Abstract
The relationship between the phenotypic and chemical composition of local Andrographis paniculata was evaluated in this study. Five seed collections were sourced from different regions of Thailand, namely Kamphaeng Saen (KS), Udon Thani (UT), Chiang Rai (CR), Chiang Mai (CM), and Ratchaburi (RB). They were cultivated in the same conditions, potted, and partially shaded (60%) in an open conventional greenhouse. The phenology and chemical composition of these plants were assessed at the commercial harvesting stage (ca. 90 days after planting). The results indicated that UT was morphologically distinctive, illustrating the highest edible biomass yield (aerial and mature leaf size). The above-ground parts (viz., leaves and stem) were then analyzed for bioactive compounds after maceration with 80% (w/w) ethanol. It was found that the highest lactone content (~14 mg/g extract) was obtained from leaf and stem extracts of all samples except KS. Nonetheless, total phenolics and flavonoids in the stem extract of KS were found to be the highest at 3.22 and 2.42 mg/g, respectively. Phytochemicals from both leaf and stem extracts were capable of high anti-oxidant activity (~70%) as determined by DPPH and ABTS assays. Chemically, RB contained the highest 14-deoxy-11,12-didehydroandrographolide (156.98 mg/g extract), while UT and CM contained up to 0.68 mg/g extract of neoandrographolide. Classification of the samples indicated a clear relationship between the morphological traits and chemical compositions. In conclusion, our findings suggest the variations in phenotypic and chemotypic relations across the different landraces of A. paniculata. In essence, the quantity of the consumable parts was essentially the marker to describe the quality of the phytochemical constituents. The overall outcome of this study was to select the physiological characteristics that could be used for further breeding programs of the ideal variety with high productivity and higher bioactive(s) content.
1. Introduction
Andrographis paniculata (Burm. f.) Wall, commonly known as “the king of bitter”, is an ethnomedicinal plant used across the Asian continent as an anti-pyretic, a detoxicant, and an analgesic. Reports also suggest that A. paniculata was traditionally utilized to alleviate dermatological, respiratory, and gastrointestinal complications caused by pathogenic bacteria and viruses [1,2,3,4,5]. The biomedical significance of A. paniculata is mainly attributed to the presence of andrographolides, a group of diterpenoid lactones widely recognized for their immunostimulant, anti-urolithiatic, anti-diabetic, anti-obesity, and anticancer activities [4,6,7,8,9,10,11,12,13]. In addition, diterpenoid derivatives, such as dehydroandrographolide, neoandrographolide, and andrographiside, are believed to contribute directly or synergistically to the observed medicinal properties of A. paniculata [14,15]. The phytochemicals from this plant have been one of the key active ingredients in several pharmaceutical formulations for the treatment of colds, HIV infection, hepatitis, diabetes, cancer, nephro-urological disorders, and even COVID-19 [3,16,17]. In Thailand, personal administration of dried aerial parts of A. paniculata has been suggested for use in primary health care to ease the symptoms associated with the common cold, including sore throat and non-infectious diarrhea [18,19] resulting in an ever-growing demand for pharmaceutical grade raw-material(s), and subsequent large-scale cultivation of A. paniculata in recent years [3,20,21,22]. Currently, the quality of A. paniculata is determined merely based on the active constituents with only one or two diterpenoid lactone constituents as marker(s). There have been no studies carried out on the biogenetic route between andrographolide and neoandrographolide or other related bioactive(s) present in this plant [23,24]. Dong et al. [25] demonstrated that the cumulative chemical profiling of A. paniculata was influenced by growth conditions, notably the habitat and geographical region of cultivation. Interestingly, these two factors also influenced biomass and secondary metabolite accumulation in the plants [23,26]. The variations in A. paniculata production characteristics and diterpene lactone concentration were also confirmed amongst different plant accessions obtained from Thailand, Indonesia, Vietnam, and India [18,27]. Notwithstanding, little is known about the relationships between their morphological and phytochemical variations. Therefore, it is deemed essential to establish a fingerprint that can help differentiate and distinguish the A. paniculata samples based on their geographical origins for a plant breeding program. The aim of the current study was to evaluate the phenotypic trait(s) that could wholly describe the plant’s yield and chemical constituents. There have not been many reports on the A. paniculata landraces grown in Thailand. Therefore, for future benefit, this research was conducted to explain the relationship and differences. The findings of this study will aid both growers and the pharmaceutical industry in selecting suitable varieties for processing and developing effective plant breeding programs that promote the commercial use of local crops as sustainable crops.
2. Materials and Methods
2.1. Plant Materials
The cultivating trial was conducted for a 3-month period, from July 2021 to September 2021. Dried seeds of A. paniculata were collected from different locations as shown in Supplementary Table S1. They were soaked in deionized water (DI) water for 48 hrs and thereafter placed on moist paper and maintained at room temperature (29 °C, 57.8% RH). After 15 days, the number of germinations was counted to calculate the percentage of seed germination [28]. The seedling(s) were transferred into 15 cm wells filled with a mixture of soil:sand media (2:1) and maintained at 27.4 °C, 65% RH for 15 days. The seedlings (~15 cm tall) were transplanted into 10-inch pots with the same growing media, each containing 2 plants, with n = 15 pots for each plant line. Plants were watered daily (at 100% evapotranspiration crops; etc.) and in the second week of cultivation, each pot was fertilized with 100 mL of liquid fertilizer (Chia Tai, Thailand, 15-15-15, 10 g/L) while maintained under 60% shade throughout the growth period [10]. The study was conducted in the nursery at the research farm of the Chiang Mai University, Faculty of Agriculture’s Mae Hia Agricultural Demonstration Research and Training Center located at 18°45′31.1″ N 98°55′47.1″ E with an elevation of 310 m above mean sea level. After collection, the plant specimens were separated and their morphological appearances were recorded with the voucher specimen numbers as shown in Supplementary Table S1 prior to sending them to the Department of Biology, Faculty of Agriculture, Chiang Mai University for taxonomic confirmation. The specimens were stored at the Plant Bioactive Compound Laboratory, Faculty of Agriculture, Chiang Mai University.
2.2. Growth and Morphology Parameters
To evaluate the various morphological parameters, we measured growth-attributing characteristics such as shoot length and the number of branches/plants. The fresh/dry weight of the separated leaves and stems of the whole plant was determined. When samples attained a constant weight while drying in the shade, their dry weights were measured. Additionally, the total fresh and dry herbage yield per plant was measured. The growth and yield attributes, viz., plant height (cm), stem diameter (mm), number of shoots, number of leaves, number of branches, number, and length (cm) of internodes of second-pair leaves, and canopy width (cm) of the main shoot were recorded before harvesting (ca. 90 days after planting). The leaf area was determined using a leaf area meter (LI-3100C, LI-COR, Nebraska, USA). Plants were removed from the pots, cleaned, and the biomass yield was recorded. They were then shade-dried at room temperature for 5 days until constant moisture content was attained (~6 ± 0.40% MC) [29]. The dried material was then ground to powder using a spice grinder (Spring Green Evolution, PG2500).
