Consideration of Maillard Reaction-Based Time–Temperature Indicator (TTI) to Visualize Shelf Life of Cold-Stored Strawberries
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Low-Temperature Storage
2.2. Preparation of Maillard Reaction Solution for TTI
2.3. Color Variation of the Reaction Solution
2.4. Activation Energy
2.5. Statistical Analysis
3. Results and Discussion
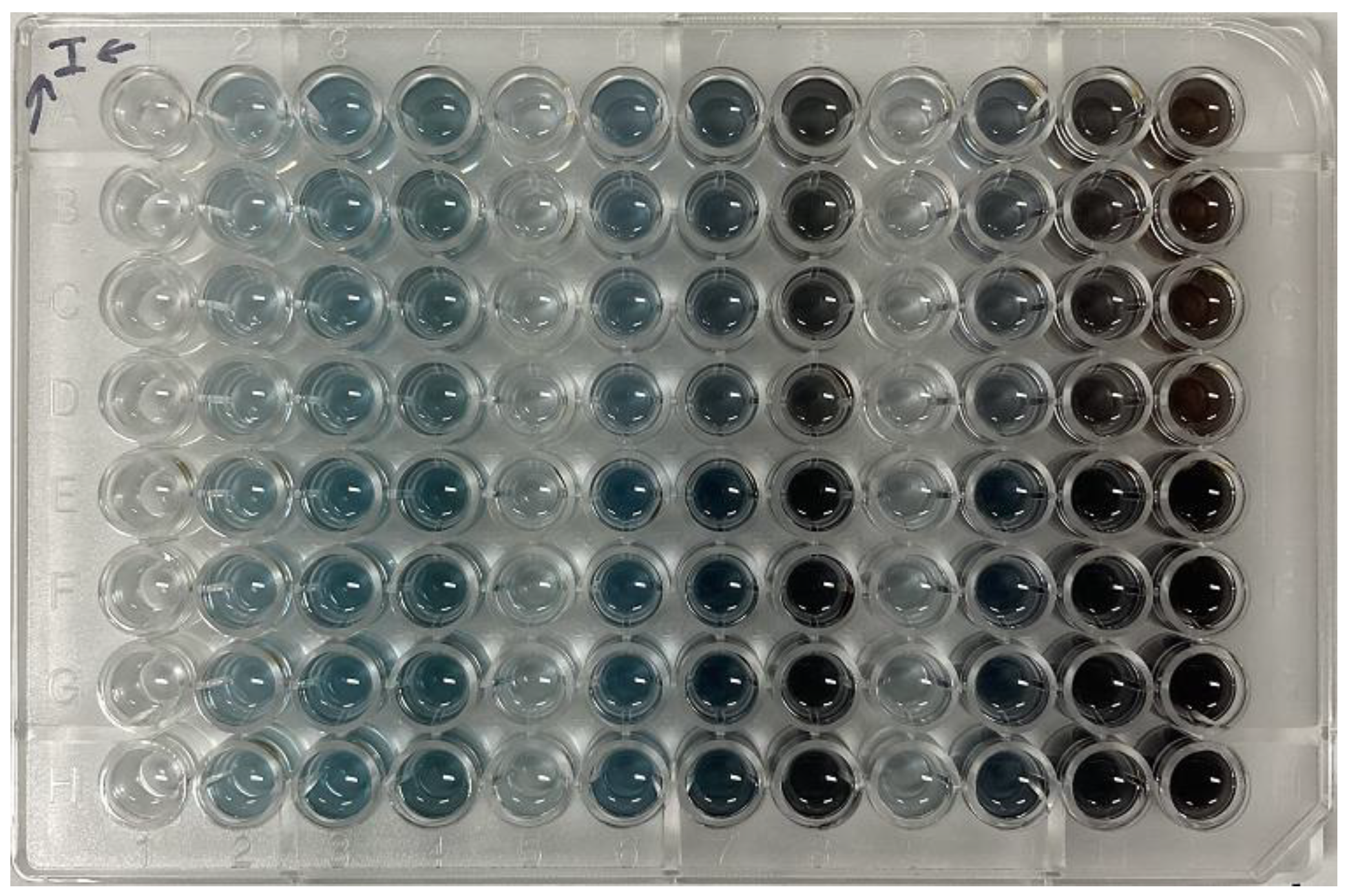
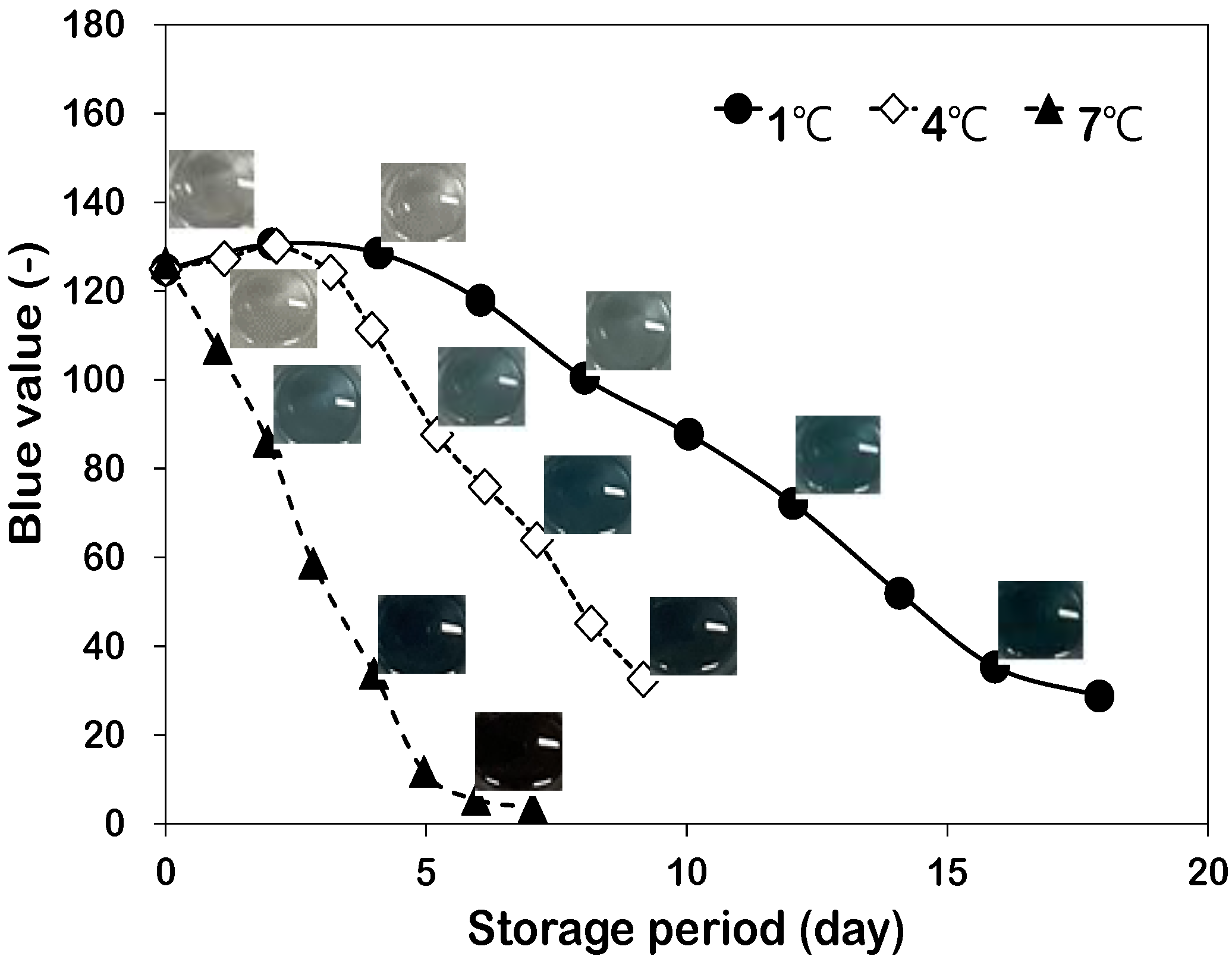
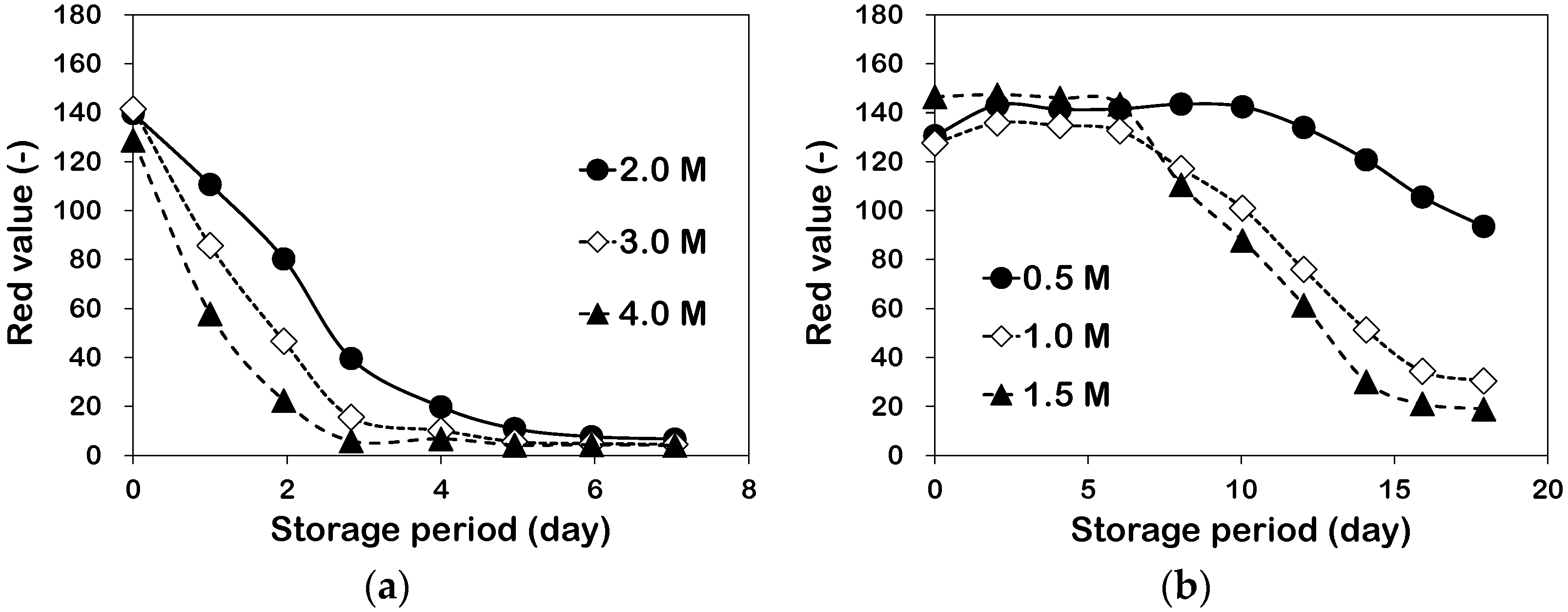
3.1. Color Variation of Maillard Reaction Solution
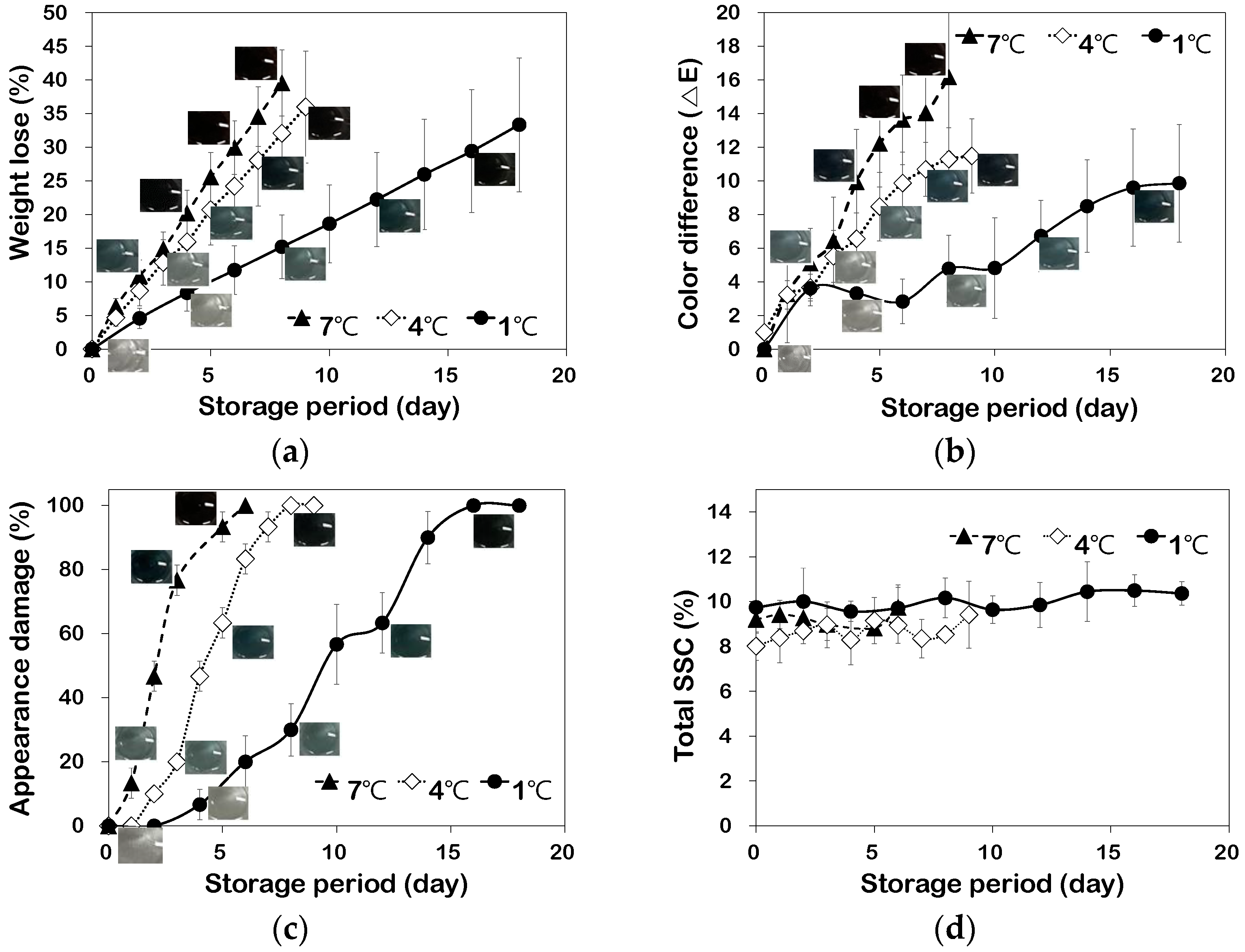
3.2. Relationship between Quality Characteristics and TTI
3.3. Activation Energy,
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, J.; Xu, Y. Prediction of fruit quality based on the RGB values of time–temperature indicator. J. Food Sci. 2021, 86, 932–941. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, G.; Xiao, X.; Liu, Y.; Zheng, X. Application of microbial TTIs as smart label for food quality: Response mechanism, application and research trends. Trends Food Sci. Technol. 2016, 51, 12–23. [Google Scholar] [CrossRef]
- Hao, C.; Hao, X.; David, J.M.C.; Long, C.; Aiquan, J.; Yaoqi, T.; Ming, M.; Zhengyu, J. Recent advances in intelligent food packaging materials: Principles, preparation and applications. Food Chem. 2022, 375, 131738. [Google Scholar] [CrossRef]
