Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Pinus mugo Samples, Fungi Strains, and Culture Media
2.2. Chemical and Reagents
2.3. Extraction Procedure
Extraction Procedure for GC/MS
2.4. Identification and Quantification of Volatile Compounds by the GC/MS Method
2.5. Determination of Total Phenolic Content (TPC)
2.6. Determination of Total Flavonoid Content (TFC)
2.7. Assessment of Antioxidant Activity
2.8. Identification of Ascorbic Acid
2.9. Evaluation of Antifungal Activity
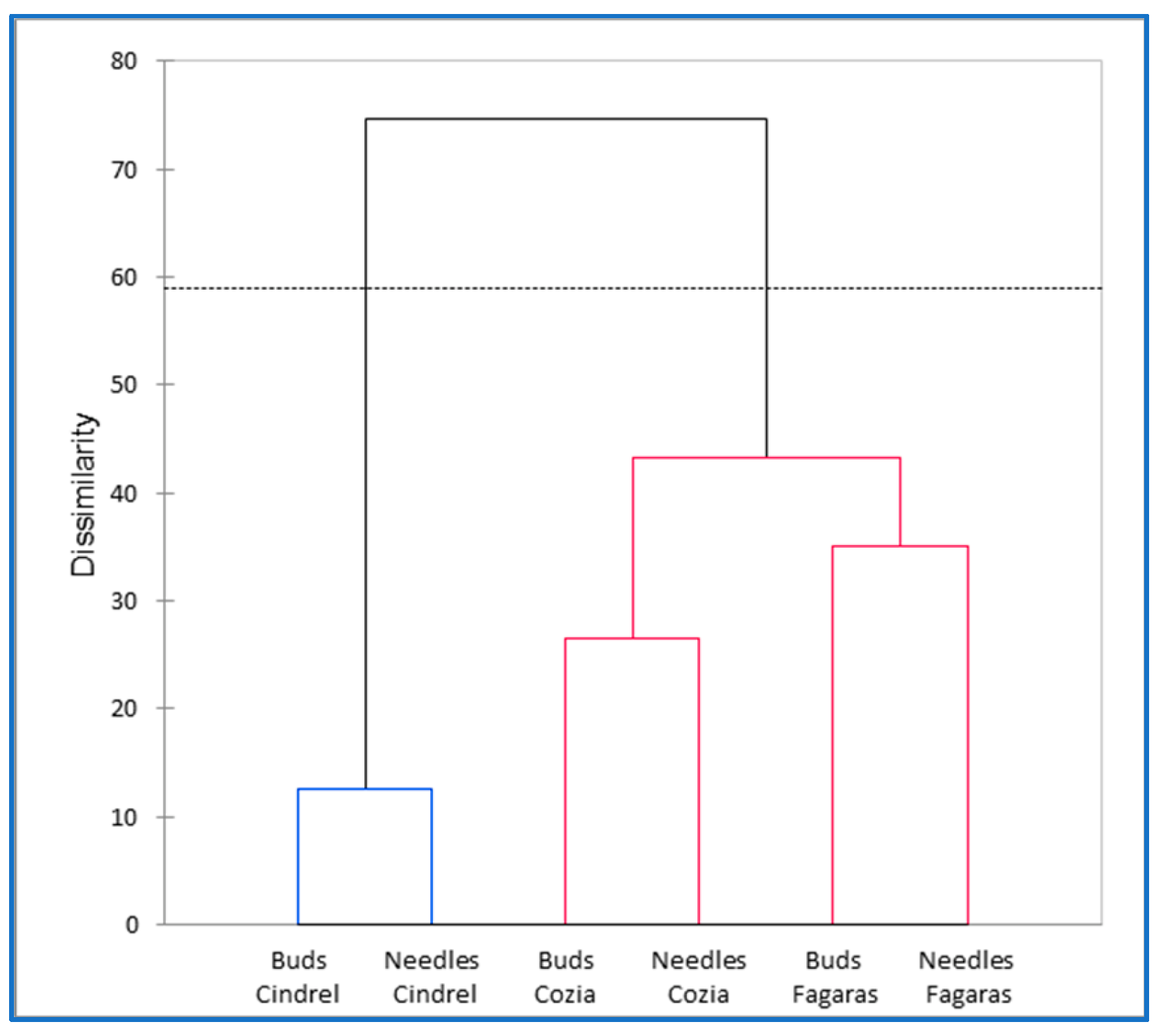
2.10. Multivariate Analysis
3. Results
3.1. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC) and Evaluation of Antioxidant Activity
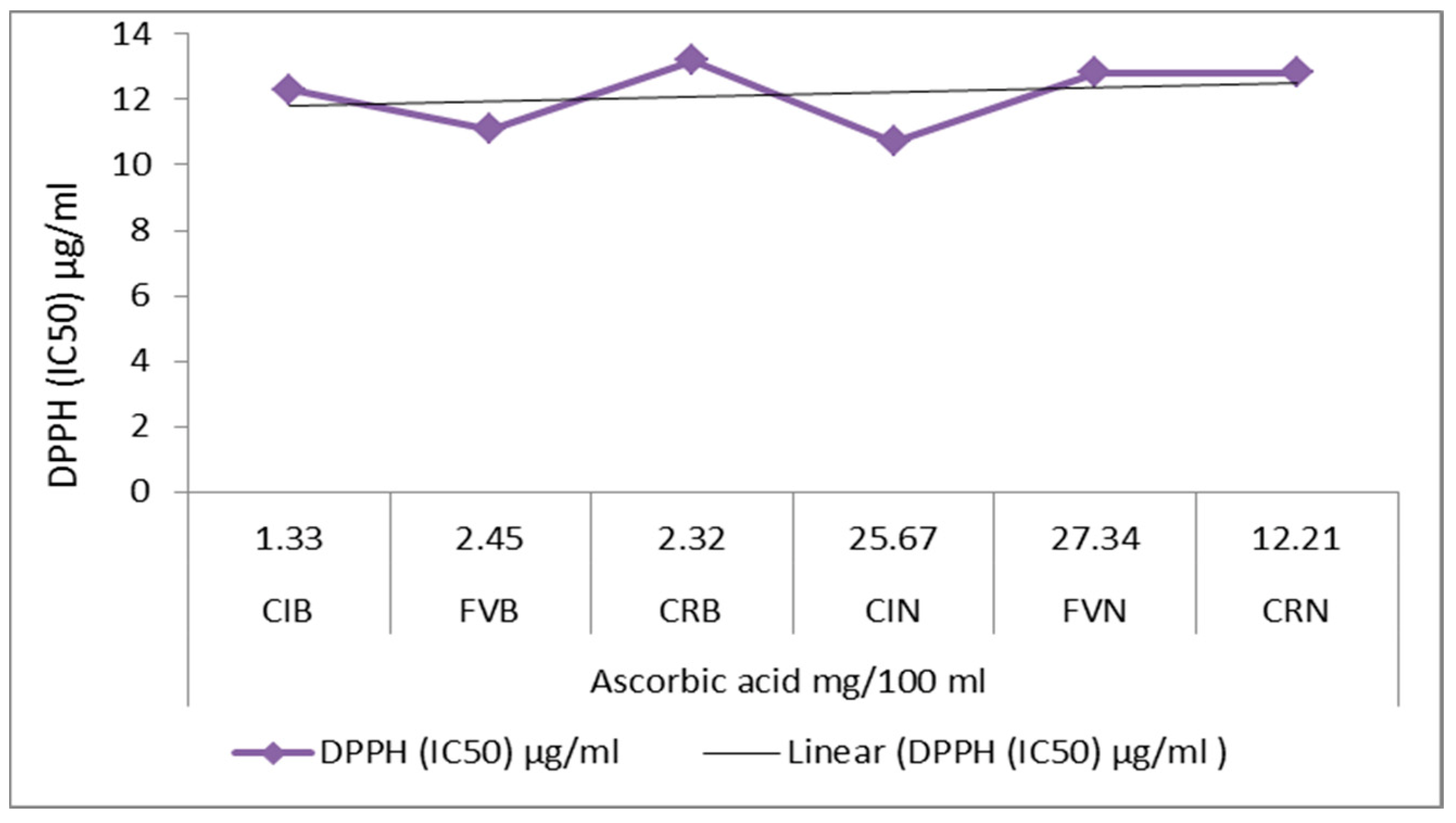
3.2. Identification and Quantification of Ascorbic Acid and Evaluation of Antioxidant Activity
3.3. Antifungal Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farjon, A.; Filer, D. An Atlas of the World’s Conifers: An Analysis of Their Distribution, Biogeography, Diversity, and Conservation Status; Brill: Leiden, The Netherlands, 2013; ISBN 978-90-04-21180-3. [Google Scholar]
- Jin, W.-T.; Gernandt, D.S.; Wehenkel, C.; Xia, X.-M.; Wei, X.-X.; Wang, X.-Q. Phylogenomic and Ecological Analyses Reveal the Spatiotemporal Evolution of Global Pines. Proc. Natl. Acad. Sci. USA 2021, 118, e2022302118. [Google Scholar] [CrossRef] [PubMed]
- Tsaryk, I.; Didukh, Y.P.; Tasenkevich, L.; Waldon, B.; Boratyński, A. Pinus mugo Turra (Pinaceae) in the Ukrainian Carpathians. Dendrobiology 2006, 55, 39–49. [Google Scholar]
- Jørgensen, H. Online Database of the European Network on Invasive Alien Species—NOBANIS. Available online: www.nobanis.org (accessed on 10 July 2022).
