The Impacts of the Emerging Climate Change on Broccoli (Brassica oleracea L. var. italica Plenck.) Crop
Abstract
:1. Introduction
2. Plant Growth and Development of Broccoli
2.1. Head Initiation
2.2. Head Growth
3. Climate Change
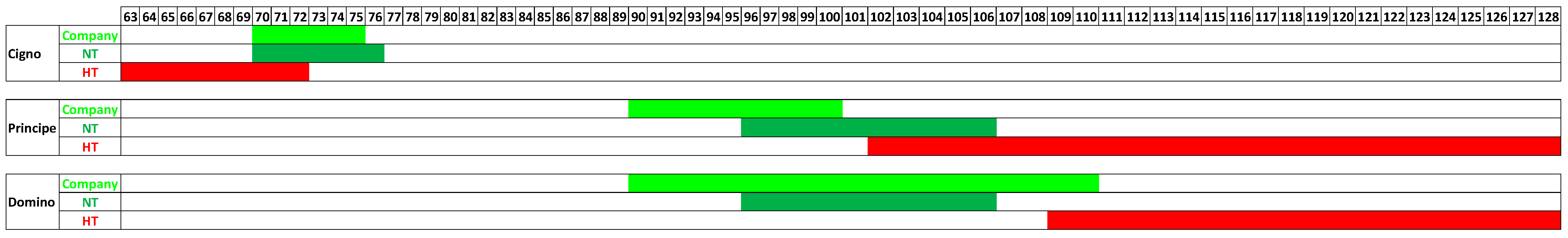
4. The Impacts of Emerging Climate Change on Broccoli Crop
5. Challenges and Future Perspectives
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dixon, G.R. Vegetable Brassicas and Related Crucifers; Crop Production Science in Horticulture Series 14; CABI Publishing: Oxfordshire, UK, 2007. [Google Scholar]
- Branca, F. Cauliflower and Broccoli. In Handbook of Plant Breeding. Vegetables I: Asteraceae, Brassicaceae, Chenopodiaceae, and Cucurbitaceae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; Volume 1, pp. 151–186. [Google Scholar]
- Carr, S.M.; Irish, V.F. Floral homeotic gene expression defines developmental arrest stages in Brassica oleracea L. vars. botrytis and italica. Planta 1997, 201, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Duclos, D.V.; Björkman, Τ. Meristem identity gene expression during curd proliferation and flower initiation in Brassica oleracea. J. Exp. Bot. 2008, 59, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irwin, J.A.; Soumpourou, E.; Lister, C.; Ligthart, J.; Kennedy, S.; Dean, C. Nucleotide polymorphism affecting FLC expression underpins heading date variation in horticultural brassicas. Plant J. 2016, 87, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef] [Green Version]
- Food and Agricultural Organization of the United Nations (FAO). FAOSTAT. Crops and Livestock Products. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 July 2022).
- Diputado, M.T., Jr.; Nichols, M.A. The effect of sowing date and cultivar on the maturity characteristics of broccoli (Brassica oleraceae var. italica). Acta Hortic. 1989, 247, 59–66. [Google Scholar] [CrossRef]
- Hadley, P.; Pearson, S. Effects of environmental factors on progress to crop maturity in selected Brassica crops. Acta Hortic. 1998, 459, 61–70. [Google Scholar] [CrossRef]
- Wiebe, H.J. The morphological development of cauliflower and broccoli cultivars depending on temperature. Sci. Hortic. 1975, 3, 95–101. [Google Scholar] [CrossRef]
- Yang, Y.W.; Tsai, C.C.; Wang, T.T. A heat-tolerant broccoli F1 hybrid, “Ching-Long 45”. HortScience 1998, 33, 1090–1091. [Google Scholar] [CrossRef] [Green Version]
- Stansell, Z.; Farnham, M.; Björkman, T. Complex horticultural quality traits in broccoli are illuminated by evaluation of the immortal BolTBDH mapping population. Front. Plant Sci. 2019, 10, 1104. [Google Scholar] [CrossRef] [Green Version]
- Wurr, D.C.E.; Fellows, J.R.; Hambidge, A.J. The influence of field environmental conditions on calabrese growth and development. J. Hortic. Sci. 1991, 66, 495–504. [Google Scholar] [CrossRef]
- Wurr, D.C.E.; Fellows, J.R.; Phelps, K.; Reader, R.J. Vernalization in calabrese (Brassica oleracea var. italica)—A model for apex development. J. Exp. Bot. 1995, 46, 1487–1496. [Google Scholar] [CrossRef]
- Grevsen, K. Effects of temperature on head growth of broccoli (Brassica oleracea L. var. italica): Parameter estimates for a predictive model. J. Hortic. Sci. Biotechnol. 1998, 73, 235–244. [Google Scholar] [CrossRef]
- Mourão, I.M.G.; Hadley, P. Environmental control of plant growth development and yield in broccoli (Brassica oleracea L. var. italica Plenk): Crop responses to light regime. Acta Hortic. 1998, 459, 71–78. [Google Scholar] [CrossRef]
- Fyffe, D.C.; Titley, M.E. Phenology studies and the prediction of harvest dates of broccoli in the Lockyer valley. Acta Hortic. 1989, 247, 53–58. [Google Scholar] [CrossRef]
