Effect of Exogenous Calcium on the Heat Tolerance in Rosa hybrida ‘Carolla’
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Treatments
2.2. Determination of Photosynthetic Responses
2.2.1. Determination of Calcium Treatment on Chlorophyll Fluorescence Parameters during Heat Stress
2.2.2. Determination of Calcium Treatment on Chlorophyll Content during Heat Stress
2.2.3. Determination of Calcium Treatment on Photosynthetic Indexes during Heat Stress
2.3. Determination of Enzymatic Activity and Malondialdehyde (MDA) Content
2.4. Determination of Relative Electrical Conductivity (REC) and Osmotic Substance Contents
2.5. Determination of Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results
3.1. Effects of Calcium Treatment on Photosynthetic Responses during Heat Stress
3.1.1. Effects of Calcium Treatment on Chlorophyll Fluorescence Parameters during Heat Stress
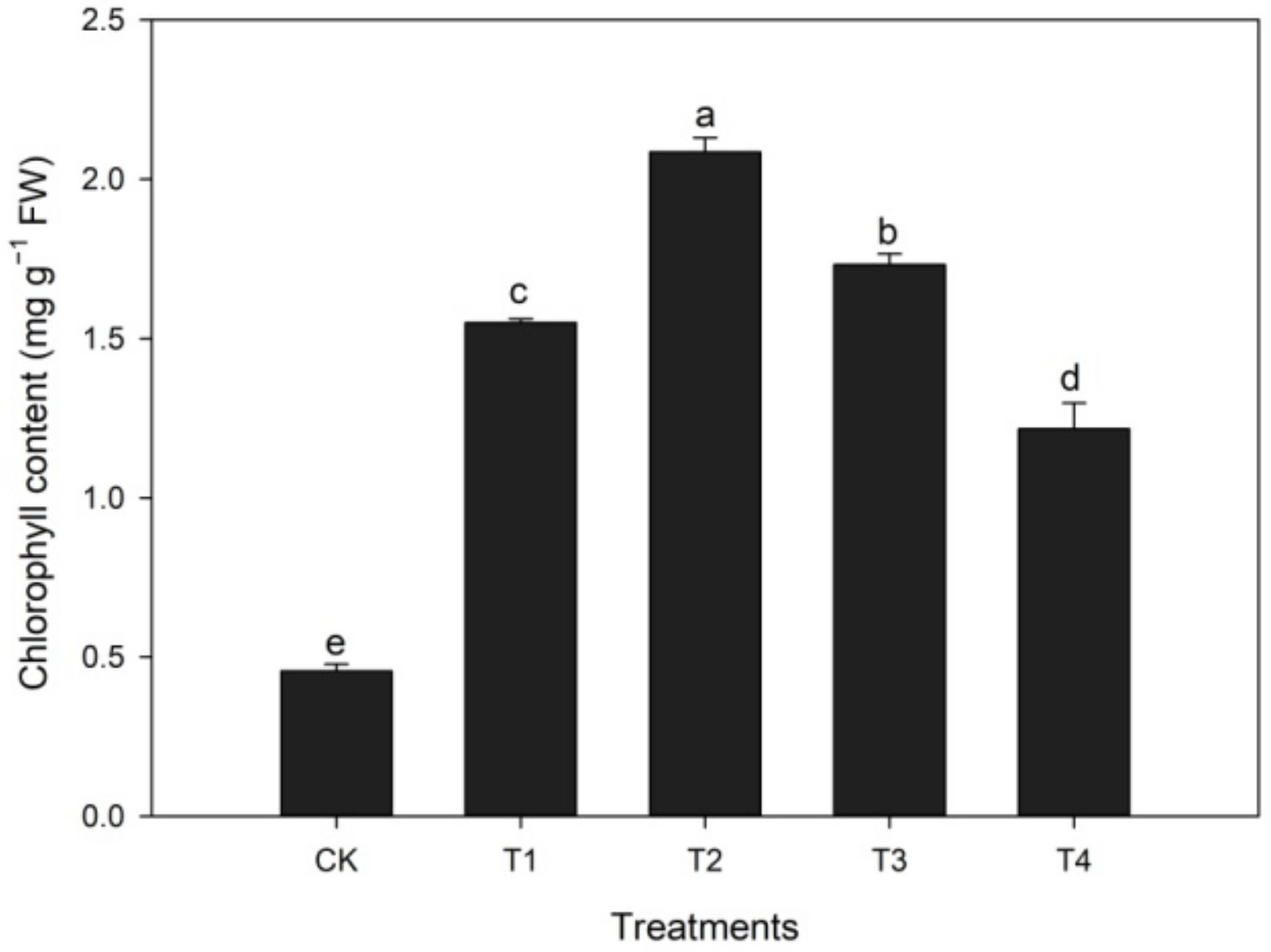
3.1.2. Effects of Calcium Treatment on Chlorophyll Content during Heat Stress
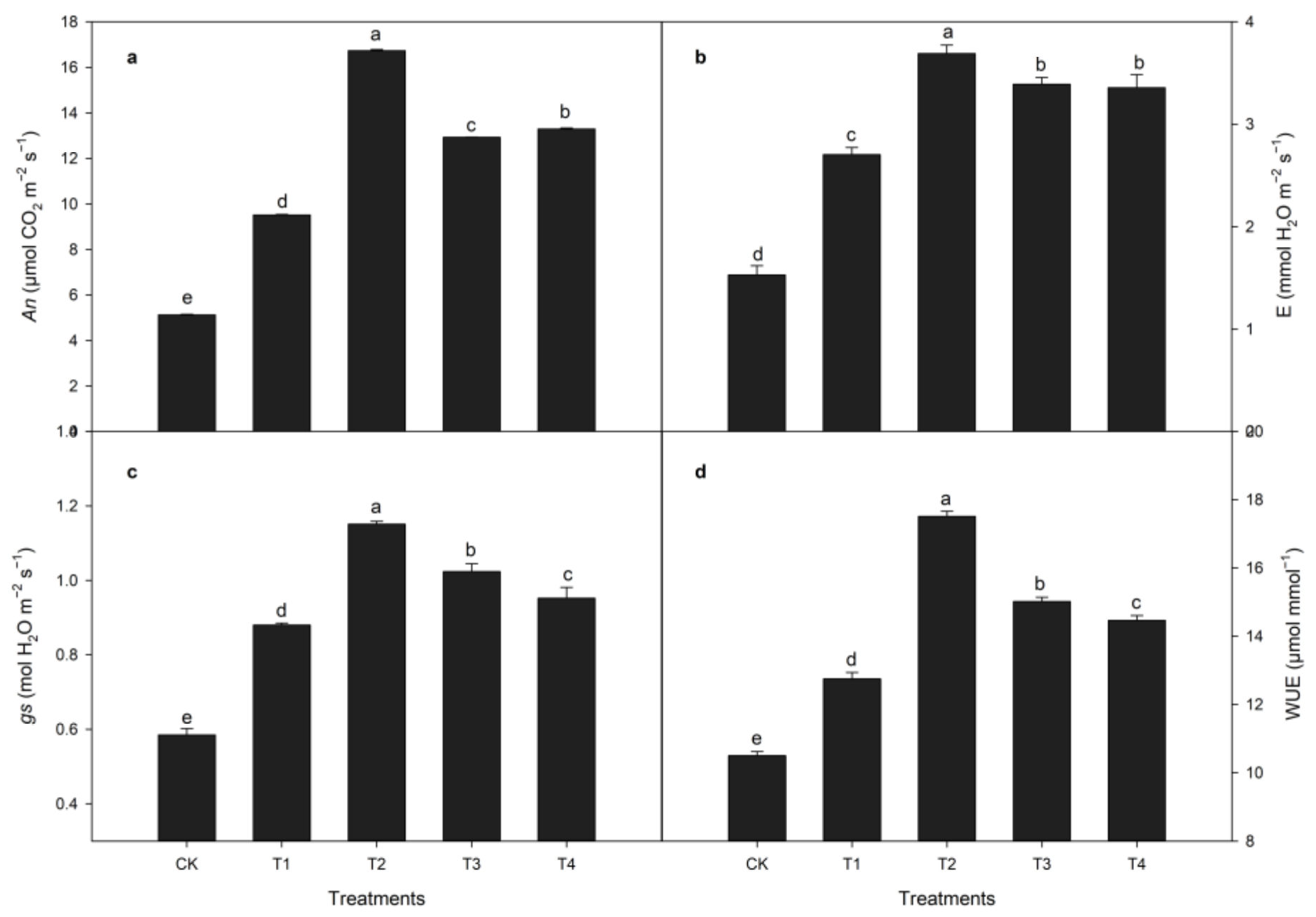
3.1.3. Effects of Calcium Treatment on Photosynthetic Indexes during Heat Stress
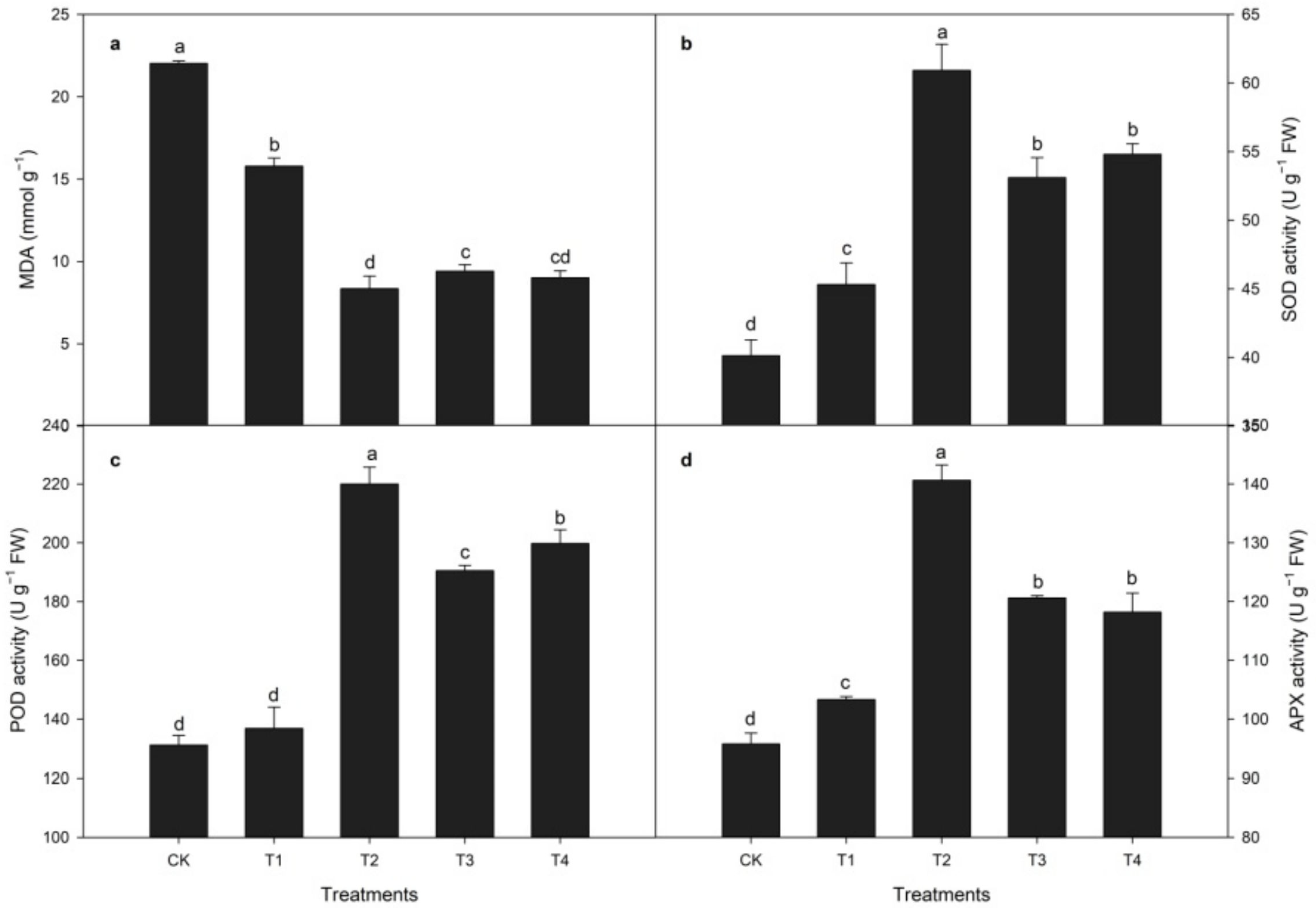
3.2. Effects of CaCl2 Treatment on Antioxidant Enzyme Activities and MDA Activity during Heat Stress
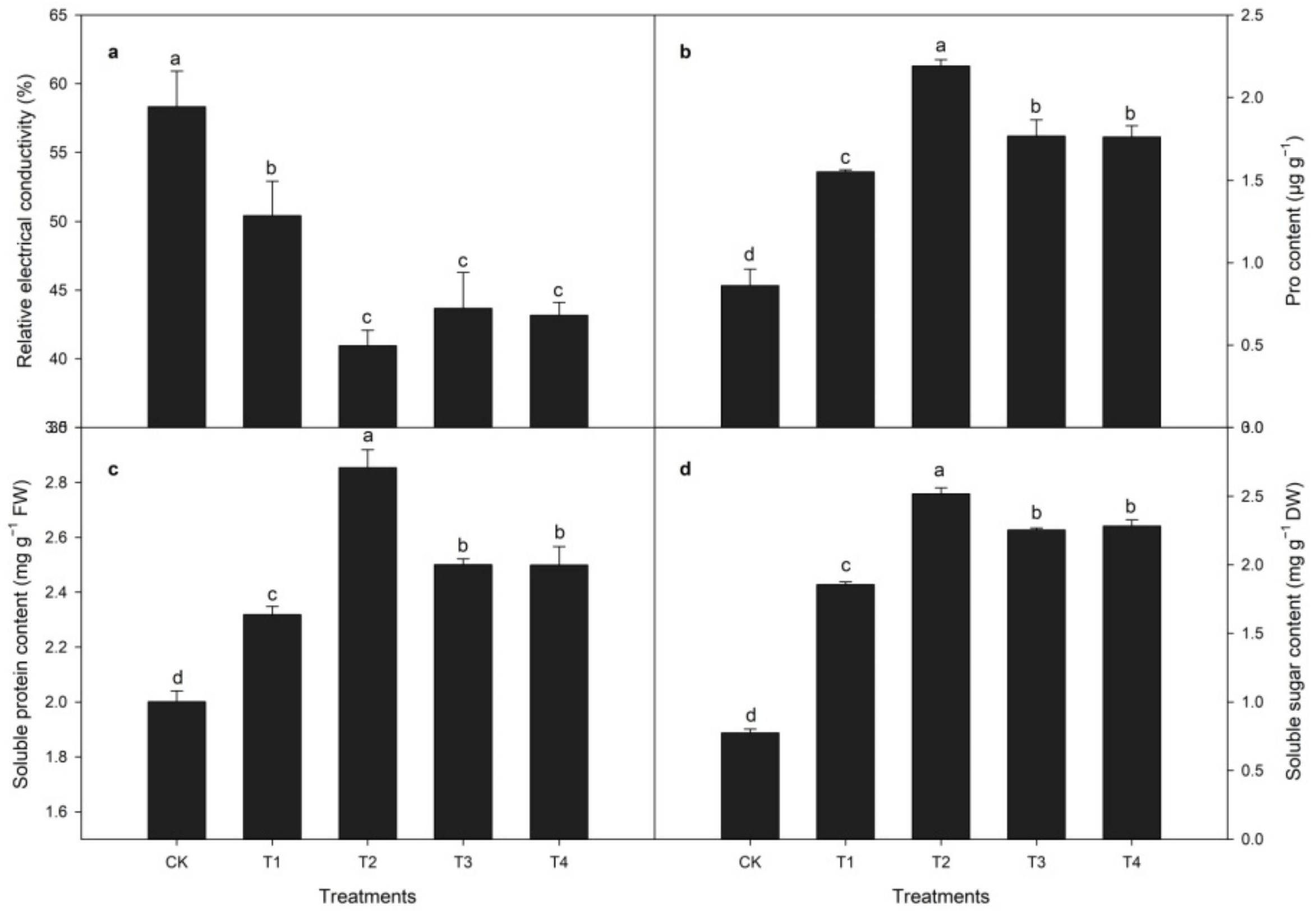
3.3. Relative Electrical Conductivity (REC) and Osmotic Substance Contents
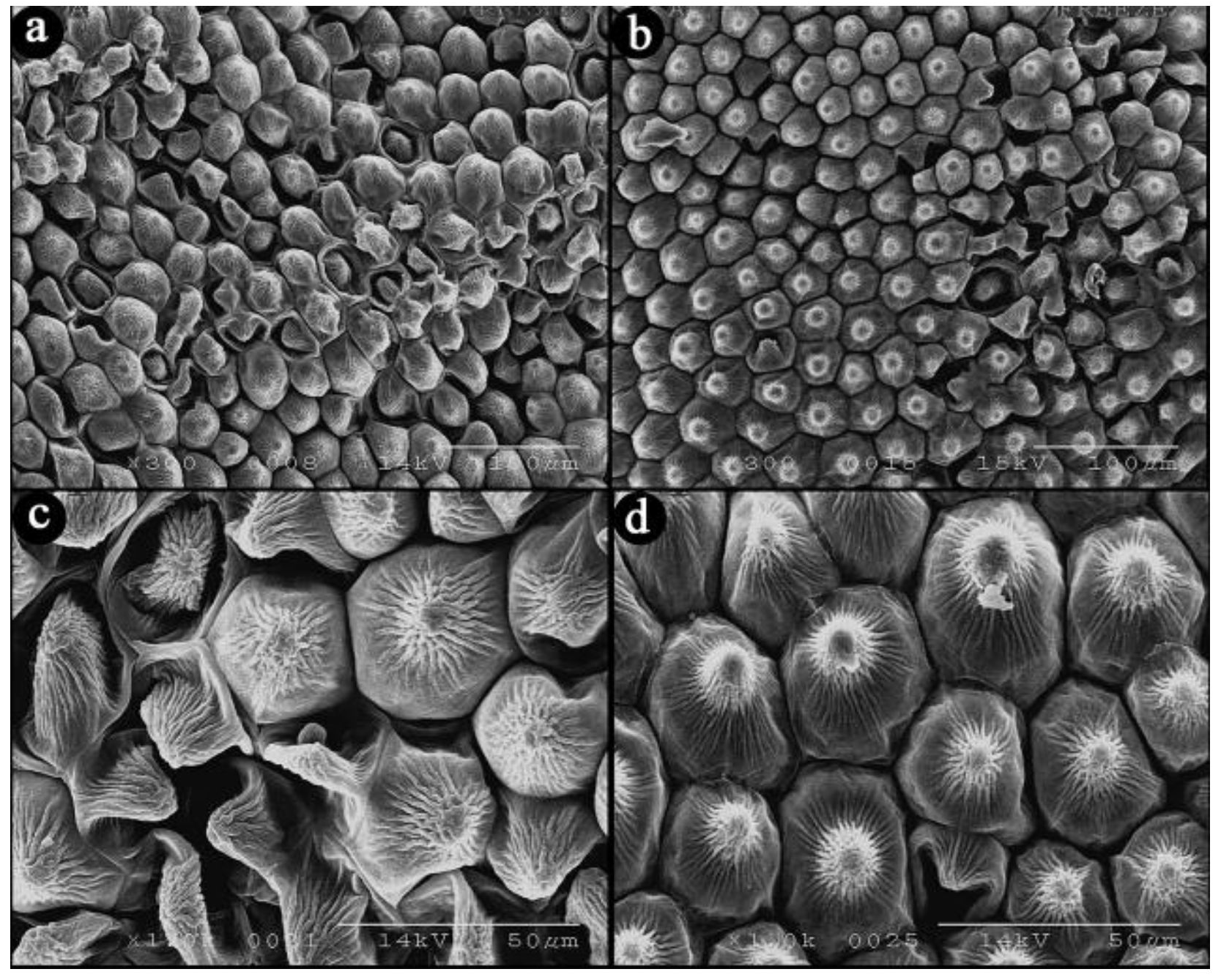
3.4. Effects of Calcium Treatment on Upper Epidermal Cells in Rosa hybrida ‘Carolla’ during Heat Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Zhao, B. Physiological and microstructural responses of two Rhododendron cultivars to high temperature and low light. Hortic. Environ. Biotechnol. 2020, 61, 445–458. [Google Scholar] [CrossRef]
- Wu, X.; Yao, X.; Chen, J.; Zhu, Z.; Zhang, H.; Zha, D. Brassinosteroids protect photosynthesis and antioxidant system of eggplant seedlings from high-temperature stress. Acta Physiol. Plant. 2014, 36, 251–261. [Google Scholar] [CrossRef]
