Chemometric Comparison and Classification of 22 Apple Genotypes Based on Texture Analysis and Physico-Chemical Quality Attributes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physico-Chemical Quality Attributes
2.3. Texture Analysis
2.4. Statistical Analyses
3. Results and Discussion
3.1. Physico-Chemical Properties
3.2. Peel Color
3.3. Texture Profile
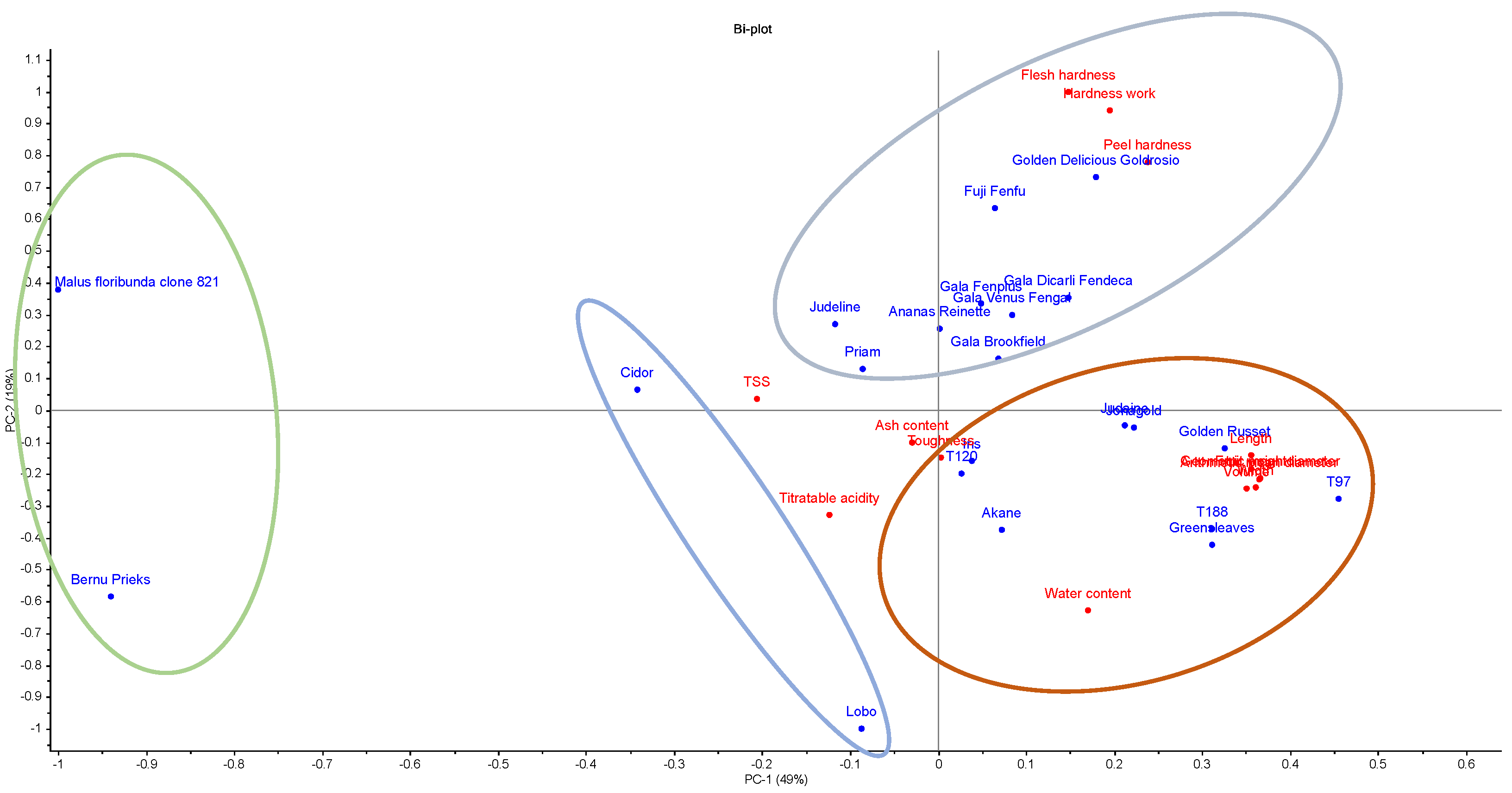
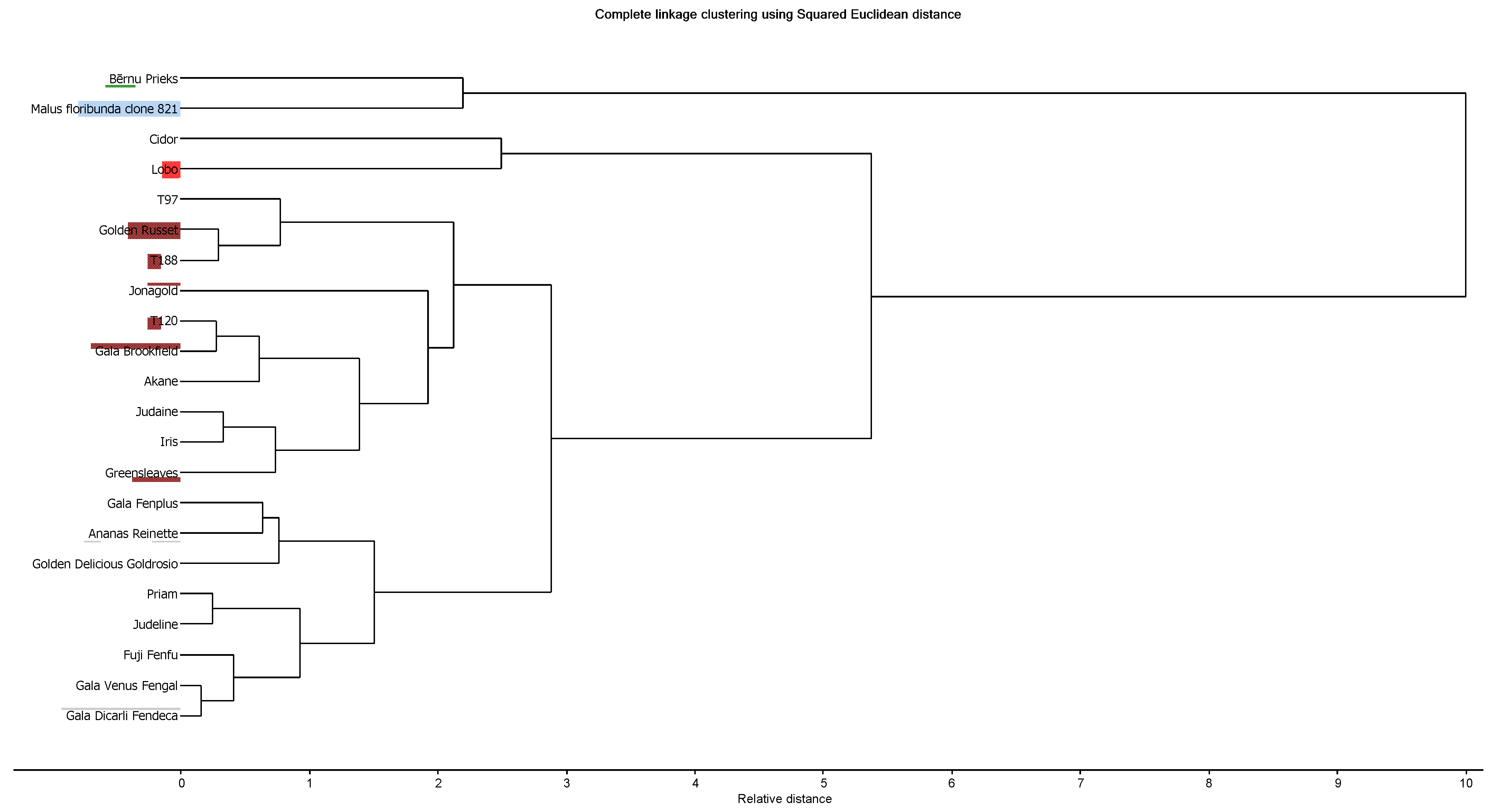
3.4. Chemometric Comparison and Classification
3.5. Final Considerations and Verification of Hypotheses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Data. Available online: http://www.fao.org/faostat (accessed on 29 December 2021).
