyanins from haskap berries (Lonicera caerulea L.) using response surface methodology. Ultrason. Sonochem. 2015, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tang, X.; Liu, C.; Dong, B.; Shao, Y.; Liu, B.; Yue, H. Ultrasonic extraction of anthocyanins from Lycium ruthenicum Murr. and its antioxidant activity. Food Sci. Nutr. 2020, 8, 2642–2651. [Google Scholar] [CrossRef]
- Liu, P.; Li, W.; Hu, Z.; Qin, X.; Liu, G. Isolation, purification, identification, and stability of anthocyanins from Lycium ruthenicum Murr. LWT—Food Sci. Technol. 2020, 126, 109334. [Google Scholar] [CrossRef]
- Qin, B.; Liu, X.; Cui, H.; Ma, Y.; Wang, Z.; Han, J. Aqueous two phase assisted by ultrasound for the extraction of anthocyanins from Lycium ruthenicum Murr. Prep. Biochem. Biotech. 2017, 47, 881–888. [Google Scholar] [CrossRef]
- He, B.; Zhang, L.L.; Yue, X.Y.; Liang, J.; Jiang, J.; Gao, X.L.; Xue, P.X. Optimization of ultrasound-assisted extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem. 2016, 204, 70–76. [Google Scholar] [CrossRef]
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical water extraction, UPLC-Triple-TOF/MS analysis and antioxidant activity of anthocyanins from Lycium ruthenicum Murr. Food Chem. 2018, 249, 119–126. [Google Scholar] [CrossRef]
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction optimization and purification of anthocyanins from Lycium ruthenicum Murr. and evaluation of tyrosinase inhibitory activity of the anthocyanins. J. Food Sci. 2020, 85, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, F.; Sang, J. Green approach for sample preparation and determination of anthocyanins from Lycium ruthenicum Murr. using a β-cyclodextrin-based extraction method coupled with UPLC-DAD analysis. Food Anal. Method. 2018, 11, 2141–2148. [Google Scholar] [CrossRef]
- Wang, H.; Xia, X.; Yu, H.; Zhao, X.; Zhong, X.; Li, Q.; Tang, J.; Zhao, Y. Effect of liquid fermentation on bread fortified with Lycium ruthenicum: A quality attribute and in vitro digestibility study. Food Chem. 2019, 299, 125131. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Saona, L.E.; Wrolstad, R.E. Extraction, isolation, and purification of anthocyanin. Curr. Protoc. Food Anal. Chem. 2001, 1, F1.1.1–F1.1.11. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T. Chemical stability of acai fruit (Euterpe oleracea Mart.) anthocyanins as influenced by naturally occurring and externally added polyphenolic cofactors in model systems. Food Chem. 2010, 18, 17–25. [Google Scholar] [CrossRef]
- Liobikas, J.; Skemiene, K.; Trumbeckaite, S.; Borutaite, V. Anthocyanins in cardioprotection: A path through mitochondria. Pharmacol. Res. 2016, 113, 808–815. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X. Anthocyanins: Structural characteristics that result in unique metabolic patterns and biological activities. Free Radical Res. 2006, 40, 1014–1028. [Google Scholar] [CrossRef]