- Fujime, Y.; Hirose, Τ. Studies on thermal conditions of curd formation and development in cauliflower and broccoli. II. Effects of diurnal variation of temperature on curd formation. J. Jpn. Soc. Hortic. Sci. 1980, 49, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.H. Diurnal temperature cycling influences flowering and node numbers of broccoli. HortScience 1988, 23, 873–875. [Google Scholar] [CrossRef]
- Fellows, J.R.; Reader, R.J.; Wurr, D.C.E. A model for leaf production and apex development in calabrese. J. Hortic. Sci. Biotechnol. 1997, 72, 327–337. [Google Scholar] [CrossRef]
- Gauss, J.F.; Taylor, G.A. Environmental factors influencing reproductive differentiation and the subsequent formation of the inflorescence of Brassica oleracea L. var. italica, Plenck, cv. ‘Coastal’. J. Am. Soc. Hortic. Sci. 1969, 94, 275–280. [Google Scholar] [CrossRef]
- Lindemann-Zutz, Κ.; Fricke, A.; Stützel, H. Prediction of time to harvest and its variability in broccoli (Brassica oleracea var. italica). Part I. Plant developmental variation and forecast of time to head induction. Sci. Hortic. 2016, 198, 424–433. [Google Scholar] [CrossRef]
- Wiebe, H.J. Vernalization of vegetable crops—A review. Acta Hortic. 1990, 267, 323–328. [Google Scholar] [CrossRef]
- Tan, D.K.Y.; Birch, C.J.; Wearing, A.H.; Rickert, K.G. Predicting broccoli development I. Development is predominantly determined by temperature rather than photoperiod. Sci. Hortic. 2000, 84, 227–243. [Google Scholar] [CrossRef] [Green Version]
- Fontes, M.R.; Ozbun, J.L.; Sadik, S. Influence of temperature on initiation of floral primordia in green sprouting broccoli. Proc. Am. Soc. Hortic. Sci. 1967, 91, 315–320. [Google Scholar]
- Francescangeli, Ν.; Sangiacomo, M.A.; Martí, H.R. Vegetative and reproductive plasticity of broccoli at three levels of incident photosynthetically active radiation. Span. J. Agric. Res. 2007, 5, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Fujime, Y.; Saito, Y.; Nakayama, Y. Photothermal induction of flower head formation in broccoli plants. J. Jpn. Soc. Hortic. Sci. 1988, 57, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Fujime, Y.; Okuda, N. The physiology of flowering in brassicas, especially about cauliflower and broccoli. Acta Hortic. 1996, 407, 247–254. [Google Scholar] [CrossRef]
- Tan, D.K.Y.; Wearing, A.H.; Rickert, K.G.; Birch, C.J. A Systems Approach to Developing a Model that Predicts Crop Ontogeny and Maturity in Broccoli in South-East Queensland. In Proceedings of the Third Australia New Zealand Systems Conference: Linking People, Nature, Business and Technology, Gatton, Australia, 1–4 October 1997; pp. 179–187. [Google Scholar]
- Grevsen, K.; Olesen, J.E. Modelling development of broccoli (Brassica oleracea L. var. italica) from transplanting to head initiation. J. Hortic. Sci. Biotechnol. 1999, 74, 698–705. [Google Scholar] [CrossRef]
- Tan, D.K.Y.; Birch, C.J.; Wearing, A.H.; Rickert, K.G. Predicting broccoli development II. Comparison and validation of thermal time models. Sci. Hortic. 2000, 86, 89–101. [Google Scholar] [CrossRef]
- Uptmoor, R.; Schrag, T.; Stützel, H.; Esch, E. Crop model based QTL analysis across environments and QTL based estimation of time to floral induction and flowering in Brassica oleracea. Mol. Breed. 2008, 21, 205–216. [Google Scholar] [CrossRef]
- Uptmoor, R.; Li, J.; Schrag, T.; Stützel, H. Prediction of flowering time in Brassica oleracea using a quantitative trait loci-based phenology model. Plant Biol. 2011, 14, 179–189. [Google Scholar] [CrossRef]
- Fujime, Y.; Okuda, N. Method for prediction of budding and harvest time of broccoli under field condition. Acta Hortic. 1994, 371, 355–362. [Google Scholar] [CrossRef]
- Mourão, I.M.; Brito, L.M. Empirical models for harvest date prediction in broccoli (Brassica oleracea L. var. italica Plenck). Acta Hortic. 2000, 539, 47–53. [Google Scholar] [CrossRef]