- Musacchi, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Wuqian, W.; Jean-Marc, C.; Gerhard, B.-S.; Sandrine, B.; Etienne, B.; François, L. Skin Color in Apple Fruit (Malus × domestica): Genetic and Epigenetic Insights. Epigenomes 2020, 4, 13. [Google Scholar]
- Kunihisa, M.; Hayashi, T.; Hatsuyama, Y.; Fukasawa-Akada, T.; Uenishi, H.; Matsumoto, T.; Kon, T.; Kasai, S.; Kudo, T.; Oshino, H.; et al. Genome-wide association study for apple flesh browning: Detection, validation, and physiological roles of QTLs. Tree Genet. Genomes 2021, 17, 11. [Google Scholar] [CrossRef]
- Igarashi, M.; Hatsuyama, Y.; Harada, T.; Fukasawa-Akada, T. Biotechnology and apple breeding in Japan. Breed Sci 2016, 66, 18–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oluwole, O.O.; Olajide, I.F. Effect of pre-storage hot air and hot water treatments on post-harvest quality of mango (Mangifera indica Linn.) fruit. Not. Sci. Biol. 2020, 12, 634. [Google Scholar] [CrossRef]
- Kingston, C.M. Maturity indices for apple and pear. Hortic. Rev. 1993, 13, 407–432. [Google Scholar]
- Shewfelt, R.L. What is quality? Postharvest Biol. Technol. 1999, 15, 197–200. [Google Scholar] [CrossRef]
- Vanoli, M.; Buccheri, M. Overview of the methods for assessing harvest maturity. Stewart Postharvest Rev. 2012, 8, 1–11. [Google Scholar] [CrossRef]
- Dobrzański, B.; Rybczyński, R. Colour change of apple as a result of storage, shelf-life, and bruising. Int. Agrophys. 2002, 16, 261–268. [Google Scholar]
- Bae, R.N.; Lee, S.K. Influence of chlorophyll, internal ethylene, and PAL on anthocyanin synthesis in ‘Fuji’ apple. J. Korean Soc. Hortic. Sci. 1995, 36, 361–370. [Google Scholar]
- Malcolm Brown, R.; Saxena, I.M.; Kudlicka, K. Cellulose biosynthesis in higher plants. Trends Plant Sci. 1996, 1, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Hampson, C.R.; Quamme, H.A.; Hall, J.W.; MacDonald, R.A.; King, M.C.; Cliff, M.A. Sensory evaluation as a selection tool in apple breeding. Euphytica 2000, 111, 79–90. [Google Scholar] [CrossRef]
- Duizer, L. A review of acoustic research for studying the sensory perception of crisp, crunchy and crackly textures. Trends Food Sci. Technol. 2001, 12, 17–24. [Google Scholar] [CrossRef]
- Tabatabaeefar, A.; Rajabipour, A. Modeling the mass of apples by geometrical attributes. Sci. Hortic. 2005, 105, 373–382. [Google Scholar] [CrossRef]
- Armin, Z.; Mohsen, A.; Azim, G. Modeling of volume and surface area of apple from their geometric characteristics and artificial neural network. Int. J. Food Prop. 2017, 20, 1532–2386. [Google Scholar]
- Hurtado, G.; Lüdeke, P.; Knoche, M. Nondestructive Determination of Fruit Surface Area Using Archimedean Buoyancy. HortScience Horts 2020, 55, 1647–1653. [Google Scholar] [CrossRef]
- FAO/IPGRI. FAO/IPGRI Multi-Crop Passport Descriptors; Food and Agriculture Organization of the United Nations and International Plant Genetic Resources Institute: Rome, Italy, 2001. [Google Scholar]
- Bramel, P.; Volk, G.M. A Global Strategy for the Conservation and Use of Apple Genetic Resources; Global Crop Diversity Trust: Bonn, Germany, 2019. [Google Scholar]
- Watkins, R.; Smith, R.A. Descriptor List for Apple (Malus); International Board for Plant Genetic Resources, Rome/Commission of European Communities: Brussels, Belgium, 1982. [Google Scholar]
- UPOV. Apple, Fruit Varieties, Malus domestica Borkh. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability TG/14/9; International Union for the Protection of New Varieties of Plants: Geneva, Switzerland, 2005. [Google Scholar]
- Maggioni, L.; Janes, R.; Hayes, A.; Swinburne, T.; Lipman, E. Report of a Working Group on Malus/Pyrus: First Meeting, 15–17 May 1997, Dublin, Ireland; International Plant Genetic Resources Institute: Rome, Italy, 1998. [Google Scholar]
