Chemical Composition, Antioxidant, Antimicrobial, Antibiofilm and Anti-Insect Activities of Jasminum grandiflorum Essential Oil
Abstract
1. Introduction
2. Materials and Methods
2.1. Essential Oil
2.2. Microorganisms
2.3. Identification of Volatile Constituents by Gas Chromatography (GC) and Gas Chromatography/Mass Spectrometry (GC/MS)
2.4. Antioxidant Activity
2.5. Antimicrobial Activity of J. grandiflorum EO
2.5.1. Disc Diffusion Method
2.5.2. Minimum Inhibitory Concentration (MIC)
2.6. Antibiofilm Activity
2.7. In Situ Antimicrobial Activity
2.8. Insecticidal Activity
2.9. Statistical Data Processing
3. Results
3.1. Identification of Volatile Constituents by Gas Chromatography (GC) and Gas Chromatography/Mass Spectrometry (GC/MS)
3.2. Antioxidant Activity
3.3. Disc Diffusion Method
3.4. Minimum Inhibitory Concentration (MIC)
3.5. Antibiofilm Activity
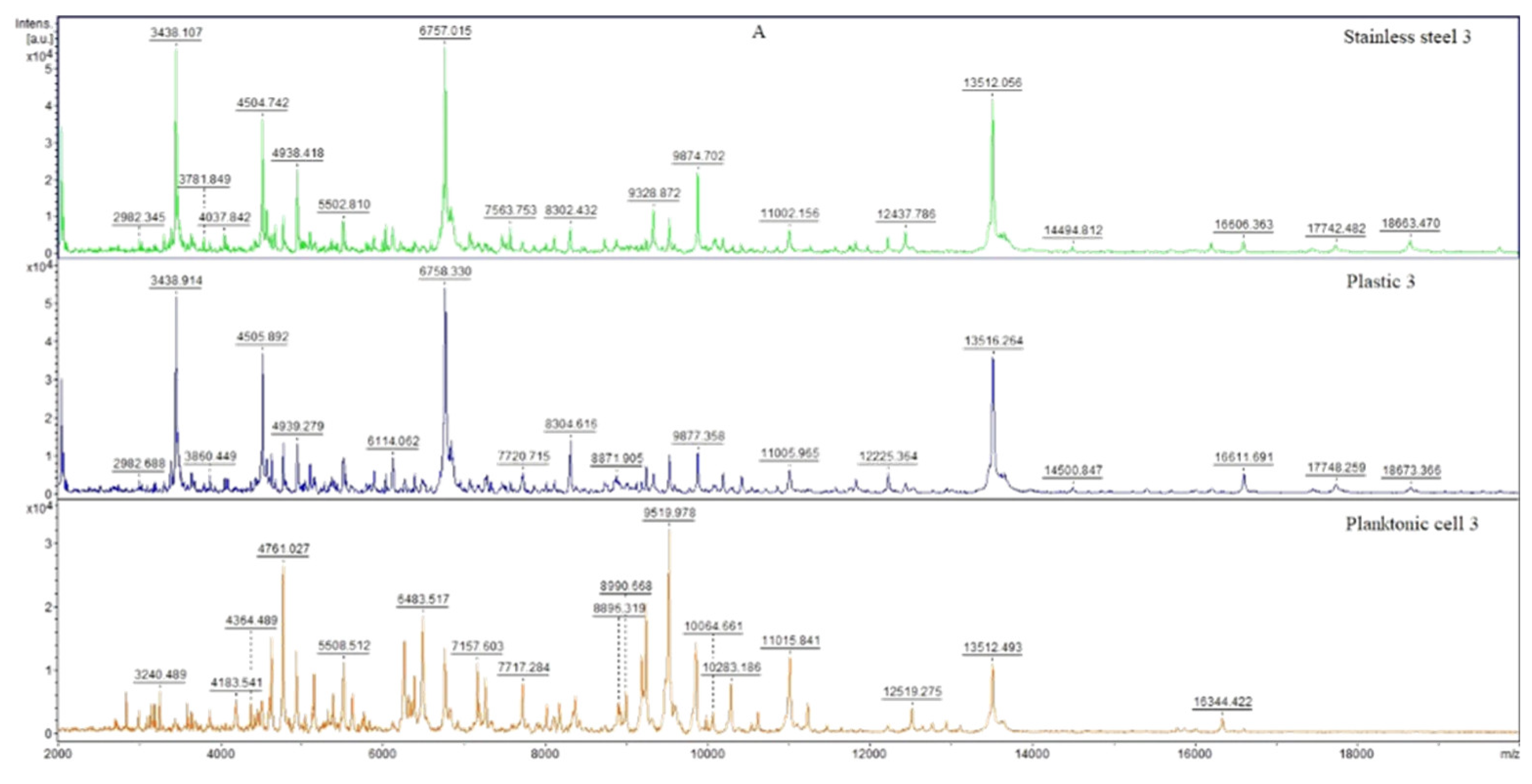
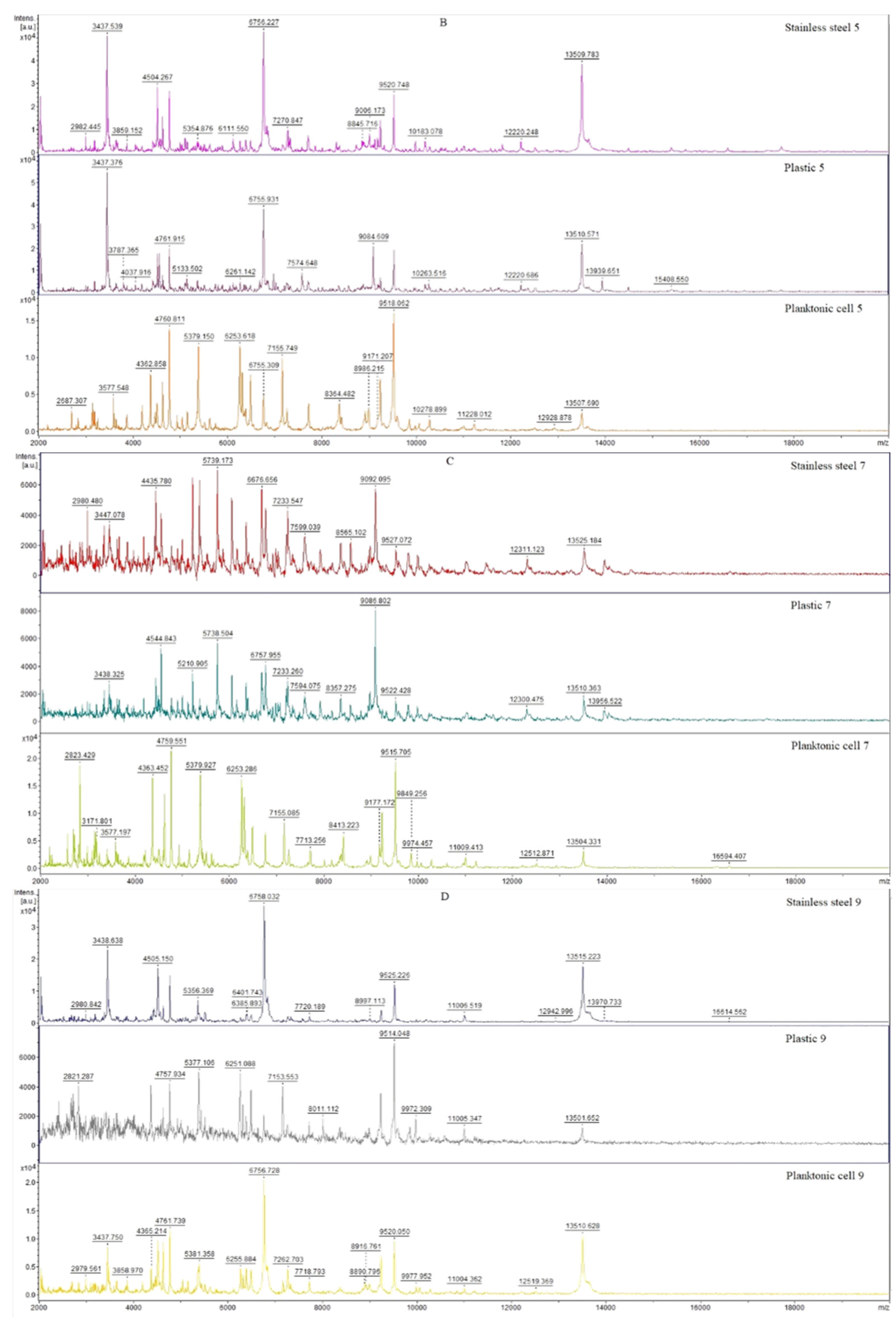
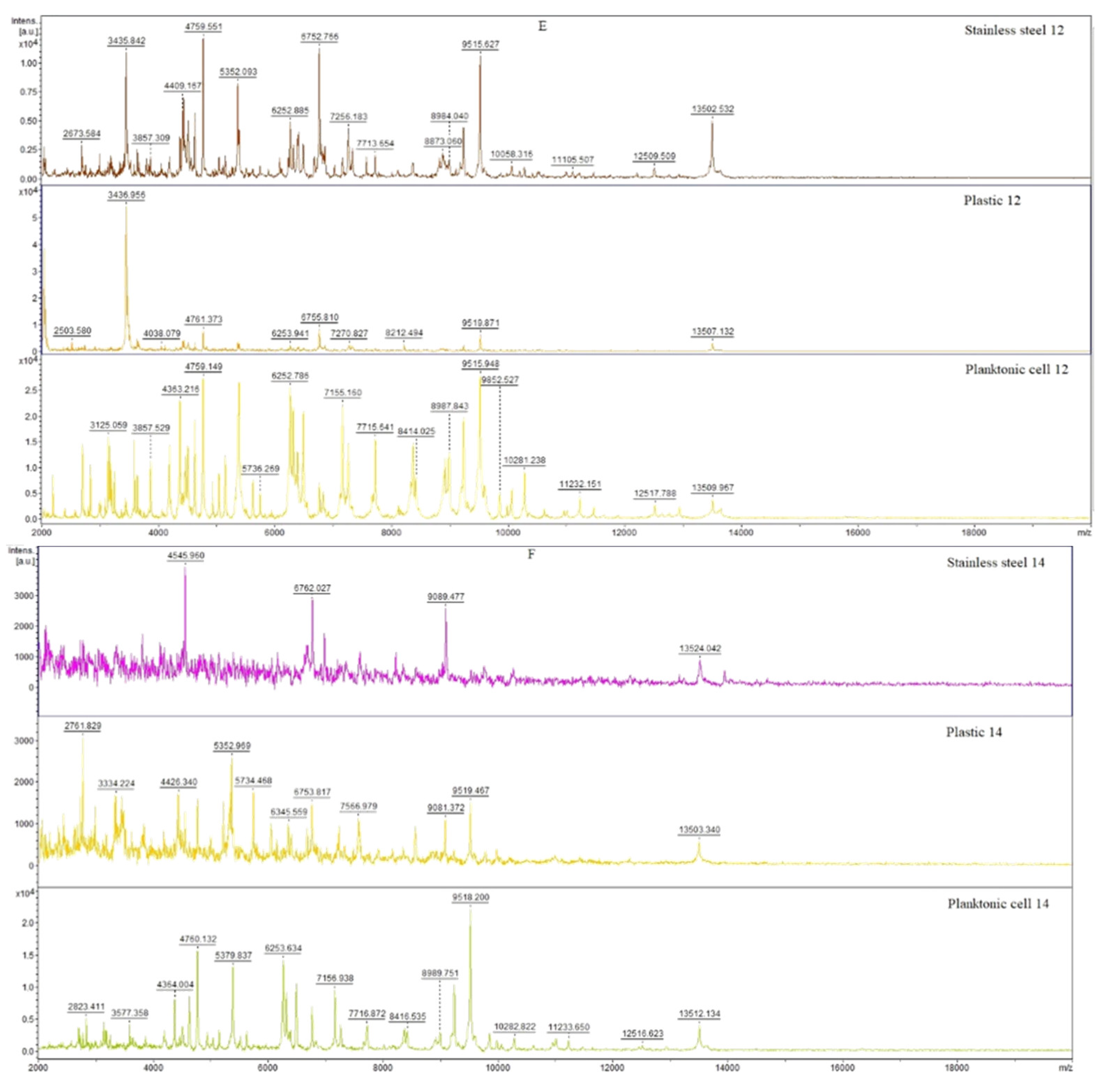
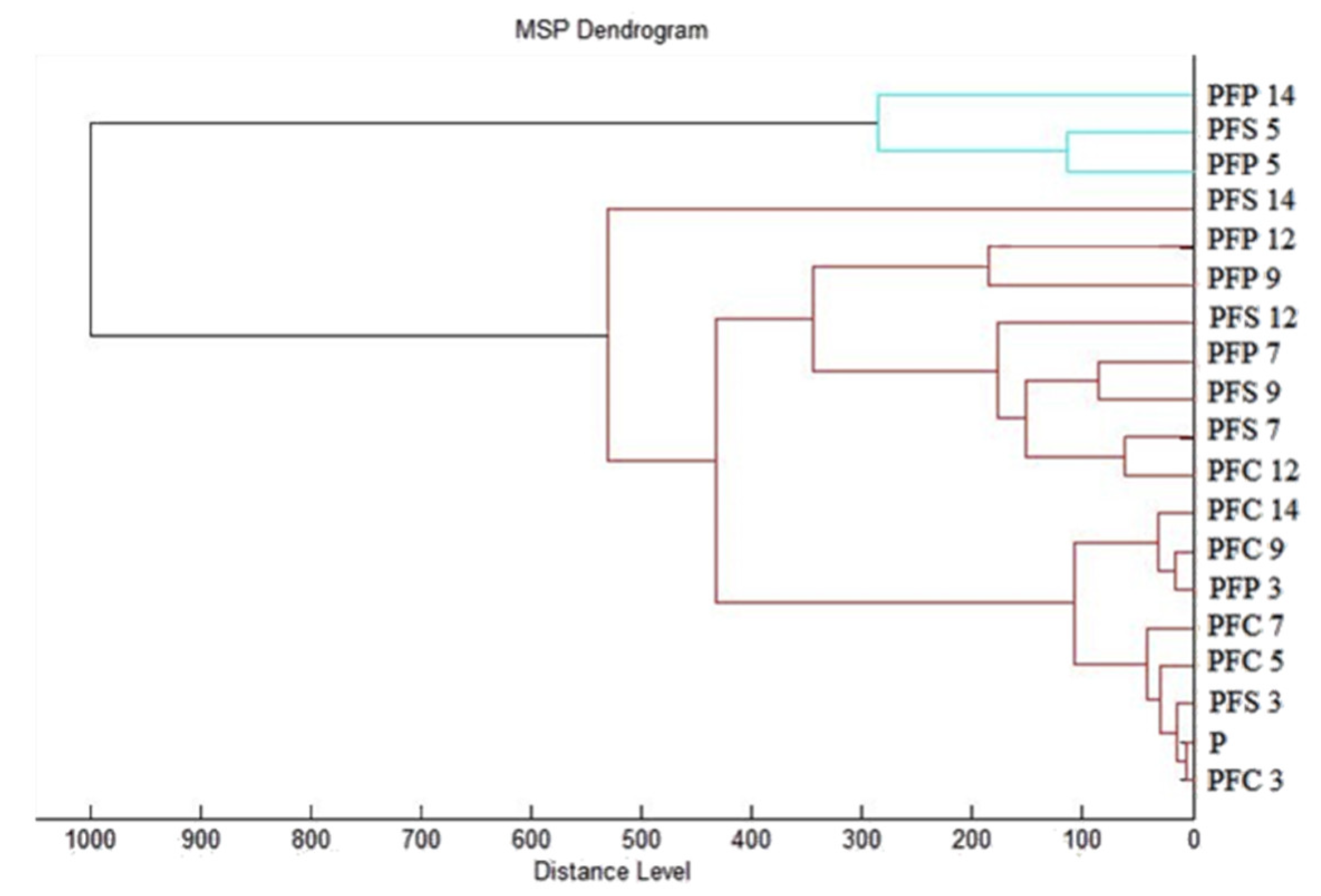
3.6. In Situ Antimicrobial Activity
3.7. Insecticidal Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stringaro, A.; Colone, M.; Angiolella, L. Antioxidant, Antifungal, Antibiofilm, and Cytotoxic Activities of Mentha Spp. Essential Oils. Medicines 2018, 5, 112. [Google Scholar] [CrossRef] [PubMed]
- Baj, T.; Sieniawska, E.; Kowalski, R.; Wesolowskp, M.; Ulewicz-Magulska, B. Effectiveness of the deryng and clevenger-type apparatus in isolation of various types of components of essential oil from the mutelina purpurea thell. Flowers. Acta Pol. Pharm. 2015, 72, 507–515. [Google Scholar] [PubMed]
- Brnawi, W.I.; Hettiarachchy, N.S.; Horax, R.; Kumar-Phillips, G.; Ricke, S. Antimicrobial Activity of Leaf and Bark Cinnamon Essential Oils against Listeria Monocytogenes and Salmonella Typhimurium in Broth System and on Celery. J. Food Process. Preserv. 2019, 43, e13888. [Google Scholar] [CrossRef]
- Benzaid, C.; Belmadani, A.; Djeribi, R.; Rouabhia, M. The Effects of Mentha × Piperita Essential Oil on C. Albicans Growth, Transition, Biofilm Formation, and the Expression of Secreted Aspartyl Proteinases Genes. Antibiotics 2019, 8, 10. [Google Scholar] [CrossRef]
- Joulain, D. Jasminum Grandiflorum Flowers—Phytochemical Complexity and Its Capture in Extracts: A Review. Flavour Fragr. J. 2021, 36, 526–553. [Google Scholar] [CrossRef]
- Arun, M.; Satish, S.; Anima, P. Phytopharmacological Profile of Jasminum Grandiflorum Linn. (Oleaceae). Chin. J. Integr. Med. 2016, 22, 311–320. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Lai, X.; Peng, X.; Wen, S.; Zhang, Z.; Xie, Y.; Li, Q.; Chen, R.; Zheng, X.; et al. Gastroprotective Effects of Extract of Jasminum Grandiflorum L. Flower in HCl/EtOH-Induced Gastric Mucosal Ulceration Mice. Biomed. Pharmacother. 2021, 144, 112268. [Google Scholar] [CrossRef]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. The Antimicrobial Efficacy of Plant Essential Oil Combinations and Interactions with Food Ingredients. Int. J. Food Microbiol. 2008, 124, 91–97. [Google Scholar] [CrossRef]