- Okazaki, K.; Sakamoto, K.; Kikuchi, R.; Saito, A.; Togashi, E.; Kuginuki, Y.; Matsumoto, S.; Hirai, M. Mapping and characterization of FLC homologs and QTL analysis of flowering time in Brassica oleracea. Theor. Appl. Genet. 2007, 114, 595–608. [Google Scholar] [CrossRef]
- Grevsen, K. Modelling plant development of broccoli. Acta Hortic. 2000, 533, 567–574. [Google Scholar] [CrossRef]
- Lin, C.W.; Fu, S.F.; Liu, Y.J.; Chen, C.C.; Chang, C.H.; Yang, Y.W.; Huang, H.J. Analysis of ambient temperature-responsive transcriptome in shoot apical meristem of heat-tolerant and heat-sensitive broccoli inbred lines during floral head formation. BMC Plant Biol. 2019, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Heather, D.W.; Sieczka, J.B.; Dickson, M.H.; Wolfe, D.W. Heat tolerance and holding ability in broccoli. J. Am. Soc. Hortic. Sci. 1992, 117, 887–892. [Google Scholar] [CrossRef] [Green Version]
- Björkman, T.; Pearson, K.J. High temperature arrest of inflorescence development in broccoli (Brassica oleracea var. italica L.). J. Exp. Bot. 1998, 49, 101–106. [Google Scholar] [CrossRef]
- Schranz, M.E.; Quijada, P.; Sung, S.B.; Lukens, L.; Amasino, L.; Osborn, T.C. Characterization and eVects of the replicated flowering time gene FLC in Brassica rapa. Genetics 2002, 162, 1457–1468. [Google Scholar] [CrossRef]
- Razi, H.; Howell, E.C.; Newbury, H.J.; Kearsey, M.J. Does sequence polymorphism of FLC paralogues underlie flowering time QTL in Brassica oleracea? Theor. Appl. Genet. 2008, 116, 179–192. [Google Scholar] [CrossRef]
- Lin, H.H.; Lin, K.H.; Chen, S.C.; Shen, Y.H.; Lo, H.F. Proteomic analysis of broccoli (Brassica oleracea) under high temperature and waterlogging stresses. Bot. Stud. 2015, 56, 18. [Google Scholar] [CrossRef] [Green Version]
- Lan, T.H.; Paterson, A.H. Comparative mapping of quantitative trait loci sculpting the curd of Brassica oleracea. Genetics 2000, 155, 1927–1954. [Google Scholar] [CrossRef]
- Labate, J.; Robertson, J.; Baldo, A.; Björkman, T. Inflorescence identity genes alleles are poor predictors of inflorescence type in broccoli and cauliflower. J. Am. Soc. Hortic. Sci. 2006, 131, 667–673. [Google Scholar] [CrossRef]
- Gao, M.; Li, G.; Yang, B.; Qiu, D.; Farnham, M.; Quiros, C. Highdensity Brassica oleracea linkage map: Identification of useful new linkages. Theor. Appl. Genet. 2007, 115, 277–287. [Google Scholar] [CrossRef]
- Lin, Y.R.; Lee, J.Y.; Tseng, M.C.; Lee, C.Y.; Shen, C.H.; Wang, C.S.; Liou, C.C.; Shuang, L.S.; Paterson, A.H.; Hwu, K.K. Subtropical adaptation of a temperate plant (Brassica oleracea var. Italica) utilizes non-vernalization-responsive QTLs. Sci. Rep. 2018, 8, 13609. [Google Scholar] [CrossRef] [Green Version]
- Lindemann-Zutz, Κ.; Fricke, A.; Stützel, H. Prediction of time to harvest and its variability in broccoli (Brassica oleracea var. italica). Part II. Growth model description, parameterisation and field evaluation. Sci. Hortic. 2016, 200, 151–160. [Google Scholar] [CrossRef]
- Wurr, D.C.E.; Hambidge, A.J.; Smith, G.P. Studies of the cause of blindness in brassicas. J. Hortic. Sci. 1996, 71, 415–426. [Google Scholar] [CrossRef]
- Forsyth, J.L.; Pearson, S.; Hadley, P.; Barnett, J.R. Apical abortion in calabrese is induced by periods of low temperature and results in premature differentiation of apical meristem cells. J. Exp. Bot. 1999, 50, 861–868. [Google Scholar] [CrossRef]
- Marshall, B.; Thompson, R. A model of the influence of air temperature and solar radiation on the time to maturity of calabrese Brassica oleracea var. italica. Ann. Bot. 1987, 60, 513–519. [Google Scholar]
- Marshall, B.; Thompson, R. Applications of a model to predict the time to maturity of calabrese Brassica oleracea. Ann. Bot. 1987, 60, 521–529. [Google Scholar] [CrossRef]
- Scaife, A.; Cox, E.F.; Morris, G.E.L. The relationship between shoot weight, plant density and time during the propagation of four vegetable species. Ann. Bot. 1987, 59, 325–334. [Google Scholar] [CrossRef]