- Coldea, G. Prodrome Des Associations Vegetales Des Carpates Du Sud-Est (Carpates Roumaines). Doc. Phytosociol. Nouv. Ser. 1991, 13, 317–539. [Google Scholar]
- Roșca, S.; Șimonca, V.; Bilașco, Ș.; Vescan, I.; Fodorean, I.; Petrea, D. The Assessment of Favourability and Spatio-Temporal Dynamics of Pinus mugo in the Romanian Carpathians Using GIS Technology and Landsat Images. Sustainability 2019, 11, 3678. [Google Scholar] [CrossRef]
- De Rigo, D.; San-Miguel-Ayanz, J.; Caudullo, G.; Durrant, T.H.; Mauri, A. (Eds.) European Atlas of Forest Tree Species; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-36740-3.
- Parobeková, Z.; Bugala, M.; Kardoš, M.; Dovciak, M.; Lukáčik, I.; Saniga, M. Long-Term Changes in Dwarf Pine (Pinus mugo Turra) Cover and Growth in the Orava Beskid Mountains, Slovakia. Mt. Res. Dev. 2018, 38, 342. [Google Scholar] [CrossRef]
- Krüssmann, G.; Krüssmann, G. Manual of Cultivated Conifers; Timber Press: Portland, OR, USA, 1985; ISBN 978-0-88192-007-9. [Google Scholar]
- Hamerník, J.; Musil, I. The Pinus mugo Complex & minus; Its Structuring and General Overview of the Used Nomenclature. J. For. Sci. 2008, 53, 253–266. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Zanuso, E.; Genisheva, Z.; Rocha, C.M.R.; Teixeira, J.A. Green and Sustainable Valorization of Bioactive Phenolic Compounds from Pinus By-Products. Molecules 2020, 25, 2931. [Google Scholar] [CrossRef]
- Șandru, D. Identification and Quantification of Valuable Phenolic Compounds from Red Wines from Western Part of Romania. World J. Adv. Res. Rev. 2019, 2, 28–33. [Google Scholar] [CrossRef]
- Neamtu, A.-A.; Szoke-Kovacs, R.; Mihok, E.; Georgescu, C.; Turcus, V.; Olah, N.K.; Frum, A.; Tita, O.; Neamtu, C.; Szoke-Kovacs, Z.; et al. Bilberry (Vaccinium myrtillus L.) Extracts Comparative Analysis Regarding Their Phytonutrient Profiles, Antioxidant Capacity along with the In Vivo Rescue Effects Tested on a Drosophila melanogaster High-Sugar Diet Model. Antioxidants 2020, 9, 1067. [Google Scholar] [CrossRef]
- Dziedziński, M.; Kobus-Cisowska, J.; Stachowiak, B. Pinus Species as Prospective Reserves of Bioactive Compounds with Potential Use in Functional Food—Current State of Knowledge. Plants 2021, 10, 1306. [Google Scholar] [CrossRef]
- Venditti, A.; Serrilli, A.M.; Vittori, S.; Papa, F.; Maggi, F.; Di Cecco, M.; Ciaschetti, G.; Bruno, M.; Rosselli, S.; Bianco, A. Secondary Metabolites from Pinus mugo Turra Subsp. Mugo Growing in the Majella National Park (Central Apennines, Italy). Chem. Biodivers. 2013, 10, 2091–2100. [Google Scholar] [CrossRef]
