Abstract
Ficus carica L. is a common fig that is an incredibly nutritional fruit, well-known for its medicinal and economic values. This study aims to establish an efficient protocol for the mass propagation of fig plantlets (Ficus carica L.) for the cultivar ‘Violette de Solliès’. Surface-sterilized shoot-tip explants were cultured on Murashige and Skoog (MS) medium supplemented with different concentrations of cytokinins (6-benzylaminopurine, BAP; thidiazuron, TDZ; kinetin, Kn; and zeatin, Zea). Induced shoots were rooted on Woody Plant Medium (WPM) with different concentrations of auxins (naphthalene-acetic acid, NAA; indole-3-acetic acid, IAA; and indole-3-butyric acid, IBA). Rooted explants were acclimatized in eight different soil substrates prior to cultivation in a commercial plot. The propagated plantlets were analyzed for genetic stability and clonal fidelity using RAPD and SCoT molecular markers, whereas scanning electron microscopy (SEM) was performed to observe the stomata morphology of post-acclimatized plants. MS media supplemented with 5.0 mg/L BAP was the optimal treatment for multiple shoot induction (15.20 ± 1.03 shoots), whereas the highest percentage of rooting (93.33%) was achieved in WPM supplemented with 3.0 mg/L IBA. Plantlets were successfully acclimatized in biochar soil with a survival rate of 100%. RAPD and SCoT analysis showed no polymorphism occurrences across six subculture cycles, whereas observations via SEM indicated normal stomata structures on the leaves of acclimatized plantlets. This study documents an efficient micropropagation protocol for Ficus carica cv. ‘Violette de Solliès’ for the production of uniformed and true-to-type plant stocks suitable for commercial propagation.
1. Introduction
Ficus is a genus from the family of Moraceae, consisting of over 800 species including Ficus carica L., the common edible fig []. The Ficus carica plant is characterized by its smooth grey bark, lobed leaves, and fibrous roots. The skin color of the fig fruits is different between cultivars, varying from yellowish green to copper, bronze, or dark purple []. This deciduous fruit tree is native to the Southwest regions of Asia and the eastern Mediterranean [], which was later introduced and cultivated abundantly in Turkey, Egypt, Greece, Iran, and Morocco []. As a top producer and exporter of figs, Turkey contributed up to 27% of fig production worldwide in 2018, producing a total of 306,000 tons of figs and generating approximately $286 million in exports for both dried and fresh figs []. Ficus carica is not native to the tropical regions of the world, but has recently been introduced as a superfruit in Malaysia, where most of its cultivation is still at a small scale of 50–1500 plants per farm []. The price of cuttings range between RM 30 and RM 100 (USD 6 and USD 22) per plant, depending on the cultivar type, and is in high demand for commercial farm establishments and home gardening []. Fig cultivation in Malaysia is still new and is currently expanding under the Indonesia, Malaysia, and Thailand Growth Triangle (IMT-GT) agriculture program, which comprises an area of approximately 10 hectares in the northern region of the country [].
The fig fruit has been a common food source and folk remedy since ancient times due to its high nutritional and medicinal values. The fruit consists of mainly fiber, potassium, calcium, and iron, which is higher than in a banana, orange, or apple []. The fig plant also produces a variety of secondary metabolites that are known for their potential health effects, namely, being antibacterial, antioxidant, antidiabetic, anti-inflammatory, antipyretic, and anticancer []. Studies have also shown that both ethanolic or methanolic extracts of the fruits, leaves, and stem barks possess antidiabetic activity [,,]. Previous studies also reported that diabetic complications such as cholesterolemia and hypoglycemia were successfully controlled in streptozotocin-induced diabetic rats via feeding with extracts of fruits and leaves of fig, indicating the strong inhibitory activity against α-amylase and α-glucosidase enzymes in mammals [,,,]. Furthermore, the efficiency of ethyl acetate extract of fig leaves exhibited significant antidiabetic activity, successfully stimulating the production of insulin from regenerated pancreatic beta cells that resulted in the reduction in blood glucose levels of diabetic rats []. Similarly, the blood glucose level of alloxan-induced diabetic rats was reduced after feeding on Ficus carica leaf extract [,].
To date, there are over 750 varieties or more of figs in the world, mostly thriving in the Western Asia and Mediterranean region, as well as in countries with a mild climate []. One of the highly sought-after fig cultivars is ‘Violette de Solliès’, which is also deemed as a premium fig, from the Solliès region of France, as it is recognized for being the sweetest fig available. ‘Violette de Solliès’ or VDS produces relatively large, dark, purple-colored fruits with a soft, pinkish-red pulp and are sweet with a slight hint of cherry. However, its availability in the market is still relatively low as there are shortages in planting materials due to the production of nonviable seeds and the inefficiency of conventional propagation methods. Fig fruits are not dependent on pollination to fully set and develop; thus, the probability of obtaining viable seeds is very low, limiting the propagation via seed. The other alternative is propagation through cuttings, which is commonly marred by poor rooting efficiency, limiting the chance of survival to only 20 to 30% []. Additionally, cuttings are more sensitive to ecological changes such as drastic shift in temperature and moisture levels, further limiting their survival rate [].
Plant tissue culture is an alternative propagation method whereby plant cells, tissues, or organs are cultured under aseptic controlled conditions. It is an efficient and widely adopted tool for the propagation of many fruit crops of economic importance, such as apples, bananas, pineapples, and citrus, in commercial nursery production []. This alternative method can be manipulated to continuously and consistently produce good-quality pathogen-free planting materials at a competitive price for large-scale production. Previous studies on the tissue culture and micropropagation of figs have reported the use of single shoot-tip explants [], nodal explants [], leaves [,,], apical meristems, and axillary buds [,,], which have successfully propagated figs from different cultivars. Up to now, there have been no tissue culture studies and micropropagation methods being reported for the cultivar of ‘Violette de Solliès’, albeit on its significance as one of the sweetest fig cultivars in the world. The present study aims to establish an efficient micropropagation protocol for the cultivar of ‘Violette de Solliès’ for the large-scale production of high-quality planting materials suitable for commercial field establishments. This study also looks to confirm if plantlets generated are true-to-type via molecular markers used to identify polymorphism, and microscopy analysis is used to observe the morphological stomata structures between the plantlets and the mother plant.
2. Materials and Methods
2.1. Establishment of In Vitro Cultures
The mother plants were obtained from the Malaysian Superfruits Valley, Perlis at an age of approximately one year, after being grown from air layering, with a height of approximately 1.5 m above the ground level; they were maintained at the Herbarium Unit of Universiti Sains Malaysia. Young shoot tips of ‘Violette de Solliès’ (VDS), approximately 3 cm in length, were collected fresh from healthy growing mother plants prior to the surface sterilization step. Shoot-tip explants were washed with 2% Sunlight® dishwashing liquid, which was followed by rinsing under running tap water for ten minutes. The explants were then surface sterilized with 70% ethanol for 5 min, which was followed by a gentle agitation with 25% Clorox® containing 2 to 3 drops of Tween 20 for 5 min. Explants were then rinsed with sterile distilled water eight times before being dried on sterile filter paper prior to culture.
2.2. Induction of Multiple Shoots
Sterile shoot-tip explants were cultured on MS [] basal medium with 2% (w/v) sucrose solidified with 0.8% (w/v) plant agar (Duchefa, Haarlem, The Netherlands) and supplemented with different plant growth regulators, namely, 6-benzylaminopurine (BAP), kinetin (Kn), thidiazuron (TDZ), and zeatin (Zea) (Duchefa Biochemie, Haarlem, The Netherlands), at various concentrations of 1.0, 2.0, 3.0, 4.0, 5.0, and 6.0 mg/L. Shoot-tip explants cultured in MS medium without the supplementation of cytokinin were used as the control treatment. Cultures were maintained under white LED (light-emitting diode) light (Philips TLD 36 W/865–6500 K, 3070 lm) with a 16/8 h photoperiod at a temperature of 25 ± 2 °C and relative humidity of 50 ± 10%. A total of three experimental replications were carried out with each replication consisting of five explant replicates. Parameters such as the percentage of shoot induction, average number, and length of adventitious shoots per explant were measured after 6 weeks of culture.