- Lin, K.; Huang, S.; Wu, C.; Chang, Y. Effects of salicylic acid and calcium chloride on heat tolerance of poinsettia. HortScience 2018, 54, 499–504. [Google Scholar] [CrossRef]
- Han, Y.; Fan, S.; Zhang, Q.; Wang, Y. Effect of heat stress on the MDA, proline and soluble sugar content in leaf lettuce seedlings. Agric. Sci. 2013, 4, 112–115. [Google Scholar] [CrossRef]
- Nadeem, M.; Khan, M.A.; Riaz, A.; Ahmed, R. Evaluation of growth and flowering potential of Rosa hybrida cultivars under faisalabad climatic conditions. Pak. J. Agric. Res. 2011, 48, 283–288. [Google Scholar]
- Cho, S.-C.; Chao, Y.-Y.; Kao, C.H. Calcium deficiency increases Cd toxicity and Ca is required for heat-shock induced Cd tolerance in rice seedlings. J. Plant Physiol. 2012, 169, 892–898. [Google Scholar] [CrossRef]
- Yan, H.; Jiang, Y.; Huang, C.; Deng, J.; He, J.; Wang, X.; Bu, Z. The Tissue Culture and Rapid Regeneration of Rosa hybrida ‘Carolla’. Chin. J. Trop. Crops 2016, 37, 1741–1746. [Google Scholar]
- Shi, L.; Wang, Z.; Kim, W.S. Effect of drought stress on shoot growth and physiological response in the cut rose ‘charming black’ at different developmental stages. Hortic. Environ. Biotechnol. 2019, 60, 1–8. [Google Scholar] [CrossRef]
- Zaccai, M.; Ackerman, R.; Genis, O.; Riov, J.; Zik, M. The bent peduncle phenomenon in roses is a developmental process involving auxin. Plant Sci. 2009, 176, 736–743. [Google Scholar] [CrossRef]
- Shin, H.; Lieth, J.; Kim, S. Effects of temperature on leaf area and flower size in rose. Acta Hortic. 2001, 547, 185–191. [Google Scholar] [CrossRef]
- Qi, W.; Zhang, C.; Wang, W.; Cao, Z.; Li, S.; Li, H.; Zhu, W.; Huang, Y.; Bao, M.; He, Y.; et al. Comparative transcriptome analysis of different heat stress responses between self-root grafting line and heterogeneous grafting line in rose. Hortic. Plant J. 2021, 7, 243–255. [Google Scholar] [CrossRef]
- Jiang, C.; Bi, Y.; Zhang, R.; Feng, S. Expression of RcHSP70, heat shock protein 70 gene from Chinese rose, enhances host resistance to abiotic stresses. Sci. Rep. 2020, 10, 2445. [Google Scholar] [CrossRef]