- Adams, R.; Tashev, A. Composition of the Leaf Volatile Terpenoids of Pinus mugo Turra from Bulgaria Compared with Oils from Other Regions. Phytologia 2019, 101, 74–80. [Google Scholar]
- Ioannou, E.; Koutsaviti, A.; Tzakou, O.; Roussis, V. The Genus Pinus: A Comparative Study on the Needle Essential Oil Composition of 46 Pine Species. Phytochem. Rev. 2014, 13, 741–768. [Google Scholar] [CrossRef]
- Amri, I.; Hamrouni, L.; Hanana, M.; Gargouri, S.; Fezzani, T.; Jamoussi, B. Chemical Composition, Physico-Chemical Properties, Antifungal and Herbicidal Activities of Pinus halepensis Miller Essential Oils. Biol. Agric. Hortic. 2013, 29, 91–106. [Google Scholar] [CrossRef]
- Fekih, N.; Allali, H.; Merghache, S.; Chaïb, F.; Merghache, D.; El Amine, M.; Djabou, N.; Muselli, A.; Tabti, B.; Costa, J. Chemical Composition and Antibacterial Activity of Pinus halepensis Miller Growing in West Northern of Algeria. Asian Pac. J. Trop. Dis. 2014, 4, 97–103. [Google Scholar] [CrossRef]
- Hajdari, A.; Mustafa, B.; Ahmeti, G.; Pulaj, B.; Lukas, B.; Ibraliu, A.; Stefkov, G.; Quave, C.L.; Novak, J. Essential Oil Composition Variability among Natural Populations of Pinus mugo Turra in Kosovo. SpringerPlus 2015, 4, 828. [Google Scholar] [CrossRef]
- Mitić, Z.S.; Jovanović, S.Č.; Zlatković, B.K.; Nikolić, B.M.; Stojanović, G.S.; Marin, P.D. Needle Terpenes as Chemotaxonomic Markers in Pinus: Subsections Pinus and Pinaster. Chem. Biodivers. 2017, 14, e1600453. [Google Scholar] [CrossRef]
- Bojović, S.; Jurc, M.; Ristić, M.; Popović, Z.; Matić, R.; Vidaković, V.; Stefanović, M.; Jurc, D. Essential-Oil Variability in Natural Populations of Pinus mugo Turra from the Julian Alps. Chem. Biodivers. 2016, 13, 181–187. [Google Scholar] [CrossRef]
- Graßmann, J.; Hippeli, S.; Spitzenberger, R.; Elstner, E.F. The Monoterpene Terpinolene from the Oil of Pinus mugo L. in Concert with α-Tocopherol and β-Carotene Effectively Prevents Oxidation of LDL. Phytomedicine 2005, 12, 416–423. [Google Scholar] [CrossRef]
- Tsitsimpikou, C.; Petrakis, P.V.; Ortiz, A.; Harvala, C.; Roussis, V. Volatile Needle Terpenoids of Six Pinus Species. J. Essent. Oil Res. 2001, 13, 174–178. [Google Scholar] [CrossRef]
- Karapandzova, M.; Stefkov, G.; Cvetkovikj, I.; Stanoeva, J.P.; Stefova, M.; Kulevanova, S. Flavonoids and Other Phenolic Compounds in Needles of Pinus peuce and Other Pine Species from the Macedonian Flora. Nat. Prod. Commun. 2015, 10, 987–990. [Google Scholar] [CrossRef] [PubMed]
- Basim, E.; Basim, H. Chemical Composition, Antibacterial and Antifungal Activities of Turpentine Oil of Pinus sylvestris L. Against Plant Bacterial and Fungal Pathogens. J. Food Agric. Environ. 2013, 11, 2261–2264. [Google Scholar]