- Fraige, K.; Pereira-Filho, E.R.; Carrilho, E. Fingerprinting of anthocyanins from grapes produced in Brazil using HPLC-DAD-MS and exploratory analysis by principal component analysis. Food Chem. 2014, 145, 395–403. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap (Lonicera caerulea L.): Role of cyanidin-3-O-glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Phenolic compounds profile and antioxidant properties of six sweet cherry (Prunus avium) cultivars. Food Res. Int. 2017, 97, 15–26. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.D.; Gebhardt, S.E.; Prior, R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef] [PubMed]
- Ichiyanagi, T.; Kashiwada, Y.; Shida, Y.; Ikeshiro, Y.; Kaneyuki, T.; Konishi, T. Nasunin from eggplant consists of cis-trans isomers of delphinidin 3-[4-(p-coumaroyl)-l-rhamnosyl (1→6)glucopyranoside]-5-glucopyranoside. J. Agric. Food Chem. 2005, 53, 9472–9477. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yue, T.; Yuan, Y.; Wang, Y. Chemometric classification of apple juices according to variety and geographical origin based on polyphenolic profile. J. Agric. Food Chem. 2013, 61, 6949–6963. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Fan, Y.; Qin, H.; Dai, G.; Li, G.; Li, Y. Transcriptome and flavonoids metabolomic analysis identifies regulatory networks and hub genes in black and white fruits of Lycium ruthenicum Murray. Front. Plant Sci. 2020, 11, 1256. [Google Scholar] [CrossRef]
- Qi, Y.; Wei, H.; Gu, W.; Shi, W.; Jiang, L.; Deng, L. Transcriptome profiling provides insights into the fruit color development of wild Lycium ruthenicum Murr. from Qinghai-Tibet Plateau. Protoplasma 2021, 258, 33–43. [Google Scholar] [CrossRef]
- Zong, Y.; Zhu, X.; Liu, Z.; Xi, X.; Li, G.; Cao, D.; Wei, L.; Li, J.; Liu, B. Functional MYB transcription factor encoding gene AN2 is associated with anthocyanin biosynthesis in Lycium ruthenicum Murray. BMC Plant Biol. 2019, 19, 169. [Google Scholar] [CrossRef]
- Liu, Z.; Shu, Q.; Wang, L.; Yu, M.; Hu, Y.; Zhang, H.; Tao, Y.; Shao, Y. Genetic diversity of the endangered and medically important Lycium ruthenicum Murr. revealed by sequence-related amplified polymorphism (SRAP) markers. Biochem. Syst. Ecol. 2012, 45, 86–97. [Google Scholar] [CrossRef]
- Wang, Z.; Yan, Y.; Nisar, T.; Sun, L.; Zeng, Y.; Guo, Y.; Wang, H.; Fang, Z. Multivariate statistical analysis combined with e-nose and e-tongue assays simplifies the tracing of geographical origins of Lycium ruthenicum Murray grown in China. Food Control 2019, 98, 457–464. [Google Scholar] [CrossRef]
- Li, Y.; Zou, X.; Shen, T.; Shi, J.; Zhao, J.; Mel, H. Determination of geographical origin and anthocyanin content of black goji berry (Lycium ruthenicum Murr.) using near-infrared spectroscopy and chemometrics. Food Anal. Method. 2017, 10, 1034–1044. [Google Scholar]
- Barba-Espín, G.; Glied, S.; Crocoll, C.; Dzhanfezova, T.; Joernsgaard, B.; Okkels, F.; Lütken, H.; Müller, R. Foliar-applied ethephon enhances the content of anthocyanin of black carrot roots (Daucus carota ssp. sativus var. atrorubens Alef.). BMC Plant Biol. 2017, 17, 70. [Google Scholar] [CrossRef] [PubMed]
- Barba-Espín, G.; Lütken, H.; Glied, S.; Crocoll, C.; Joernsgaard, B.; Müller, R. Anthocyanin elicitation for bio-sustainable colourant production in carrot. Acta Hortic. 2019, 1242, 87–92. [Google Scholar] [CrossRef]
- Reis, L.; Forney, C.F.; Jordan, M.; Munro Pennell, K.; Fillmore, S.; Schemberger, M.O.; Ayub, R.A. Metabolic profile of strawberry fruit ripened on the plant following treatment with an ethylene elicitor or inhibitor. Front. Plant Sci. 2020, 11, 995. [Google Scholar] [CrossRef] [PubMed]