- Bheemanahalli, R.; Gajanayake, B.; Lokhande, S.; Singh, K.; Seepaul, R.; Collins, P.; Reddy, K.R. Physiological and pollen-based screening of shrub roses for hot and drought environments. Sci. Hortic. 2021, 282, 110062. [Google Scholar] [CrossRef]
- Xie, L.; Zhang, H.; Li, D. Physiological responses of garden roses to hot and humid conditions. Hortic. Sci. 2019, 46, 26–33. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, X.; Li, Q.; Chen, X.; Li, X. Comparative transcriptome analysis to elucidate the enhanced thermotolerance of tea plants (Camellia sinensis) treated with exogenous calcium. Planta 2019, 249, 775–786. [Google Scholar] [CrossRef]
- Nuzhyna, N.V.; Tkachuk, O.O. Various antioxidant responses to hyperthermia in anatomically different species of the genus Rosa. Biosyst. Divers. 2019, 27, 193–199. [Google Scholar] [CrossRef]
- Li, Z.Q.; Xing, W.; Luo, P.; Zhang, F.J.; Jin, X.L.; Zhang, M.H. Comparative transcriptome analysis of Rosa chinensis ‘Slater’s crimson China’ provides insights into the crucial factors and signaling pathways in heat stress response. Plant Physiol. Biochem. 2019, 142, 312–331. [Google Scholar] [CrossRef]
- Senthil-Kumar, M.; Kumar, G.; Srikanthbabu, V.; Udayakumar, M. Assessment of variability in acquired thermotolerance: Potential option to study genotypic response and the relevance of stress genes. J. Plant Physiol. 2007, 164, 111–125. [Google Scholar] [CrossRef]
- Larkindale, J.; Knight, M.R. Protection against heat stress-induced oxidative damage in Arabidopsis involves calcium, abscisic acid, ethylene, and salicylic acid. Plant Physiol. 2002, 128, 682–695. [Google Scholar] [CrossRef]
- Shen, H.; Zhao, B.; Xu, J.; Zheng, X.; Huang, W. Effects of salicylic acid and calcium chloride on heat tolerance in Rhododendron ‘Fen Zhen Zhu’. J. Am. Soc. Hortic. Sci. 2016, 141, 363–372. [Google Scholar] [CrossRef]
- Bhatia, S.; Asthir, B. Calcium mitigates heat stress effect in wheat seeding growth by altering carbohydrate metabolism. Indian J. Plant Physiol. 2014, 19, 138–143. [Google Scholar] [CrossRef]