- Wurr, D.C.E.; Fellows, J.R.; Hambidge, A.J. The effect of plant density on calabrese head growth and its use in a predictive model. J. Hortic. Sci. 1992, 67, 77–85. [Google Scholar] [CrossRef]
- Arias, P.A.; Bellouin, N.; Coppola, E.; Jones, R.G.; Krinner, G.; Marotzke, J.; Naik, V.; Palmer, M.D.; Plattner, G.K.; Rogelj, J.; et al. Technical Summary. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 33–144. [Google Scholar] [CrossRef]
- Guiot, J.; Wolfgang, C. Climate change: The 2015 Paris agreement thresholds and Mediterranean basin ecosystems. Science 2016, 354, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Praveen, B.; Sharma, P. A review of literature on climate change and its impacts on agriculture productivity. J. Public Aff. 2019, 19, e1960. [Google Scholar] [CrossRef]
- Naik, P.S.; Singh, M.; Ranjan, J.K. Impact of Climate Change on Vegetable Production and Adaptation Measures. In Abiotic Stress Management for Resilient Agriculture; Minhas, P., Rane, J., Pasala, R., Eds.; Springer Nature Singapore Pte Ltd.: Singapore, 2017; pp. 413–428. [Google Scholar]
- Committee for the Study of the Effects of Climate Change (CSECC). The Environmental, Economic and Social Impacts of Climate Change in Greece; Bank of Greece: Athens, Greece, 2011. [Google Scholar]
- Yohannes, H. A review on relationship between climate change and agriculture. J. Earth Sci. Clim. Chang. 2016, 7, 335. [Google Scholar] [CrossRef]
- Koundinya, A.; Sidhya, P.; Pandit, M.K. Impact of climate change on vegetable cultivation—A review. Int. J. Environ. Agric. Biotechnol. 2014, 7, 145–155. [Google Scholar]
- Abewoy, D. Review on impacts of climate change on vegetable production and its management practices. Adv. Crop Sci. Technol. 2018, 6, 330. [Google Scholar] [CrossRef]
- Bisbis, M.B.; Gruda, N.; Blanke, M. Potential impacts of climate change on vegetable production and product quality—A review. J. Clean. Prod. 2018, 170, 1602–1620. [Google Scholar] [CrossRef]
- Dong, J.; Gruda, N.; Li, X.; Tang, Y.; Zhang, P.; Duan, Z. Sustainable vegetable production under changing climate: The impact of elevated CO2 on yield of vegetables and the interactions with environments—A review. J. Clean. Prod. 2020, 253, 119920. [Google Scholar] [CrossRef]
- Singh, D.P.; Kumari, M.; Prakash, H.G. Climate Change Impact on Cole Crops and Mitigation Strategies. In Advances in Research on Vegetable Production Under a Changing Climate; Solankey, S.S., Kumari, M., Kumar, M., Eds.; Advances in Olericulture; Springer: Cham, Switzerland, 2021; Volume 1, pp. 113–123. [Google Scholar]
- Solankey, S.S.; Kumari, M.; Akhtar, S.; Singh, H.K.; Ray, P.K. Challenges and Opportunities in Vegetable Production in Changing Climate: Mitigation and Adaptation Strategies. In Advances in Research on Vegetable Production under a Changing Climate; Solankey, S.S., Kumari, M., Kumar, M., Eds.; Advances in Olericulture; Springer: Cham, Switzerland, 2021; Volume 1, pp. 13–59. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture—Trends and Challenges; FAO: Rome, Italy, 2017. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). The Future of Food and Agriculture—Alternative Pathways to 2050; FAO: Rome, Italy, 2018. [Google Scholar]
- Dong, J.; Gruda, N.; Lam, S.K.; Li, X.; Duan, Z. Effects of elevated CO2 on nutritional quality of vegetables: A review. Front. Plant Sci. 2018, 9, 924. [Google Scholar] [CrossRef]
- Soares, J.C.; Santos, C.S.; Carvalho, S.M.P.; Pintado, M.M.; Vasconcelos, M.W. Pre-serving the nutritional quality of crop plants under a changing climate: Importance and strategies. Plant Soil 2019, 443, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Dufault, R.J. Dynamic relationships between field temperatures and broccoli head quality. J. Am. Soc. Hortic. Sci. 1996, 121, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J.E.; Grevsen, K. Effects of temperature and irradiance on vegetative growth of cauliflower (Brassica oleracea L. botrytis) and broccoli (Brassica oleracea L. italica). J. Exp. Bot. 1997, 48, 1591–1598. [Google Scholar] [CrossRef] [Green Version]