- Nadjib, B.M.; Amine, F.M.; Abdelkrim, K.; Fairouz, S.; Maamar, M. Liquid and vapour phase antibacterial activity of eucalyptus globulus essential oil = susceptibility of selected respiratory tract pathogens. Am. J. Infect. Dis. 2014, 10, 105–117. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 978-1-932633-21-4. [Google Scholar]
- Vandendool, H.; Kratz, P.D. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas-Liquid Partition Chromatography. J. Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Kačániová, M.; Terentjeva, M.; Galovičová, L.; Ivanišová, E.; Štefániková, J.; Valková, V.; Borotová, P.; Kowalczewski, P.Ł.; Kunová, S.; Felšöciová, S.; et al. Biological Activity and Antibiofilm Molecular Profile of Citrus aurantium Essential Oil and Its Application in a Food Model. Molecules 2020, 25, 3956. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2001; ISBN 978-3-527-30364-9. [Google Scholar]
- Rout, P.K.; Naik, S.N.; Rao, Y.R. Composition of Absolutes of Jasminum Sambac L. Flowers Fractionated with Liquid CO 2 and Methanol and Comparison with Liquid CO2 Extract. J. Essent. Oil Res. 2010, 22, 398–406. [Google Scholar] [CrossRef]
- Rao, Y.R.; Rout, P.K. Geographical Location and Harvest Time Dependent Variation in the Composition of Essential Oils of Jasminum Sambac. (L.) Aiton. J. Essent. Oil Res. 2003, 15, 398–401. [Google Scholar] [CrossRef]
- Tamogami, S.; Awano, K.; Kitahara, T. Analysis of the Enantiomeric Ratios of Chiral Components in Absolute Jasmine. Flavour Fragr. J. 2001, 16, 161–163. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schweiger, T.; Denkova, Z.; Slavchev, A.; Stoyanova, A.; Schmidt, E.; Geissler, M. Chemical Composition, Olfactory Evaluation and Antimicrobial Activities of Jasminum Grandiflorum L. Absolute from India. Nat. Prod. Commun. 2007, 2, 1934578X0700200411. [Google Scholar] [CrossRef]
- Luo, H.; Lin, S.; Ren, F.; Wu, L.; Chen, L.; Sun, Y. Antioxidant and Antimicrobial Capacity of Chinese Medicinal Herb Extracts in Raw Sheep Meat. J. Food Prot. 2007, 70, 1440–1445. [Google Scholar] [CrossRef]
- Wang, H.-F.; Yih, K.-H.; Huang, K.-F. Comparative Study of the Antioxidant Activity of Forty-Five Commonly Used Essential Oils and Their Potential Active Components. J. Food Drug Anal. 2020, 18, 8. [Google Scholar] [CrossRef]
- Balkrishna, A.; Rohela, A.; Kumar, A.; Kumar, A.; Arya, V.; Thakur, P.; Oleksak, P.; Krejcar, O.; Verma, R.; Kumar, D.; et al. Mechanistic Insight into Antimicrobial and Antioxidant Potential of Jasminum Species: A Herbal Approach for Disease Management. Plants 2021, 10, 1089. [Google Scholar] [CrossRef]