2.3. Root Induction from Shoot Explants
Shoots generated in vitro (from the treatment of 6.0 mg/L BAP) at the length of approximately 2 cm were excised and cultured in Woody Plant Medium (WPM) [] supplemented with auxins, namely, indole-3-acetic acid (IAA), indole-3-butric acid (IBA), and naphthalene-acetic acid (NAA), at the concentrations of 0.0, 1.0, 2.0, 3.0, and 4.0 mg/L. Parameters such as the percentage of root induction, and average root number and length per explant were measured after 6 weeks of culture. The experiment was repeated thrice with five replicates for each replication.
2.4. Acclimatization on Different Soil Substrates
Shoot explants with well-grown roots (approximately 9 weeks in WPM supplemented with 3.0 mg/L IBA) were gently removed from the culture media, with the remaining agar rinsed off with tap water. Plantlets were transferred to germination pots (5 cm in diameter) filled with different soil substrates, namely, Jiffy pellet (Jiffy International, Stange, Norway), peat, perlite, vermiculite, a peat and perlite mixture (2:1), a peat and vermiculite mixture (2:1), and biochar soil (Serbajadi, Malaysia), with a garden soil mixture (black compost, coco peat, and red soil mixture in the ratio of 3:1:1) as the control. The plantlets were irrigated with tap water once every 3 days and kept in closed transparent containers under 25 ± 2 °C with a total of 15 replicates per soil substrate evaluated. The cover of the container was gradually removed within 30 days to decrease the humidity inside the container. The survival rate of the plantlets (%) was recorded after 6 weeks. Successfully acclimatized plantlets were transferred to polybags (10 cm × 12 cm) containing the garden soil mixture and maintained under a shaded roof with normal cultivation practices for approximately 8 weeks prior to planting in the commercial plot.
2.5. RAPD- and SCoT-PCR Molecular Analysis
The genomic DNA was isolated from leaf samples of three randomly selected regenerated tissue-cultured plantlets from the first up to the sixth subculture cycle, following the protocols of the Wizard® Genomic DNA purification kit (Promega, Madison, WI, USA). DNA quality and concentrations were measured via the NanoDrop Spectrophotometer (ASP-2680, ACTGene Inc., Piscataway, NJ, USA) and DNA samples were diluted to a fixed concentration of 20 ng/µL prior to storage at −20 °C for the subsequent steps. The reaction mixtures were prepared by mixing 50 ng of genomic DNA (20 ng/µL), 1.0 µM of primer (Operon Technologies Inc., Alameda, CA, USA), and 10 µL of 2X GoTaq® Green Master Mix (Promega, Madison, WI, USA), with a total reaction volume of 20 µL. The PCR reaction was performed in the T100TM Thermal Cycler (Bio-Rad, Hercules, CA, USA) and initiated with a 4 min denaturation step at 94 °C; subsequently, another 40 cycles of the 1 min denaturation step at 94 °C were set up before the annealing step. An annealing step was performed at approximately 5 °C below the derived melting temperature of the primers for 1 min, followed by 1 min of the extension step at 72 °C. Lastly, a final extension of 10 min at 72 °C was carried out at the end of the PCR reaction. The amplified DNA samples were loaded into a 1.0% agarose gel with 1x TBE (Tris-Borate-EDTA) and RedSafeTM (iNtRON Biotechnology, Seongnam-si, South Korea) as the buffer and nucleic acid staining reagent, respectively. The voltage for agarose gel electrophoresis was set at 70 volts for 1.5 h, and the results were visualized and analyzed using a gel documentation system, UVIdoc HD5 Gel Imaging System (UVITec Limited, Cambridge, UK). A total of 60 RAPD primers from OPC, OPK, and OPU series and 36 SCoT primers [] were tested. Seven primers which produced clear and reproducible bands from each marker were selected and used in the assessment of genetic stability. Both RAPD and SCoT reactions were repeated for a minimum of three replications to ensure reproducible bands and consistency of the band patterns.
2.6. Scanning Electron Microscopy (SEM) Analysis
Leaf samples were collected from the in vitro cultures, acclimatized plantlets (20 and 60 days old), and field-grown plants (control), and excised into pieces 1 cm2 in size. The leaf samples were fixed in McDowell–Trump fixative and prepared in a 0.1 M phosphate buffer for 2 to 24 h at 4 °C, which was followed by rinsing thrice under the same buffer for 10 min. Next, the leaves were kept in 1% osmium tetroxide (OsO4) that was prepared in a 0.1 M phosphate buffer for 2 h at room temperature. All samples were then washed twice with distilled water for 10 min. Following this, the leaves were dehydrated with ethanol at increasing concentrations (50, 75, 95, and 99.5%). The dehydrated samples were then immersed in 2 mL of hexamethyldisilazane (HMDS) for 10 min and air-dried in a desiccator. The dried leaves were adhered to a SEM sample stub and sputtered with gold prior to viewing in the SUPRA® 55VP field-emission scanning electron micrograph (FE-SEM, Carl Zeiss, Jena, Germany).
2.7. Statistical Analysis
The experimental design applied in this study was a completely randomized design with three replications containing five explants per replicate. The data were analyzed using a one-way analysis of variance (ANOVA) via SPSS statistical software version 26, followed by a post hoc test, Duncan’s multiple range test, where p < 0.05.
3. Results and Discussion
3.1. Multiple Shoot Induction
In the current study, MS media supplemented with 2.0, 4.0, and 6.0 mg/L BAP, 5.0 and 6.0 mg/L Kn, and all concentrations of TDZ and Zea (except Zea at 2.0 mg/L) generated the maximum shoot induction with a percentage of 100%, which was higher than the control at only 40% (Table 1). It was also evident that the number of adventitious shoots induced was directly proportional to the concentration of plant growth regulators used. In the present study, BAP was identified as the most potent plant growth regulator in the induction of multiple shoots, followed by Zea, TDZ, and Kn. The treatment of 6.0 mg/L BAP generated the highest number of adventitious shoots with the value of 16.40 ± 2.11 shoots per explant (Figure 1a), indicating that BAP alone is efficient enough for rapid shoot proliferation in this cultivar. However, this value is not significant for the treatment of 5.0 mg/L BAP, which also resulted in a higher number of induced shoots (15.20 ± 1.03). On the other hand, treatments with Zea were found to stimulate shoot elongation. The optimal value in the increment of shoot length was observed in explants treated with 1.0 mg/L Zea, recording an average shoot length of 1.53 ± 0.21 cm, regardless of this value not being significant for the results of Zea treatments of 2.0, 3.0, and 4.0 mg/L. In this study, short and abundant shoots with small defined leaves were observed in treatments with BAP. It was also evident that relatively low concentrations of Kn (0 to 4.0 mg/L) induced very few to no multiple shoots, as illustrated in Figure 1b. Nevertheless, the production of multiple shoots increased as Kn concentration increased. Abnormal shoot formation (short and fasciated shoots) was noticed in all the TDZ treatments, particularly for high TDZ concentrations (5.0 and 6.0 mg/L), as shown in Figure 1c.

Table 1.
The effects of different concentrations of plant growth regulators on shoot regeneration after 6 weeks of culture.