- Liu, Y.-F.; Zhang, J.-H.; Lu, B.; Yang, X.-H.; Li, Y.-G.; Wang, Y.; Wang, J.-M.; Zhang, H.; Guan, J.-J. Statistic Analysis on Quantitative Characteristics for Developing the DUS Test Guideline of Ranunculus asiaticus L. J. Integr. Agric. 2013, 12, 971–978. [Google Scholar] [CrossRef]
- Janes, R.; Jones, J.I. The use of multivariate discriminant analysis in compiling minimum descriptor lists for Malus. In Report of a Working Group on Malus/Pyrus. First Meeting, Dublin, Ireland, 15–17 May 1997; International Plant Genetic Resources Institute: Rome, Italy, 1998; pp. 24–30. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants. Bbch Monograph, 2nd ed.; Federal Biological Research Centre for Agriculture and Forestry: Bonn, Germany, 2001. [Google Scholar]
- Mohsenin, N.N. Physical Properties of Plant and Animal Materials; Gordon and Breach Science Publisher: New York, NY, USA, 1986; pp. 841–881. [Google Scholar]
- Qiu, X.; Zhang, H.; Zhang, H.; Duan, C.; Xiong, B.; Wang, Z. Fruit Textural Characteristics of 23 Plum (Prunus salicina Lindl) Cultivars: Evaluation and Cluster Analysis. HortScience Horts 2021, 56, 816–823. [Google Scholar] [CrossRef]
- Bejaei, M.; Stanich, K.; Cliff, M. Modelling and Classification of Apple Textural Attributes Using Sensory, Instrumental and Compositional Analyses. Foods 2021, 10, 384. [Google Scholar] [CrossRef]
- Jan, I.; Rab, A.; Sajid, M.; Sciences, P. Storage performance of apple cultivars harvested at different stages of maturity. J. Anim. Plant Sci. 2012, 22, 438–447. [Google Scholar]
- Kumar, P.; Sethi, S.; Sharma, R.R.; Singh, S.; Saha, S.; Sharma, V.K.; Verma, M.K.; Sharma, S.K. Nutritional characterization of apple as a function of genotype. J. Food Sci. Technol. 2018, 55, 2729–2738. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Gao, H.; Zhao, L.; Liao, X.; Chen, F.; Wang, Z.; Hu, X. Chemical compositional characterization of some apple cultivars. Food Chem. 2007, 103, 88–93. [Google Scholar] [CrossRef]
- Beyer, M.; Hahn, R.; Peschel, S.; Harz, M.; Knoche, M. Analysing fruit shape in sweet cherry (Prunus avium L.). Sci. Hortic. 2002, 96, 139–150. [Google Scholar] [CrossRef]
- Ma, T.; Sun, X.; Zhao, J.; You, Y.; Lei, Y.; Gao, G.; Zhan, J. Nutrient compositions and antioxidant capacity of kiwifruit (Actinidia) and their relationship with flesh color and commercial value. Food Chem. 2017, 218, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, L.; Yaniv, Y.; Kaplunov, T.; Doron-Faigenboim, A.; Carmi, N.; Porat, R. Diversity in sensory quality and determining factors influencing mandarin flavor liking. J. Food Sci. 2015, 80, S418–S425. [Google Scholar] [CrossRef]
- Nardozza, S.; Gamble, J.; Axten, L.G.; Wohlers, M.W.; Clearwater, M.J.; Feng, J.; Harker, F.R. Dry matter content and fruit size affect flavour and texture of novel Actinidia deliciosa genotypes. J. Sci. Food Agric. 2011, 91, 742–748. [Google Scholar] [CrossRef]
- AMcClure, K.; Gong, Y.H.; Song, J.; Vinqvist-Tymchuk, M.; Palmer, L.C.; Fan, L.; Burgher-MacLellan, K.; Zhang, Z.; Celton, J.M.; FForney, C.; et al. Genome-wide association studies in apple reveal loci of large effect controlling apple polyphenols. Hortic. Res. 2019, 6, 444–455. [Google Scholar]
- Ayour, J.; Le Bourvellec, C.; Gouble, B.; Audergon, J.M.; Benichou, M.; Renard, C. Changes in cell wall neutral sugar composition related to pectinolytic enzyme activities and intra-flesh textural property during ripening of ten apricot clones. Food Chem. 2021, 339, 128096. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; George, N.; Sobti, B.; AlRashidi, N.; Ghnimi, S.; Ali, A.A.; Andersson, A.A.M.; Andersson, R.; Antony, A.P.; Hamed, F. Dietary fiber components, microstructure, and texture of date fruits (Phoenix dactylifera, L.). Sci. Rep. 2020, 10, 21767. [Google Scholar] [CrossRef]