- Jirovetz, L. Antimicrobial Testings, Gas Chromatographic Analysis and Olfactory Evaluation of an Essential Oil of Hop Cones (Humulus Lupulus L.) from Bavaria and Some of Its Main Compounds. Sci. Pharm. 2006, 74, 189–201. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Denkova, Z.; Stoyanova, A.; Murgov, I.; Schmidt, E.; Geissler, M. Antimicrobial Testinas and Gas Chromatoaraphic Analvsis of Pure Oxvaenated Monoterpenes 1.8-Cineole, α-Terpineol, Terpinen-4-Ol and Camphor as Well as Target Comoounds in Essential Oils of Pine (Pinus Pinaster), Rosemary (Rosmarinus Officinalis), Tea Tree (Melaleuca Alternifolia). Sci. Pharm. 2005, 73, 27–39. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Schmidt, E.; Stoyanova, A.S.; Denkova, Z.; Nikolova, R.; Geissler, M. Purity, Antimicrobial Activities and Olfactoric Evaluations of Geraniol/Nerol and Various of Their Derivatives. J. Essent. Oil Res. 2007, 19, 288–291. [Google Scholar] [CrossRef]
- Bharathi, P.R.; Sripathi, S.K.; Lakshmi, A.N. Jasminum grandiflorum linn.–an update review. Int. J. Pharm. Sci. Res. 2020, 11, 1994–2010. [Google Scholar]
- Ali, S.T.; Ayub, A.; Ali, S.N. Antibacterial Activity Of Methanolic Extracts From Some Selected Medicinal Plants. Fuuast J. Biol. 2017, 7, 123–125. [Google Scholar]
- Shekhar, S. Evaluation of antimicrobial activity of jasminum species using solvent extracts against clinical pathogens. World J. Pharm. Pharm. Sci. 2015, 4, 11. [Google Scholar]
- Nagarajappa, R.; Batra, M.; Sharda, A.J.; Asawa, K.; Sanadhya, S.; Daryani, H.; Ramesh, G. Antimicrobial Effect of Jasminum grandiflorum L. and Hibiscus Rosa-Sinensis L. Extracts Against Pathogenic Oral Microorganisms--An In Vitro Comparative Study. Oral Health Prev. Dent. 2015, 13, 341–348. [Google Scholar] [CrossRef]
- Britto, A.J.; Grecelin, H. Efficacy of Fruits of Jasminum grandiflorum Linn. against Plant and Animal Pathogens. Asian J. Pharm. Clin. Res. 2011, 4, 74–75. [Google Scholar]
- Nidiry, E.; Srivastava, H. A Comparative Study of the Antifungal Activities of Absolutes of Jasmine and Tuberose and Their Constituents. Indian Perfum. 2007, 51, 53–54. [Google Scholar]
- Khond, M.; Bhosale, J.D.; Arif, T.; Padhi, M.M.; Dabur, R. Screening of Some Selected Medicinal Plants Extracts for In-Vitro Antimicrobial Activity. Middle East J. Sci. Res. 2009, 4, 271–278. [Google Scholar]
- El-Tarabily, K.A.; El-Saadony, M.T.; Alagawany, M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Elwan, H.A.M.; Elnesr, S.S.; El-Hack, M.E.A. Using Essential Oils to Overcome Bacterial Biofilm Formation and Their Antimicrobial Resistance. Saudi J. Biol. Sci. 2021, 28, 5145–5156. [Google Scholar] [CrossRef]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to Nature: Combating Candida Albicans Biofilm, Phospholipase and Hemolysin Using Plant Essential Oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- de Oliveira Carvalho, I.; Purgato, G.A.; Píccolo, M.S.; Pizziolo, V.R.; Coelho, R.R.; Diaz-Muñoz, G.; Alves Nogueira Diaz, M. In Vitro Anticariogenic and Antibiofilm Activities of Toothpastes Formulated with Essential Oils. Arch. Oral Biol. 2020, 117, 104834. [Google Scholar] [CrossRef]