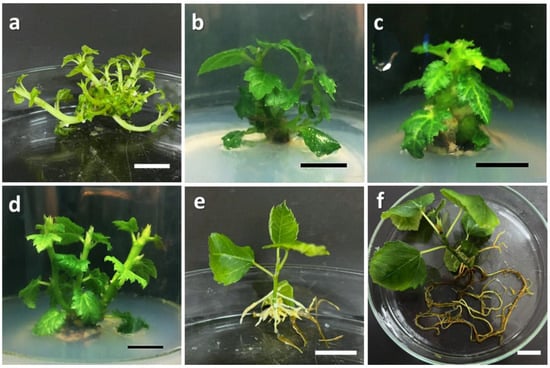
Figure 1.
Shoot explant of Ficus carica cv. ‘Violette de Solliès’ at different stages of micropropagation: (a) multiple shoot induction in MS medium supplemented with 6.0 mg/L BAP; (b) multiple shoot induction in MS medium supplemented with 5.0 mg/L Kn; (c) multiple shoot induction in MS medium supplemented with 5.0 mg/L TDZ; (d) multiple shoot induction in MS medium supplemented with 6.0 mg/L Zea; (e) root induction in WPM supplemented with 3.0 mg/L IBA; (f) root morphology prior to acclimatization in biochar soil. Scale bars = 1 cm.
Cytokinin is known for its promotive effects on shoot multiplication, as it functions as a signaling molecule involved directly in the cell division of meristematic cells [,]. It controls the size of the shoot meristem, the number of leaf primordia, shoot growth, leaf production, and the development of axillary buds, as well as mediating apical dominance []. However, the response of tissues towards exogenous plant growth regulators can be due to the physiological status of the plant and/or the interaction between the endogenous regulators, resulting in a different response depending on the growth regulator supplemented. In this study, BAP was found to be the most effective cytokinin in stimulating multiple shoot production of ‘Violette de Solliès’. The effectiveness of BAP in inducting multiple shoots is credited to its ability in reducing apical dominance in explants, leading to lateral shoot production []. In addition, BAP was also reported to be more stable in tissue culture when compared to other cytokinins due to its slower rate of metabolism. The results in this study corroborate the previous finding, whereby the maximum number of shoots (4.43 shoots per explant) were produced by explants of the “Bursa Siyahi” fig cultivar when supplemented with 1.0 mg/L BAP []. Additionally, the treatment with BAP at 2.5 mg/L was effective in inducing multiple shoots (19.7 shoots) on fig cultivars “Sultany”, “Aboudi”, and “White Adcy” []. Furthermore, the comparison of the effectiveness of different cytokinins on the shoot multiplication of the fig cultivar “Salti Kodari” found BAP to be the most effective cytokinin in comparison to Kn and Zea []. The highest number of multiple shoots (4.15 ± 0.43) was induced in the treatment of 0.8 mg/L BAP, which was higher than other cytokinin treatments of zeatin and thidiazuron for the Golden Orphan cultivar []. On the other hand, the treatments of BAP resulted in a higher number of multiple shoots in comparison to Zea induced in shoot-tip explants for the cultivar of Japanese BTM 6 []. In the current study, the 5.0 mg/L BAP was selected as the optimal concentration of BAP for shoot multiplication based on the high response in shoot number and shoot length for micropropagation purposes.
The results in the current study highlighted the potential of Zea in the elongation of shoots for the explants of the fig cultivar ‘Violette de Solliès’. A previous study via transcriptome analysis mentioned that the expression of genes linked to zeatin biosynthesis in sugarcane is upregulated and is associated with the elongation of internodes []. Shoot clusters of lingonberry (Vaccinium vitis-idaea L.) were also successfully elongated when supplemented with Zea []. Besides, Zea at 0.5 mg/L effectively promoted shoot elongation in Magnolia sp. var. “Vulcan”, as explants recorded a maximum average shoot length of 4.9 ± 0.2 cm []. These studies further indicated the efficiency of BAP in shoot proliferation and the potential elongation effects of Zeatin for the species of Ficus carica. On the contrary, treatments with Kn and TDZ were observed to be less effective in promoting multiple shoots and shoot elongation for the current cultivar of ‘Violette de Solliès’. Another study reported similar results with the treatment of Kn producing only 1.415 shoots per explant in the micropropagation of figs native to Kurdistan, Iraq []. Kn (0.8 and 1.2 mg/L) had no effect on the shoot multiplication for the fig cultivar of “Salti Kodari”, whereby the highest number of shoots induced was only at an average of 1.2 shoots []. However, a different observation was reported for the fig cultivar “Roxo de Valinhos’”, whereby Kn at 2.0 mg/L was found to induce the highest number of shoots (4.25 shoots per explant) with no signs of vitrification []. The difference in the response to supplementation of exogenous plant growth regulators could be potentially influenced by the genotype of the plant itself. This was previously proven as figs from the cultivar “Deanna” achieved a shoot multiplication rate of 60% when treated with 3.5 mg/L BAP in combination with 0.5 mg/L gibberellic acid, whereas no shoots were induced by explants from fig cultivars “Poona Fig” and “Brown Turkey” []. In the current study, TDZ was discovered to exhibit abnormal effects on ‘Violette de Solliès’ explants as the shoots induced were found to be fasciated, preventing healthy explant growth. TDZ-induced deformed shoots with hyperhydration is possible, especially when TDZ is supplemented at excessive concentrations or under a prolonged culture duration [,]. The regulation of endogenous hormone levels could be the reason for abnormal shoot formation []. Additionally, the hyperhydricity and fasciation of shoots might be due to the latent consequences of TDZ on endogenous auxin such as IAA, and certain cytokinins, particularly BAP []. In the micropropagation of Chinese skullcap (Scutellaria baicalensis Georgi), the concentration of BAP in the MS medium decreased drastically when supplemented with TDZ at high concentrations (5.0 mg/L), which led to the increase in the IAA:BAP ratio []. High TDZ concentrations, therefore, caused an imbalance in the auxin/cytokinin ratio, which is essential for effective shoot proliferation. In the current experiment, it was evident that Kn and TDZ were not favorable for the induction of multiple shoots for the cultivar of ‘Violette de Solliès’.
3.2. Root Induction via the Supplementation of Auxins
Of the three auxins evaluated in this study, IBA was observed to be the most effective in inducing roots for the in vitro explants of Ficus carica cv. ‘Violette de Solliès’. With reference to Table 2, the highest rooting percentage and number of induced roots was observed in the treatment of 3.0 mg/L IBA with the values of 93.33% and 6.80 ± 1.43, respectively. Even though the treatment of 4.0 mg/L IAA resulted in the highest number of induced roots (7.27 ± 1.56 roots per shoot), this value is not significant in comparison to the treatment of 3.0 mg/L IBA. Similarly, all IBA treatments (1.0 mg/L up to 4.0 mg/L) also resulted in the stimulation of root elongation, as the values recorded were not significantly different from the values of the highest elongation observed in the treatment of 2.0 mg/L IAA. There were no morphological abnormalities or differences observed in the roots induced for all auxin treatments. The roots induced exhibited two different root types; aerial roots that were thin and hairy formed above the culture medium, and thick, brittle roots were embedded in the culture medium (Figure 1e). Figure 1f demonstrates the root morphology after 9 weeks of culture prior to acclimatization.

Table 2.
The effects of IAA, IBA, and NAA on root regeneration after 6 weeks of culture.