- Rastegar, S.; Shojaie, A.; Koy, R.A.M. Foliar application of salicylic acid and calcium chloride delays the loss of chlorophyll and preserves the quality of broccoli during storage. J. Food Biochem. 2022, 46, e14154. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Effects of calcium on antioxidant activities and water relations associated with heat tolerance in two cool-season grasses. J. Exp. Bot. 2001, 52, 341–349. [Google Scholar] [CrossRef]
- Zaman, Q.U.; Abbasi, A.; Tabassum, S.; Ashraf, K.; Ahmad, Z.; Siddiqui, M.H.; Alamri, S.; Maqsood, S.; Sultan, K. Calcium induced growth, physio-biochemical, antioxidants, osmolytes adjustments and phytoconstituents status in spinach under heat stress. S. Afr. J. Bot. 2022, 149, 701–711. [Google Scholar] [CrossRef]
- Shi, L.; He, S.; Wang, Z.; Kim, W.S. Stomatal and non-stomatal limitations of Rosa hybrida ‘Pink Bell’ under drought stress. Acta Hortic. 2020, 1291, 117–130. [Google Scholar] [CrossRef]
- Wang, J.; Yu, G.; Wang, B.; Qi, H.; Xu, Z. Response of photosynthetic rate and stomatal conductance of rice to light intensity and CO2 concentration in Northern China. Chin. J. Plant Ecol. 2005, 29, 16–25. [Google Scholar]
- Wu, J.; Xu, G.; Li, H.; Zeng, X.; Jiang, J.; Liu, Y.; Wei, Y.; Ren, H. Effects of Heat Stress on Chlorophyll Fluorescence and Photosynthetic Characteristic Parameters in Grape (Vitisvinifera L. ’Manicure finger’). Xinjiang Agric. Sci. 2021, 58, 2274–2281. [Google Scholar]
- Shi, L.; Kim, W.S. Effect of drought stress during supplemental lighting on diurnal photosynthesis of cut rose ‘Charming Black’. Hortic. Environ. Biotechnol. 2015, 56, 582–587. [Google Scholar] [CrossRef]
- Erinle, K.O.; Jiang, Z.; Ma, B.; Li, J.; Chen, Y.; Ur-Rehman, K.; Shahla, A.; Zhang, Y. Exogenous calcium induces tolerance to atrazine stress in Pennisetum seedlings and promotes photosynthetic activity, antioxidant enzymes and psbA gene transcripts. Ecotoxicol. Environ. Saf. 2016, 132, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Song, F.; Xu, H. Influence of arbuscular mycorrhiza on lipid peroxidation and antioxidant enzyme activity of maize plants under temperature stress. Mycorrhiza 2010, 20, 325–332. [Google Scholar] [CrossRef]