- Galovičová, L.; Borotová, P.; Valková, V.; Vukovic, N.L.; Vukic, M.; Terentjeva, M.; Štefániková, J.; Ďúranová, H.; Kowalczewski, P.Ł.; Kačániová, M. Thymus serpyllum Essential Oil and Its Biological Activity as a Modern Food Preserver. Plants 2021, 10, 1416. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Galovičová, L.; Borotová, P.; Vukovic, N.L.; Vukic, M.; Kačániová, M. Cymbopogon citratus Essential Oil: Its Application as an Antimicrobial Agent in Food Preservation. Agronomy 2022, 12, 155. [Google Scholar] [CrossRef]
- López, P.; Sánchez, C.; Batlle, R.; Nerín, C. Vapor-Phase Activities of Cinnamon, Thyme, and Oregano Essential Oils and Key Constituents against Foodborne Microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef]
- Nedorostova, L.; Kloucek, P.; Kokoska, L.; Stolcova, M.; Pulkrabek, J. Antimicrobial Properties of Selected Essential Oils in Vapour Phase against Foodborne Bacteria. Food Control 2009, 20, 157–160. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial Potential and Chemical Composition of Eucalyptus Globulus Oil in Liquid and Vapour Phase against Food Spoilage Microorganisms. Food Chem. 2011, 126, 228–235. [Google Scholar] [CrossRef]
- Ahmed, N.; Hanani, Y.A.; Ansari, S.Y.; Anwar, S. Jasmine (Jasminum Sambac L., Oleaceae) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Elsevier: Amsterdam, The Netherlands, 2016; pp. 487–494. ISBN 978-0-12-416641-7. [Google Scholar]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal Activity of Lemon (Citrus lemon L.), Mandarin (Citrus reticulata L.), Grapefruit (Citrus paradisi L.) and Orange (Citrus sinensis L.) Essential Oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Synergistic Interactions of Plant Essential Oils with Antimicrobial Agents: A New Antimicrobial Therapy. Crit. Rev. Food Sci. Nutr. 2022, 62, 1740–1751. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Malik, A. Antimicrobial Potential and Chemical Composition of Mentha Piperita Oil in Liquid and Vapour Phase against Food Spoiling Microorganisms. Food Control 2011, 22, 1707–1714. [Google Scholar] [CrossRef]
- Combrinck, S.; Regnier, T.; Kamatou, G.P.P. In Vitro Activity of Eighteen Essential Oils and Some Major Components against Common Postharvest Fungal Pathogens of Fruit. Ind. Crops Prod. 2011, 33, 344–349. [Google Scholar] [CrossRef]
- Stević, T.; Berić, T.; Šavikin, K.; Soković, M.; Gođevac, D.; Dimkić, I.; Stanković, S. Antifungal Activity of Selected Essential Oils against Fungi Isolated from Medicinal Plant. Ind. Crops Prod. 2014, 55, 116–122. [Google Scholar] [CrossRef]
- Hernandez, E. Essential oils | Distillation. In Encyclopedia of Separation Science; Elsevier: Amsterdam, The Netherlands, 2000; pp. 2739–2744. ISBN 978-0-12-226770-3. [Google Scholar]
- Noosidum, A.; Chareonviriyaphap, T.; Chandrapatya, A. Synergistic Repellent and Irritant Effect of Combined Essential Oils on Aedes Aegypti (L.) Mosquitoes. J. Vector Ecol. 2014, 39, 298–305. [Google Scholar] [CrossRef]