2.3. Moisture Content
The moisture content of the A. paniculata samples was obtained by measuring the percent weight after 12 h at 105 °C in a hot-air oven, as determined by the method of the Association of Official Analytical Chemists [30].
2.4. Chemical Analyses
The sample powder (20 g) was macerated with 500 mL of 80% (w/w) ethanol thrice at room temperature, and the supernatant was combined. The solvent was removed by high centrifugation, resulting in a concentrated extract. In the last step, the extract (1 g) was redissolved with 1.5 mL of 80% ethanol and used for further chemical analysis [30].
2.5. Total Phenolic Content
The total phenolic content was determined using the method described by Sunanta, Chung [30]. The ethanolic extract (30 µL) was mixed with 150 µL of Folin–Ciocalteu reagent and 120 µL of 7.5% (w/v) NaCO3 solution. After incubating for 60 min, a UV-Vis spectrophotometer (SPECTROstar Nano, BMG LABTECH, Ortenberg, Germany) with a single UV-visible beam was used to determine the absorbance at 765 nm, and calibration standards using gallic acid were prepared at the concentration range of 30–300 mg/mL. The total phenolic content was given as the equivalent amount of gallic acid in milligrams per gram of the extract.
2.6. Total Flavonoid Content
Total flavonoid content was determined according to Sunanta, Chung [30] by adding 25 µL of the extract to 125 µL of distilled water. Following this, 7.5 µL of a 5% NaNO2 solution was added to the mixture. After allowing the mixture to react for 5 min, 15 µL of 10% AlCl3·6H2O solution was added. Thereafter, 1 M NaOH solution (50 µL) and distilled water (27.5 µL) were added. The absorbance of the test solution was determined using the spectrophotometer at a wavelength of 510 nm. The calibration standards were prepared using catechin at a concentration of 15 mg/mL. The total amount of flavonoids was given in milligrams of catechin equivalent per gram of the extract.
2.7. Anti-Oxidant Activities
2.7.1. Free Radical Scavenging Activity
The method for analyzing free radical scavenging activity was followed according to Sunanta, Chung [30]. Experimentally, 25 µL of the crude extract was mixed with 250 µL of 0.20 mM DPPH (2,2-diphenyl-1-picrylhydrazyl) and incubated at room temperature, in the dark, for 30 min. The absorbance at 517 nm was read, and DPPH radical scavenging performance was calculated using the following formula:
where Abscontrol is the absorbance of DPPH radical mixed with methanol and Abssample is the absorbance of DPPH radical reacted with sample extract/standard.
DPPH radical scavenging activity (%) = [(Abscontrol − Abssample)]/(Abscontrol)] × 100
2.7.2. Radical Cation Decolorization Assay
The method of Sangta, Wongkaew [31] was used and modified accordingly. The ABTS [2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid)] (1 mL) was diluted in 60 mL of 80% methanol, resulting in an absorbance of 0.7 ± 0.02 units at 734 nm. Equal volumes of 7.00 mM ABTS and 2.45 mM K2S2O8 solution were made into the working solution. The extract (200 µL) was pipetted into microtiter wells filled with 500 µL ABTS and 500 µL of the working solution, agitated, and left to stand at room temperature for 30 min (the mixture was incubated in the dark, at room temperature, for 12–16 h). The ABTS scavenging potential was estimated using the equation:
where Abscontrol is the absorbance of ABTS radical mixed with 80% methanol and Abssample is the absorbance of ABTS radical reacted with sample extract.
ABTS radical scavenging activity (%) = [(Abscontrol − Abssample)]/(Abscontrol)] × 100
2.8. Total Lactone
The total lactone content was analyzed using andrographolide as a standard according to the methodology described by Gajbhiye and Khristi [32]. For the spectrophotometric determination of total lactones, a 3,5-dinitro benzoic acid reagent was prepared by dissolving 1.0 g of the compound in 100 mL of ethanol. A potassium hydroxide reagent was prepared by dissolving 0.5 g in 100 mL of ethanol. Both reagents were prepared fresh during the analysis and stored in amber-colored bottles. For the spectrophotometric determination of the total lactones, UV-visible spectrophotometry was employed according to Aromdee [33]. Aliquots of the standard A. paniculata supplement (20 mg, Yanhee Fa Thalai Chon, Bangkok, Thailand) ranging from 0.10 to 1.00 mL were transferred to a test tube and made up to 2 mL with ethanol. Afterward, 0.5 mL of 3,5-dinitro benzoic acid and potassium hydroxide reagents were added to each aliquot and thoroughly mixed using a vortex. After 10 min, the absorbance was measured against a blank at 536 nm and used for sample analysis. Plant extracts were analyzed using the same procedure, and the andrographolide content of plant samples was calculated using the standard curve regression equation.
2.9. High Performance Liquid Chromatography (HPLC)
The HPLC conditions were as described by Jirakiattikul, Rithichai [34]. The sample extract was sonicated for 10 min and then passed through a 0.22 µm filter membrane. Ultra-high performance liquid chromatography was performed using a Nova-Pak C18 column (150 mm × 3.9 mm × 4 µm) (UHPLC, Nexera LC-30 A, Shimadzu) with a guard column. A mobile phase of phosphoric acid (A)-to-acetonitrile (B) at a volume ratio of 5 min, % A, % B; 4.5 min, % A, % B; 5.0 min, % A, % B; and 4.0 min, % B was delivered at a flow rate of 1.5 mL/min, over a total run time of 14 min. A sample (20 µL) was injected into the system. The concentrations of a standard (750 ppm and 1000 ppm) mixture containing 14-deoxyandrographolide and neoandrographolide in the range of 1.0 g were freshly prepared and injected. As a function of concentration, the peak area for each compound was plotted against calibration curves. To figure out how much of each compound was recovered, known amounts of reference standards (1 mg/mL) were added to the crude extracts.
2.10. Metabolite Profiling
The LC-MS/MS analysis was performed on an Agilent LC-QTOF 6500 system with an Agilent ZORBAX Eclipse XDB column-C18 (2.1 mm × 50 mm × 1.8 μm). Water containing 0.1% formic acid was used as the mobile phase, while acetonitrile containing 0.1% formic acid was used as the eluent in a gradient mode. The injection volume was 20 μL, and the temperature of the column was maintained at 30 °C. The flow rate remained constant across the gradient. The UPLC system was coupled to a QTOF mass spectrometer (6500 series: Model-G6545B) with an AP-ESI Dual Spray source. The parameters for the analysis were set using the positive ion mode, and spectra were acquired over the mass range of 120–1000 m/z. The MS data were processed with MassHunter v B.08.00, Rapid Control version 2.9 (Agilent Technologies). This software is integrated with libraries to give a list of possible elemental formulas. The MassHunter software (Agilent Technologies, Santa Clara, CA, USA) was used to refine the data. An MS/MS fragment matching and an error of less than 5 ppm were adjusted to determine the precision of the compounds’ confirmation [35,36].