- Min, D.; Dong, L.; Shu, P.; Cui, X.; Zhang, X.; Li, F. The application of carbon dioxide and 1-methylcyclopropene to maintain fruit quality of ‘Niuxin’ persimmon during storage. Sci. Hortic. 2018, 229, 201–206. [Google Scholar] [CrossRef]
- Fan, H.; Li, T.; Sun, X.; Sun, X.; Zheng, C. Effects of humic acid derived from sediments on the postharvest vase life extension in cut chrysanthemum flowers. Postharvest Biol. Technol. 2015, 101, 82–87. [Google Scholar] [CrossRef]
- Sairam, R.K.; Rao, K.V.; Srivastava, G.C. Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 2002, 163, 1037–1046. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.; Li, Y.; Tan, Y.; Kong, J.; Li, H.; Li, Y.; Zhu, Y. Overexpression of SBPase enhances photosynthesis against high temperature stress in transgenic rice plants. Plant Cell Rep. 2007, 26, 1635–1646. [Google Scholar] [CrossRef]
- Jahan, M.S.; Wang, Y.; Shu, S.; Zhong, M.; Chen, Z.; Wu, J.; Sun, J.; Guo, S. Exogenous salicylic acid increases the heat tolerance in Tomato (Solanum lycopersicum L.) by enhancing photosynthesis efficiency and improving antioxidant defense system through scavenging of reactive oxygen species. Sci. Hortic. 2019, 247, 421–429. [Google Scholar] [CrossRef]
- Oh, S.; Moon, K.H.; Son, I.C.; Song, E.Y.; Moon, Y.E.; Koh, S.C. Growth, photosynthesis and chlorophyll fluorescence of Chinese Cabbage in response to high temperature. Korean J. Hortic. Sci. Technol. 2014, 32, 318–329. [Google Scholar] [CrossRef]
- Hu, W.; Tian, S.; Di, Q.; Duan, S.; Dai, K. Effects of exogenous calcium on mesophyll cell ultrastructure, gas exchange, and photosystem II in tobacco (Nicotiana tabacum Linn.) under drought stress. Photosynthetica 2018, 56, 1204–1211. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Sakran, A.M.; Basalah, M.O.; Ali, H.M. Effect of Calcium and Potassium on Antioxidant System of Vicia faba L. Under Cadmium Stress. Int. J. Mol. Sci. 2012, 13, 6604–6619. [Google Scholar] [CrossRef]