The efficiency of IBA in stimulating in vitro root formation was also reported for other fig cultivars. The addition of IBA increased the root number and length of “Sultany”, “Gizy”, and “Aboudi” fig cultivars []. The percentage of rooting in the cultivars “Brown Turkey” and “Brunswick”, as well as “Zidi”, were improved with IBA supplementation [,]. The treatment of 0.4 mg/L IBA resulted in the optimal percentage of rooting (83.33%) in the micropropagation of the yellow fig of the Golden Orphan cultivar []. Exogenous IBA was the main regulator of adventitious root induction, particularly in plant species with a very low content of endogenous auxin, such as Arabidopsis thaliana []. The supplementation of exogenous IBA is crucial as it leads to the conversion of IBA into IAA, which is necessary for the formation and stimulation of adventitious shoots []. The ability of IBA to promote roots could be credited to its relatively high stability []. IBA was also found to be more stable than IAA following exposure to high pressure and temperature during autoclave sterilization []. Moreover, IBA also acts as a slow-releasing hormone, enabling its effects to remain longer in the culture medium and resulting in the constant promotion of roots []. IBA can also be converted to free IAA at a continuous rate in the media, further enabling the constant presence of IAA rather than direct supplementation of IAA for plant regeneration []. These studies further indicated the efficiency of IBA in root induction, as observed in the current study for Ficus carica.
In the current study, auxin IAA was also observed to support the induction of in vitro roots for ‘Violette de Solliès’ as the supplementation at 2.0 and 4.0 mg/L recorded the highest average root number and length. In the rooting of fig from the cultivar “Conadria”, the addition of IAA (0.1 mg/L) alone or in combination with IBA (1.0 mg/L) generated the maximum rooting percentage (100%). IAA was also found to be efficient at promoting root formation for other plant species. Orthosiphon stamineus explants produced the highest number of roots (11.9 ± 4.1 roots per explants) with the treatment of 3.0 mg/L IAA []. Auxin IAA can stimulate cell differentiation in the section responsible for rooting in cultures prior to root formation, further indicating its essential role in the aspect of the development of primary roots and lateral roots, as well as for the root apical meristem (RAM) and root vascular differentiation [,]. Moreover, the accumulation of IAA in the phloem was discovered to also induce the formation of adventitious roots in plants [].
The treatment of auxin NAA, however, did not result in much significance in terms of root induction for the in vitro cultures of ‘Violette de Solliès’, as the roots formed rapidly grew in the treatments of IBA and IAA. NAA is another type of auxin commonly used in the rooting of different plant species. Rooting studies on Ficus carica of the Golden Orphan cultivar revealed that treatments of NAA in the rooting media resulted in callus formation, indirectly inhibiting the formation of roots for micropropagation []. NAA was observed to be inferior to IBA with regard to the rooting of fig, as the highest rooting percentage recorded in the treatment with NAA was 76.66%, whereas with IBA, the highest rate recorded was 91.11% []. NAA was also less efficient in rooting when compared to IBA for Ficus carica explants, recording a rooting rate as low as 8.34% [].
Auxin NAA was also found to be unsuitable for the regeneration of certain plant tissues due to its low absorbance efficiency that could potentially reduce the rooting efficiency in culture []. However, NAA was effective at inducing roots from in vitro clones of Ficus carica []. Additionally, NAA was found to have successfully induced the highest average number of roots (4.87) and length (4.07 cm) for Ficus carica cv. Sultani explants []. This further indicated that the reactivity towards different types of auxins is species or cultivar specific, thus cementing the importance of an optimal rooting evaluation to ensure the production of healthy plantlets with higher survival rates.
3.3. Acclimatization and Effect of Different Soil Substrates on the Survival of Plantlets
In the present study, Jiffy pellets and biochar soil were the most effective soil substrates for the acclimatization of ‘Violette de Solliès’ plantlets, with both substrates recording the highest plantlet survival rate of 100% (Table 3). Vermiculite was the least suitable soil substrate as plantlets recorded the lowest survival rate (66.66%), which was even lower than the control (garden soil mix) at 73.33%. There were no morphological abnormalities observed in acclimatized plantlets (Figure 2a,b). Healthy acclimatized Ficus carica cv. ‘Violette de Solliès’ plantlets were then potted and supplemented with fertilizers of equal NPK ratios for eight weeks before being planted in the greenhouse. The images for the acclimatized plantlets, acclimatized plants grown in the commercial greenhouse, and fruits produced from the tissue-cultured plants after a year of being established in the greenhouse are shown in Figure 2.

Table 3.
Percentage of survival rates of rooted plantlets of Ficus carica cv. ‘Violette de Solliès’ after 6 weeks of acclimatization in garden soil mixture (control) and different types of soil substrates.
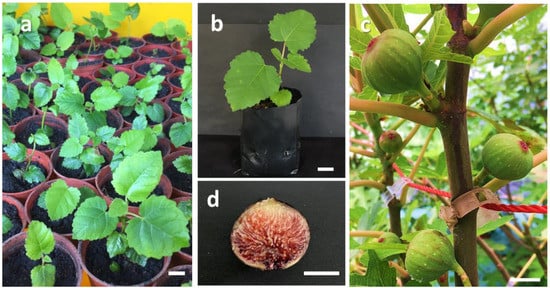
Figure 2.
Ex vitro plantlets and fruits of Ficus carica cv. ‘Violette de Solliès’: (a) acclimatized plants in biochar soil after 2 weeks (scale bar = 1 cm); (b) acclimatized plants in polybags after 8 weeks (scale bar = 5 cm); (c) acclimatized plants grown in commercial greenhouse (scale bar = 2 cm); (d) fruit produced from tissue-cultured plants 10 months after acclimatization (scale bar = 1 cm).
The efficiency of the Jiffy pellets in aiding the acclimatization step in this study was attributed to its high porosity that enables rapid root penetration, allowing better nutrient transport and uptake from the soil []. Jiffy pellets are made of Sphagnum peat moss or coco fibers with high water retention capacities, which provides an ongoing supply of moisture essential for continuous plant growth and survival. The results from this study are in agreement with the findings from Mengesha et al. (2013), where pineapple plantlets were reported to show improved growth when acclimatized in Jiffy pellets, reporting a survival rate as high as 98%. Similarly, Jiffy pellets were also successfully utilized for the acclimatization of hazelnut plantlets, as plantlets had developed well-grown roots and recorded the highest survival rate of 97% []. As for the cultivar of ‘Violette de Solliès’, plantlets also recorded the maximum survival (100%) when acclimatized in biochar soil. Biochar soil is made up of carbon-rich organic materials, such as tree bark, wood chips, rice husk, and other agricultural wastes through pyrolysis, and its effectiveness is credited to its ability for improving soil structure as well as increasing pH and water retention capacities, leading to optimal plant growth [,]. Biochar soil was also used to improve the growth of maize [] and mung bean [], leading to healthy crop production. Both soil substrates were found most suitable for the acclimatization of ‘Violette de Solliès’ plantlets, but biochar soil is preferred due to its lower cost and easy market availability.
Based on current studies, it was also observed that ‘Violette de Solliès’ plantlets generated from the optimum tissue culture protocol were vigorous and fast-growing compared to conventionally grown figs. Fig fruits were observed to successfully produce from tissue-cultured plants approximately 10 months after acclimatization, which is approximately 2 to 3 times faster than the conventionally grown figs in the same cultivation plot. The tissue culture protocol generated from this study produced healthy growing plantlets that are disease-free and had a complete whole-plant system prior to acclimatization, which consequently, promotes vigorous plant growth when transferred to the commercial cultivation plot.
3.4. Polymorphism Analysis via RAPD and SCoT Molecular Markers
The seven RAPD primers generated a total of 47 bands ranging from 140 bp (OPU_20) to 1250 bp (OPU_12). In this study, there were no polymorphic bands detected via RAPD analysis through the comparison of banding patterns between the mother plant and micropropagated plant DNA samples, ranging from the first up to the sixth subculture cycle (Table 4). As for the SCoT analysis, 51 bands ranging from 200 bp (SCoT_22) to 2100 bp (SCoT_29) were obtained with an average of 7.29 bands per primer. Similar to the observation through RAPD analysis, no polymorphism was observed from the first up to the sixth subculture cycle of micropropagated plants (Table 4). The banding results revealed a total of 98 scorable bands from the RAPD and SCoT primers, where all the bands were monomorphic amongst the micropropagated plants and the mother plant (control) (Figure 3).