- Jirakanjanakit, N.; Leemingsawat, S.; Thongrungkiat, S.; Apiwathnasorn, C.; Singhaniyom, S.; Bellec, C.; Dujardin, J.P. Influence of Larval Density or Food Variation on the Geometry of the Wing of Aedes (Stegomyia) Aegypti: Geometry of the Wing of Aedes (Stegomyia) Aegypti. Trop. Med. Int. Health 2007, 12, 1354–1360. [Google Scholar] [CrossRef] [PubMed]
- Thanispong, K.; Sathantriphop, S.; Chareonviriyaphap, T. Insecticide Resistance of Aedes Aegypti and Culex Quinquefasciatus in Thailand. J. Pestic. Sci. 2008, 33, 351–356. [Google Scholar] [CrossRef]
- Chanda, E. Optimizing Strategic Insecticide Resistance Management Planning in Malaria Vectors. In Insecticides Resistance; Trdan, S., Ed.; InTech: London, UK, 2016; ISBN 978-953-51-4591-2. [Google Scholar]
- Park, B.-S.; Choi, W.-S.; Kim, J.-H.; Kim, K.-H.; Lee, S.-E. Monoterpenes from thyme (Thymus vulgaris) as potential mosquito repellents. J. Am. Mosq. Control Assoc. 2005, 21, 80–83. [Google Scholar] [CrossRef]
- Yang, P.; Ma, Y. Repellent Effect of Plant Essential Oils against Aedes albopictus. J. Vector Ecol. J. Soc. Vector Ecol. 2005, 30, 231–234. [Google Scholar]
- Fradin, M.S.; Day, J.F. Comparative Efficacy of Insect Repellents against Mosquito Bites. N. Engl. J. Med. 2002, 347, 13–18. [Google Scholar] [CrossRef]
- Sritabutra, D.; Soonwera, M.; Waltanachanobon, S.; Poungjai, S. Evaluation of Herbal Essential Oil as Repellents against Aedes aegypti (L.) and Anopheles dirus Peyton & Harrion. Asian Pac. J. Trop. Biomed. 2011, 1, S124–S128. [Google Scholar] [CrossRef]
- Sachan, S.; Paarakh, P.; Gavani, U. Antibacterial Activity of Jasminum Grandiflorum Linn Leaves. J. Pharm. Res. 2009, 2, 1206–1207. [Google Scholar]
- Noosidum, A.; Prabaripai, A.; Chareonviriyaphap, T.; Chandrapatya, A. Excito-Repellency Properties of Essential Oils from Melaleuca leucadendron L., Litsea cubeba (Lour.) Persoon, and Litsea salicifolia (Nees) on Aedes aegypti (L.) Mosquitoes. J. Vector Ecol. 2008, 33, 305–312. [Google Scholar] [CrossRef]
- de Boer, H.; Vongsombath, C.; Pålsson, K.; Björk, L.; Jaenson, T.G.T. Botanical Repellents and Pesticides Traditionally Used Against Hematophagous Invertebrates in Lao People’s Democratic Republic: A Comparative Study of Plants Used in 66 Villages. J. Med. Entomol. 2010, 47, 400–414. [Google Scholar] [CrossRef]
- Suwansirisilp, K.; Visetson, S.; Prabaripai, A.; Tanasinchayakul, S.; Grieco, J.P.; Bangs, M.J.; Chareonviriyaphap, T. Behavioral Responses of Aedes Aegypti and Culex Quinquefasciatus (Diptera: Culicidae) to Four Essential Oils in Thailand. J. Pest Sci. 2013, 86, 309–320. [Google Scholar] [CrossRef]
- Tawatsin, A.; Wratten, S.D.; Scott, R.R.; Thavara, U.; Techadamrongsin, Y. Repellency of Volatile Oils from Plants against Three Mosquito Vectors. J. Vector Ecol. J. Soc. Vector Ecol. 2001, 26, 76–82. [Google Scholar]