- Bejaei, M.; Cliff, M.A.; Singh, A. Multiple Correspondence and Hierarchical Cluster Analyses for the Profiling of Fresh Apple Customers Using Data from Two Marketplaces. Foods 2020, 9, 873. [Google Scholar] [CrossRef]
- Hooks, T.; Niu, G.; Masabni, J.; Sun, Y.; Ganjegunte, G. Performance and Phytochemical Content of 22 Pomegranate (Punica granatum) Varieties. HortScience 2021, 56, 217–225. [Google Scholar] [CrossRef]
- Wang, F.; Huang, Y.; Wu, W.; Zhu, C.; Zhang, R.; Chen, J.; Zeng, J. Metabolomics Analysis of the Peels of Different Colored Citrus Fruits (Citrus reticulata cv. ‘Shatangju’) During the Maturation Period Based on UHPLC-QQQ-MS. Molecules 2020, 25, 396. [Google Scholar] [CrossRef] [Green Version]
- Kuras, A.; Antonius, K.; Kalendar, R.; Kruczy ´nska, D.; Korbin, M. Application of five DNA marker techniques to distinguish between five apple (Malus × domestica Borkh.) cultivars and their sports. J. Hortic. Sci. Biotechnol. 2013, 88, 790–794. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, I.; Echeverría, G.; Soria, Y. Differences in fruit colour development, anthocyanin content, fruit quality and consumer acceptability of eight ‘Gala’ apple strains. Sci. Hortic. 2008, 119, 32–40. [Google Scholar] [CrossRef]
- Dan, C.; Șerban, C.; Sestras, A.F.; Militaru, M.; Morariu, P.; Sestras, R.E. Consumer Perception Concerning Apple Fruit Quality, Depending on Cultivars and Hedonic Scale of Evaluation—A Case Study. Not. Sci. Biol. 2015, 7, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Sestras, R.E.; Pamfil, D.; Ardelean, M.; Botez, C.; Sestras, A.F.; Mitre, I.; Dan, C.; Mihalte, L. Use of Phenotypic and MAS Selection Based on Bulk Segregant Analysis to Reveal the Genetic Variability Induced by Artificial Hybridization in Apple. Not. Bot. Horti Agrobot. Cluj-Napoca 2009, 37, 135. [Google Scholar] [CrossRef]
- Laurens, F.; Aranzana, M.J.; Arus, P.; Bassi, D.; Bink, M.; Bonany, J.; Caprera, A.; Corelli-Grappadelli, L.; Costes, E.; Durel, C.-E.; et al. An integrated approach for increasing breeding efficiency in apple and peach in Europe. Hortic. Res. 2018, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dan, C.; Sestras, A.F.; Bozdog, C.; Sestras, R.E. Investigation of wild species potential to increase genetic diversity useful for apple breeding. Genetika 2015, 47, 993–1011. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Fischer, M. Breeding Apple (Malus × Domestica Borkh). In Breeding Plantation Tree Crops: Temperate Species; Gradziel, T.M., Ed.; Springer: New York, NY, USA, 2009; pp. 33–81. [Google Scholar]
- Sestras, A.F.; Pamfil, D.; Dan, C.; Bolboaca, S.D.; Jäntschi, L.; Sestras, R.E. Possibilities to improve apple scab (Venturia inaequalis (Cke.) Wint.) and powdery mildew (Podosphaera leucotricha (Ell. et Everh.) Salm.) resistance on apple by increasing genetic diversity using potentials of wild species. Aust. J. Crop Sci. 2011, 5, 748–755. [Google Scholar]
- Crosby, J.A.; Janick, J.; Pecknold, P.C.; Korban, S.S.; Oconnor, P.A.; Ries, S.M.; Goffreda, J.; Voordeckers, A. Breeding apples for scab resistance: 1945–1990. Fruit Var. J. 1992, 46, 145–166. [Google Scholar] [CrossRef]
- Radenkovs, V.; Kviesis, J.; Juhnevica-Radenkova, K.; Valdovska, A.; Püssa, T.; Klavins, M.; Drudze, I. Valorization of Wild Apple (Malus spp.) By-Products as a Source of Essential Fatty Acids, Tocopherols and Phytosterols with Antimicrobial Activity. Plants 2018, 7, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, F.; Evans, K.; Norelli, J.L.; Zhang, Z.; Peace, C. Prospects for achieving durable disease resistance with elite fruit quality in apple breeding. Tree Genet. Genomes 2020, 16, 21. [Google Scholar] [CrossRef]