- Naeem, M.; Traub, J.R.; Athar, H.R.; Loescher, W. Exogenous calcium mitigates heat stress effects in common bean: A coordinated impact of photoprotection of PSII, up-regulating antioxidants, and carbohydrate metabolism. Acta Physiol. Plant. 2020, 42, 180. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Guo, F.; Meng, J.-J.; Li, X.-G.; Wan, S.-B. Calcium contributes to photoprotection and repair of photosystem II in peanut leaves during heat and high irradiance. J. Integr. Plant Biol. 2015, 57, 486–495. [Google Scholar] [CrossRef]
- Tan, W.; Meng, Q.w.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, G.; Qi, M.; Li, T. Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress. J. Plant Growth Regul. 2015, 34, 263–273. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B. Lipid- and calcium-signaling regulation of HsfA2c -mediated heat tolerance in tall fescue. Environ. Exp. Bot. 2017, 136, 59–67. [Google Scholar] [CrossRef]
- Larkindale, J.; Huang, B. Thermotolerance and antioxidant systems in Agrostis stolonifera: Involvement of salicylic acid, abscisic acid, calcium, hydrogen peroxide, and ethylene. J. Plant Physiol. 2004, 161, 405–413. [Google Scholar] [CrossRef]
- Shanbehpour, F.; Rastegar, S.; Ghasemi, M. Effect of preharvest application of calcium chloride, putrescine, and salicylic acid on antioxidant system and biochemical changes of two Indian jujube genotypes. J. Food Biochem. 2020, 44, e13474. [Google Scholar] [CrossRef]
- Gordeeva, A.V.; Zvyagilskaya, R.A.; Labas, Y.A. Cross-talk between reactive oxygen species and calcium in living cells. Biochemistry 2003, 68, 1077–1080. [Google Scholar] [CrossRef]
- Ahmad, P.; Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Kumar, A.; Ashraf, M. Upregulation of antioxidant and glyoxalase systems mitigates NaCl stress in Brassica juncea by supplementation of zinc and calcium. J. Plant Interact. 2018, 13, 151–162. [Google Scholar] [CrossRef]