2.11. Statistical Analysis
The experiment utilized a completely randomized design (CRD) with ten replicates. All experimental data were expressed as the mean plus the standard deviation (SD). A one-way analysis of variance (ANOVA) and Duncan’s multiple range tests at a significance level of 0.05 were conducted to determine the significance of the difference between samples of each type of A. paniculata (SPSS Institute, Armonk, NY, USA). Principal component analysis (PCA) was utilized to summarize the visual differences between the chemical components of the extracts, utilizing XLSTAT version 2018.5 (Suite NY, New York, NY, USA). To display the metabolite profile clustering, the m/z data were subjected to an online web server program, Heatmapper (heatmapper.ca) [37].
3. Results and Discussion
3.1. Taxonomical and Physiological Characteristics
The taxonomical characteristics of the A. paniculata collection are shown in Figure 1. Overall, they were branched, herbaceous plants growing to a height of 30–110 cm. All samples had stems that were acutely quadrangular, slender, highly branched, easily broken, dark green in color, square in cross section with longitudinal furrows and wing-like projections along the angles. The leaves were simple, opposite, lanceolate, glabrous, and lance-shaped. The leaf margins were found to be acute, entirely or slightly undulating, and the upper leaves were typically bract-like with a short petiole. The flowers were small and borne in spreading racemes. The plant’s inflorescence was present, terminal and axillary in the panicle, usually 10–30 mm long; the bracts were small; and the pedicel short. The results of morpho-physiological evaluation(s) are also mentioned in Table 1. Among all accessions, UT was found to be the smallest in plant height (~15 cm), stem diameter (~12 cm), and number of shoots, leaves, and branches, with the smallest canopy size of ~14.6 cm. CR and CM were found to be the tallest plants (~22 cm) with the largest stem diameter (~15 mm). KS and UT had the same stem diameter, number of shoots, number of leaves, number of branches, and length of internodes of the second-pair leaves (~12 mm, ~11, ~23, ~6, and ~6 cm, respectively), but less than those of the CR, CM, and RB. In CM, the leaf area index in young leaves and old leaves was the smallest (~9 and ~16 cm, respectively). In KS, the diameter of the stem, the number of shoots, the number of branches, and the length of the internodes of the second-pair leaves were found to be the smallest, approx. 12 mm, 11 cm, and 6 cm, respectively (Table 1).
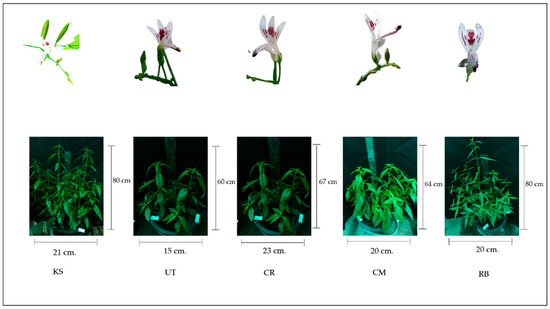
Figure 1.
Taxonomical characteristics of 5 collections of Andrographis paniculata. KS = Kamphaeng Saen, UT = Udon Thani, CR = Chiang Rai, CM = Chiang Mai, RB = Ratchaburi.

Table 1.
Physiological characteristics of Andrographis paniculata collected from various locations.
In concurrence with the findings mentioned in Table 1, the A. paniculata leaf and stem per plant had a significant direct effect on its phytochemical, particularly total andrographolide, yield. Aerial fresh weight yield (per plant) and dried leaf biomass (per plant) of the A. paniculata varieties 90 days after planting were found to be significantly different (Table 1). The highest values were 37.83 and 14.61 g/plant, respectively, found in CM and RB, which had fresh leaf weight yields of 27.75 and 26.6 g/plant and dry leaf weight yields of 11.01 and 10.65 g/plant, respectively. KS had the lowest fresh and dry leaf weight yields of 21.16 and 7.97 g/plant for the A. paniculata planted at different planting distances. It was found that the yield of fresh and dry leaf weights also differed statistically. A. paniculata yield per plant was determined by the number of primary branches and plant spread. The plant height and number of secondary branches had a negligible indirect negative effect on the total yield per plant [38]. Optimizing plant growth and the biosynthesis of desirable secondary metabolites has been a primary objective in commercial plant production. However, environmental factors and agricultural practices can have a substantial effect on plant growth and crop quality [39,40,41,42]. Plant growth optimization is also deemed essential to improve the secondary metabolite quality [43,44].
3.2. Chemical Properties
The presence of multiple pharmacological properties makes andrographolide a potential therapeutic agent [45]. Andrographolide contains an α-alkylidene-butyrolactone moiety and three hydroxyls at C-3, C-19, and C-14 that are responsible for its cytotoxic effects against numerous cancer cell lines [45]. Andrographolide is abundant in leaves and can be readily isolated as a crystalline solid from crude plant extracts [6,46,47,48,49,50]. In CM for both edible parts shown in Table 2, the total lactone was ~9.00–14.00 mg/g, while KS had the lowest. The amount of total lactone did not differ much between these utilizable parts. The phenolic and flavonoid contents were significantly higher in the KS, CM, and RB (3.00–5.00 mg/g and 2.50–3.00 mg/g, respectively), and the stem part contained slightly higher amounts. Flavonoids are phenolic group constituents, and flavones were found to be more prevalent in fresh A. paniculata. The total flavonoid content of this plant collection ranged between 3.40 and 1.06 mg/g. The antioxidant activities as determined by DPPH• and ABTS•+ were around 73% of crude extract, while the activities were significantly lower in the CM for both edible parts. It is also worth highlighting that ethanol was chosen in the extraction process due to its higher solubility, as reported previously [51].

Table 2.
Chemical properties of Andrographis paniculata.
The results of the andrographolide contents from different parts are mentioned in Table 2. The HPLC chromatograms are displayed in Supplementary Figure S1. The number of diterpene lactones, namely 14-deoxy-11,12-didehydroandrographolide (>100 mg/g) and neo-andrographolide (>0.50 mg/g), was much higher in the stem than contained in the leaf tissue. The leaf ethanolic fraction from landraces KS, UT, and CM contained 14-deoxy-11,12-didehydroandrographolide at ~0.01 mg/g, while neo-andrographolide was the highest in the KS (~0.11 mg/g), then in the UT, CR, and RB (0.06–0.09 mg/g) and the lowest in the CM (~0.04 mg/g). In stem, the UT sample illustrated the lowest content of 14-deoxy-11,12-didehydroandrographolide 105 mg/g), whereas neo-andrographolide was the highest (~0.68 mg/g). The neo-andrographolide was found in the highest amount in UT and CM (~0.7 mg/g), followed by the KS and CR (~0.6 mg/g), and the RB was the lowest (0.09 mg/g). Chromatography and spectroscopic techniques play a major role in phytochemical profiling and are routinely utilized for qualitative and quantitative analysis of pharmaceutically and biologically active materials [52,53]. Changes in the amount of andrographolide could be caused by the way samples are prepared for HPLC measurement, but the overall trend is the same. The variation in the proportion of andrographolides may also be attributable to differences in sample origin, their respective genotypes, and variable expression of genes such as the WRKY (“Worky”) transcription factors [32,54,55].