- Felföldi, Z.; Ranga, F.; Socaci, S.A.; Farcas, A.; Plazas, M.; Sestras, A.F.; Vodnar, D.C.; Prohens, J.; Sestras, R.E. Physico-Chemical, Nutritional, and Sensory Evaluation of Two New Commercial Tomato Hybrids and Their Parental Lines. Plants 2021, 10, 2480. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.L.; Kostick, S.; Brutcher, L.; Schonberg, B.; Barritt, B.; Evans, K. Trends in Fruit Quality Improvement From 15 Years of Selection in the Apple Breeding Program of Washington State University. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Teh, S.L.; Kostick, S.A.; Evans, K.M. Genetics and Breeding of Apple Scions. In The Apple Genome; Korban, S.S., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 73–103. [Google Scholar]

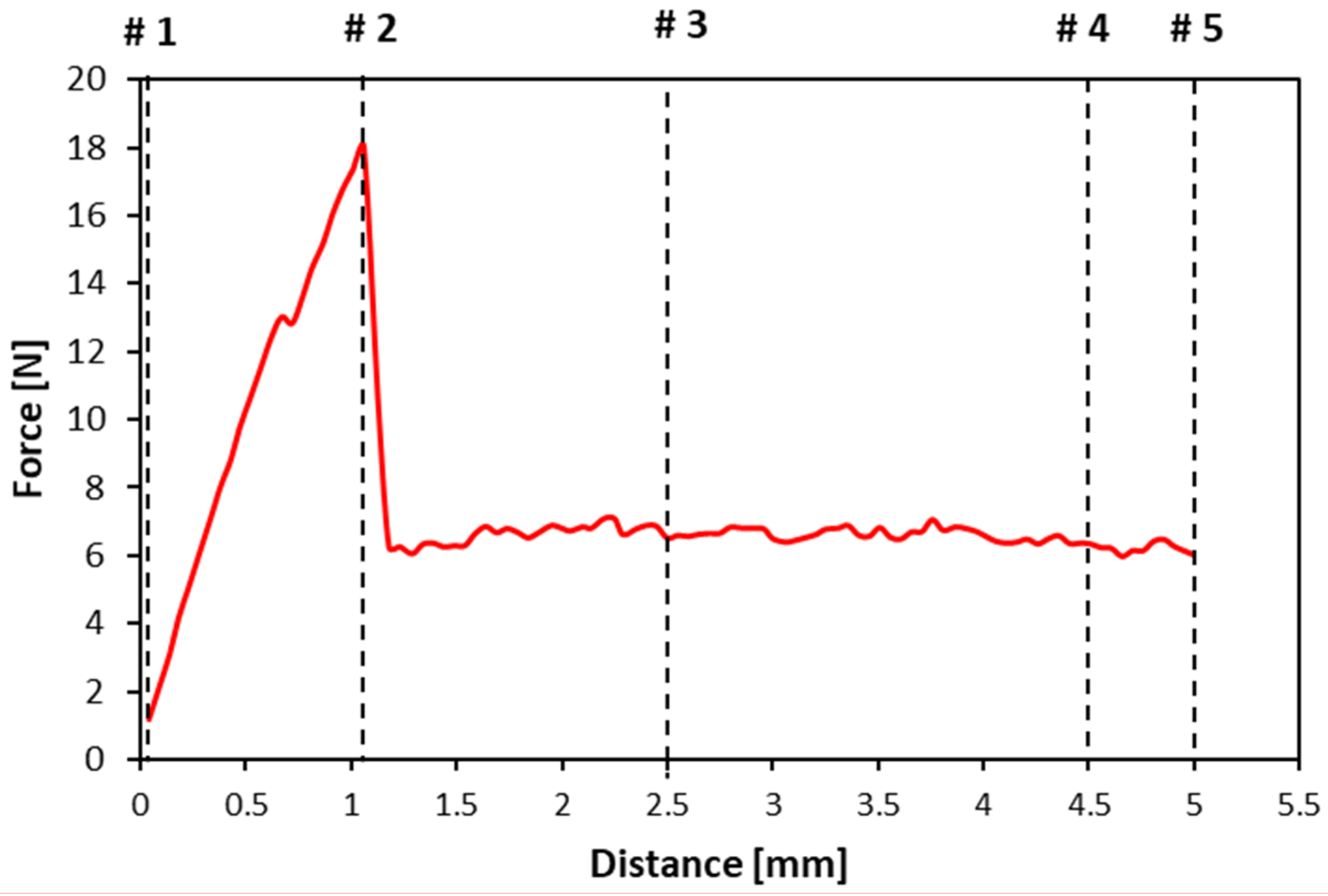
| Parameters | Units | Definition | Method of Calculation and Location on the Force-Distance Textural Curve |
|---|---|---|---|
| Peel hardness | N | The force at the maximum peak during the puncture testing | Shown by anchor #2 on Figure 2 |
| Toughness | mm | The displacement at the maximum force (peel hardness) during the puncture process | Measured from the distance between anchors #1 and #2 on the “Distance (mm)” x-axis of Figure 2 |
| Hardness work | mJ | The mechanical work conducted to rupture the peel and flesh to the target distance value (5 mm) | Calculated from the total area under the red textural curve between anchors #1 and #5 on Figure 2. The hardness work is calculated to the target distance, even though the hardness point may occur at the fracture |
| Flesh hardness | N | The average force between 2.5 and 4.5 mm depth of the probe where the steady state force (plateau) is reached | Calculated by the average of force recorded between anchor #3 and #4 on Figure 2 |
| Genotype | Fruit Weight (g) | Volume (mL) | Water Content (%) | Ash Content (%) | Total Soluble Solids (%) | Titratable Acidity (% Malic Acid) |
|---|---|---|---|---|---|---|
| M. floribunda clone 821 | 23.95 ± 3.90 f | 32.50 ± 5.00 e | 79.069 ± 1.24 h | 2.17 ± 0.94 a | 24.03 ± 0.60 a | 1.00 ± 0.02 c |
| Greensleaves | 161.29 ± 46.53 a,b | 237.50 ± 70.42 a | 88.063 ± 0.09 a | 1.55 ± 0.99 a | 12.97 ± 0.76 i,j | 0.40 ± 0.02 g,h,i |
| T188 | 171.42 ± 35.53 a,b | 225.00 ± 52.60 a,b | 85.980 ± 0.20 b,c,d | 2.38 ± 0.87 a | 14.63 ± 0.93 g,h,i,j | 0.79 ± 0.02 d |
| Fuji Fenfu | 100.52 ± 10.97 c,d,e | 127.50 ± 15.00 b,c,d,e | 85.315 ± 0.88 c,d,e | 1.93 ± 0.42 a | 12.80 ± 0.69 j,k | 0.35 ± 0.01 h,i |
| Iris | 127.75 ± 14.52 b,c,d,e | 155.00 ± 38.73 a,b,c | 86.454 ± 0.21 a,b,c,d | 1.83 ± 0.36 a | 16.80 ± 1.01 c,d,e,f,g | 0.61 ± 0.04e |
| Bērnu Prieks | 29.54 ± 5.69 f | 45.00 ± 12.91 d,e | 86.042 ± 0.07 b,c,d | 2.69 ± 0.15 a | 16.07 ± 1.33 d,e,f,g,h | 1.28 ± 0.02 a |
| Lobo | 112.92 ± 24.13 b,c,d,e | 162.50 ± 45.73 a,b,c | 86.671 ± 0.24 a,b,c,d | 2.50 ± 0.18 a | 16.63 ± 0.70 c,d,e,f,g | 0.55 ± 0.01 e,f |
| Gala Dicarli Fendeca | 134.48 ± 25.84 b,c | 167.50 ± 33.04 a,b,c | 86.657 ± 0.21 a,b,c,d | 1.88 ± 0.24 a | 16.63 ± 0.84 c,d,e,f,g | 0.20 ± 0.01 j,k |
| Judaine | 142.14 ± 24.38 a,b,c | 177.50 ± 35.94 a,b,c | 85.990 ± 0.92 b,c,d | 1.87 ± 0.28 a | 13.77 ± 0.12 h,i,j | 0.56 ± 0.02 e,f |
| Judeline | 75.16 ± 17.00d e,f | 102.50 ± 26.30 c,d,e | 85.102 ± 0.23 d,e | 1.85 ± 0.59 a | 15.07 ± 0.38 f,g,h,i,j | 0.49 ± 0.02 f,g |
| Golden Delicious Goldrosio | 127.81 ± 30.75 b,c,d,e | 175.00 ± 42.03 a,b,c | 81.339 ± 0.23 g | 2.91 ± 0.95 a | 15.63 ± 0.12 e,f,g,h,i | 0.61 ± 0.07 e |
| Golden Russet | 167.88 ± 39.97 a,b | 220.00 ± 52.28 a,b | 82.756 ± 0.81 f,g | 2.68 ± 0.65 a | 16.47 ± 2.16 c,d,e,f,g | 0.65 ± 0.01 e |
| Ananas Reinette | 127.70 ± 33.81 b,c,d,e | 165.00 ± 61.91 a,b,c | 82.173 ± 0.18 f,g | 2.79 ± 0.36 a | 19.73 ± 1.37 b | 0.40 ± 0.02 g,h,i |
| Cidor | 69.29 ± 18.65 e,f | 97.50 ± 17.08 c,d,e | 81.627 ± 0.17 g | 2.66 ± 0.39 a | 19.03 ± 0.29 b,c | 0.23 ± 0.03 j,k |
| Akane | 129.93 ± 16.65 b,c,d | 150.00 ± 40.82 a,b,c | 87.548 ± 0.07 a,b | 3.09 ± 0.49 a | 18.67 ± 0.45 b,c,d | 0.24 ± 0.01 j,k |