- Maynard, D.N.; Hochmuth, G.J. Knott’s Handbook for Vegetable Growers, 5th ed.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2007. [Google Scholar]
- Branham, S.E.; Stansell, Z.J.; Couillard, D.M.; Farnham, M.W. Quantitative trait loci mapping of heat tolerance in broccoli (Brassica oleracea var. italica) using genotyping-by-sequencing. Theor. Appl. Genet. 2017, 130, 529–538. [Google Scholar] [CrossRef]
- Luo, Q. Temperature thresholds and crop production: Α review. Clim. Chang. 2011, 109, 583–598. [Google Scholar] [CrossRef]
- Koularmanis, K. Study of the Impacts of Emerging Climate Change on Broccoli Crop. Master’s Thesis, Aristotle University, Thessaloniki, Greece, 8 July 2022. [Google Scholar]
- Kop, E.P.; Teakle, G.R.; McClenaghan, R.; Lynn, J.R.; King, G.J. Genetic analysis of the bracting trait in cauliflower and broccoli. Plant Sci. 2003, 164, 803–808. [Google Scholar] [CrossRef]
- Farnham, M.W.; Stansell, Z.J.; Griffiths, P.D.; Davis, J.M.; Hutton, M.; Bjorkman, T. Using Regional Broccoli Trial Data to Select Experimental Hybrids for Input into Advanced Yield Trials. HortScience 2014, 49, S242–S243. [Google Scholar]
- Farnham, M.W.; Bjorkman, T. Breeding vegetables adapted to high temperatures: A case study with broccoli. HortScience 2011, 46, 1093–1097. [Google Scholar] [CrossRef] [Green Version]
- Stansell, Z.; Björkman, Τ.; Branham, S.; Couillard, D.; Farnham, M.W. Use of a quality trait index to increase the reliability of phenotypic evaluations in broccoli. HortScience 2017, 52, 1490–1495. [Google Scholar] [CrossRef] [Green Version]
- Stansell, Z.; Björkman, T. From landrace to modern hybrid broccoli: The genomic and morphological domestication syndrome within a diverse B. oleracea collection. Hortic. Res. 2020, 7, 159. [Google Scholar] [CrossRef]
- Farnham, M.; Björkman, T. Evaluation of experimental broccoli hybrids developed for summer production in the Eastern United States. HortScience 2011, 46, 858–863. [Google Scholar] [CrossRef] [Green Version]
- Axelsson, T.; Shavorskaya, O.; Lagercrantz, U. Multiple flowering time QTLs within several brassica species could be the result of duplicated copies of one ancestral gene. Genome 2001, 44, 856–864. [Google Scholar] [CrossRef]
- Li, G.; Gao, M.; Yang, B.; Quiros, C.F. Gene for gene alignment between the Brassica and Arabidopsis genomes by direct transcriptome mapping. Theor. Appl. Genet. 2003, 107, 168–180. [Google Scholar] [CrossRef]
- Lin, K.H.; Chang, L.C.; Lai, C.D.; Lo, H.F. AFLP mapping of quantitative trait loci influencing seven head-related traits in broccoli (Brassica oleracea var. italica). J. Hortic. Sci. Biotechnol. 2013, 88, 257–268. [Google Scholar] [CrossRef]
- Chen, C.C.; Fu, S.F.; Norikazu, M.; Yang, Y.W.; Liu, Y.J.; Ikeo, K.; Gojobori, T.; Huang, H.J. Comparative miRNAs analysis of two contrasting broccoli inbred lines with divergent head-forming capacity under temperature stress. BMC Genom. 2015, 16, 1026. [Google Scholar] [CrossRef]
- Parkin, I.P.; Koh, C.; Tang, H.; Robinson, S.J.; Kagale, S.; Clarke, W.E.; Town, C.D.; Nixon, J.; Krishnakumar, V.; Bidwell, S.L.; et al. Transcriptome and methylome profiling reveals relics of genome dominance in the mesopolyploid Brassica oleracea. Genome Biol. 2014, 15, R77. [Google Scholar] [CrossRef] [Green Version]
- Slocum, M.K.; Figdore, S.S.; Kennard, W.C.; Suzuki, J.Y.; Osborn, T.C. Linkage arrangement of restriction fragment length polymorphism loci in Brassica oleracea. Theor. Appl. Genet. 1990, 80, 57–64. [Google Scholar] [CrossRef]