While the entire plant has been reported to be of therapeutic value, the leaves were found to contain the highest concentration of useful terpenoids, including andrographolide, followed by the stems, roots, and lastly the seeds [56,57]. However, it is known that all plant parts contain extractable bioactive active compounds [47,58,59]. The major bioactive classes of A. paniculata are terpenoid lactones and flavonoids, which are responsible for pharmacological activities such as analgesic, anti-cancer, anti-diabetic, anti-fertility, anti-inflammatory, anti-malarial, anti-microbial, anti-oxidant, anti-pyretic, anti-viral, anti-retroviral, anti-venom, cardioprotective, hepatoprotective, immunomodulatory, and neuroprotective properties [60,61]. Reportedly, A. paniculata contains over 20 diterpenoids and over 10 flavonoids [62,63], while andrographolide is the most common diterpenoid found in A. paniculata, making up about 1–6% of the dried whole plant, stem and leaf extracts, respectively [42,58,59]. Deoxyandrographolide, neoandrographolide, 14-deoxy-11,12-didehydroandrographide, and isoandrographolide are the other principal diterpenoids along with flavonoids and polyphenols [42,58,64,65,66]. In vitro study advised that 14-deoxy-11,12-didehydroandrographolide and neoandrographolide possess immunostimulatory, anti-infectious, anti-atherosclerotic, anti-inflammatory, anti-microbial, and anti-hepatotoxic properties [6,67]. In addition, 5-hydroxy-7,8-dimethoxyflavone, 5-hydroxy-7,8,2′,5′-tetramethoxyflavone, 5-hydroxy-7,8,2′,3′-tetramethoxyflavone, and 5-hydroxy-7,8,2′-trimethoxyflavone are structurally related flavonoids. The principal flavonoids isolated were 7-O-methylwogonin and 2′-methyl ether [48,64,68,69].
Flavones are the most prevalent naturally occurring, widely dispersed group of low molecular weight, benzo-γ-pyrone-structured phenolic compounds. Several studies suggest that flavones are protective against numerous infectious and degenerative diseases. Their activity against microbial infections is dependent on their structural category, hydroxylation, conjugations, and degree of polymerization, and they are known to be produced by plants [70,71,72]. The antioxidant activities of these polyphenolic compounds are due to the presence of a functional hydroxyl group, making them molecules of interest to the nutraceutical, pharmaceutical, and medical industries as therapeutic agents for a variety of human diseases, including cardiovascular, cancerous, and age-related diseases [73,74]. Flavones can induce protective enzymatic pathways and play a significant role in inflammation suppression by inhibiting xanthine oxidase, cyclo-oxygenase, lipoxygenase, and phosphoinositide 3-kinase [75,76]. It has been reported that 14-deoxy-11,12-didehydroandrographolide has promising anti-steatohepatitis, anti-liver fibrosis, antioxidant, and anti-inflammatory properties [77]. The 14-dehydroxy-11,12-didehydroandrographolide is also known to prevent the development of multiple drug resistance (MDR) when combined with azithromycin and gentamicin [76,78]. Andrographolactone was recently identified and characterized [79]; no biological activity has been reported to date.
3.3. Chemometric Relationship
Several chemical variables were used to understand the relationship between the morphological data of the five A. paniculata varieties used in this study and their chemical properties. These variables are shown in Table 1 and Table 2. The morphological scale is represented in the graph in Figure 2A. As shown, representing the bioactive compound relationship as a score curve, all patterns were evenly distributed in the scatter plot, with 95.47% in PC1 and 7.98% in PC2. As shown in Figure 2C, all patterns were evenly distributed across the plot, with 99.98% in PC1 and 1.34% in PC2.
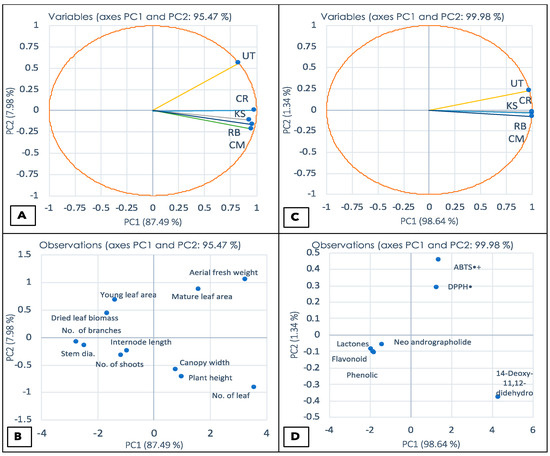
Figure 2.
Principal Component Analysis (PCA) of physical characteristics of Andrographis paniculata between sample variables (A) and observations (B) and PCA of general chemical analyses between sample variables (C) and observations (D). The sample accessions were taken from KS = Kamphaeng Saen, UT = Udon Thani, CR = Chiang Rai, CM = Chiang Mai, RB = Ratchaburi.
As mentioned in Table 1, the physiological data were computed using principal component analysis (Figure 2). The figures A and B variables of morphology were divided into two categories: UT observed in the mature leaf area (14.61 g) and aerial fresh weight (37.7 g) was the greatest and thus distinguished from the other variants by the data. The biplot analysis in Figure 2B revealed that UT was distinguished by variables such as aerial fresh weight and mature leaf area. The other four species were associated with young leaf area, dried leaf biomass, No. of branches, stem diameter, No. of shoots, internode length, and young leaf area to a lesser extent.
For the chemical analysis (Figure 2C), the UT had the highest antioxidant potential while illustrating low phytochemical content. However, the other samples corresponded with higher concentrations of the observed chemical compounds (Figure 2D). Obviously, the PCA patterns of the morphological and phytochemical data were in-line, indicating that the plants with smaller physiological characteristics illustrated higher amounts of active ingredients. However, the antioxidant activities were conversely lower. Chlorophyll, which is the primary pigment used in photosynthesis, is frequently used as a marker to determine plant biomass [12]. Multiple studies have demonstrated a significant correlation between chlorophyll content and secondary metabolites such as total phenolic compounds, flavonoid content, and anthocyanin content [9,10]. The research conducted by Stratil, Klejdus [80] revealed a highly significant correlation between total phenolic content and antioxidant activity. In the common case, the DPPH- and ABTS-scavenging activity were related to the total amount of phenolic and flavonoid compounds [81]. Antioxidant compounds are key elements for the prevention of diseases caused by free radicals and for protecting the nervous system and memory [82]. The main antioxidants in A. paniculata are phenolic acids, flavonoids, and lactones that possess anti-cancer, neuroprotective, and neurotrophic properties [30,51,83,84]. More work regarding the metabolomic profiling of the biosynthesis of active ingredients in this plant needs to be further investigated.