- Tsujihashi, M.; Tanaka, S.; Koayama, K.; Koseki, S. Application of Time–Temperature Indicator/Integrator Based on the Maillard Reaction to Frozen Food Distribution. Food Bioprocess Technol. 2022, 15, 1343–1358. [Google Scholar] [CrossRef]
- Pandian, A.T.; Chaturvedi, S.; Chakraborty, S. Applications of enzymatic time–temperature indicator (TTI) devices in quality monitoring and shelf-life estimation of food products during storage. Food Meas. 2021, 15, 1523–1540. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Yang, M.; Zhang, Y.; Xiang, K.; Tang, R. Review of Time Temperature Indicators as Quality Monitors in Food Packaging. Packag. Technol. Sci. 2015, 28, 839–867. [Google Scholar] [CrossRef]
- Maillard, L.C. Action des acides amines sur les sucres: Formation des melanoidines par voie methodique. CR Acad. Sci. 1912, 154, 66–68. [Google Scholar]
- Martins, S.I.F.S.; Jongen, W.M.F.; Boekel, M.A.J.S. A Review of Maillard Reaction in Food and Implications to Kinetic Modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Lee, J.H.; Harada, R.; Kawamura, S.; Koseki, S. Development of a Novel Time-Temperature Integrator/Indicator (TTI) Based on the Maillard Reaction for Visual Thermal Monitoring of the Cooking Process. Food Bioprocess Technol. 2018, 11, 185–193. [Google Scholar] [CrossRef]
- Lee, J.H.; Morita, A.; Kuroshima, M.; Kawamura, S.; Koseki, S. Development of a novel time–temperature integrator/indicator (TTI) based on the maillard reaction for visual monitoring of melon (Cucumis melo L.) maturity during cultivation. Food Meas. 2018, 12, 2899–2904. [Google Scholar] [CrossRef]