- Scavroni, J.; Ferreira, L.C.; Ferrarese, M.d.L.L.; Ono, E.O.; Rodrigues, J.D. Ethephon and calcium chloride, a combination that improves skin color of ‘Rubi’ table grape. Rev. Bras. Frutic. 2018, 40, e777. [Google Scholar] [CrossRef]
- De Santis, D.; Bellincontro, A.; Forniti, R.; Botondi, R. Time of postharvest ethylene treatments affects phenols, anthocyanins, and volatile compounds of Cesanese red wine grape. Foods 2021, 10, 322. [Google Scholar] [CrossRef] [PubMed]
- Awad, M.A.; de Jager, A.; van der Plas, L.H.; van der Krol, A.R. Flavonoid and chlorogenic acid changes in skin of ’Elstar’ and ’Jonagold’ apples during development and ripening. Sci. Hortic. 2001, 90, 69–83. [Google Scholar] [CrossRef]
- Ma, Y.J.; Duan, H.R.; Zhang, F.; Li, Y.; Yang, H.S.; Tian, F.P.; Zhou, X.H.; Wang, C.M.; Ma, R. Transcriptomic analysis of Lycium ruthenicum Murr. during fruit ripening provides insight into structural and regulatory genes in the anthocyanin biosynthetic pathway. PLoS ONE 2018, 13, e0208627. [Google Scholar] [CrossRef]
- Zong, Y.; Li, S.; Xi, X.; Cao, D.; Wang, Z.; Wang, R.; Liu, B. Comprehensive influences of overexpression of a MYB transcriptor regulating anthocyanin biosynthesis on transcriptome and metabolome of tobacco leaves. Int. J. Mol. Sci. 2019, 20, 5123–5135. [Google Scholar] [CrossRef]
- Fang, T.; Zhen, Q.L.; Liao, L.; Owiti, A.; Zhao, L.; Korban, S.S.; Han, Y. Variation of ascorbic acid concentration in fruits of cultivated and wild apples. Food Chem. 2017, 225, 132–137. [Google Scholar] [CrossRef]
- Kovačević, D.B.; Putnik, P.; Dragović-Uzelac, V.; Vahčić, N.; Babojelić, M.S.; Levaj, B. Influences of organically and conventionally grown strawberry cultivars on anthocyanins content and color in purees and low-sugar jams. Food Chem. 2015, 181, 94–100. [Google Scholar] [CrossRef]
- Tan, L.; Dong, Q.; Cao, J.; Gen, D.; Hu, F. Extraction and identification of anthocyanins in Lycium ruthenicum Murr. Nat. Prod. Res. Dev. 2014, 26, 1797–1802. [Google Scholar]
- Lu, Y.; Kong, X.; Zhang, J.; Guo, C.; Qu, Z.; Jin, L.; Wang, H. Composition changes in Lycium ruthenicum fruit dried by different methods. Front. Nutr. 2021, 8, 737521. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, J.; Yang, D. In situ stability of anthocyanins in Lycium ruthenicum Murray. Molecules 2021, 26, 7073. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agr. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Ouyang, J.; Hu, N.; Meng, J.; Su, C.; Wang, J.; Wang, H. Improved colorimetric analysis for subtle changes of powdered anthocyanins extracted from Lycium ruthenicum Murr. Food Chem. 2022, 371, 131080. [Google Scholar] [CrossRef]
- Qin, X.; Yuan, D.; Wang, Q.; Hu, Z.; Wu, Y.; Cai, J.; Huang, Q.; Li, S.; Liu, G. Maillard-reacted whey protein isolates enhance thermal stability of anthocyanins over a wide pH range. J. Agric. Food Chem. 2018, 66, 9556–9564. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Mi, J.; Lu, L.; Chen, F.; Luo, Q.; Li, X.; Yan, Y.; Cao, Y.; Huang, Q. Preparation of anthocyanin-loaded nanoparticles from Lycium ruthenium Murr. and its protective effect on oxidative damage of EAhy926 cells induced by oxidized low-density lipoprotein. Food Sci. 2019, 40, 162–168. [Google Scholar]