3.4. Metabolite Profiling of A. paniculata Leaf Constituents
In the present study, A. paniculata was analyzed for metabolite profiling using LC-ESI-MS/MS. A total of 110 metabolites were identified in Supplementary Table S2. The heatmap displays 2 major distinctive clusters with the UT, projected separately from the others (Figure 3A). The CM and RB were closely related in the metabolite profile. Similarly, in the bi-plot analysis, only 44.25% across PC1 (23.05%) and PC2 (21.20%) were considered (Figure 3B), commercial (Figure 3C) mass spectral data of supplementary Table S2 mass spectral libraries and from literature. The samples of CM and RB were cast away from the others by compounds of positive fragment dodemorph (282.28 m/z) (3), phytosphingosine (318.30 m/z), C16 Sphinganine (274.27 m/z), Xylostasin (212.21 m/z) (30,31,39), Triethyl phosphate (205.06 m/z) (63) and(S)-2-Methylbutanal 121.09 m/z) B (105). The CR, KS, and RB were projected with the compounds Oleamide, 6,10,14-Trimethyl-5,9,13-pentadecatrien-2-one, and Inulobiose with a 282.28 m/z of the positive ion of 280.26 m/z and 166.06 m/z, respectively. The rest of the metabolites were shared across the samples, which were general chemotypes of A. paniculata. In other words, terpenoid lactones and flavonoids were commonly identified using LC–MS/MS analysis [61], as previously reported. Terpenoid lactones such as neoandrographolide (510.29 m/z), 14-deoxyandrographolide (340.21 m/z), andrograpanin (453.13 m/z), andrographin (382.42 m/z), and andrographolide (476.26 m/z) were identified from the ethanolic extract. Andrographidine F (527.65 m/z), andrographiside (542.46 m/z), andrographidine B (484.43 m/z), andrographidine A (472.53 m/z), 5-hydroxy-7,8,2′-trimethoxyflavone-5-glucoside (489.16 m/z), and andrographidine D (460.38 m/z) were identified as new flavone glycosides.
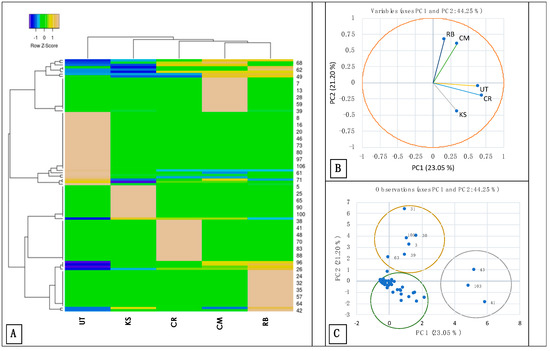
Figure 3.
Heatmap analysis of metabolite profiling of the mass spectrometry, (A) PCA–biplot analysis, (B) and score plot with the principal compounds (C). The compound numbers are according to the data presented in Supplementary Table S2. The sample accessions were taken from KS = Kamphaeng Saen, UT = Udon Thani, CR = Chiang Rai, CM = Chiang Mai, RB = Ratchaburi.
The observed diterpenes were categorized as monomers and polymers of diterpene lactones and as pentacyclic or macrocyclic diterpenoids based on their skeletal core [85]. The monomers contain multiple hydroxyl groups that exhibit a unique fragmentation behavior characterized by the successive loss of one or more H2O molecules [85]. The positive ion mode was significantly more effective for analyzing these types of compounds. Kumar et al. [61] also reported fragmentation of andrographolide from the methanol extract of A. paniculata aerial parts, but the fragmentation pattern differed at m/z 303 and 275 due to the loss of CO in reverse Diels-Alder (RDA). Due to their diverse nature and often restricted distribution, the biological function of a particular diterpenoid cannot be generalized to the entire class of molecules. A. paniculata is an active ingredient in the numerous formulations listed in the Thai national herbal medicine list. Because of this, A. paniculata has been chosen as a marker for standardizing raw and commercialized herbal products using HPLC and LC-MS methods for quality control and quality assurance [86].
4. Conclusions
The present study evaluated physiological traits that could be used for further propagation of high-yielding ideal varieties and found high bioactive content differences between A. paniculata cultivars. To establish this, the relationship between the phenotypic and chemical composition of local A. paniculata varieties was evaluated. The results indicated that the species contained a substantial amount of total lactone, while phenolic and flavonoid content varied among the tested samples. The amounts of 14-deoxy-11,12-didehydroandrographolide and neo-andrographolide had a better influence on the antioxidant potential than other phytochemical ingredients. Overall, morphological traits (i.e., higher aerial fresh weight and larger mature leaf size) are indicative of higher phytochemical yield for its utilization for consumption and pharmaceutical purposes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/horticulturae8100978/s1, Supplementary Table S1. Andrographis paniculata seed information. Supplementary Table S2. liquid chromatography electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) analysis of Andrographis paniculata leaf. Supplementary Figure S1. HPLC chromatogram of lactone standards.
Author Contributions
Conceptualization, S.R.S.; methodology, N.E.O. and S.R.S.; validation, S.K.P.; formal analysis, N.E.O.; investigation, N.E.O.; resources, N.E.O., S.K.P., T.C. and C.L.; data curation, N.E.O.; writing—original draft preparation, N.E.O., writing—review and editing, S.K.P. and S.R.S.; visualization, S.K.P. and S.R.S.; supervision, S.R.S.; project administration, T.C. and C.L.; funding acquisition, C.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research project was partially supported by Chiang Mai University.
Data Availability Statement
Not applicable.
Acknowledgments
N.E.O., S.K.P., T.C., C.L. and S.R.S. thank Chiang Mai University, Thailand, for the support and infrastructure provided for the conduct of this research. S.K.P. thanks the JSS Academy of Higher Education and Research, Mysore, India, for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wen, L.; Xia, N.; Chen, X.; Li, Y.; Hong, Y.; Liu, Y.; Wang, Z.; Liu, Y. Activity of antibacterial, antiviral, anti-inflammatory in compounds andrographolide salt. Eur. J. Pharmacol. 2014, 740, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Raina, A.P.; Gupta, V.; Sivaraj, N.; Dutta, M. Andrographis paniculata (Burm. f.) Wall. ex Nees (kalmegh), a traditional hepatoprotective drug from India. Genet. Resour. Crop Evol. 2013, 60, 1181–1189. [Google Scholar] [CrossRef]
- Valdiani, A.; Kadir, M.A.; Tan, S.G.; Talei, D.; Abdullah, M.P.; Nikzad, S. Nain-e Havandi Andrographis paniculata present yesterday, absent today: A plenary review on underutilized herb of Iran’s pharmaceutical plants. Mol. Biol. Rep. 2012, 39, 5409–5424. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Sheng, F.; Zhang, Z.; Ma, X.; Gao, T.; Fu, C.; Li, P. Andrographis paniculata (Burm. f.) Nees and its major constituent andrographolide as potential antiviral agents. J. Ethnopharmacol. 2021, 272, 113954. [Google Scholar] [CrossRef]