- Kianian, S.F.; Quiros, C.F. Generation of a Brassica oleracea composite RFLP map: Linkage arrangements among various populations and evolutionary implications. Theor. Appl. Genet. 1992, 84, 544–554. [Google Scholar] [CrossRef]
- Camargo, L.; Williams, P.; Osborn, T. Mapping of quantitative trait loci controlling resistance of Brassica oleracea to Xanthomonas campestris pv. campestris in the field and greenhouse. Phytopathology 1995, 85, 1296–1300. [Google Scholar] [CrossRef]
- Bohuon, E.J.R.; Keith, D.J.; Parkin, I.A.P.; Sharpe, A.G.; Lydiate, D.J. Alignment of the conserved C genomes of Brassica oleracea and Brassica napus. Theor. Appl. Genet. 1996, 93, 833–839. [Google Scholar] [CrossRef]
- Brown, A.F.; Jeffery, E.; Juvik, J.A. A polymerase chain reaction-based linkage map of broccoli and identification of quantitative trait loci associated with harvest date and head weight. J. Am. Soc. Hortic. Sci. 2007, 132, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Liu, Y.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H.; Wang, Y.; Ji, J.; Li, Z. Advances in genetics and molecular breeding of broccoli. Horticulturae 2021, 7, 280. [Google Scholar] [CrossRef]
- Liu, C.; Yao, X.; Li, G.; Huang, L.; Wu, X.; Xie, Z. Identification of major loci and candidate genes for anthocyanin biosynthesis in broccoli using QTL-Seq. Horticulturae 2021, 7, 246. [Google Scholar] [CrossRef]
- Walley, P.G.; Carder, J.; Skipper, E.; Mathas, E.; Lynn, J.; Pink, D.; Buchanan-Wollaston, V. A new broccoli × broccoli immortal mapping population and framework genetic map: Tools for breeders and complex trait analysis. Theor. Appl. Genet. 2012, 124, 467–484. [Google Scholar] [CrossRef] [Green Version]
- Barham, R.; Joynt, D. Heat Tolerant Broccoli. U.S. Patent 6,294,715, 25 September 2001. [Google Scholar]
- Barham, R.; Joynt, D. Heat Tolerant Broccoli. U.S. Patent 6,784,345, 31 August 2004. [Google Scholar]
- Barham, R.; Joynt, D. Broccoli Line M7007. U.S. Patent 7,053,271, 30 May 2006. [Google Scholar]
- Barham, R.; Joynt, D. Broccoli Line M7009. U.S. Patent 7,351,884, 1 April 2008. [Google Scholar]
- Barham, R.; Joynt, D. Broccoli Line M7028. U.S. Patent 7,829,763, 9 November 2010. [Google Scholar]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Abdull Razis, A.F.; Arumugam, A.; Konsue, N. Glucosinolates and Isothiocyanates: Cancer Preventive Effects. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons Ltd.: Oxford, UK, 2018; Volume I, pp. 199–210. [Google Scholar]
- Herr, I.; Büchler, M.W. Dietary constituents of broccoli and other cruciferous vegetables: Implications for prevention and therapy of cancer. Cancer Treat. Rev. 2010, 36, 377–383. [Google Scholar] [CrossRef]
- Latté, K.P.; Appel, K.E.; Lampen, A. Health benefits and possible risks of broccoli-An overview. Food Chem. Toxicol. 2011, 49, 3287–3309. [Google Scholar] [CrossRef]
- Amjad, A.I.; Parikh, R.A.; Appleman, L.J.; Hahm, E.R.; Singh, K.; Singh, S.V. Broccoli-derived sulforaphane and chemoprevention of prostate cancer: From bench to bedside. Curr. Pharmacol. Rep. 2015, 1, 382–390. [Google Scholar] [CrossRef]
- Dacosta, C.; Bao, Y. The role of microRNAs in the chemopreventive activity of sulforaphane from cruciferous vegetables. Nutrients 2017, 9, 902. [Google Scholar] [CrossRef] [Green Version]
- Sturm, C.; Wagner, A.E. Brassica-derived plant bioactives as modulators of chemopreventive and inflammatory signaling pathways. Int. J. Mol. Sci. 2017, 18, 1890. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Liu, Y.; Ma, L.; Ji, R.; Qu, Y.; Xin, Y.; Lv, G. Chemopreventive activity of sulforaphane. Drug Des. Devel. Ther. 2018, 12, 2905–2913. [Google Scholar] [CrossRef] [Green Version]
- Soundararajan, P.; Kim, J.S. Anti-carcinogenic glucosinolates in cruciferous vegetables and their antagonistic effects on prevention of cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [Green Version]