- Han, A.; Jiang, H.; Jia, Q.; Ma, L.; Bai, H. Optimization of microencapsulation of anthocyanins from Lycium ruthenicum Murr. by response surface methodology and stability of the microcapsules. Food Sci. 2016, 37, 82–87. [Google Scholar]
- Han, F.; Wang, M.; Yan, H.; Wang, H.; Li, M. Study on microencapsulation of Lycium ruthenicum anthocyanins. J. Anhui Agric. Sci. 2017, 45, 74–77. [Google Scholar]
- Zhang, C.; Ma, Y.; Zhao, X.Y.; Mu, J. Influence of copigmentation on stability of anthocyanins from purple potato peel in both liquid state and solid state. J. Agric. Food Chem. 2009, 57, 9503–9508. [Google Scholar] [CrossRef]
- Luan, G.; Wang, Y.; Ouyang, J.; He, Y.; Zhou, W.; Dong, Q.; Wang, H.; Hu, N. Stabilization of Lycium ruthenicum Murr. anthocyanins by natural polyphenol extracts. J. Food Sci. 2021, 86, 4365–4375. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Guo, M.; Li, L.; Chen, M.; Jiang, S.; Li, X.; Jiang, S. Extract from Lycium ruthenicum Murr. incorporating κ-carrageenan colorimetric film with a wide pH-sensing range for food freshness monitoring. Food Hydrocolloid 2019, 94, 1–10. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. High-purity isolation of anthocyanins mixtures from fruits and vegetables—A novel solid-phase extraction method using mixed mode cation exchange chromatography. J. Chromatogr. A 2011, 1218, 7914–7922. [Google Scholar] [CrossRef] [PubMed]
- León-Carmona, J.R.; Galano, A.; Alvarez-Idaboy, J.R. Deprotonation routes of anthocyanidins in aqueous solution, pKa values, and speciation under physiological conditions. RSC Adv. 2016, 6, 53421–53429. [Google Scholar] [CrossRef]
- Sigurdson, G.T.; Tang, P.; Giusti, M.M. Natural colorants: Food colorants from natural sources. Annu. Rev. Food Sci. T. 2017, 8, 261–280. [Google Scholar] [CrossRef] [PubMed]
- Abuduaibifu, A.; Tamer, C.E. Evaluation of physicochemical and bioaccessibility properties of Goji Berry Kombucha. J. Food Process. Preserv. 2019, 43, 14. [Google Scholar] [CrossRef]
- Liu, B.; Xu, Q.; Sun, Y. Black goji berry (Lycium ruthenicum) tea has higher phytochemical contents and in vitro antioxidant properties than red goji berry (Lycium barbarum) berry tea. Food Qual. Saf. 2020, 4, 193–201. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of physicochemical properties, functional compounds and antioxidant capacity during spontaneous fermentation of Lycium Ruthenicum Murr. (Qinghai-Tibet Plateau) natural vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Bai, H.J.; Wang, H.B.; Luo, F. Study on extracting and scavenging activity against DPPH free radical of pigment from Lycium rethenicum. Acta Agric. Boreali-Occident. Sin. 2007, 16, 190–192. [Google Scholar]
- Li, J.; Qu, W.; Zhang, S.; Lu, H. Study on antioxidant activity of pigment of Lycium ruthenicum. China J. Chin. Mater. Med. 2006, 31, 1179–1783. [Google Scholar]
- Sarian, M.N.; Ahmed, Q.U.; Mat So’ad, S.Z.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Akilah, S.N.; Mohamad, S.; Khatib, A.; Latip, J.; et al. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef] [PubMed]