Table 4.
The nucleotide sequences of primers used for RAPD and SCoT molecular marker analysis.
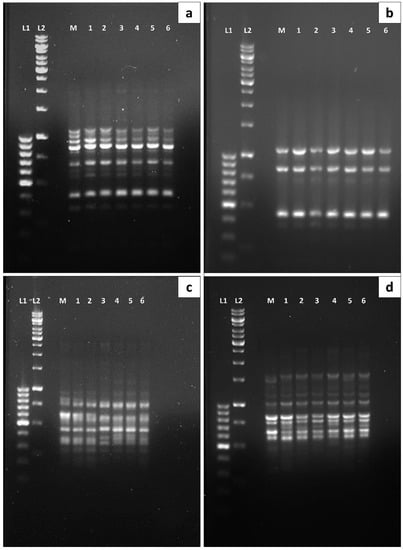
Figure 3.
RAPD and SCoT marker profiles of mother plant and micropropagated plants (first to sixth subculture cycles) of Ficus carica cv. ‘Violette de Solliès’: (a) OPU_U20; (b) OPC_20; (c) SCoT_21; (d) SCoT_24. Lane L1 and L2 represent the 100 bp and 1 kb ladder DNA, respectively; lane M represents mother plant and lanes 1–6 represent the micropropagated plants from first to sixth subculture (n = 3).
Somaclonal variation is a genetic variation associated with plant tissue culture caused by changes either at the genotypic or phenotypic level. The occurrence of mutations in plants generated via tissue culture can be triggered by numerous stress factors such as wounding and the exposure of explants to sterilizing agents, media components (plant growth regulators and salts), and in vitro culture environments (different lighting conditions, humidity, and temperature) []. Others stress factors such as the rapid multiplication of cultures [] or frequent subculturing may lead to mutations and somaclonal variations. Therefore, it is recommended that the developed micropropagation protocol should ensure the number of subcultures required is kept to a minimum []. For instance, a frequency increase in somaclonal variation from 1.3% to 3.8% between the 5th and 11th subculture of the Brazilian banana “Nanicão” could negatively impact the quality of plantlets produced [].
In this study, no polymorphism was observed from the first up to the sixth subculture cycle of micropropagated plants, as confirmed via RAPD and SCoT molecular marker analysis. A previous study utilized inter-simple sequence repeat (ISSR) markers for the identification of the genetic alteration of the fig cultivar “Black fig” exposed to different plant growth regulators, growth retardants, photoperiods, and cold acclimatization []. In addition, homogeneity between the fig mother plant and the micropropagated plantlets was successfully evaluated by El-Dessoky et al. (2016), using RAPD and ISSR markers with 45 scorable bands produced from six RAPD primers showing 6.6% polymorphism []. As opposed to the findings by El-Dessoky et al. (2016), the current study reported 100% monomorphic bands based on RAPD analysis of the total 60 RAPD primers screened, compared to only 16 RAPD primers screened by El-Dessoky et al. (2016). RAPD markers could only amplify a small region of the plant genome; therefore, the use of other molecular markers to compliment and validate the other regions of the DNA is most preferred, particularly to ensure higher reliability in the detection of polymorphism []. The current study includes the application of SCoT molecular markers which target the short, conserved regions of plant genes surrounding the ATG start codon [] to further confirm the genetic stability of micropropagated Ficus carica plantlets. SCoT is a reliable gene-targeted marker, where the molecular profiles generated by SCoT primers can be correlated to the plant’s functional genes, and thus, correspond to its respective traits [,,]. The application of SCoT molecular markers for genetic variation detection in micropropagated plantlets have been previously utilized for the species of Pittosporum eriocarpum Royle [], Ansellia africana [], and Bauhinia racemosa Lam []. However, up to now, the application of SCoT molecular markers in polymorphism detection for the Ficus genus has not been reported. The current study is the first to assess the suitability of SCoT molecular markers for polymorphism detection of micropropagated Ficus carica plantlets. In the present study, the generated SCoT molecular profile has further confirmed the clonal uniformity of the micropropagated fig plantlets up to the sixth number of the subculture cycle. This has further validated the reliability of the developed micropropagation protocol in this study for the mass production of plantlets which maintain the novel genetic make-up of the parent plant, making it suitable for commercial planting.
3.5. Observation of Stomatal Structures via SEM
In the present study, SEM was utilized to assist with further structural observations on the stomata of the in vitro regenerated plantlets. From the analysis, it was evident that there were no significant differences observed in the stomatal structures from the abaxial epidermis of fig leaves obtained from plantlets after 20 and 60 days of acclimatization in comparison to the field-grown plants. However, a crystal-like formation was prominent in the stomata of in vitro explants, as illustrated in Figure 4a. These crystalline structures completely disappeared post-acclimatization (Figure 4b,c) and were also absent in field-grown plants (Figure 4d). This observation further validates that the exposure to in vitro culture conditions and subculture durations did not result in alterations in the morphological characteristics of the fig plantlets grown post-acclimatization, regardless of being previously subjected to culture conditions. The stomata of the leaf samples that were 20 and 60 days old and from acclimatized plantlets when observed under SEM were deemed to be normal with kidney-shaped guard cells and external periclinal walls that were well formed without signs of deformations; this was the same as for field-grown plants. Normal stomata formation is crucial to prevent disruption of the gaseous exchange channels that facilitate the plant respiration, photosynthesis, and transpiration required for optimal plant growth. Besides, the stomata also play a key role in inducing plant responses and their ability to adapt, as it is highly sensitive to environmental changes []. However, structural abnormalities in the stomata may be environmentally induced, resulting in the breakdown of stomatal functions which could negatively impact plant growth and development, leading to eventual death. Based on our observations and screening of the leaf samples under SEM in this study (Figure 4), the in vitro and acclimatized plant stomatal complexes were of the actinocytic type. The actinocytic type of stomata was observed in all plants of the Ficus genus and is found only on the abaxial surface (hypostomatic) of the leaf [].
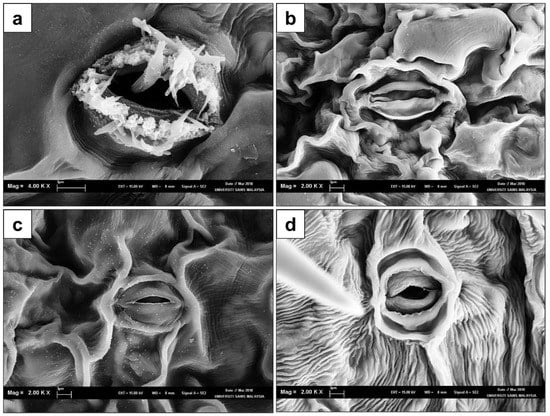
Figure 4.
Scanning electron microscopy images of leaves of Ficus carica cv. ‘Violette de Solliès’ pre- and post-acclimatization: (a) in vitro leaf; (b) 20 days after acclimatization; (c) 40 days after acclimatization; (d) field-grown plant.
On the other hand, crystalline-like structures were also observed on the leaves of in vitro explants in this study. It was hypothesized that these structures are cell-mediated calcium oxalate (CaOx) crystals. Calcium is known to be abundantly found in the environment and is a vital element for plant growth and development, mainly as a structural component of the cell wall that functions as an osmoticum, as well as playing a pivotal role in the signaling of developmental pathways []. However, calcium at high cytosolic free levels with concentrations exceeding 10−7 M could result in detrimental effects on the calcium signaling pathway, energy metabolism, and microskeletal dynamics []. Therefore, the uptake, distribution, and storage of calcium within the cell wall and the vacuole needs to be carefully regulated in plants []. Calcium oxalate crystals are often formed in plant cells (bundle sheath cells), tissues (epidermal, ground, and vascular tissues), or organs (leaves, roots, and stems) when the levels of calcium within the cell is present in excess (Webb, 1999). The leaves of plants from the Ficus genus were reported to accumulate minerals in the form of amorphous calcium carbonate cystoliths, calcium oxalates, and silica phytoliths []. This report potentially indicates that the observed crystalline-like structures of the in vitro leaf samples were associated with the deposition of these minerals.