- Tuetun, B.; Choochote, W.; Kanjanapothi, D.; Rattanachanpichai, E.; Chaithong, U.; Chaiwong, P.; Jitpakdi, A.; Tippawangkosol, P.; Riyong, D.; Pitasawat, B. Repellent Properties of Celery, Apium graveolens L., Compared with Commercial Repellents, against Mosquitoes under Laboratory and Field Conditions. Trop. Med. Int. Health 2005, 10, 1190–1198. [Google Scholar] [CrossRef]
- Kim, B.-S.; Yi, H.; Chun, J.; Cha, C.-J. Genome Sequence of Type Strain of Staphylococcus aureus Subsp. aureus. Gut Pathog. 2014, 6, 6. [Google Scholar] [CrossRef]
| No. | Compound | % | RI (lit.) | RI (calc.) |
|---|---|---|---|---|
| 1 | (Z)-b-Ocimene | 1.1 | 801 | 801 |
| 2 | m-Methylphenol | 0.3 | 855 | 855 |
| 3 | (E)-Hexenyl propionate | 0.2 | 902 | 900 |
| 4 | Linalool | 9.6 | 960 | 965 |
| 5 | Benzyl acetate | 37.0 | 973 | 976 |
| 6 | 2-Undecanone | 0.5 | 977 | 978 |
| 7 | Eugenol | 2.1 | 979 | 981 |
| 8 | (Z)-Jasmone | 5.0 | 985 | 986 |
| 9 | (E,E)-a-Farnesene | 0.9 | 988 | 991 |
| 10 | Caryophyllenyl alcohol | 1.9 | 991 | 997 |
| 11 | (Z)-Methyl jasmonate | 0.3 | 998 | 1004 |
| 12 | Benzyl benzoate | 34.7 | 1024 | 1026 |
| 13 | (Z,Z)-Farnesyl acetone | 0.6 | 1031 | 1035 |
| 14 | Methyl hexadecanoate | 0.8 | 1035 | 1037 |
| 15 | Isophytol | 3.3 | 1042 | 1046 |
| 16 | (E)-Phytol acetate | 1.4 | 1096 | 1099 |
| ∑ | 99.7 |
| Microorganisms | Inhibition Zone (mm) | Activity of EO | ATB (mm) |
|---|---|---|---|
| Yersinia enterocolitica | 3.00 ± 0.00 | * | 26 ± 2.00 |
| Haemophilus influenzae | 2.33 ± 0.58 | * | 30 ± 3.00 |
| Escherichia coli | 3.67 ± 1.15 | * | 27 ± 0.50 |
| Listeria monocytogenes | 5.33 ± 0.58 | ** | 24 ± 1.50 |
| Staphylococcus aureus | 3.67 ± 0.58 | * | 29 ± 1.00 |
| Streptococcus pneumoniae | 2.33 ± 0.58 | * | 27 ± 2.00 |
| Candida albicans | 2.67 ± 0.58 | * | 24 ± 2.00 |
| Candida tropicalis | 2.33 ± 0.58 | * | 30 ± 1.00 |
| Candida glabrata | 3.33 ± 0.58 | * | 32 ± 1.50 |
| Pseudomonas fluorescens biofilm | 3.67 ± 0.58 | * | 26 ± 1.00 |
| Microorganism | MIC 50 | MIC 90 |
|---|---|---|
| Yersinia enterocolitica | 6.43 | 8.73 |
| Haemophilus influenzae | 11.36 | 15.26 |
| Escherichia coli | 6.43 | 8.73 |
| Listeria monocytogenes | 6.43 | 8.73 |
| Staphylococcus aureus | 11.36 | 15.26 |
| Streptococcus pneumoniae | 11.36 | 15.26 |
| Candida albicans | 3.18 | 5.24 |
| Candida tropicalis | 3.18 | 5.24 |
| Candida glabrata | 0.65 | 0.97 |
| Pseudomonas fluorescens biofilm | 11.36 | 15.26 |
| Food Model | Bacteria | Microbial Growth Inhibition (%) | |||
|---|---|---|---|---|---|
| Concentration of Leaves EO | |||||
| 62.5 (μL/L) | 125 (μL/L) | 250 (μL/L) | 500 (μL/L) | ||
| Apple | Gram-negative | ||||
| E. coli | −66.70 ± 0.98 a | 74.45 ± 0.88 b,a | 54.48 ± 0.84 c,b,a | 25.00 ± 2.45 d,c,b,a | |
| H. influenzae | −64.91 ± 2.40 a | −54.63 ± 1.58 b,a | −25.43 ± 1.62 c,b,a | −16.06 ± 0.26 d,c,b,a | |
| Y. enterocolitica | 34.37 ± 0.61 a | 2.96 ± 0.62 b,a | 56.63 ± 1.13 c,b,a | 63.69 ± 1.85 d,c,b,a | |
| Gram-positive | |||||