- Song, J.L.; Gao, Y.; Xu, J. Protective effects of methanolic extract form fruits of Lycium ruthenicum Murr. on 2,2′-azobis (2-amidinopropane) dihydrochloride-induced oxidative stress in LLC-PK1 cells. Pharmacogn. Mag. 2014, 10, 522–528. [Google Scholar] [PubMed]
- You, Q.; Chen, F.; Wang, X.; Luo, P.G.; Jiang, Y. Inhibitory effects of muscadine anthocyanins on alpha-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011, 59, 9506–9511. [Google Scholar] [CrossRef] [PubMed]
- Lo Piparo, E.; Scheib, H.; Frei, N.; Williamson, G.; Grigorov, M.; Chou, C.J. Flavonoids for controlling starch digestion: Structural requirements for inhibiting human α-amylase. J. Med. Chem. 2008, 51, 3555–3561. [Google Scholar] [CrossRef]
- Li, D.; Yang, Y.; Sun, L.; Fang, Z.; Chen, L.; Zhao, P.; Wang, Z.; Guo, Y. Effect of young apple (Malus domestica Borkh. cv. Red Fuji) polyphenols on alleviating insulin resistance. Food Biosci. 2020, 36, 100637. [Google Scholar]
- Tian, B.; Zhao, J.; Xie, X.; Chen, T.; Yin, Y.; Zhai, R.; Wang, X.; An, W.; Li, J. Anthocyanins from the fruits of Lycium ruthenicum Murray improve high-fat diet-induced insulin resistance by ameliorating inflammation and oxidative stress in mice. Food Funct. 2021, 12, 3855–3871. [Google Scholar] [CrossRef]
- Ren, Z.; Xie, Z.; Cao, D.; Gong, M.; Yang, L.; Zhou, Z.; Ou, Y. C-Phycocyanin inhibits hepatic gluconeogenesis and increases glycogen synthesis via activating Akt and AMPK in insulin resistance hepatocytes. Food Funct. 2018, 9, 2829–2839. [Google Scholar] [CrossRef]
- Luan, G.; Wang, Y.; Wang, Z.; Zhou, W.; Hu, N.; Li, G.; Wang, H. Flavonoid glycosides from fenugreek seeds regulate glycolipid metabolism by improving mitochondrial function in 3T3-L1 adipocytes in vitro. J. Agric. Food Chem. 2018, 66, 3169–3178. [Google Scholar] [CrossRef]
- Abu Bakar, M.H.; Tan, J.S. Improvement of mitochondrial function by celastrol in palmitate-treated C2C12 myotubes via activation of PI3K-AKT signaling pathway. Biomed. Pharmacother. 2017, 93, 903–912. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Xue, J.; Liu, Z.; Zhu, H.; Niu, X.; Jing, N. PW289 Anthocyanins extracted from Lycium Ruthenicum Murray alleciate cardiac cardiomyopathy in experimental diabetic eats. Glob. Heart 2014, 9, e317. [Google Scholar] [CrossRef]
- Kuntz, S.; Asseburg, H.; Dold, S.; Roempp, A.; Froehling, B.; Kunz, C.; Rudloff, S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015, 6, 1136–1149. [Google Scholar] [CrossRef] [PubMed]
- Koloverou, E.; Panagiotakos, D.B. Inflammation: A new player in the link between mediterranean diet and diabetes mellitus: A review. Curr. Nutr. Rep. 2017, 6, 247–256. [Google Scholar] [CrossRef]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317. [Google Scholar] [CrossRef] [PubMed]
- Zand, H.; Morshedzadeh, N.; Naghashian, F. Signaling pathways linking inflammation to insulin resistance. Diabetes-Metab. Res. 2017, 11, S307–S309. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Mowat, C.; Cole, A.; Windsor, A.; Ahmad, T.; Arnott, I.; Driscoll, R.; Mitton, S.; Orchard, T.; Rutter, M.; Younge, L.; et al. Guidelines for the management of inflammatory bowel disease in adults. Gut 2011, 60, 571–607. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, Q.; Yin, J.; Zhang, Z.; Bao, H.; Wang, X. Anthocyanins attenuate neuroinflammation through the suppression of MLK3 activation in a mouse model of perioperative neurocognitive disorders. Brain Res. 2020, 1726, 146504. [Google Scholar] [CrossRef]