- Sakai, K.; Lee, J.H.; Kocharunchitt, C.; Ross, T.; Jeoson, I.; Koayama, K.; Koseki, S. Development of a Maillard Reaction–Based Time-Temperature Integrator/indicator (TTI) for Visual Monitoring of Chilled Beef During Long-term Storage and Distribution. Food Bioprocess Technol. 2020, 13, 2094–2103. [Google Scholar] [CrossRef]
- Lee, J.H.; Kawamura, S.; Koseki, S. Quantitative Evaluation of Changes in Color during Maillard Reaction for Development of Novel Time-Temperature Integrators/Indicators. Food Sci. Technol. Res. 2018, 24, 283–287. [Google Scholar] [CrossRef]
- Lee, K.H.; Bong, S.J.; Yoon, Y.J.; Lee, B.; Kwak, I.H.; Min, K.H.; Kim, H.K. Quality Changes of Strawberry by Slow-released ClO2 Gas Gel-pack during Storage. Korean J. Food Nutr. 2017, 30, 591–598. [Google Scholar] [CrossRef]
- Feliziani, E.; Romanazzi, G. Postharvest Decay of Strawberry Fruit: Etiology, Epidemiology, and Disease Management. J. Berry Res. 2016, 6, 47–63. [Google Scholar] [CrossRef]
- Darwish, O.S.; Ali, M.R.; Khojah, E.; Samra, B.N.; Ramadan, K.M.A.; El-Mogy, M.M. Pre-Harvest Application of Salicylic Acid, Abscisic Acid, and Methyl Jasmonate Conserve Bioactive Compounds of Strawberry Fruits during Refrigerated Storage. Horticulturae 2021, 7, 568. [Google Scholar] [CrossRef]