- Celiński, K.; Bonikowski, R.; Wojnicka-Półtorak, A.; Chudzińska, E.; Maliński, T. Volatiles as Chemosystematic Markers for Distinguishing Closely Related Species within the Pinus mugo Complex. Chem. Biodiv. 2015, 12, 1208–1213. [Google Scholar] [CrossRef] [PubMed]
- Ciuman, R.R. Phytotherapeutic and Naturopathic Adjuvant Therapies in Otorhinolaryngology. Eur. Arch. Otorhinolaryngol. 2012, 269, 389–397. [Google Scholar] [CrossRef][Green Version]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical Composition, Anti-Inflammatory Activity and Cytotoxic Effects of Essential Oils from Three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Grassmann, J.; Hippeli, S.; Vollmann, R.; Elstner, E.F. Antioxidative Properties of the Essential Oil from Pinus mugo. J. Agric. Food Chem. 2003, 51, 7576–7582. [Google Scholar] [CrossRef]
- Garzoli, S.; Masci, V.L.; Caradonna, V.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Liquid and Vapor Phase of Four Conifer-Derived Essential Oils: Comparison of Chemical Compositions and Antimicrobial and Antioxidant Properties. Pharmaceuticals 2021, 14, 134. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils—A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Yesil-Celiktas, O.; Ganzera, M.; Akgun, I.; Sevimli, C.; Korkmaz, K.S.; Bedir, E. Determination of Polyphenolic Constituents and Biological Activities of Bark Extracts from Different Pinus Species: Biological Activities of Pine Bark Extracts. J. Sci. Food Agric. 2009, 89, 1339–1345. [Google Scholar] [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Mármol, I.; Quero, J.; Jiménez-Moreno, N.; Rodríguez-Yoldi, M.J.; Ancín-Azpilicueta, C. A Systematic Review of the Potential Uses of Pine Bark in Food Industry and Health Care. Trends Food Sci. Technol. 2019, 88, 558–566. [Google Scholar] [CrossRef]
- Milić, N.; Milanović, M.; Četojević-Simin, D.; Malenčić, Đ.; Prvulović, D.; Pavkov, N.; Radulović, Z.; Milošević, N.; Rašković, A.; Mandić, A. Phytochemical Characterization and Effects on Cell Proliferation of Pinus nigra Arn. Bark. Arch. Pharm. 2021, 354, 2000416. [Google Scholar] [CrossRef]
- Kurti, F.; Giorgi, A.; Beretta, G.; Mustafa, B.; Gelmini, F.; Testa, C.; Angioletti, S.; Giupponi, L.; Zilio, E.; Pentimalli, D.; et al. Chemical Composition, Antioxidant and Antimicrobial Activities of Essential Oils of Different Pinus Species from Kosovo. J. Essent. Oil Res. 2019, 31, 263–275. [Google Scholar] [CrossRef]