- Wu, X.L.; Li, X.X.; Jia, S.L.; Gao, Z.L.; Lu, Z.; Dai, X.L.; Sun, Y.X. Memory enhancing and antioxidant activities of Lycium ruthenicum Murray anthocyanin extracts in an Aβ 42-induced rat model of dementia. Mod. Food Sci. Technol. 2017, 33, 29–34. [Google Scholar]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radical Bio. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Subedi, B.H.; Joshi, P.H.; Jones, S.R.; Martin, S.S.; Blaha, M.J.; Michos, E.D. Current guidelines for high-density lipoprotein cholesterol in therapy and future directions. Vasc. Health Risk Man. 2014, 10, 205–216. [Google Scholar]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Jamar, G.; Estadella, D.; Pisani, L.P. Contribution of anthocyanin-rich foods in obesity control through gut microbiota interactions. Biofactors 2017, 43, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zhao, J.; Zhang, M.; Chen, Z.; Ma, Q.; Liu, H.; Nie, C.; Zhang, Z.; An, W.; Li, J. Lycium ruthenicum anthocyanins attenuate high-fat diet-Induced colonic barrier dysfunction and inflammation in mice by modulating the gut microbiota. Mol. Nutr. Food Res. 2021, 65, e2000745. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal short chain fatty acids and their link with diet and human health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Zhu, W.; Gregory, J.; Org, E.; Buffa, J.; Gupta, N.; Wang, Z.; Li, L.; Fu, X.; Wu, Y.; Mehrabian, M.; et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016, 165, 111–124. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Wang, X.; Wang, W. Lycium ruthenicum extract alleviates high-fat diet-induced nonalcoholic fatty liver disease via enhancing the AMPK signaling pathway. Mol. Med. Rep. 2015, 12, 3835–3840. [Google Scholar] [CrossRef]
- Ma, X.; Fan, X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Luca, S.V.; Macovei, I.; Bujor, A.; Miron, A.; Skalicka-Woźniak, K.; Aprotosoaie, A.C. Bioactivity of dietary polyphenols: The role of metabolites. Crit. Rev. Food Sci. 2020, 60, 626–659. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bioaccessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Norberto, S.; Silva, S.; Meireles, M.; Faria, A.; Pintado, M.; Calhau, C. Blueberry anthocyanins in health promotion: A metabolic overview. J. Funct. Foods 2013, 5, 1518–1528. [Google Scholar] [CrossRef]
- Zhu, F. Dietary fiber polysaccharides of amaranth, buckwheat and quinoa grains: A review of chemical structure, biological functions and food uses. Carbohyd. Polym. 2020, 248, 116819. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- Tang, H.-Y.; Fang, Z.; Ng, K. Dietary fiber-based colon-targeted delivery systems for polyphenols. Trends Food Sci. Technol. 2020, 100, 333–348. [Google Scholar] [CrossRef]
- Li, Z.L.; Mi, J.; Lu, L.; Luo, Q.; Liu, X.; Yan, Y.M.; Jin, B.; Cao, Y.L.; Zeng, X.X.; Ran, L.W. The main anthocyanin monomer of Lycium ruthenicum Murray induces apoptosis through the ROS/PTEN/PI3K/Akt/caspase 3 signaling pathway in prostate cancer DU-145 cells. Food Funct. 2021, 12, 1818–1828. [Google Scholar] [CrossRef]