- Mehmet, S.A.; Cengiz, C. Individual and combined effects of ultrasound, ozone and chlorine dioxide on strawberry storage life. LWT-Food Sci. Technol. 2014, 57, 244–251. [Google Scholar] [CrossRef]
- Mehmet, S.A.; Riza, T.; Mehmet, B.B.; Cengiz, C. An innovative technique for extending shelf life of strawberry: Ultrasound. LWT-Food Sci. Technol. 2013, 52, 93–101. [Google Scholar] [CrossRef]
- Al-Dairi, M.; Pathare, P.B.; Al-Yahyai, R. Effect of Postharvest Transport and Storage on Color and Firmness Quality of Tomato. Horticulturae 2021, 7, 163. [Google Scholar] [CrossRef]
- Laroque, D.; Inisan, C.; Berger, C.; Vouland, E.; Dufossé, L.; Guérard, F. Kinetic Study on the Maillard Reaction. Consideration of Sugar Reactivity. Food Chem. 2008, 111, 1032–1042. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, M.J.; Shim, S.D.; Lee, S.J. Application of fuzzy reasoning to prediction of beef sirloin quality using time temperature integrators (TTIs). Food Control 2012, 24, 148–153. [Google Scholar] [CrossRef]
- Kim, K.H.; Kim, E.J.; Lee, S.J. New Enzymatic Time-Temperature Integrator (TTI) That Uses Laccase. J. Food Eng. 2012, 113, 118–123. [Google Scholar] [CrossRef]
- Wanihsuksombat, C.; Hongtrakul, V.; Suppakul, P. Development and Characterization of a Prototype of a Lactic Acid-based Time-Temperature Indicator for Monitoring Food Product Quality. J. Food Eng. 2010, 100, 427–434. [Google Scholar] [CrossRef]