4. Conclusions
The current study is the first to report on an efficient and effective micropropagation protocol of Ficus carica cv. ‘Violette de Solliès’. The MS medium supplemented with 5.0 mg/L BAP was identified to be the optimal media for multiple shoot formation, whereas the WPM supplemented with 3.0 mg/L IBA induced the highest percentage of rooting. On the other hand, biochar soil was found to be the optimal soil substrate for fig acclimatization. The polymorphism analysis via RAPD and SCoT molecular techniques indicated no genetic variation occurrence between plantlets up to the sixth subculture cycle, whereas the normal structural development of stomata was observed in acclimatized plantlets as confirmed by SEM analysis, further indicating that the micropropagated plants are genetically uniform and true-to-type.
Author Contributions
Conceptualization, B.L.C., W.T.L. and S.S.; methodology, W.T.L. and L.V.T.; validation, S.P.K., L.V.T. and D.S.; resources, B.L.C.; data curation, S.P.K.; writing—original W.T.L. and L.V.T.; writing—B.L.C., D.S. and S.P.K.; visualization, B.L.C.; supervision, B.L.C.; project administration, B.L.C., W.T.L. and L.V.T.; funding acquisition, B.L.C. All authors have read and agreed to the published version of the manuscript.
Funding
The Economic Planning Unit of the Prime Minister’s Department, Malaysia (Grant code: 304/PBIOLOGI/6501099/U120).
Acknowledgments
The authors would like to acknowledge the Economic Planning Unit of the Prime Minister’s Department, Malaysia for funding the project (Grant code: 304/PBIOLOGI/6501099/U120). They also thank Universiti Sains Malaysia, FigDirect Sdn. Bhd, and the Agricultural Crop Trust (Malaysia) for their support of this project.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Dhage, S.; Pawar, B.; Chimote, V.; Jadhav, S.; Kale, A.A. In vitro callus induction and plantlet regeneration in Fig (Ficus carica L.). J. Cell Tissue Res. 2012, 12, 3395–3400. [Google Scholar]
- Danial, G.H.; Ibrahim, D.A.; Brkat, S.A.; Khalil, B.M. Multiple shoots production from shoot tips of Fig tree (Ficus carica L.) and callus induction from leaf segments. Int. J. Pure Appl. Sci. Technol. 2014, 9, 117–124. [Google Scholar]
- Chawla, A.; Kaur, R.; Sharma, A.K. Ficus carica Linn.: A review on its pharmacognostic, phytochemical and pharmacological aspects. Int. J. Pharmacol. Res. 2012, 2012, 215–232. [Google Scholar]
- Flaishman, M.A.; Rodov, V.; Stover, E. The Fig: Botany, Horticulture, and Breeding. In Horticultural Reviews; Janick, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 113–196. ISBN 978-0-470-38014-7. [Google Scholar]
- Daily Sabah with AA Turkey Top Producer, Exporter of Hazelnuts, Cherries, Figs, Apricots. Dly Sabah. 19 August 2019. Available online: https://www.dailysabah.com/business/2019/08/19/turkey-top-producer-exporter-of-hazelnuts-cherries-figs-apricots (accessed on 1 November 2022).
- Moniruzzaman, M.; Yaakob, Z.; Khatun, R.; Awang, N. Mealybug (Pseudococcidae) Infestation and organic control in Fig (Ficus carica) orchards of Malaysia. Biol. Environ. Proc. R. Ir. Acad. 2017, 117, 25–32. [Google Scholar] [CrossRef]
- Kamarubahrin, A.; Haris, A.; Abdul Shukor, S.; Mohd Daud, S.N.; Ahmad, N.; Zulkefli, Z.; Muhamed, N.A.; Makmun, A.H. An overview Malaysia as a hub of planting prophetic fruits. Malays. J. Sustain. Agric. 2019, 3, 13–19. [Google Scholar] [CrossRef]
- Crisosto, H.; Ferguson, L.; Bremer, V.; Stover, E.; Colelli, G. Fig (Ficus carica L.). In Postharvest Biology and Technology of Tropical and Subtropical Fruits; Elsevier: Amsterdam, The Netherlands, 2011; pp. 134–160. ISBN 978-1-84569-735-8. [Google Scholar]
- Stalin, C.; Dineshkumar, P.; Nithiyananthan, K. Evaluation of antidiabetic activity of methanolic leaf extract of Ficus carica in alloxan - induced diabetic rats. Asian J. Pharm. Clin. Res. 2012, 5, 85–87. [Google Scholar]
- Ahmad, M.Z.; Ali, M.; Mir, S.R. Anti-diabetic activity of Ficus carica L. stem barks and isolation of two new flavonol esters from the plant by using spectroscopical techniques. Asian J. Biomed. Pharm. Sci. 2013, 3, 7. [Google Scholar]
- Mopuri, R.; Islam, M.S. Antidiabetic and anti-obesity activity of Ficus carica: In vitro experimental studies. Diabetes Metab. 2016, 42, 300. [Google Scholar] [CrossRef]
- Canal, J.; Torres, M.; Romero, A.; Perez, C. Chloroform extract obtained from a decoction of Ficus carica leaves improves the cholesterolaemic status of rats with streptozotocin-induced diabetes. Acta Physiol. Hung. 2000, 87, 71–76. [Google Scholar] [CrossRef]
- Rashidi, A.A.; Noureddini, M. Hypoglycemic effect of the aromatic water of leaves of Ficus carica in normal and streptozotocin induced diabetic rats. Pharmacologyonline 2011, 1, 372–379. [Google Scholar]
- Sheikh, Y.; Maibam, B.C.; Biswas, D.; Laisharm, S.; Deb, L.; Talukdar, N.C.; Borah, J.C. Anti-diabetic potential of selected ethno-medicinal plants of North East India. J. Ethnopharmacol. 2015, 171, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Stephen Irudayaraj, S.; Christudas, S.; Antony, S.; Duraipandiyan, V.; Naif Abdullah, A.-D.; Ignacimuthu, S. Protective effects of Ficus carica leaves on glucose and lipids levels, carbohydrate metabolism enzymes and β-cells in type 2 diabetic rats. Pharm. Biol. 2017, 55, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- El-Shobaki, F.; El-Bahay, A.; Esmail, R.; El-Megeid, A.A.; Esmail, N. Effect of figs fruit (Ficus carica L.) and its leaves on hyperglycemia in alloxan diabetic rats. World J. Dairy Food Sci. 2010, 5, 47–57. [Google Scholar]
- Guvenc, M.; Tuzcu, M.; Yilmaz, O. Analysis of fatty acid and some lipophilic vitamins found in the fruits of the Ficus carica variety picked from the Adiyaman district. Res. J. Biol. Sci. 2009, 4, 320–323. [Google Scholar]
- Kumar, V.; Radha, A.; Kumar Chitta, S. In vitro plant regeneration of Fig (Ficus carica L. cv. Gular) using apical buds from mature trees. Plant Cell Rep. 1998, 17, 717–720. [Google Scholar] [CrossRef]
- Dolgun, O.; Tekintas, F. Production of Fig (Ficus carica L.) nursery plants by stem layering method. Agric. Conspec. Sci. ACS 2008, 73, 157–160. [Google Scholar]
- Kajla, S.; Poonia, A.K.; Kharb, P.; Duhan, J.S. Role of biotechnology for commercial production of fruit crops. In Biotechnology: Prospects and Applications; Salar, R.K., Gahlawat, S.K., Siwach, P., Duhan, J.S., Eds.; Springer India: New Delhi, India, 2013; pp. 27–37. ISBN 978-81-322-1682-7. [Google Scholar]