- Goswami, S.; Kumar, R.R.; Sharma, S.K.; Kala, Y.K.; Singh, K.; Gupta, R.; Dhavan, G.; Rai, G.K.; Singh, G.P.; Pathak, H.; et al. Calcium triggers protein kinases-induced signal transduction for augmenting the thermotolerance of developing wheat (Triticum aestivum) grain under the heat stress. J. Plant Biochem. Biotechnol. 2015, 24, 441–452. [Google Scholar] [CrossRef]
- Zhao, H.-J.; Tan, J.-F. Role of calcium ion in protection against heat and high irradiance stress-induced oxidative damage to photosynthesis of wheat leaves. Photosynthetica 2005, 43, 473–476. [Google Scholar] [CrossRef]
- Ahmad, P.; Latef, A.A.A.; Abd_Allah, E.F.; Hashem, A.; Sarwat, M.; Anjum, N.A.; Gucel, S. Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed Chickpea (Cicer arietinum L.). Front. Plant Sci. 2016, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Marta, P.M.; Sergi, M.B. Ecophysiology of invasive plants: Osmotic adjustment and antioxidants. Trends Plant Sci. 2013, 18, 660–666. [Google Scholar] [CrossRef]
- Tang, Y.; Zhao, D.; Meng, J.; Tao, J. EGTA reduces the inflorescence stem mechanical strength of herbaceous peony by modifying secondary wall biosynthesis. Hortic. Res. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, Z.; Zhou, S.; Ou, L.; XZ Dai; Ma, Y.; Zhang, Z.; Chen, W.; Li, X.; Liang, C. Exogenous Ca2+ alleviates waterlogging-caused damages to pepper. Photosynthetica 2016, 54, 620–629. [Google Scholar] [CrossRef]
- Al-Whaibi, M.H.; Siddiqui, M.H.; Basalah, M.O. Salicylic acid and calcium-induced protection of wheat against salinity. Protoplasma 2012, 249, 769–778. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Xie, B.; Hu, S.; Zheng, Y.; Jin, P. Effects of exogenous calcium and calcium chelant on cold tolerance of postharvest loquat fruit. Sci. Hortic. 2020, 269, 109391. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Z.; Wang, C.; Lin, T. Exogenous Ca2+ alleviates nitrogen and water deficit, and improves growth of wheat (Triticum aestivum) seedlings exposed to high temperature. Plant Growth Regul. 2010, 61, 223–229. [Google Scholar] [CrossRef]