- Chen, W.; Li, P.; Liu, Y.; Yang, Y.; Ye, X.; Zhang, F.; Huang, H. Isoalantolactone induces apoptosis through ROS-mediated ER stress and inhibition of STAT3 in prostate cancer cells. J. Exp. Clin. Canc. Res. 2018, 37, 309. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Feng, M.; Hu, X.; Zhang, H.; Zhang, R.; Dong, X.; Liu, C.; Zhang, Z.; Jiang, S. Maduramicin induces cardiac muscle cell death by the ROS-dependent PTEN/Akt-Erk1/2 signaling pathway. J. Cell Physiol. 2019, 234, 10964–10976. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, L.; Wang, Y.; Luo, R.; Li, X.; Dong, J. Anti-fatigue activities of anthocyanins from Lycium ruthenicum Murry. Food Sci. Technol. 2021, 42, 242703. [Google Scholar] [CrossRef]

| No. | Anthocyanins | Molecular Formula | References |
|---|---|---|---|
| Petundin derivatives | |||
| 1 | Petundin 3-O-galactoside-5-O-glucoside | C28H33O16 + | [2,6,11,28,33] |
| 2 | Petundin 3,5-O-diglucosides | C28H33O17 + | [2,6,11,18,28] |
| 3 | Petunidin 3-O-rutinoside-5-O-glucoside | C34H43O21 + | [14,19,22,33] |
| 4 | Petunidin 3-O-glucoside (maloyl)-5-O-glucoside | C31H35O19 + | [2,6,11,40] |
| 5 | Petunidin 3-O-glucoside (feruloyl)-5-O-glucoside | C38H41O20 + | [2,6,11,40] |
| 6 | Petunidin 3-O-rutinoside (feruloyl)-5-O-glucoside | C44H51O24 + | [19,22,25] |
| 7 | Petunidin 3-O-rutinoside (caffeoyl)-5-O-glucoside | C43H37O24 + | [2,6,11,19,28] |
| 8 | Petunidin 3-O-rutinoside (cis-caffeoyl)-5-O-glucoside | C43H50O24 + | [5] |
| 9 | Petunidin 3-O-rutinoside (trans-caffeoyl)-5-O-glucoside | C43H50O24 + | [5,22] |
| 10 | Petunidin 3-O-rutinoside (p-coumaroyl)-5-O-glucoside | C43H37O23 + | [14,20,22] |
| 11 | Petunidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside | C43H37O23 + | [2,5,6,11,19,22,28,33] |
| 12 | Petunidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | C43H37O23 + | [1,2,5,6,11,18,19,22,28,33,42] |
| 13 | Petunidin 3-O-rutinoside (glucosyl-cis-p-coumaroyl)-5-O-glucoside | C49H53O28 + | [19,22] |
| 14 | Petunidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside | C49H53O28 + | [19,22] |
| 15 | Petunidin 3-O-[6-O-(4-O-(cis-p-coumaroyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C43H49O23 + | [29,34,40] |
| 16 | Petunidin 3-O-[6-O-(4-O-(trans-p-coumaroyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C43H49O23 + | [29,30,32,34,40] |
| 17 | Petunidin 3-O-[6-O-(4-O-p-caffeoyl-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C43H49O24 + | [14,40] |
| 18 | Petunidin 3-O-[6-O-(4-O-(trans-p-caffeoyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C43H49O24 + | [29,34] |
| 19 | Petunidin 3-O-[6-O-(4-O-(4-O-cis-(β-D-glucopyranoside)-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C49H59O28 + | [14,29,34,40] |
| 20 | Petunidin3-O-[6-O-(4-O-(4-O-trans-(β-D-glucopyranoside)-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C49H59O28 + | [14,29,34,40] |
| 21 | Petunidin 3-O-[6-O-(4-O-(4-O-(β-D-glucopyranosyl)-cis-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C49H59O28 + | [14,40] |
| 22 | Petunidin 3-O-[6-O-(4-O-(4-O-(β-D-glucopyranosyl)-trans-p-coumaroyl) -α-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C49H59O28 + | [14,40] |