| Gala Brookfield | 119.01 ± 26.94 b,c,d,e | 152.50 ± 28.72 a,b,c | 85.180 ± 0.34 c,d,e | 2.44 ± 0.21 a | 16.40 ± 1.14 c,d,e,f,g,h | 0.30 ± 0.02 i,j |
| T97 | 199.94 ± 29.81 a | 247.50 ± 46.46 a | 87.108 ± 0.49 a,b,c | 2.08 ± 0.21 a | 17.20 ± 0.95 b,c,d,e,f,g | 1.17 ± 0.05 b |
| Gala Fenplus | 125.51 ± 18.82 b,c,d,e | 152.50 ± 26.30 a,b,c | 83.630 ± 0.44 e,f | 2.85 ± 0.20 a | 13.17 ± 0.21 i,j | 0.57 ± 0.01 e,f |
| Gala Venus Fengal | 128.18 ± 11.70 b,c,d | 152.50 ± 17.08 a,b,c | 85.230 ± 0.13 c,d,e | 1.47 ± 0.43 a | 15.27 ± 0.31 f,g,h,i,j | 0.18 ± 0.01 k |
| Jonagold | 135.18 ± 20.07 b,c | 170.00 ± 31.62 a,b,c | 86.967 ± 0.50 a,b,c,d | 3.38 ± 0.35 a | 10.13 ± 0.12 k | 0.77 ± 0.04 d |
| Priam | 101.04 ± 15.46 c,d,e | 135.00 ± 23.80 b,c,d | 85.158 ± 0.08 c,d,e | 1.98 ± 0.91 a | 18.30 ± 0.85 b,c,d,e | 0.42 ± 0.01 g,h |
| T120 | 118.85 ± 29.66 b,c,d,e | 160.00 ± 43.20 a,b,c | 87.139 ± 0.52 a,b,c | 2.17 ± 0.45 a | 17.40 ± 0.00 b,c,d,e,f | 0.35 ± 0.01 h,i |
| Mean | 119.52 | 155.00 | 85.05 | 2.32 | 16.22 | 0.55 |
| Coefficient of Variation (%) | 35.17 | 34.58 | 2.74 | 22.1 | 17.78 | 54.3 |
| Genotype | Fruit Dimension (mm) | |||
|---|---|---|---|---|
| Width | Length | Geometric Mean Diameter | Arithmetic Mean Diameter | |
| M. floribunda clone 821 | 36.83 ± 2.19 e | 36.35 ± 2.66 d | 36.10 ± 2.09 e | 36.11 ± 0.86 d |
| Greensleaves | 74.18 ± 10.15 a,b | 59.60 ± 9.69 a,b | 67.97 ± 9.89 a,b,c,d | 68.27 ± 7.67 a,b |
| T188 | 75.84 ± 6.31 a,b | 62.05 ± 6.99 a,b | 70.06 ± 4.64 a,b,c | 70.32 ± 7.29 a,b |
| Fuji Fenfu | 63.40 ± 2.38 b,c,d | 52.08 ± 4.71 b | 58.81 ± 3.15 b,c,d | 59.03 ± 6.09 a,b |
| Iris | 68.29 ± 2.47 a,b,c,d | 55.65 ± 5.21 a,b | 62.40 ± 3.34 a,b,c,d | 62.63 ± 6.42 a,b |
| Bērnu Prieks | 38.80 ± 4.31 e | 36.70 ± 3.03 c,d | 37.52 ± 4.06 e | 37.53 ± 1.12 c,d |
| Lobo | 69.50 ± 3.94 a,b,c,d | 58.50 ± 3.45 a,b | 64.79 ± 3.09 a,b,c,d | 64.97 ± 5.75 a,b |
| Gala Dicarli Fendeca | 65.45 ± 3.90 a,b,c,d | 56.18 ± 3.59 a,b | 61.29 ± 3.85 a,b,c,d | 61.42 ± 4.75 a,b |
| Judaine | 73.60 ± 1.62 a,b | 57.35 ± 2.70 a,b | 66.16 ± 1.57 a,b,c,d | 66.52 ± 8.32 a,b |
| Judeline | 58.60 ± 5.75 c,d | 56.30 ± 5.98 a,b | 57.23 ± 6.64 c,d | 57.24 ± 1.21 a,b |
| Golden Delicious Goldrosio | 66.48 ± 7.61 a,b,c,d | 58.48 ± 7.78 a,b | 62.79 ± 7.68 a,b,c,d | 62.88 ± 4.06 a,b |
| Golden Russet | 78.90 ± 3.45 a | 62.85 ± 1.35 a,b | 71.48 ± 1.97 a,b | 71.80 ± 8.18 a,b |
| Ananas Reinette | 66.93 ± 7.73 a,b,c,d | 54.79 ± 4.92 a,b | 62.09 ± 6.67 a,b,c,d | 62.33 ± 6.58 a,b |
| Cidor | 57.58 ± 3.80 d | 50.65 ± 5.46 b,c | 54.65 ± 4.18 d | 54.73 ± 3.63 b,c |
| Akane | 71.10 ± 1.40 a,b,c | 58.00 ± 2.90 a,b | 65.90 ± 1.48 a,b,c,d | 66.17 ± 7.12 a,b |
| Gala Brookfield | 65.63 ± 2.23 a,b,c,d | 57.40 ± 1.30 a,b | 62.29 ± 1.75 a,b,c,d | 62.40 ± 4.39 a,b |
| T97 | 76.78 ± 5.67 a,b | 67.03 ± 6.30 a | 72.76 ± 6.45 a,b,c,d | 72.88 ± 5.16 a,b |
| Gala Fenplus | 63.30 ± 4.84 b,c,d | 55.88 ± 6.90 a,b | 59.87 ± 6.12 a,b,c,d | 59.95 ± 3.76 a,b |
| Gala Venus Fengal | 64.78 ± 3.87 b,c,d | 54.75 ± 4.49 a,b | 60.60 ± 4.24 a,b,c,d | 60.76 ± 5.30 a |
| Jonagold | 69.30 ± 2.98 a,b,c,d | 58.30 ± 2.37 a,b | 64.64 ± 2.55 a,b,c,d | 64.82 ± 5.78 a,b |
| Priam | 58.43 ± 2.45 c,d | 59.28 ± 2.23 a,b | 58.13 ± 2.03 b,c,d | 58.14 ± 1.30 a,b |
| T120 | 69.13 ± 9.46 a,b,c,d | 54.43 ± 9.58 a,b | 63.00 ± 9.61 a | 63.33 ± 7.83 a,b |
| Genotype | Background Color | Covering Color | Overlapping Color | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | L* | a* | b* | L* | a* | b* | |
| T97 | 66.55 ± 2.49 | −6.11 ± 0.69 | 40.85 ± 1.65 | - | - | - | |||
| T120 | 69.69 ± 2.02 | −4.12 ± 0.78 | 39.56 ± 1.12 | - | - | - | - | - | |
| Greensleaves | 65.90 ± 1.15 | −5.70 ± 0.45 | 42.08 ± 1.88 | - | - | - | - | - | - |
| T188 | 70.00 ± 1.73 | −4.66 ± 0.60 | 41.96 ± 1.30 | - | - | - | - | - | - |