- Miura, M.; Gomyo, T. Formation of Blue Pigment in the Earlier Stage of Browning a System Composed of D-Xylose and Glycine. J. Agric. Chem. Soc. Jpn. 1982, 56, 417–425. [Google Scholar]
- Andreas, A.P.; Konstantoula, A.D.; Panagiotis, D.; Kyriakos, A.R. Effect of Gaseous Ozone and Heat Treatment on Quality and Shelf Life of Fresh Strawberries during Cold Storage. Int. J. Fruit Sci. 2021, 21, 218–231. [Google Scholar] [CrossRef]
- Shehata, S.A.; Abdeldaym, E.A.; Ali, M.R.; Mohamed, R.M.; Bob, R.I.; Abdelgawad, K.F. Effect of Some Citrus Essential Oils on Post-Harvest Shelf Life and Physicochemical Quality of Strawberries during Cold Storage. Agronomy 2020, 10, 1466. [Google Scholar] [CrossRef]
- Nadim, Z.; Ahmadi, E.; Sarikhani, H.; Chayjan, R.A. Effect of methylcellulose-based edible coating on strawberry fruit’s quality maintenance during storage. J. Food Process. Preserv. 2014, 39, 80–90. [Google Scholar] [CrossRef]
- Kahramanoğlu, İ.; Panfilova, O.; Kesimci, T.G.; Bozhüyük, A.U.; Gürbüz, R.; Alptekin, H. Control of Postharvest Gray Mold at Strawberry Fruits Caused by Botrytis cinerea and Improving Fruit Storability through Origanum onites L. and Ziziphora clinopodioides L. Volatile Essential Oils. Agronomy 2022, 12, 389. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, Y.; Wang, D.; Zhang, L.; Sun, J.; Sun, L.; Zhang, B. Effect of alginate coating combined with yeast antagonist on strawberry (Fragaria × ananassa) preservation quality. Postharvest Biol. Technol. 2009, 53, 84–90. [Google Scholar] [CrossRef]