| L. monocytogenes | −45.22 ± 1.82 a | −23.99 ± 1.59 b,a | 34.52 ± 0.90 c,b,a | −66.89 ± 1.00 d,c,b,a | |
| S. aureus | 46.62 ± 1.28 a | 35.06 ± 1.35 b,a | 11.71 ± 1.06 c,b,a | 3.67 ± 0.95 d,c,b,a | |
| S. pneumoniae | −77.11 ± 1.66 a | −46.78 ± 1.11 b,a | −36.30 ± 1.66 c,b,a | −13.00 ± 0.59 d,c,b,a | |
| Yeasts | |||||
| C. albicans | 76.96 ± 1.53 a | 7.11 ± 0.47 b,a | 24.81 ± 0.89 c,b,a | 45.84 ± 0.99 d,c,b,a | |
| C. glabrata | 13.60 ± 1.06 a | 24.66 ± 1.00 b,a | 31.85 ± 1.42 c,b,a | 64.63 ± 1.44 d,c,b,a | |
| C. tropicalis | 7.03 ± 0.83 a | −5.48 ± 1.22 b,a | 43.93 ± 1.84 c,b,a | 66.32 ± 2.30 d,c,b,a | |
| Pseudomonas fluorescens biofilm | 56.96 ± 2.29 a | −7.77 ± 0.94 b,a | 32.84 ± 1.65 c,b,a | 43.91 ± 1.80 d,c,b,a | |
| Pear | Gram-negative | ||||
| E. coli | 87.86 ± 1.30 a | 24.70 ± 2.45 b,a | 53.20 ± 2.57 c,b,a | 24.18 ± 1.66 d,c,a | |
| H. influenzae | −77.98 ± 1.25 a | −54.33 ± 1.11 b,a | −33.92 ± 0.36 c,b,a | 42.70 ± 0.91 d,c,b,a | |
| Y. enterocolitica | 28.71 ± 3.47 a | −32.07 ± 0.53 b,a | 72.09 ± 3.07 c,b,a | 96.15 ± 2.31 d,c,b,a | |
| Gram-positive | |||||
| L. monocytogenes | 42.42 ± 1.14 a | 34.58 ± 0.89 b,a | −24.69 ± 1.10 c,b,a | 2.78 ± 0.56 d,c,b,a | |
| S. aureus | 7.21 ± 1.69 a | 12.82 ± 1.32 b,a | 27.43 ± 2.50 c,b,a | 35.04 ± 1.41 d,c,b,a | |
| S. pneumoniae | −34.03 ± 1.10 a | −16.22 ± 0.48 b,a | 33.27 ± 1.17 c,b,a | 86.32 ± 2.10 d,c,b,a | |
| Yeasts | |||||
| C. albicans | 13.08 ± 1.96 a | 26.57 ± 3.90 b,a | 50.31 ± 1.83 c,b,a | 96.53 ± 3.03 d,c,b,a | |
| C. glabrata | 8.30 ± 0.56 a | 18.00 ± 1.12 b,a | 31.74 ± 1.32 c,b,a | 65.04 ± 1.54 d,c,b,a | |
| C. tropicalis | 34.41 ± 2.09 a | 24.42 ± 0.66 b,a | 16.84 ± 0.98 c,b,a | 8.88 ± 0.58 d,c,b,a | |
| Pseudomonas fluorescens biofilm | −66.88 ± 0.96 a | −56.70 ± 1.23 b,a | 44.44 ± 0.88 c,b,a | 55.81 ± 1.05 d,c,b,a | |
| Carrot | Gram-negative | ||||
| E. coli | 12.32 ± 1.97 a | 24.70 ± 2.45 b,a | 53.20 ± 2.57 c,b,a | 92.35 ± 3.57 d,c,b,a | |
| H. influenzae | −78.18 ± 1.50 a | −23.56 ± 0.62 b,a | −63.93 ± 0.91 c,b,a | −44.25 ± 0.99 d,c,b,a | |
| Y. enterocolitica | 14.88 ± 2.29 a | 26.15 ± 1.92 b,a | −24.36 ± 2.11 c,b,a | −4.55 ± 0.88 d,c,b,a | |
| Gram-positive | |||||
| L. monocytogenes | −5.03 ± 0.64 a | −1.98 ± 0.29 b,a | 2.86 ± 0.46 c,b,a | 5.43 ± 0.23 d,c,b,a | |
| S. aureus | 8.67 ± 1.09 a | −46.11 ± 1.53 b,a | −55.52 ± 1.28 c,b,a | −6.07 ± 0.59 d,c,b,a | |
| S. pneumoniae | −54.41 ± 2.33 a | −66.33 ± 2.01 b,a | 67.66 ± 0.83 c,b,a | 82.52 ± 1.01 d,c,b,a | |
| Yeasts | |||||
| C. albicans | −6.25 ± 0.50 a | −11.77 ± 1.05 b,a | 54.55 ± 1.00 c,b,a | 95.30 ± 2.25 d,c,b,a | |
| C. glabrata | 25.03 ± 1.57 a | 34.00 ± 4.66 b,a | 44.62 ± 2.01 c,b,a | 68.46 ± 0.71 d,c,b,a | |
| C. tropicalis | 68.46 ± 3.13 a | 6.29 ± 0.44 b,a | 14.63 ± 0.92 c,b,a | 33.10 ± 2.00 d,c,b,a | |
| Pseudomonas fluorescens biofilm | −5.95 ± 0.24 a | 7.28 ± 0.39 b,a | 44.51 ± 1.48 c,b,a | 55.33 ± 1.49 d,c,b,a | |
| White radish | Gram-negative | ||||