- Luo, C.; Peng, S.; Ma, X. Studies on changes of calcium, calmodulin and the floral substance content during flower bud differentiation in strawberry (Fragaria ananassa Duch.). J. Mt. Agric. Biol. 2000, 19, 266–271. [Google Scholar]
- Wu, Y.-Q.; Zhao, D.-Q.; Han, C.-X.; Tao, J. Biochemical and molecular responses of herbaceous peony to high temperature stress. Can. J. Plant Sci. 2016, 96, 474–484. [Google Scholar] [CrossRef]
- Li, Y.; Li, W.; Hu, D.; Lei, T.; Shen, P.; Li, J.; Gao, S. Metabolomics analysis reveals the role of cyanidin metabolism in Plumbago auriculata flower color. J. Plant Biol. 2021, 64, 253–265. [Google Scholar] [CrossRef]
- Shi, Q.; Li, L.; Zhou, L.; Wang, Y. Morphological and biochemical studies of the yellow and purple–red petal pigmentation in Paeonia delavayi. HortScience 2018, 53, 1102–1108. [Google Scholar] [CrossRef]
- Gong, M.; Li, Y.-J.; Chen, S.-Z. Abscisic acid-induced thermotolerance in maize seedlings is mediated by calcium and associated with antioxidant systems. J. Plant Physiol. 1998, 153, 488–496. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Shen, Y.; Wang, K.; He, S.; Kim, W.-S.; Shang, W.; Wang, Z.; Shi, L. Effect of Exogenous Calcium on the Heat Tolerance in Rosa hybrida ‘Carolla’. Horticulturae 2022, 8, 980. https://doi.org/10.3390/horticulturae8100980
Wang H, Shen Y, Wang K, He S, Kim W-S, Shang W, Wang Z, Shi L. Effect of Exogenous Calcium on the Heat Tolerance in Rosa hybrida ‘Carolla’. Horticulturae. 2022; 8(10):980. https://doi.org/10.3390/horticulturae8100980
Chicago/Turabian StyleWang, Han, Yuxiao Shen, Kaixuan Wang, Songlin He, Wan-Soon Kim, Wenqian Shang, Zheng Wang, and Liyun Shi. 2022. "Effect of Exogenous Calcium on the Heat Tolerance in Rosa hybrida ‘Carolla’" Horticulturae 8, no. 10: 980. https://doi.org/10.3390/horticulturae8100980
APA StyleWang, H., Shen, Y., Wang, K., He, S., Kim, W.-S., Shang, W., Wang, Z., & Shi, L. (2022). Effect of Exogenous Calcium on the Heat Tolerance in Rosa hybrida ‘Carolla’. Horticulturae, 8(10), 980. https://doi.org/10.3390/horticulturae8100980