- Majeed, A.; Muhammad, Z.; Majid, A.; Shah, A.; Hussain, M. Impact of low doses of gamma irradiation on shelf life and chemical quality of strawberry (Fragariaxananassa) cv. ‘Corona’. J. Anim. Plant Sci. 2014, 24, 1531–1536. [Google Scholar]
- Yoon, Y.S.; Kim, J.K.; Lee, K.C.; Eun, J.B.; Park, J.H. Effects of electron-beam irradiation on postharvest strawberry quality. J. Food Process. Preserv. 2020, 44, 14665. [Google Scholar] [CrossRef]
- Taoukis, P.S. Modelling the use of time-temperature indicators in distribution and stock rotation. In Food Process Modelling; Tijkskens, L.M.M., Hertog, M.L.A.T.M., Nicolai, B.M., Eds.; Woodhead Publishing: Amsterdam, The Netherlands, 2001; pp. 402–431. [Google Scholar]
| Concentration (M) | ln A | |||||
|---|---|---|---|---|---|---|
| D-xylose | Glycine | |||||
| Color variation | Red | 3.0 | 0.5 | 181 | 77.7 | 0.99 |
| 4.0 | 170 | 73.3 | 0.99 | |||
| 2.0 | 1.0 | 135 | 58.1 | 0.99 | ||
| 3.0 | 132 | 57.3 | 0.99 | |||
| 4.0 | 152 | 65.8 | 0.98 | |||
| 2.0 | 1.5 | 126 | 54.4 | 0.99 | ||
| 3.0 | 114 | 49.3 | 0.99 | |||
| 4.0 | 143 | 62.2 | 0.99 | |||
| Green | 4.0 | 0.5 | 178 | 76.4 | 0.99 | |
| 2.0 | 1.0 | 173 | 74.1 | 0.98 | ||
| 3.0 | 138 | 59.6 | 0.99 | |||
| 4.0 | 119 | 51.2 | 0.99 | |||
| 2.0 | 1.5 | 141 | 60.8 | 0.99 | ||
| 3.0 | 108 | 46.7 | 0.99 | |||