- Parab, A.; Chew, B.; Lit chow, Y.; Subramaniam, S. Organogenesis on apical buds in common Fig (Ficus carica) var. Black Jack. Electron. J. Biotechnol. 2021, 54, 69–76. [Google Scholar] [CrossRef]
- Sriskanda, D.; Liew, Y.X.; Khor, S.P.; Merican, F.; Subramaniam, S.; Chew, B.L. An efficient micropropagation protocol for Ficus carica cv. Golden Orphan suitable for mass propagation. Biocatal. Agric. Biotechnol. 2021, 38, 102225. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, M.Y.; Yun, P.Y.; Chandrasekhar, T.; Lee, H.-Y.; Song, P.-S. Production of multiple shoots and plant regeneration from leaf segments of Fig tree (Ficus carica L.). J. Plant Biol. 2007, 50, 440–446. [Google Scholar] [CrossRef]
- Soliman, H.I.; Gabr, M.; Abdallah, N.A. Efficient transformation and regeneration of Fig (Ficus carica L.) via somatic embryogenesis. GM Crops 2010, 1, 40–51. [Google Scholar] [CrossRef]
- Al-Khaybari, A. Propagation of Fig Trees (cv. Brown Turkey) by Tissue Culture Technique; King Saud University: Riyadh, Saudi Arabia, 2008. [Google Scholar]
- Ling, W.; Liew, F.; Lim, W.; Subramaniam, S.; Chew, B.L. Shoot induction from axillary shoot tip explants of Fig (Ficus carica) cv. Japanese BTM 6. Trop. Life Sci. Res. 2018, 29, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.B.; McCown, B.H. Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot-tip culture. Comb. Proc. Int. Plant Propagators’ Soc. (USA) 1980, 30, 421–427. [Google Scholar]
- Collard, B.C.Y.; Mackill, D.J. Start Codon Targeted (SCoT) polymorphism: A simple, novel dna marker technique for generating gene-targeted markers in plants. Plant Mol. Biol. Rep. 2009, 27, 86–93. [Google Scholar] [CrossRef]
- Košir, P.; Škof, S.; Luthar, Z. Direct shoot regeneration from nodes of Phalaenopsis orchids. Acta Agric. Slov. 2004, 83, 233–242. [Google Scholar]
- Zanello, C.A.; Duarte, W.N.; Gomes, D.M.; Cardoso, J.C. Micropropagation from inflorescence nodal segments of Phalaenopsis and acclimatization of plantlets using different substrates. Horticulturae 2022, 8, 340. [Google Scholar] [CrossRef]
- Le Bris, M. Hormones in Growth and Development. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; p. B9780128096338051000. ISBN 978-0-12-809633-8. [Google Scholar]
- Seyyedyousefi, S.R.; Kaviani, B.; Dehkaei, N.P. The effect of different concentrations of NAA and BAP on micropropagation of Alstroemeria. Eur. J. Exp. Biol. 2013, 3, 133–136. [Google Scholar]
- Demiralay, A.; Yalçin-Mendi, Y.; Aka-Kaçar, Y.; Çetiner, S. In vitro Propagation of Ficus carica L. var. Bursa Siyahi through meristem culture. Acta Hortic. 1998, 480, 165–168. [Google Scholar] [CrossRef]
- Mustafa, N.; Taha, R. Influence of plant growth regulators and subculturing on in vitro multiplication of some Fig (Ficus carica) Cultivars. J. Appl. Sci. Res. 2012, 8, 4038–4044. [Google Scholar]
- Shatnawi, M.; Shibli, R.A.; Shahrour, W.G.; Al-Qudah, T.S.; Taleb, A.-Z. Micropropagation and conservation of Fig (Ficus carica L.). J. Adv. Agric. 2019, 10, 1669–1679. [Google Scholar] [CrossRef]
- Qiu, L.; Chen, R.; Fan, Y.; Huang, X.; Luo, H.; Xiong, F.; Liu, J.; Zhang, R.; Lei, J.; Zhou, H.; et al. Integrated MRNA and small RNA sequencing reveals microRNA regulatory network associated with internode elongation in sugarcane (Saccharum officinarum L.). BMC Genom. 2019, 20, 817. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.C. Micropropagation of Lingonberry: Influence of genotype, explant orientation, and overcoming TDZ-Induced inhibition of shoot elongation using zeatin. HortScience 2005, 40, 185–188. [Google Scholar] [CrossRef]
- Kim, T.-D.; Kim, J.-A.; Lee, N.-N.; Choi, C.-H. Multiple shoot induction and plant regeneration from axillary buds of Magnolia ‘Vulcan. ’ J. Plant Biotechnol. 2020, 47, 40–45. [Google Scholar] [CrossRef][Green Version]
- Fráguas, C.B.; Pasqual, M.; Dutra, L.F.; Cazetta, J.O. Micropropagation of Fig (Ficus carica L.) “Roxo de Valinhos” plants. In Vitro Cell. Dev. Biol. 2013, 40, 471–474. [Google Scholar] [CrossRef]
- Dhage, S.S.; Chimote, V.P.; Pawar, B.D.; Kale, A.A.; Pawar, S.V.; Jadhav, A.S. Development of an efficient in vitro regeneration protocol in Fig (Ficus carica L.). J. Appl. Hortic. 2015, 17, 160–164. [Google Scholar] [CrossRef]
- Sivanesan, I.; Song, J.Y.; Hwang, S.J.; Jeong, B.R. Micropropagation of Cotoneaster Wilsonii Nakai—A rare endemic ornamental plant. Plant Cell Tissue Organ Cult. PCTOC 2011, 105, 55–63. [Google Scholar] [CrossRef]
- Feng, J.-C.; Yu, X.M.; Shang, X.L.; Li, J.D.; Wu, Y.X. Factors influencing efficiency of shoot regeneration in Ziziphus jujuba Mill. ‘Huizao.’ Plant Cell Tissue Organ Cult. PCTOC 2010, 101, 111–117. [Google Scholar] [CrossRef]
- Siwach, P.; Gill, A.R. Enhanced shoot multiplication in Ficus religiosa L. in the presence of adenine sulphate, glutamine and phloroglucinol. Physiol. Mol. Biol. Plants 2011, 17, 271–280. [Google Scholar] [CrossRef]
- Zhang, C.G.; Li, W.; Mao, Y.F.; Zhao, D.L.; Dong, W.; Guo, G.Q. Endogenous hormonal levels in Scutellaria baicalensis calli induced by thidiazuron. Russ. J. Plant Physiol. 2005, 52, 345–351. [Google Scholar] [CrossRef]
- Hepaksoy, S.; Aksoy, U. Propagation of Ficus carica L. clones by in vitro culture. Biol. Plant. 2006, 50, 433–436. [Google Scholar] [CrossRef]
- Metwali, E.; Soliman, H.; Al-Zahrani, H.; Howladar, S.; Fuller, M. Influence of different concentrations of salt stress on in vitro multiplication of some Fig (Ficus carica L.) cultivars. Life Sci. J. 2014, 11. [Google Scholar]
- Bayoudh, C.; Labidi, R.; Majdoub, A.; Mars, M. In vitro propagation of caprifig and female fig varieties (Ficus carica L.) from shoot-tips. J. Agric. Sci. Technol. 2015, 17, 1597–1608. [Google Scholar]
- Fattorini, L.; Veloccia, A.; Della Rovere, F.; D’Angeli, S.; Falasca, G.; Altamura, M.M. Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. BMC Plant Biol. 2017, 17, 121. [Google Scholar] [CrossRef]
- Epstein, E.; Ludwig-Muller, J. Indole-3-butyric acid in plants: Occurrence, synthesis, metabolism and transport. Physiol. Plant. 1993, 88, 382–389. [Google Scholar] [CrossRef]
- Nissen, S.; Sutter, E. Stability of IAA and IBA in nutrient medium to several tissue culture procedures. HortScience 1990, 25, 800–802. [Google Scholar] [CrossRef]