- Chandrasekaran, C.; Thiyagarajan, P.; Sundarajan, K.; Goudar, K.S.; Deepak, M.; Murali, B.; Allan, J.J.; Agarwal, A. Evaluation of the genotoxic potential and acute oral toxicity of standardized extract of Andrographis paniculata (KalmCold™). Food Chem. Toxicol. 2009, 47, 1892–1902. [Google Scholar] [CrossRef]
- Chao, W.W.; Lin, B.F. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chin. Med. 2010, 5, 17. [Google Scholar] [CrossRef]
- McNeal, K.S.; Herbert, B.E. Volatile organic metabolites as indicators of soil microbial activity and community composition shifts. Soil Sci. Soc. Am. J. 2009, 73, 579–588. [Google Scholar] [CrossRef]
- Murthy, H.N.; Dalawai, D. Biotechnological production of diterpenoid lactones from cell and organ cultures of Andrographis paniculata. Appl. Microbiol. Biotechnol. 2021, 105, 7683–7694. [Google Scholar] [CrossRef]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of light-emitting diodes on the accumulation of phenolic compounds and glucosinolates in Brassica juncea sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Rao, M.J.; Xu, Y.; Huang, Y.; Tang, X.; Deng, X.; Xu, Q. Ectopic expression of citrus UDP-GLUCOSYL TRANSFERASE gene enhances anthocyanin and proanthocyanidins contents and confers high light tolerance in Arabidopsis. BMC Plant Biol. 2019, 19, 603. [Google Scholar] [CrossRef]
- Shen, Q.; Li, L.; Jiang, Y.; Wang, Q. Functional characterization of ent-copalyl diphosphate synthase from Andrographis paniculata with putative involvement in andrographolides biosynthesis. Biotechnol. Lett. 2016, 38, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Suryani, C.; Dwiwahyuningsih, T.; Supriyadi, S.; Santoso, U. Derivatization of chlorophyll from pandan (Pandanus amaryllifolius Roxb.) leaves and their antioxidant activity. Period. Tche Quim. 2020, 17, 1110–1126. [Google Scholar] [CrossRef]
- Swaroop, A.K.; Lalitha, C.M.V.N.; Shanmugam, M.; Subramanian, G.; Natarajan, J.; Selvaraj, J. Plant Derived Immunomodulators; A Critical Review. Adv. Pharm. Bull. 2022, 12, 712–729. [Google Scholar] [CrossRef]
- Talei, D.; Valdiani, A.; Yusop, M.K.; Abdullah, M.P. Estimation of salt tolerance in Andrographis paniculata accessions using multiple regression model. Euphytica 2013, 189, 147–160. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, T.; Kaul, S.; Kapoor, K.K.; Dhar, M.K. Anticancer potential of labdane diterpenoid lactone “andrographolide” and its derivatives: A semi-synthetic approach. Phytochem. Rev. 2017, 16, 513–526. [Google Scholar] [CrossRef]
- Xie, R.; Lin, Z.; Zhong, C.; Li, S.; Chen, B.; Wu, Y.; Huang, L.; Yao, H.; Shi, P.; Huang, J. Deciphering the potential anti-COVID-19 active ingredients in Andrographis paniculata (Burm. F.) Nees by combination of network pharmacology, molecular docking, and molecular dynamics. Rsc Adv. 2021, 11, 36511–36517. [Google Scholar] [CrossRef]
- Wanaratna, K.; Leethong, P.; Inchai, N.; Chueawiang, W.; Sriraksa, P.; Tabmee, A.; Sirinavin, S. Efficacy and safety of Andrographis paniculata extract in patients with mild COVID-19: A randomized controlled trial. MedRxiv 2021. [Google Scholar] [CrossRef]
- Prathanturarug, S.; Soonthornchareonnon, N.; Chuakul, W.; Saralamp, P. Variation in growth and diterpene lactones among field-cultivated Andrographis paniculata. J. Nat. Med. 2007, 61, 159–163. [Google Scholar] [CrossRef]
- Pholphana, N.; Panomvana, D.; Rangkadilok, N.; Suriyo, T.; Puranajoti, P.; Ungtrakul, T.; Pongpun, W.; Thaeopattha, S.; Songvut, P.; Satayavivad, J. Andrographis paniculata: Dissolution investigation and pharmacokinetic studies of four major active diterpenoids after multiple oral dose administration in healthy Thai volunteers. J. Ethnopharmacol. 2016, 194, 513–521. [Google Scholar] [CrossRef]
- Puri, A.; Saxena, R.; Saxena, R.; Saxena, K.; Srivastava, V.; Tandon, J. Immunostimulant agents from Andrographis paniculata. J. Nat. Prod. 1993, 56, 995–999. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Suwannapoom, C.; Khamyong, N.; Pintakum, D.; Lamphun, S.N.; Triwitayakorn, K.; Osathanunkul, K.; Madesis, P. Hybrid analysis (barcode-high resolution melting) for authentication of Thai herbal products, Andrographis paniculata (Burm. f.) Wall. ex Nees. Pharmacogn. Mag. 2016, 12 (Suppl. S1), S71. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, Y.; Marshall, R.L.; Mukkur, T.K. An investigation on the antimicrobial activity of Andrographis paniculata extracts and andrographolide in vitro. Asian J. Plant Sci. 2006, 5, 527–530. [Google Scholar]
- Jha, S.; Das, J.; Sharma, A.; Hazra, B.; Goyal, M.K. Probabilistic evaluation of vegetation drought likelihood and its implications to resilience across India. Glob. Planet. Chang. 2019, 176, 23–35. [Google Scholar] [CrossRef]
- Pandey, P.; Ali, S.N.; Champati Ray, P.K. Glacier-glacial lake interactions and glacial lake development in the central Himalaya, India (1994–2017). J. Earth Sci. 2021, 32, 1563–1574. [Google Scholar] [CrossRef]
- Dong, H.J.; Zhang, Z.J.; Yu, J.; Liu, Y.; Xu, F.G. Chemical Fingerprinting of Andrographis paniculata (Burm. f.) Nees by HPLC and Hierarchical Clustering Analysis; Oxford University Press: Oxford, UK, 2009; Volume 47, pp. 931–935. [Google Scholar]
- Cha, J.-H.; Yang, W.-H.; Xia, W.; Wei, Y.; Chan, L.-C.; Lim, S.-O.; Li, C.-W.; Kim, T.; Chang, S.-S.; Lee, H.-H. Metformin promotes antitumor immunity via endoplasmic-reticulum-associated degradation of PD-L1. Mol. Cell 2018, 71, 606–620.e607. [Google Scholar] [CrossRef]
- Pandey, A.; Mandal, A. Variation in morphological characteristics and andrographolide content in Andrographis paniculata (Burm. f.) Nees of Central India. Iran. J. Energy Environ. 2010, 1, 165–169. [Google Scholar]
- Kumar, B.; Verma, S.K.; Singh, H. Effect of temperature on seed germination parameters in Kalmegh (Andrographis paniculata Wall. ex Nees.). Ind. Crops Prod. 2011, 34, 1241–1244. [Google Scholar] [CrossRef]
- Wongkaew, M.; Sommano, S.R.; Tangpao, T.; Rachtanapun, P.; Jantanasakulwong, K. Mango peel pectin by microwave-assisted extraction and its use as fat replacement in dried Chinese sausage. Foods 2020, 9, 450. [Google Scholar] [CrossRef]