- Felšöciová, S.; Vukovic, N.; Jeżowski, P.; Kačániová, M. Antifungal Activity of Selected Volatile Essential Oils against Penicillium sp. Open Life Sci. 2020, 15, 511–521. [Google Scholar] [CrossRef]
- Dei Cas, L.; Pugni, F.; Fico, G. Tradition of Use on Medicinal Species in Valfurva (Sondrio, Italy). J. Ethnopharmacol 2015, 163, 113–134. [Google Scholar] [CrossRef]
- Vitalini, S.; Iriti, M.; Puricelli, C.; Ciuchi, D.; Segale, A.; Fico, G. Traditional Knowledge on Medicinal and Food Plants Used in Val San Giacomo (Sondrio, Italy)—An Alpine Ethnobotanical Study. J. Ethnopharmacol. 2013, 145, 517–529. [Google Scholar] [CrossRef]
- Constantinescu, G.; Haţieganu-Buruiană, E. Să Ne Cunoaştem Plantele Medicinale, Proprietăţile Lor Terapeutice Şi Modul de Folosire; Editura Medicală: Bucharest, Romania, 1986. [Google Scholar]
- Dumitru, E.; Dumitru, R. Terapia Naturistă. Incursiune În Farmacia Naturii; Editura Ştiinţifică: Bucharest, Romania, 1992. [Google Scholar]
- Vitalini, S.; Puricelli, C.; Mikerezi, I.; Iriti, M. Plants, People and Traditions: Ethnobotanical Survey in the Lombard Stelvio National Park and Neighbouring Areas (Central Alps, Italy). J. Ethnopharmacol. 2015, 173, 435–458. [Google Scholar] [CrossRef]
- Li, T.S.C. Medicinal Plants: Culture, Utilization and Phytopharmacology; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-367-39846-0. [Google Scholar]
- Olech, M.; Nowak, R.; Ivanova, D.; Tashev, A.; Boyadzhieva, S.; Kalotova, G.; Angelov, G.; Gawlik-Dziki, U. LC-ESI-MS/MS-MRM Profiling of Polyphenols and Antioxidant Activity Evaluation of Junipers of Different Origin. Appl. Sci. 2020, 10, 8921. [Google Scholar] [CrossRef]
- Al-Rifai, A.; Aqel, A.; Al-Warhi, T.; Wabaidur, S.M.; Al-Othman, Z.A.; Badjah-Hadj-Ahmed, A.Y. Antibacterial, Antioxidant Activity of Ethanolic Plant Extracts of Some Convolvulus Species and Their DART-ToF-MS Profiling. Evid. Based Complement. Alternat. Med. 2017, 2017, 5694305. [Google Scholar] [CrossRef]
- Stegăruș, D.I.; Lengyel, E.; Apostolescu, G.F.; Botoran, O.R.; Tanase, C. Phytochemical Analysis and Biological Activity of Three Stachys Species (Lamiaceae) from Romania. Plants 2021, 10, 2710. [Google Scholar] [CrossRef]
- Meos, A.; Zaharova, I.; Kask, M.; Raal, A. Content of Ascorbic Acid in Common Cowslip (Primula veris L.) Compared to Common Food Plants and Orange Juices. Acta Biol. Crac. Ser. Bot. 2017, 59, 113–120. [Google Scholar] [CrossRef]
- Gorunescu, F. Exploratory Data Analysis. In Data Mining; Intelligent Systems Reference Library; Springer: Berlin/Heidelberg, Germany, 2011; Volume 12, pp. 57–157. ISBN 978-3-642-19720-8. [Google Scholar]