| 23 | Petunidin 3-O-[6-O-α-L-rhamnopyranosyl-β-D-glucopyranoside]-5-O- [β-D-glucopyranoside] | C34H43O21 + | [4] |
| Malvidin derivatives | |||
| 24 | Malvidin 3-O-rutinoside-5-O-glucoside | C35H45O21 + | [19] |
| 25 | Malvidin 3-O-rutinoside (feruloyl)-5-O-glucoside | C45H53O24 + | [19] |
| 26 | Malvidin 3-O-rutinoside (p-coumaroyl)-5-O-glucoside | C44H51O23 + | [35] |
| 27 | Malvidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside | C44H51O23 + | [2,6,11,20,29,41] |
| 28 | Malvidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | C44H51O23 + | [20,22,23] |
| 29 | Malvidin 3-O-rutinoside (glucosyl-cis-p-coumaroyl)-5-O-glucoside | C50H61O28 + | [20] |
| 30 | Malvidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside | C50H61O28 + | [20] |
| 31 | Malvidin 3-O-[6-O-(4-O-p-coumaroyl-α-L-rhamnosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C44H51O23 + | [15,41] |
| 32 | Malvidin 3-O-[6-O-(4-O-(4-O-trans-(β-D-glucopyranoside)-p- coumaroyl)-a-L-rhamnopyranosyl)-β-D-glucopyranoside]-5-O- [β-D-glucopyranoside] | C44H51O23 + | [20] |
| Delphinidin derivatives | |||
| 33 | Delphinidin 3-O-rutinoside (cis-p-coumaroyl)-5-O-glucoside | C42H47O23 + | [2,6,11,20,29] |
| 34 | Delphinidin 3-O-rutinoside (trans-p-coumaroyl)-5-O-glucoside | C42H47O23 + | [2,6,11,22,29,34] |
| 35 | Delphinidin 3-O-rutinoside (glucosyl-trans-p-coumaroyl)-5-O-glucoside | C48H57O28 + | [20] |
| 36 | Delphinidin 3-O-[6-O-(4-O-p-coumaroyl-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C42H47O23 + | [15,41] |
| 37 | Delphinidin 3-O-[6-O-(4-O-(trans-p-coumaroyl)-α-L-rhamnopyranosyl) -β-D-glucopyranoside]-5-O-[β-D-glucopyranoside] | C42H47O23 + | [30,35] |
| Peonidin derivatives | |||
| 38 | Peonidin 3-O-[6-O-(4-O-E-p-coumaroyl-O-α-rhamnopyranosyl) -β-glucopyranoside]-5-O-[β-glucopyranoside] | C43H48O22 + | [27] |
| 39 | Peonidin 3-O-[6-O-(4-O-E-p-coumaroyl-O-α-rhamnopyranosyl) -β-glucopyranoside]-5-O-[β-glucopyranoside] | C43H48O22 + | [27] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Nisar, T.; Fang, Z.; Wang, L.; Wang, Z.; Gu, H.; Wang, H.; Wang, W. Current Developments on Chemical Compositions, Biosynthesis, Color Properties and Health Benefits of Black Goji Anthocyanins: An Updated Review. Horticulturae 2022, 8, 1033. https://doi.org/10.3390/horticulturae8111033
Yan Y, Nisar T, Fang Z, Wang L, Wang Z, Gu H, Wang H, Wang W. Current Developments on Chemical Compositions, Biosynthesis, Color Properties and Health Benefits of Black Goji Anthocyanins: An Updated Review. Horticulturae. 2022; 8(11):1033. https://doi.org/10.3390/horticulturae8111033
Chicago/Turabian StyleYan, Yuzhen, Tanzeela Nisar, Zhongxiang Fang, Lingling Wang, Zichao Wang, Haofeng Gu, Huichun Wang, and Wenying Wang. 2022. "Current Developments on Chemical Compositions, Biosynthesis, Color Properties and Health Benefits of Black Goji Anthocyanins: An Updated Review" Horticulturae 8, no. 11: 1033. https://doi.org/10.3390/horticulturae8111033
APA StyleYan, Y., Nisar, T., Fang, Z., Wang, L., Wang, Z., Gu, H., Wang, H., & Wang, W. (2022). Current Developments on Chemical Compositions, Biosynthesis, Color Properties and Health Benefits of Black Goji Anthocyanins: An Updated Review. Horticulturae, 8(11), 1033. https://doi.org/10.3390/horticulturae8111033