| E. coli | −35.32 ± 1.67 a | 54.88 ± 0.94 b,a | 22.59 ± 0.95 c,b,a | 12.93 ± 0.57 d,c,b,a | |
| H. influenzae | 6.33 ± 0.51 a | 16.15 ± 1.11 b,a | 35.27 ± 0.86 c,b,a | 77.26 ± 1.03 d,c,b,a | |
| Y. enterocolitica | 76.63 ± 2.26 a | 54.33 ± 1.01 b,a | 33.44 ± 1.12 c,b,a | 15.60 ± 1.22 d,c,b,a | |
| Gram-positive | |||||
| L. monocytogenes | 7.96 ± 0.69 a | −23.53 ± 2.16 b,a | −44.66 ± 1.00 c,b,a | −34.26 ± 0.70 d,c,b,a | |
| S. aureus | 77.11 ± 1.67 a | 21.63 ± 1.06 b,a | −36.07 ± 1.50 c,b,a | −23.37 ± 1.47 d,c,b,a | |
| S. pneumoniae | 8.19 ± 0.56 a | 13.18 ± 1.41 b,a | 21.85 ± 1.72 c,b,a | 32.63 ± 0.90 d,c,b,a | |
| Yeasts | |||||
| C. albicans | 5.15 ± 0.18 a | 15.62 ± 1.06 b,a | 26.43 ± 1.05 c,b,a | 35.65 ± 1.12 d,c,b,a | |
| C. glabrata | 73.99 ± 1.42 a | 65.76 ± 1.64 b,a | 84.04 ± 1.54 c,b,a | 93.45 ± 1.90 d,c,b,a | |
| C. tropicalis | 56.96 ± 2.29 a | −7.77 ± 0.94 b,a | 32.84 ± 1.65 c.b.a | 43.91 ± 1.80 d,c,b,a | |
| Pseudomonas fluorescens biofilm | 5.54 ± 0.90 a | 7.85 ± 0.28 b | 13.03 ± 0.37 c,b,a | 88.40 ± 1.50 d,c,b,a | |
| Concentration (%) | Number of Living Individuals | Number of Dead Individuals | Insecticidal Activity (%) |
|---|---|---|---|
| 100 | 0 | 30 | 100 |
| 50 | 0 | 30 | 100 |
| 25 | 0 | 30 | 100 |
| 12.5 | 0 | 30 | 100 |
| 6.25 | 12 | 18 | 60 |
| Control group | 30 | 0 | 0 |
| Concentration (%) | Number of Living Individuals | Number of Dead Individuals | Insecticidal Activity (%) |
|---|---|---|---|
| 100 | 0 | 30 | 100 |
| 50 | 3 | 27 | 90 |
| 25 | 9 | 21 | 70 |
| 12.5 | 15 | 15 | 50 |
| 6.25 | 18 | 12 | 40 |
| Control group | 30 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galovičová, L.; Čmiková, N.; Vukovic, N.; Vukic, M.; Kowalczewski, P.Ł.; Bakay, L.; Kačániová, M. Chemical Composition, Antioxidant, Antimicrobial, Antibiofilm and Anti-Insect Activities of Jasminum grandiflorum Essential Oil. Horticulturae 2022, 8, 953. https://doi.org/10.3390/horticulturae8100953
Galovičová L, Čmiková N, Vukovic N, Vukic M, Kowalczewski PŁ, Bakay L, Kačániová M. Chemical Composition, Antioxidant, Antimicrobial, Antibiofilm and Anti-Insect Activities of Jasminum grandiflorum Essential Oil. Horticulturae. 2022; 8(10):953. https://doi.org/10.3390/horticulturae8100953
Chicago/Turabian StyleGalovičová, Lucia, Natália Čmiková, Nenad Vukovic, Milena Vukic, Przemysław Łukasz Kowalczewski, Ladislav Bakay, and Miroslava Kačániová. 2022. "Chemical Composition, Antioxidant, Antimicrobial, Antibiofilm and Anti-Insect Activities of Jasminum grandiflorum Essential Oil" Horticulturae 8, no. 10: 953. https://doi.org/10.3390/horticulturae8100953
APA StyleGalovičová, L., Čmiková, N., Vukovic, N., Vukic, M., Kowalczewski, P. Ł., Bakay, L., & Kačániová, M. (2022). Chemical Composition, Antioxidant, Antimicrobial, Antibiofilm and Anti-Insect Activities of Jasminum grandiflorum Essential Oil. Horticulturae, 8(10), 953. https://doi.org/10.3390/horticulturae8100953