- Sancho-Knapik, D.; Gil-Pelegrín, E.; Ferrio, J.P.; Alonso-Forn, D.; Martín-Sánchez, R.; dos Santos Silva, J.V.; Imanishi, J.; Peguero-Pina, J.J.; Sanz, M.Á. Changes in the Abundance of Monoterpenes from Breathable Air of a Mediterranean Conifer Forest: When Is the Best Time for a Human Healthy Leisure Activity? Forests 2022, 13, 965. [Google Scholar] [CrossRef]
- Hazrati, S.; Hosseini, S.J.; Ebadi, M.-T.; Nicola, S. Evolution of Phytochemical Variation in Myrtle (Myrtus communis L.) Organs during Different Phenological Stages. Horticulturae 2022, 8, 757. [Google Scholar] [CrossRef]
- Guri, A.; Kefalas, P.; Roussis, V. Antioxidant Potential of Six Pine Species. Phytother. Res. 2006, 20, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kang, S.; Jeon, J.; Um, B. A Comparison of Pycnogenol® and Bark Extracts from Pinus Thunbergii and Pinus Densiflora: Extractability, Antioxidant Activity and Proanthocyanidin Composition. J. Med. Plants Res. 2012, 6, 2839–2849. [Google Scholar]
- Ustun, O.; Senol, F.; Kurkcuoglu, M.; Orhan, I.; Kartal, M.; Baser, K. Investigation on Chemical Composition, Anticholinesterase and Antioxidant Activities of Extracts and Essential Oils of Turkish Pinus Species and Pycnogenol. Ind. Crops Prod. 2012, 38, 115–123. [Google Scholar] [CrossRef]
- Apetrei, C.L.; Tuchilus, C.; Aprotosoaie, A.C.; Oprea, A.; Malterud, K.E.; Miron, A. Chemical, Antioxidant and Antimicrobial Investigations of Pinus cembra L. Bark and Needles. Molecules 2011, 16, 7773–7788. [Google Scholar] [CrossRef]
- Carr, A.; Vissers, M. Synthetic or Food-Derived Vitamin C—Are They Equally Bioavailable? Nutrients 2013, 5, 4284–4304. [Google Scholar] [CrossRef]
- Raal, A.; Nisuma, K.; Meos, A. Pinus sylvestris L. and Other Conifers as Natural Sources of Ascorbic Acid. J. Pharm. Pharmacogn. Res. 2018, 6, 89–95. [Google Scholar]
- Koutsaviti, A.; Toutoungy, S.; Saliba, R.; Loupassaki, S.; Tzakou, O.; Roussis, V.; Ioannou, E. Antioxidant Potential of Pine Needles: A Systematic Study on the Essential Oils and Extracts of 46 Species of the Genus Pinus. Foods 2021, 10, 142. [Google Scholar] [CrossRef]
- Krauze-Baranowska, M.; Mardarowicz, M.; Wiwart, M.; Pobłocka, L.; Dynowska, M. Antifungal Activity of the Essential Oils from Some Species of the Genus Pinus. Z. Nat. C 2002, 57, 478–482. [Google Scholar] [CrossRef]
- Erland, L.A.; Bitcon, C.R.; Lemke, A.D.; Mahmoud, S.S. Antifungal Screening of Lavender Essential Oils and Essential Oil Constituents on Three Post-Harvest Fungal Pathogens. Nat. Prod. Commun. 2016, 11, 523–527. [Google Scholar] [CrossRef]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) Essential Oils and Their Main Components to Enhance Itraconazole Activity against Azole Susceptible/Not-Susceptible Cryptococcus neoformans Strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef]