| Gala Fenplus | 38.80 ± 2.78 | 34.75 ± 1.22 | 12.92 ± 1.29 | - | - | - | |||
| Gala Dicarli Fendeca | 42.76 ± 3.16 | 34.35 ± 2.56 | 14.85 ± 1.89 | 51.45 ± 5.22 | 30.09 ± 3.92 | 19.259 ± 1.4 | - | ||
| Gala Venus Fengal | 50.89 ± 3.51 | 40.60 ± 2.13 | 19.31 ± 0.93 | 60.45 ± 5.93 | 23.63 ± 5.17 | 24.79 ± 3.46 | |||
| M. floribunda clone 821 | 68.04 ± 1.95 | 26.54 ± 2.05 | 35.24 ± 1.70 | 42.46 ± 2.79 | 39.88 ± 2.51 | 16.56 ± 2.96 | - | - | - |
| Bērnu Prieks | 53.54 ± 3.73 | 30.21 ± 3.19 | 14.039 ± 2.12 | 38.28 ± 2.76 | 34.47 ± 3.26 | 10.58 ± 1.41 | |||
| Golden Russet | 65.30 ± 1.47 | −7.63 ± 0.51 | 40.44 ± 1.29 | 65.24 ± 1.48 | 5.388 ± 1.87 | 39.28 ± 3.62 | - | - | - |
| Jonagold | 72.73 ± 1.84 | 1.68 ± 0.47 | 35.58 ± 1.54 | 46.52 ± 2.34 | 37.3 ± 1.30 | 17.87 ± 2.71 | - | - | - |
| Fuji Fenfu | 67.87 ± 1.79 | 0.82 ± 0.02 | 31.05 ± 1.03 | - | - | - | 57.14 ± 5.3 | 15.76 ± 6.37 | 22.18 ± 5.28 |
| Lobo | 77.44 ± 1.03 | −0.45 ± 0.09 | 33.39 ± 2.14 | - | - | - | 65.34 ± 2.52 | 22.32 ± 3.7 | 25.21 ± 2.28 |
| Judeline | 73.14 ± 2.04 | −6.51 ± 0.98 | 37.31 ± 1.47 | - | - | - | 60.95 ± 3.08 | 20.46 ± 6.75 | 25.38 ± 3.80 |
| Golden Delicious Goldrosio | 71.17 ± 2.43 | −2.74 ± 0.16 | 39.56 ± 1.42 | - | - | - | 68.17 ± 0.61 | 12.32 ± 4.35 | 32.71 ± 0.98 |
| Gala Brookfield | 74.313.60 | 0.37 ± 0.48 | 33.68 ± 3.34 | 60.99 ± 7.95 | 22.33 ± 8.49 | 26.53 ± 3.26 | |||
| Ananas Reinette | 62.19 ± 1.55 | −3.99 ± 0.17 | 42.04 ± 2.55 | 51.78 ± 1.76 | 11.83 ± 1.25 | 26.85 ± 1.77 | 58.12 ± 1.3 | 3.14 ± 3.06 | 35.55 ± 1.54 |
| Cidor | 70.37 ± 2.94 | −0.13 ± 1.61 | 43.97 ± 1.40 | 59.14 ± 3.33 | 15.22 ± 2.27 | 34.00 ± 1.57 | 63.11 ± 3.93 | 12.1 ± 2.59 | 37.37 ± 2.77 |
| Iris | 74.84 ± 1.65 | −0.74 ± 0.09 | 40.88 ± 3.62 | 41.81 ± 2.56 | 40.42 ± 1.65 | 18.73 ± 3.08 | 61.26 ± 6.64 | 24.17 ± 5.58 | 29.81 ± 2.97 |
| Judaine | 73.44 ± 1.71 | 1.17 ± 0.37 | 39.69 ± 1.44 | 58.02 ± 1.07 | 33.11 ± 4.27 | 24.75 ± 3.15 | 59.45 ± 5.96 | 25.29 ± 4.26 | 27.96 ± 3.26 |
| Akane | 74.74 ± 1.91 | 0.58 ± 0.67 | 31.07 ± 3.27 | 33.90 ± 1.51 | 30.18 ± 2.79 | 9.41 ± 1.35 | 50.04 ± 5.37 | 31.29 ± 2.67 | 15.98 ± 0.70 |
| Priam | 79.01 ± 1.09 | 0.77 ± 0.62 | 33.13 ± 1.23 | 46.83 ± 1.62 | 37.69 ± 0.91 | 16.71 ± 0.81 | 67.18 ± 1.46 | 13.84 ± 2.97 | 27.47 ± 2.21 |
| Genotype | Peel Hardness (N) | Toughness (mm) | Flesh Hardness (N) | Hardness Work (mJ) |
|---|---|---|---|---|
| M. floribunda clone 821 | 5.80 ± 0.52 f,g | 1.17 ± 0.13 b | 3.08 ± 0.39 b,c,d | 16.26 ± 1.01 f |
| Greensleaves | 7.90 ± 0.85 e,f | 1.30 ± 0.26 b | 2.79 ± 0.55 c,d | 17.60 ± 1.65 d,e |
| T188 | 8.88 ± 1.19 d,e,f | 1.29 ± 0.10 b | 2.92 ± 0.45 b,c,d | 18.33 ± 2.62 c,d,e |
| Fuji Fenfu | 13.69 ± 1.36 a,b | 1.23 ± 0.09 b | 4.47 ± 0.52 a,b | 27.84 ± 1.38 a,b |
| Iris | 9.35 ± 2.78 d,e | 1.49 ± 0.36 a,b | 3.09 ± 0.83 b,c,d | 18.36 ± 3.70 d,e |
| Bērnu Prieks | 3.80 ± 0.39 g | 1.16 ± 0.29 b | 1.06 ± 0.30 e | 7.08 ± 1.48 f |
| Lobo | 3.98 ± 0.78 g | 1.44 ± 0.22 a,b | 0.97 ± 0.17 e | 6.88 ± 0.87 f |
| Gala Dicarli Fendeca | 11.76 ± 1.70 a,b,c,d | 1.22 ± 0.09 b | 4.20 ± 0.85 a,b,c | 25.08 ± 2.14 a,b,c |
| Judaine | 10.06 ± 1.36 c,d,e | 1.29 ± 0.14 b | 3.34 ± 0.43 a,b,c,d | 21.42 ± 2.03 b,c,d,e |
| Judeline | 10.19 ± 1.49 c,d,e | 1.42 ± 0.14 a,b | 3.70 ± 0.77 a,bc,d | 22.62 ± 3.28 a,bc,d,e |
| Golden Delicious Goldrosio | 15.00 ± 2.59 a | 1.30 ± 0.16 b | 4.76 ± 1.14 a | 28.90 ± 5.15 a |
| Golden Russet | 10.58 ± 1.42 b,cd,e | 1.25 ± 0.27 b | 3.07 ± 0.87 b,c,d | 19.90 ± 4.71 c,d,e |
| Ananas Reinette | 9.67 ± 1.20 d,e | 1.26 ± 0.06 b | 3.85 ± 1.05 a,b,c,d | 21.90 ± 3.02 b,c,d,e |
| Cidor | 7.58 ± 1.83 e,f | 1.87 ± 0.22 a | 2.86 ± 0.66 c,d | 17.60 ± 3.91 d,e |
| Akane | 8.51 ± 1.30 d,e,f | 1.28 ± 0.31 b | 2.36 ± 0.38 d,e | 16.38 ± 1.39 e |
| Gala Brookfield | 10.65 ± 1.36 b,c,d,e | 1.16 ± 0.08 b | 3.42 ± 0.38 a,b,c,d | 22.12 ± 2.80 b,c,d,e |