- Yagishita, Y.; Fahey, J.W.; Dinkova-Kostova, A.T.; Kensler, T.W. Broccoli or sulforaphane: Is it the source or dose that matters? Molecules 2019, 24, 3593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandini, D.B.; Rao, R.S.; Deepak, B.S.; Reddy, P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020, 24, 405. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.E.; Baniasadi, M.; Giansiracusa, D.; Giansiracusa, M.; Garcia, M.; Fryda, Z.; Wong, T.L.; Bishayee, A. Sulforaphane: A broccoli bioactive phytocompound with cancer preventive potential. Cancers 2021, 13, 4796. [Google Scholar] [CrossRef] [PubMed]
- Mahn, A.; Castillo, A. Potential of sulforaphane as a natural immune system enhancer: A review. Molecules 2021, 26, 752. [Google Scholar] [CrossRef] [PubMed]
- Surh, Y.J. Nrf2 paradox: Can cancer patients eat broccoli? Food Front. 2021, 2, 25–28. [Google Scholar] [CrossRef]
- Djaldetti, M. Sulforaphane: The principal broccoli phytochemical as a cancer challenger. Rec. Progr. Nutr. 2022, 2, 008. [Google Scholar] [CrossRef]
- Witzel, K.; Kurina, A.B.; Artemyeva, Α.M. Opening the treasure chest: The current status of research on Brassica oleracea and B. rapa vegetables from ex situ germplasm collections. Front. Plant Sci. 2021, 12, 643047. [Google Scholar] [CrossRef]
- dos Santos, J.B.; Nienhuis, J.; Skroch, P.; Tivang, J.; Slocum, M.K. Comparison of RAPD and RFLP genetic markers in determining genetic similarity among Brassica oleracea L. genotypes. Theor. Appl. Genet. 1994, 87, 909–915. [Google Scholar] [CrossRef]
- Ciancaleoni, S.; Chiarenza, G.L.; Raggi, L.; Branca, F.; Negri, V. Diversity characterization of broccoli (Brassica oleracea L. var. italica Plenck) landraces for their on-farm (in situ) safeguard and use in breeding programs. Genet. Resour. Crop Evol. 2014, 61, 451–464. [Google Scholar] [CrossRef]
- Torricelli, R.; Ciancaleoni, S.; Negri, V. Performance and stability of homogeneous and heterogeneous broccoli (Brassica oleracea L. var. italica Plenck) varieties in organic and low-input conditions. Euphytica 2014, 199, 385–395. [Google Scholar] [CrossRef]
- Branca, F.; Chiarenza, G.L.; Cavallaro, C.; Gu, H.; Zhao, Z.; Tribulato, A. Diversity of Sicilian broccoli (Brassica oleracea var. italica) and cauliflower (Brassica oleracea var. botrytis) landraces and their distinctive bio-morphological, antioxidant, and genetic traits. Genet. Resour. Crop Evol. 2018, 65, 485–502. [Google Scholar] [CrossRef]
- Braun, H.J.; Atlin, G.; Payne, T. Multi-Location Testing as a Tool to Identify Plant Response to Global Climate Change. In Climate Change and Crop Production; Reynolds, M.P., Ed.; CABI Publishers: Wallingford, UK, 2010; pp. 115–138. [Google Scholar]
- Ciancaleoni, S.; Onofri, A.; Torricelli, R.; Negri, V. Broccoli yield response to environmental factors in sustainable agriculture. Eur. J. Agron. 2016, 72, 1–9. [Google Scholar] [CrossRef]
- Alfaro, E.J.; Gershunov, A.; Cayan, D. Prediction of summer maximum and minimum temperature over the Central and Western United States: The roles of soil moisture and sea surface temperature. J. Clim. 2006, 19, 1407–1421. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Thomson, A.M.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef] [Green Version]
- Kałużewicz, A.; Krzesiński, W.; Knaflewski, Μ.; Lisiecka, J.; Spiżewski, T.; Frąszczak, B. Effect of temperature on the growth of broccoli (Brassica oleracea var. italica Plenck) cv. Fiesta. J. Fruit Ornam. Plant Res. 2012, 77, 129–141. [Google Scholar] [CrossRef]
- Shapla, S.A.; Hussain, M.A.; Mandal, M.S.H.; Mehraj, H.; Jamal Uddin, A.F.M. Growth and yield of broccoli (Brassica oleracea var. Italica L.) to different planting times. Int. J. Bus. Soc. Sci. Res. 2014, 2, 95–99. [Google Scholar]