| 4.0 | 147 | 63.9 | 0.99 | |||
| Blue | 4.0 | 0.5 | 192 | 82.3 | 0.99 | |
| 2.0 | 1.0 | 187 | 79.9 | 0.96 | ||
| 3.0 | 134 | 57.5 | 0.99 | |||
| 4.0 | 113 | 48.8 | 0.99 | |||
| 2.0 | 1.5 | 143 | 61.7 | 0.99 | ||
| 3.0 | 108 | 46.6 | 0.99 | |||
| 4.0 | 140 | 60.9 | 0.99 | |||
| Weight loss | 105 | 46.9 | 0.90 | |||
| Color difference | 145 | 63.1 | 0.97 | |||
| Appearance damage | 103 | 47.5 | 0.93 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, B.-H.; Lee, J.-H. Consideration of Maillard Reaction-Based Time–Temperature Indicator (TTI) to Visualize Shelf Life of Cold-Stored Strawberries. Horticulturae 2022, 8, 979. https://doi.org/10.3390/horticulturae8100979
Cho B-H, Lee J-H. Consideration of Maillard Reaction-Based Time–Temperature Indicator (TTI) to Visualize Shelf Life of Cold-Stored Strawberries. Horticulturae. 2022; 8(10):979. https://doi.org/10.3390/horticulturae8100979
Chicago/Turabian StyleCho, Byeong-Hyo, and Jung-Hyun Lee. 2022. "Consideration of Maillard Reaction-Based Time–Temperature Indicator (TTI) to Visualize Shelf Life of Cold-Stored Strawberries" Horticulturae 8, no. 10: 979. https://doi.org/10.3390/horticulturae8100979
APA StyleCho, B.-H., & Lee, J.-H. (2022). Consideration of Maillard Reaction-Based Time–Temperature Indicator (TTI) to Visualize Shelf Life of Cold-Stored Strawberries. Horticulturae, 8(10), 979. https://doi.org/10.3390/horticulturae8100979