- Sunanta, P.; Chung, H.H.; Kunasakdakul, K.; Ruksiriwanich, W.; Jantrawut, P.; Hongsibsong, S.; Sommano, S.R. Genomic relationship and physiochemical properties among raw materials used for Thai black garlic processing. Food Sci. Nutr. 2020, 8, 4534–4545. [Google Scholar] [CrossRef]
- Sangta, J.; Wongkaew, M.; Tangpao, T.; Withee, P.; Haituk, S.; Arjin, C.; Sringarm, K.; Hongsibsong, S.; Sutan, K.; Pusadee, T. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants 2021, 10, 1422. [Google Scholar] [CrossRef]
- Gajbhiye, N.; Khristi, S. Distribution pattern of andrographolide and total lactones in different parts of Kalmegh plant. Indian J. Hortic. 2010, 64, 591–593. [Google Scholar]
- Aromdee, C. Andrographolide: Progression in its modifications and applications–A patent review (2012–2014). Expert Opin. Ther. Pat. 2014, 24, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Jirakiattikul, Y.; Rithichai, P.; Prachai, R.; Itharat, A. Elicitation enhancement of bioactive compound accumulation and antioxidant activity in shoot cultures of Boesenbergia rotunda L. Agric. Nat. Resour. 2021, 55, 456–463. [Google Scholar]
- Li, F.; Cao, J.; Liu, Q.; Hu, X.; Liao, X.; Zhang, Y. Acceleration of the Maillard reaction and achievement of product quality by high pressure pretreatment during black garlic processing. Food Chem. 2020, 318, 126517. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Kar, A.; Mukherjee, P.K.; Haldar, P.K.; Sharma, N.; Katiyar, C.K. Immunoprotective potential of Ayurvedic herb Kalmegh (Andrographis paniculata) against respiratory viral infections–LC–MS/MS and network pharmacology analysis. Phytochem. Anal. 2021, 32, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Maison, T.; Volkaert, H.; Boonprakob, U.; Paisooksantivatana, Y. Genetic Diversity of Andrographis paniculataWall. ex Nees as Revealed by Morphological Characters and Molecular Markers. Agric. Nat. Resour. 2005, 39, 388–399. [Google Scholar]
- Kumar, R.A.; Sridevi, K.; Kumar, N.V.; Nanduri, S.; Rajagopal, S. Anticancer and immunostimulatory compounds from Andrographis paniculata. J. Ethnopharmacol. 2004, 92, 291–295. [Google Scholar] [CrossRef]
- Rao, Y.K.; Vimalamma, G.; Rao, C.V.; Tzeng, Y.-M. Flavonoids and andrographolides from Andrographis paniculata. Phytochemistry 2004, 65, 2317–2321. [Google Scholar] [CrossRef]
- Xu, C.; Chou, G.X.; Wang, Z.T. A new diterpene from the leaves of Andrographis paniculata Nees. Fitoterapia 2010, 81, 610–613. [Google Scholar] [CrossRef]
- Burgos, R.; Caballero, E.; Sanchez, N.; Schroeder, R.; Wikman, G.; Hancke, J. Testicular toxicity assesment of Andrographis paniculata dried extract in rats. J. Ethnopharmacol. 1997, 58, 219–224. [Google Scholar] [CrossRef]
- Guo, L.-P.; Zhou, L.-Y.; Kang, C.-Z.; Wang, H.-Y.; Zhang, W.-J.; Wang, S.; Wang, R.-S.; Wang, X.; Han, B.-X.; Zhou, T. Strategies for medicinal plants adapting environmental stress and “simulative habitat cultivation” of Dao-di herbs. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2020, 45, 1969–1974. [Google Scholar]
- Hossain, M.; Urbi, Z.; Sule, A.; Rahman, K. Andrographis paniculata (Burm. f.) Wall. ex Nees: A review of ethnobotany, phytochemistry, and pharmacology. Sci. World J. 2014, 2014, 274905. [Google Scholar] [CrossRef] [PubMed]
- Varma, A.; Padh, H.; Shrivastava, N. Andrographolide: A new plant-derived antineoplastic entity on horizon. Evid.-Based Complement. Altern. Med. 2011, 2011, 815390. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.-W.; Kuo, Y.-H.; Li, W.-C.; Lin, B.-F. The production of nitric oxide and prostaglandin E2 in peritoneal macrophages is inhibited by Andrographis paniculata, Angelica sinensis and Morus alba ethyl acetate fractions. J. Ethnopharmacol. 2009, 122, 68–75. [Google Scholar] [CrossRef]
- Matsuda, T.; Kuroyanagi, M.; Sugiyama, S.; Umehara, K.; Ueno, A.; Nishi, K. Cell differentiation-inducing diterpenes from Andrographis paniculata Nees. Chem. Pharm. Bull. 1994, 42, 1216–1225. [Google Scholar] [CrossRef]
- Chao, W.-W.; Kuo, Y.-H.; Lin, B.-F. Anti-inflammatory activity of new compounds from Andrographis paniculata by NF-κB transactivation inhibition. J. Agric. Food Chem. 2010, 58, 2505–2512. [Google Scholar] [CrossRef]
- Patra, A.; Mitra, A.K.; Biswas, S.; Gupta, C.D.; Chatterjee, T.K.; Basu, K.; Barua, A. Carbon-13 NMR spectra of some labdane diterpenoids. Org. Magn. Reson. 1981, 17, 301–302. [Google Scholar] [CrossRef]
- Fujita, T.; Fujitani, R.; Takeda, Y.; Takaishi, Y.; Yamada, T.; Kido, M.; Miura, I. On the diterpenoids of Andrographis paniculata: X-ray crystallographic analysis of andrographolide and structure determination of new minor diterpenoids. Chem. Pharm. Bull. 1984, 32, 2117–2125. [Google Scholar] [CrossRef]
- Arash, R.; Koshy, P.; Sekaran, M. Antioxidant potential and content of phenolic compounds in ethanolic extracts of selected parts of Andrographis paniculata. J. Med. Plants Res. 2010, 4, 197–202. [Google Scholar]
- Chin, Y.-W.; Balunas, M.J.; Chai, H.B.; Kinghorn, A.D. Drug discovery from natural sources. AAPS J. 2006, 8, E239–E253. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R. In Vitro Activities of Andrographolide and Its Derivative on Cell Lines Thesis. Ph.D. Thesis, Department of Biotechnology, University of Calicut, Malappuram, India, 2014. [Google Scholar]
- Sharma, S.N.; Sinha, R.K.; Sharma, D.; Jha, Z. Assessment of intra-specific variability at morphological, molecular and biochemical level of Andrographis paniculata (Kalmegh). Curr. Sci. 2009, 96, 402–408. [Google Scholar]
- Zhang, R.; Chen, Z.; Zhang, L.; Yao, W.; Xu, Z.; Liao, B.; Mi, Y.; Gao, H.; Jiang, C.; Duan, L. Genomic characterization of WRKY transcription factors related to andrographolide biosynthesis in Andrographis paniculata. Front. Genet. 2021, 11, 601689. [Google Scholar] [CrossRef]
- Mishra, S.; Tiwari, S.; Kakkar, A.; Pandey, A. Chemoprofiling of Andrographis paniculata (Kalmegh) for its andrographolide content in Madhya Pradesh, India. Int. J. Pharma Bio. Sci. 2010, 1. [Google Scholar]
- Parasher, R.; Upadhyay, A.; Khan, N.A.; Dwivedi, S.K. Biochemical estimation and quantitative determination of medicinally important andrographolide in Andrographis peniculata at different growth stages. Electron. J. Environ. Agric. Food Chem. 2011, 10, 2479–2486. [Google Scholar]