| T97 | 10.95 ± 0.76 b,c,d,e | 1.28 ± 0.19 b | 3.46 ± 0.53 a,b,c,d | 21.80 ± 2.45 b,c,d,e |
| Gala Fenplus | 10.97 ± 0.73 b,c,d,e | 1.07 ± 0.06 b | 4.01 ± 0.86 a,b,c | 23.72 ± 3.10 a,b,c,d |
| Gala Venus Fengal | 11.44 ± 1.24 b,c,d | 1.29 ± 0.32 b | 3.72 ± 0.65 a,b,c,d | 22.96 ± 3.06 a,b,c,d |
| Jonagold | 13.22 ± 0.78 a,b,c | 1.48 ± 0.23 a,b | 3.04 ± 0.48 b,c,d | 22.38 ± 2.47 b,c,d,e |
| Priam | 8.51 ± 2.13 d,e,f | 1.34 ± 0.13 b | 3.69 ± 0.75 a,b,c,d | 20.70 ± 1.65 c,d,e |
| T120 | 8.52 ± 0,94 d,e,f | 1.09 ± 0.27 b | 2.83 ± 0.44 c,d | 17.50 ± 1.25 d,e |
| Physico-Chemical Quality Attributes | Fruit Weight (g) | Volume (mL) | Water Content (%) | Ash Content (%) | Total Soluble Solids (%) | Titratable Acidity (% Malic Acid) | Width (mm) | Length (mm) | Geometric Mean Diameter (mm) | Arithmetic Mean Diameter (mm) | Peel Hardness (N) | Toughness (mm) | Hardness Work (mJ) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Volume (mL) | 0.981 | ||||||||||||
| Water Content (%) | 0.443 | 0.435 | |||||||||||
| Ash Content (%) | −0.056 NS | −0.076 NS | −0.237 NS | ||||||||||
| Total Soluble Solids (%) | −0.411 NS | −0.423 NS | −0.524 | −0.012 NS | |||||||||
| Titratable Acidity (%Malic Acid) | −0.115 NS | −0.108 NS | −0.087 NS | 0.205 NS | 0.052 NS | ||||||||
| Width (mm) | 0.940 | 0.935 | 0.465 | −0.013 NS | −0.441 | −0.298 NS | |||||||
| Length (mm) | 0.899 | 0.895 | 0.417 NS | −0.027 NS | −0.400 NS | −0.264 NS | 0.919 | ||||||
| Geometric Mean Diameter (mm) | 0.943 | 0.938 | 0.463 | −0.011 NS | −0.434 | −0.300 NS | 0.992 | 0.960 | |||||
| Arithmetic Mean Diameter (mm) | 0.943 | 0.938 | 0.464 | −0.011 NS | −0.434 | −0.301 NS | 0.993 | 0.958 | 1.000 | ||||
| Peel Hardness (N) | 0.463 | 0.395 NS | −0.042 NS | 0.038 NS | −0.476 | −0.283 NS | 0.429 | 0.471 | 0.452 | 0.449 | |||
| Toughness (mm) | −0.085 NS | −0.054 NS | −0.075 NS | 0.074 NS | 0.009 NS | −0.182 NS | 0.061 NS | 0.116 NS | 0.080 NS | 0.078 NS | −0.077 NS | ||
| Hardness Work (mJ) | 0.345 NS | 0.283 NS | −0.196 NS | −0.116 NS | −0.297 NS | −0.357 NS | 0.291 NS | 0.373 NS | 0.323 NS | 0.321 NS | 0.941 | −0.110 NS | |
| Flesh Hardness (N) | 0.242 NS | 0.187 NS | −0.318 NS | −0.198 NS | −0.134 NS | −0.343 NS | 0.167 NS | 0.271 NS | 0.202 NS | 0.199 NS | 0.849 | −0.148 NS | 0.972 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mureșan, A.E.; Sestras, A.F.; Militaru, M.; Păucean, A.; Tanislav, A.E.; Pușcaș, A.; Mateescu, M.; Mureșan, V.; Marc, R.A.; Sestras, R.E. Chemometric Comparison and Classification of 22 Apple Genotypes Based on Texture Analysis and Physico-Chemical Quality Attributes. Horticulturae 2022, 8, 64. https://doi.org/10.3390/horticulturae8010064
Mureșan AE, Sestras AF, Militaru M, Păucean A, Tanislav AE, Pușcaș A, Mateescu M, Mureșan V, Marc RA, Sestras RE. Chemometric Comparison and Classification of 22 Apple Genotypes Based on Texture Analysis and Physico-Chemical Quality Attributes. Horticulturae. 2022; 8(1):64. https://doi.org/10.3390/horticulturae8010064
Chicago/Turabian StyleMureșan, Andruța E., Adriana F. Sestras, Mădălina Militaru, Adriana Păucean, Anda E. Tanislav, Andreea Pușcaș, Mădălina Mateescu, Vlad Mureșan, Romina A. Marc (Vlaic), and Radu E. Sestras. 2022. "Chemometric Comparison and Classification of 22 Apple Genotypes Based on Texture Analysis and Physico-Chemical Quality Attributes" Horticulturae 8, no. 1: 64. https://doi.org/10.3390/horticulturae8010064
APA StyleMureșan, A. E., Sestras, A. F., Militaru, M., Păucean, A., Tanislav, A. E., Pușcaș, A., Mateescu, M., Mureșan, V., Marc, R. A., & Sestras, R. E. (2022). Chemometric Comparison and Classification of 22 Apple Genotypes Based on Texture Analysis and Physico-Chemical Quality Attributes. Horticulturae, 8(1), 64. https://doi.org/10.3390/horticulturae8010064












.jpg)