| Mean Values | Change (Δ) | Change (%) | ||||
|---|---|---|---|---|---|---|
| Emission Scenarios | Emission Scenarios | Emission Scenarios | ||||
| Periods | A2 | B2 | A2 | B2 | A2 | B2 |
| Air temperature 2 m above the ground (°C) | ||||||
| 1961–1990 | 16.17 ± 0.68 | 16.14 ± 0.56 | ||||
| 2071–2080 | 19.58 ± 0.80 | 18.81 ± 0.67 | 3.41 ± 0.42 | 2.66 ± 0.19 | 21.1 ± 2.8 | 16.5 ± 1.0 |
| 2081–2090 | 19.93 ± 0.82 | 18.94 ± 0.71 | 3.76 ± 0.49 | 2.80 ± 0.34 | 23.3 ± 3.2 | 17.3 ± 2.1 |
| 2091–2100 | 20.64 ± 0.80 | 19.25 ± 0.72 | 4.46 ± 0.38 | 3.11 ± 0.39 | 27.6 ± 2.6 | 19.3 ± 2.5 |
| Air relative humidity 2 m above the ground (%) | ||||||
| 1961–1990 | 68.47 ± 4.27 | 69.49 ± 4.63 | ||||
| 2071–2080 | 66.45 ± 2.99 | 68.42 ± 5.02 | −2.02 ± 2.28 | −1.07 ± 0.79 | −2.8 ± 2.9 | −1.6 ± 1.2 |
| 2081–2090 | 65.50 ± 3.04 | 68.14 ± 5.03 | −2.97 ± 2.20 | −1.35 ± 0.72 | −4.2 ± 2.7 | −2.0 ± 1.0 |
| 2091–2100 | 65.23 ± 2.99 | 68.68 ± 4.80 | −3.24 ± 2.09 | −0.81 ± 0.76 | −4.6 ± 2.6 | −1.2 ± 1.1 |
| Precipitation (mm/year) | ||||||
| 1961–1990 | 510.1 ± 108.0 | 524.1 ± 113.8 | ||||
| 2071–2080 | 442.7 ± 112.9 | 497.4 ± 108.6 | −67.4 ± 34,6 | −26.7 ± 50.2 | −13.8 ± 7.6 | −4.6 ± 9.8 |
| 2081–2090 | 397.1± 99.6 | 475.7 ± 109.0 | −113.0 ± 29.5 | −48.4 ± 36.4 | −22.6 ± 5.5 | −9.2 ± 8.2 |
| 2091–2100 | 437.7 ± 126.6 | 525.2 ± 138.0 | −72.4 ± 51.1 | 1.1 ± 54.5 | −15.2 ± 10.9 | −0.4 ± 11.2 |
| Incoming at the surface total short wavelength radiation (W/m2) | ||||||
| 1961–1990 | 196.1 ± 20.8 | 203.0 ± 21.9 | ||||
| 2071–2080 | 199.0 ± 19.9 | 206.0 ± 18.3 | 2.9 ± 4.2 | 3.0 ± 5.3 | 1.6 ± 2.5 | 1.7 ± 3.2 |
| 2081–2090 | 201.0 ± 19.6 | 207.2 ± 18.5 | 4.9 ± 4.9 | 4.2 ± 4.8 | 2.6 ± 3.1 | 2.3 ± 3.0 |
| 2091–2100 | 200.5 ± 20.0 | 205.2 ± 18.4 | 4.5 ± 5.4 | 2.3 ± 5.2 | 2.4 ± 3.3 | 1.4 ± 3.1 |
| Cloud cover fraction (%) | ||||||
| 1961–1990 | 35.8 ± 4.4 | 36.4 ± 2.1 | ||||
| 2071–2080 | 31.7 ± 4.3 | 33.3 ± 3.1 | −4.0 ± 1.6 | −3.1 ± 1.4 | −11.3 ± 4.3 | −8.8 ± 4.2 |
| 2081–2090 | 30.2 ± 4.2 | 32.7 ± 2.6 | −5.5 ± 1.7 | −3.8 ± 1.0 | −15.5 ± 4.3 | −10.4 ± 2.9 |
| 2091–2100 | 30.0 ± 4.1 | 33.6 ± 3.1 | −5.7 ± 1.8 | −2.9 ± 1.7 | −16.1 ± 4.8 | −8.0 ± 4.8 |
| Impact Groups | Specific Impacts |
|---|---|
| Plant growth and development | Most parameters (plant height, plant leaf number and weight, stem length and weight) except of the number of lateral shoots |
| The rate of plant growth | |
| Head | Initiation of head formation |
| Diameter | |
| Weight | |
| Characteristics | |
| Quality of the produce | Presence of leaves between flower buds |
| Uneven flower bud size | |
| Uneven head surface | |
| Undesirable head coloration | |
| Crop productivity | Marketable crop yield |
| Non-marketable production | |
| Cultivation | Days from planting to harvest |
| Duration of the harvesting period |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siomos, A.S.; Koularmanis, K.; Tsouvaltzis, P. The Impacts of the Emerging Climate Change on Broccoli (Brassica oleracea L. var. italica Plenck.) Crop. Horticulturae 2022, 8, 1032. https://doi.org/10.3390/horticulturae8111032
Siomos AS, Koularmanis K, Tsouvaltzis P. The Impacts of the Emerging Climate Change on Broccoli (Brassica oleracea L. var. italica Plenck.) Crop. Horticulturae. 2022; 8(11):1032. https://doi.org/10.3390/horticulturae8111032
Chicago/Turabian StyleSiomos, Anastasios S., Konstantinos Koularmanis, and Pavlos Tsouvaltzis. 2022. "The Impacts of the Emerging Climate Change on Broccoli (Brassica oleracea L. var. italica Plenck.) Crop" Horticulturae 8, no. 11: 1032. https://doi.org/10.3390/horticulturae8111032
APA StyleSiomos, A. S., Koularmanis, K., & Tsouvaltzis, P. (2022). The Impacts of the Emerging Climate Change on Broccoli (Brassica oleracea L. var. italica Plenck.) Crop. Horticulturae, 8(11), 1032. https://doi.org/10.3390/horticulturae8111032