- Cheung, H.; Cheung, C.; Kong, C. Determination of bioactive diterpenoids from Andrographis paniculata by micellar electrokinetic chromatography. J. Chromatogr. A 2001, 930, 171–176. [Google Scholar] [CrossRef]
- Pholphana, N.; Rangkadilok, N.; Thongnest, S.; Ruchirawat, S.; Ruchirawat, M.; Satayavivad, J. Determination and variation of three active diterpenoids in Andrographis paniculata (Burm. f.) Nees. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2004, 15, 365–371. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, B.; Bajpai, V. Andrographis paniculata (Burm. f.) Nees: Traditional uses, phytochemistry, pharmacological properties and quality control/quality assurance. J. Ethnopharmacol. 2021, 275, 114054. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A.; Bajpai, V.; Sharma, K.R.; Kumar, B. Identification and characterization of terpenoid lactones and flavonoids from ethanolic extract of Andrographis paniculata (Burm. f.) Nees using liquid chromatography/tandem mass spectrometry. Sep. Sci. Plus 2018, 1, 762–770. [Google Scholar] [CrossRef]
- Kishore, P.H.; Reddy, M.V.B.; Reddy, M.K.; Gunasekar, D.; Caux, C.; Bodo, B. Flavonoids from Andrographis lineata. Phytochemistry 2003, 63, 457–461. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Zhang, H.; Wang, X.; Zhou, H. Synthesis of andrographolide derivatives and their TNF-α and IL-6 expression inhibitory activities. Bioorg. Med. Chem. Lett. 2007, 17, 6891–6894. [Google Scholar] [CrossRef]
- Bhaskar Reddy, M.V.; Kishore, P.H.; Rao, C.V.; Gunasekar, D.; Caux, C.; Bodo, B. New 2 ‘-Oxygenated Flavonoids from Andrographis affinis. J. Nat. Prod. 2003, 66, 295–297. [Google Scholar] [CrossRef] [PubMed]
- Dua, V.; Ojha, V.; Roy, R.; Joshi, B.; Valecha, N.; Devi, C.U.; Bhatnagar, M.; Sharma, V.; Subbarao, S. Anti-malarial activity of some xanthones isolated from the roots of Andrographis paniculata. J. Ethnopharmacol. 2004, 95, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Sareer, O.; Ahmad, S.; Umar, S. Andrographis paniculata: A critical appraisal of extraction, isolation and quantification of andrographolide and other active constituents. Nat. Prod. Res. 2014, 28, 2081–2101. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.K.; Roy, S.; Dey, S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 2003, 74, 692–694. [Google Scholar] [CrossRef]
- Kuroyanagi, M.; Sato, M.; Ueno, A.; Nishi, K. Flavonoids from Andrographis paniculata. Chem. Pharm. Bull. 1987, 35, 4429–4435. [Google Scholar] [CrossRef]
- Radhika, P.; Prasad, Y.R.; Lakshmi, K.R. Flavones from the stem of Andrographis paniculata Nees. Nat. Prod. Commun. 2010, 5, 59–60. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C.; Theoharides, T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000, 52, 673–751. [Google Scholar]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Murković, M. Phenolic Compounds. U: Encyclopedia of Food Sciences and Nutrition; Caballer, B., Fingla, P., Toldra, F., Eds.; Academic Press: Cambridge, MA, USA, 2003; Volume 2, pp. 4507–4513. [Google Scholar]
- Kumar, S.; Mishra, A.; Pandey, A.K. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med. 2013, 13, 120. [Google Scholar] [CrossRef]
- Pandey, A.K. Anti-staphylococcal activity of a pan-tropical aggressive and obnoxious weed Parthenium histerophorus: An in vitro study. Natl. Acad. Sci. Lett. 2007, 30, 383–386. [Google Scholar]
- Walker, E.H.; Pacold, M.E.; Perisic, O.; Stephens, L.; Hawkins, P.T.; Wymann, M.P.; Williams, R.L. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol. Cell 2000, 6, 909–919. [Google Scholar] [CrossRef]
- Dwivedi, M.K.; Sonter, S.; Mishra, S.; Singh, P.; Singh, P.K. Secondary metabolite profiling and characterization of diterpenes and flavones from the methanolic extract of Andrographis paniculata using HPLC-LC-MS/MS. Future J. Pharm. Sci. 2021, 7, 184. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Chen, H.-W.; Lii, C.-K.; Jhuang, J.-H.; Huang, C.-S.; Li, M.-L.; Yao, H.-T. A diterpenoid, 14-deoxy-11, 12-didehydroandrographolide, in Andrographis paniculata reduces steatohepatitis and liver injury in mice fed a high-fat and high-cholesterol diet. Nutrients 2020, 12, 523. [Google Scholar] [CrossRef]
- Majumdar, M.; Misra, T.K.; Roy, D.N. In vitro anti-biofilm activity of 14-deoxy-11, 12-didehydroandrographolide from Andrographis paniculata against Pseudomonas aeruginosa. Braz. J. Microbiol. 2020, 51, 15–27. [Google Scholar] [CrossRef]
- Wang, G.-C.; Wang, Y.; Williams, I.D.; Sung, H.H.-Y.; Zhang, X.-Q.; Zhang, D.-M.; Jiang, R.-W.; Yao, X.-S.; Ye, W.-C. Andrographolactone, a unique diterpene from Andrographis paniculata. Tetrahedron Lett. 2009, 50, 4824–4826. [Google Scholar] [CrossRef]
- Stratil, P.; Klejdus, B.; Kubáň, V. Determination of total content of phenolic compounds and their antioxidant activity in vegetables evaluation of spectrophotometric methods. J. Agric. Food Chem. 2006, 54, 607–616. [Google Scholar] [CrossRef]
- Tummanichanont, C.; Phoungchandang, S.; Srzednicki, G. Effects of pretreatment and drying methods on drying characteristics and quality attributes of Andrographis paniculata. J. Food Process. Preserv. 2017, 41, e13310. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef]
- Naheed, Z.; Cheng, Z.; Wu, C.; Wen, Y.; Ding, H. Total polyphenols, total flavonoids, allicin and antioxidant capacities in garlic scape cultivars during controlled atmosphere storage. Postharvest Biol. Technol. 2017, 131, 39–45. [Google Scholar] [CrossRef]
- Lin, F.; Wu, S.; Lee, S.; Ng, L. Antioxidant, antioedema and analgesic activities of Andrographis paniculata extracts and their active constituent andrographolide. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2009, 23, 958–964. [Google Scholar]
- Yang, T.; Xu, C.; Wang, Z.T.; Wang, C.H. Comparative pharmacokinetic studies of andrographolide and its metabolite of 14-deoxy-12-hydroxy-andrographolide in rat by ultra-performance liquid chromatography–mass spectrometry. Biomed. Chromatogr. 2013, 27, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Trugo, L.C.; Finglas, P.M. Encyclopedia of Food Sciences and Nutrition; Academic Press: Cambridge, MA, USA, 2003. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).