- Epstein, E.; Sagee, O.; Zelcer, A. Uptake and metabolism of indole-3-butyric acid and indole-3-acetic acid by Petunia cell suspension culture. Plant Growth Regul. 1993, 13, 31–40. [Google Scholar] [CrossRef]
- Pick, A.; Ling, A.; Kok, K.; Hussein, S.; Ling, S.; Ong, S.L. Effects of plant growth regulators on adventitious roots induction from different explants of Orthosiphon stamineus. Am. -Eurasian J. Sustain. Agric. 2009, 3, 493–501. [Google Scholar]
- Ponce, G.; Barlow, P.W.; Feldman, L.J.; Cassab, G.I. Auxin and ethylene interactions control mitotic activity of the quiescent centre, root cap size, and pattern of cap cell differentiation in maize. Plant Cell Environ. 2005, 28, 719–732. [Google Scholar] [CrossRef]
- Aloni, R.; Aloni, E.; Langhans, M.; Ullrich, C.I. Role of cytokinin and auxin in shaping root architecture: Regulating vascular differentiation, lateral root initiation, root apical dominance and root gravitropism. Ann. Bot. 2006, 97, 883–893. [Google Scholar] [CrossRef]
- Wakle, A.G.; Shinde, S.J.; Jadhav, S.D.; Gharate, P.S. Influence of different levels of IAA and NAA growth regulators on air layers in Fig (Ficus carica L.) cv. Dinkar. J. Pharm. Innov. 2021, 10, 119–124. [Google Scholar]
- Hausman, J.F. Changes in peroxidase activity, auxin level and ethylene production during root formation by poplar shoots raised in vitro. Plant Growth Regul. 1993, 13, 263–268. [Google Scholar] [CrossRef]
- Mengesha, A.; Ayenew, B.; Tadesse, T. Acclimatization of in vitro propagated pineapple (Ananas comosus (L.), var. Smooth cayenne) plantlets to ex vitro condition in Ethiopia. Am. J. Plant Sci. 2013, 4, 317–323. [Google Scholar] [CrossRef]
- Nas, M.; Read, P. Improved rooting and acclimatization of micropropagated hazelnut shoots. HortScience Publ. Am. Soc. Hortic. Sci. 2004, 39, 1688–1690. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using poultry litter biochars as soil amendments. Soil Res. 2008, 46, 437. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Uzoma, K.C.; Inoue, M.; Andry, H.; Fujimaki, H.; Zahoor, A.; Nishihara, E. Effect of cow manure biochar on maize productivity under sandy soil condition: Cow manure biochar agronomic effects in sandy soil. Soil Use Manag. 2011, 27, 205–212. [Google Scholar] [CrossRef]
- Saxena, J.; Rawat, J.; Kumar, R. Conversion of biomass waste into biochar and the effect on mung bean crop production. CLEAN—Soil Air Water 2017, 45, 1501020. [Google Scholar] [CrossRef]
- Krishna, H.; Alizadeh, M.; Singh, D.; Singh, U.; Chauhan, N.; Eftekhari, M.; Sadh, R.K. Somaclonal variations and their applications in horticultural crops improvement. 3 Biotech 2016, 6, 54. [Google Scholar] [CrossRef]
- Israeli, Y.; Lahav, E.; Reuveni, O. In Vitro Culture of Bananas. In Bananas and Plantains; Gowen, S., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1995; pp. 147–178. ISBN 978-94-011-0737-2. [Google Scholar]
- Bhatia, S.; Sharma, K. Chapter 13—Technical Glitches in Micropropagation. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia, S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 393–404. ISBN 978-0-12-802221-4. [Google Scholar]
- Rodrigues, P.; Tulmann Neto, A.; Cassieri Neto, P.; Mendes, B. Influence of the Number of Subcultures on Somaclonal Variation in Micropropagated Nanicã O (Musa spp., Aaa Group). Acta Hortic. 1997, 490, 469–474. [Google Scholar]
- Fatah, N.; Abouarab, M.; Amin, A.; Diab, A. Short term preservation for fig (Ficus carica cv. Black Fig) by different osmotic stabilizers. Egypt. J. Genet. Cytol. 2016, 45, 47–61. [Google Scholar] [CrossRef]
- Dessoky, E.S.; Attia, A.O.; Mohamed, E.A.M. An efficient protocol for in vitro propagation of fig (Ficus carica sp.) and evaluation of genetic fidelity using RAPD and ISSR markers. J. Appl. Biol. Biotechnol. 2016, 4, 057–063. [Google Scholar]
- Khor, S.P.; Yeow, L.C.; Poobathy, R.; Zakaria, R.; Chew, B.L.; Subramaniam, S. Droplet-vitrification of Aranda Broga Blue orchid: Role of ascorbic acid on the antioxidant system and genetic fidelity assessments via RAPD and SCoT markers. Biotechnol. Rep. 2020, 26, e00448. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High Frequency regeneration protocol for Dendrobium nobile: A model tissue culture approach for propagation of medicinally important orchid species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Kumar, V.; Van Staden, J. Assessment of genetic stability amongst micropropagated Ansellia africana, a vulnerable medicinal orchid species of Africa using SCoT markers. S. Afr. J. Bot. 2017, 108, 294–302. [Google Scholar] [CrossRef]
- Thakur, J.; Dwivedi, M.D.; Sourabh, P.; Uniyal, P.L.; Pandey, A.K. Genetic Homogeneity revealed using SCoT, ISSR and RAPD markers in micropropagated Pittosporum eriocarpum Royle-An endemic and endangered medicinal plant. PLoS ONE 2016, 11, e0159050. [Google Scholar] [CrossRef]
- Sharma, U.; Rai, M.K.; Shekhawat, N.S.; Kataria, V. Genetic homogeneity revealed in micropropagated Bauhinia racemosa Lam. using gene targeted markers CBDP and SCoT. Physiol. Mol. Biol. Plants 2019, 25, 581–588. [Google Scholar] [CrossRef]
- Zhu, J.; Yu, Q.; Xu, C.; Li, J.; Qin, G. Rapid estimation of stomatal density and stomatal area of plant leaves based on object-oriented classification and its ecological trade-off strategy analysis. Forests 2018, 9, 616. [Google Scholar] [CrossRef]
- Shakir, H.M.; Baji, S.H. Anatomical study of some characters in certain species of genus Ficus L. growing in Iraq. J. Biol. Agric. Healthc. 2016, 6, 98–105. [Google Scholar]
- Webb, M.A. Cell-mediated crystallization of calcium oxalate in plants. Plant Cell 1999, 11, 751–761. [Google Scholar] [CrossRef][Green Version]
- Kretsinger, R.H. Evolution of the Informational Role of Calcium in Eukaryotes. In Calcium-Binding Proteins and Calcium Function; North Holland Publishing: New York, NY, USA, 1977; pp. 63–72. [Google Scholar]
- Leigh, R.A.; Tomos, A.D.; Mansfield, T.A.; Davies, W.J.; Leigh, R.A. Ion distribution in cereal leaves: Pathways and mechanisms. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1993, 341, 75–86. [Google Scholar]
- Pierantoni, M.; Tenne, R.; Rephael, B.; Brumfeld, V.; van Casteren, A.; Kupczik, K.; Oron, D.; Addadi, L.; Weiner, S. Mineral deposits in Ficus leaves: Morphologies and locations in relation to function. Plant Physiol. 2018, 176, 1751–1763. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).