- Mačionienė, I.; Čepukoit, D.; Šalomskienė, J.; Černauskas, D.; Burokienė, D.; Šalaševičienė, A. Effects of Natural Antimicrobials on Xanthomonas Strains Growth. Horticulturae 2021, 8, 7. [Google Scholar] [CrossRef]

| Local Harvest | Location in the Southern Carpathians | Altitude (m) | Climate | Average Annual Temperatures (°C) | Annual Average of Precipitation (mm3) | Geological Structure |
|---|---|---|---|---|---|---|
| Cindrel-Iujbea | Southwestern | 1750 | Temperate moderate | 4–6 | 800–1400 | Crystalline shales |
| Făgăraș-Viștea | Central–eastern | 1800 | Temperate continental | 6–8 | 1100 | Crystalline shales |
| Cozia-Rotunda | Central–southern | 1550 | Temperate moderate | +3 | 1200 | Hard gneiss rocks |
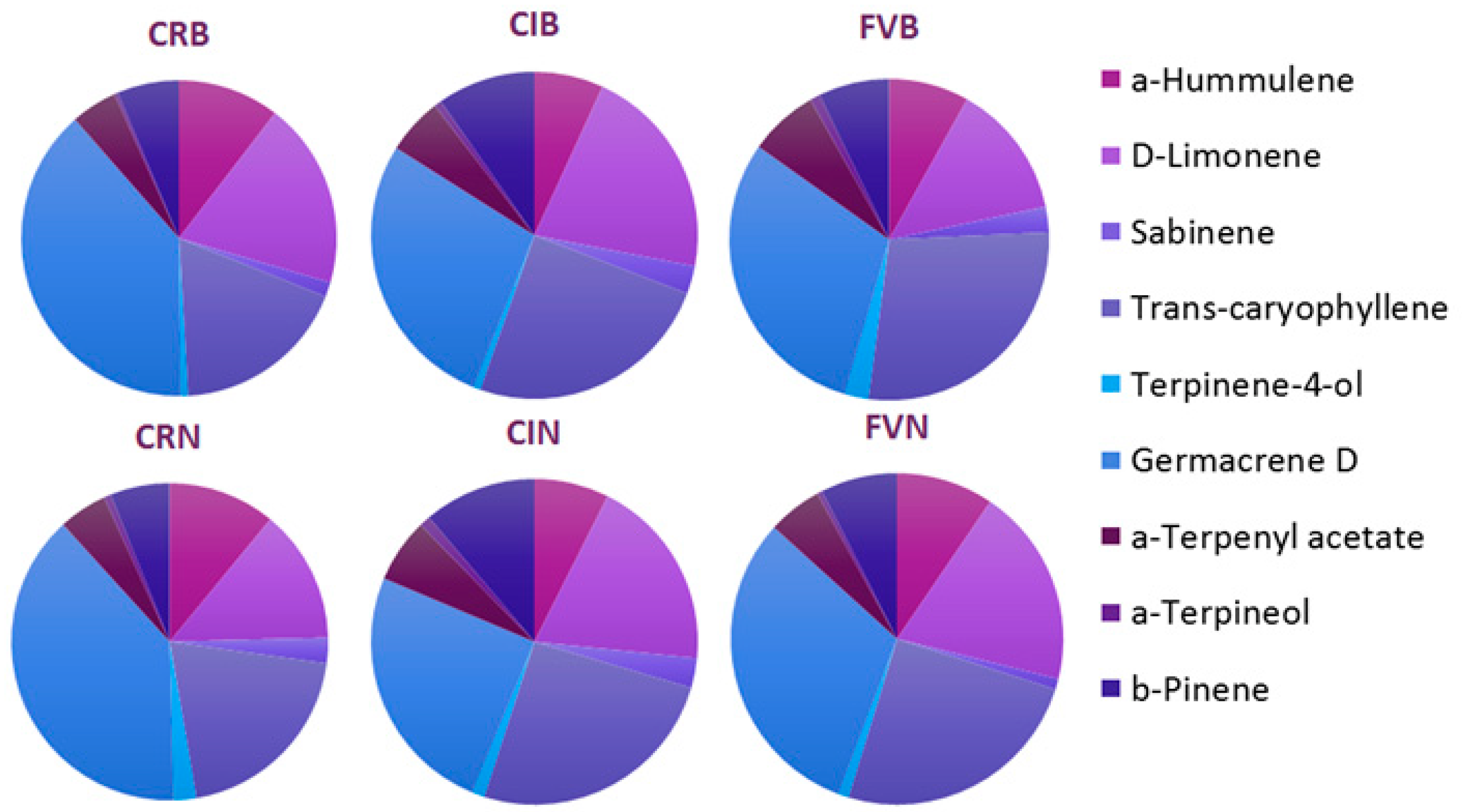
| Compound | RI | Ions (m/z) | Cindrel-Iujbea (%) | Făgăraș-Viștea (%) | Cozia-Rotunda (%) | |||
|---|---|---|---|---|---|---|---|---|
| Buds | Needles | Buds | Needles | Buds | Needles | |||
| Hexanal | 6.369 | 44.56.100 | 0.01 | 0.01 | - | - | - | - |
| 1-Propanol | 7.146 | 31.59.60 | - | - | 0.01 | - | - | 0.02 |
| α-Pinene | 8.236 | 93.121.136 | 14.77 | 15.27 | 14.35 | 16.31 | 16.05 | 15.99 |
| Camphene | 8.916 | 93.121.136 | 6.78 | 5.96 | 4.13 | 6.32 | 5.98 | 4.27 |
| Sabinene | 9.003 | 77.93.136 | 0.88 | 0.91 | 0.78 | 0.34 | 0.47 | 0.82 |
| β-Pinene | 10.138 | 41.93.136 | 3.11 | 3.48 | 2.17 | 2.45 | 1.99 | 1,91 |
| β-Myrcene | 11.005 | 69.93.136 | 1.45 | 1.76 | 2.12 | 2.31 | 2.17 | 1.99 |
| Octanal | 12.127 | 43.84.128 | 1.01 | 0.92 | 0.23 | 0.41 | 0.16 | 0.17 |
| δ-3-Carene | 13.892 | 79.93.136 | 21.42 | 19.83 | 20.71 | 20.94 | 19.66 | 18.28 |
| p-Cymene | 14.243 | 91.191.34 | 1.1 | 0.92 | 0.18 | 1.23 | - | 0.45 |
| D-Limonene | 15.891 | 43.67.168 | 6.78 | 5.96 | 4.13 | 6.32 | 5.98 | 4.27 |
| Linalool acetate | 16.008 | 93.121.196 | - | - | 0.12 | 0.11 | - | 0.17 |
| 2-Methyl-1-butanol nerolidol | 17.087 | 57.70.88 | - | - | - | 0.01 | - | - |
| 3-Methyl-1-butanol | 17.319 | 55.77.80 | 0.01 | 0.01 | - | - | - | - |
| Germacrene D | 18.006 | 105.121.204 | 8.93 | 7.81 | 9.22 | 10.12 | 12.14 | 12.13 |
| α-Terpenyl acetate | 18.62 | 43.121.196 | 1.79 | 1.98 | 2.12 | 1.73 | 1.49 | 1.56 |
| α-Terpinene | 18.718 | 93.121.136 | 1.01 | 0.99 | 0.19 | 1.13 | 0.45 | 0.38 |
| Terpinene-4-ol | 18.906 | 71.111.154 | 0.21 | 0.37 | 0.71 | 0.32 | 0.22 | 0.71 |
| α-Terpineol | 19.005 | 59.93.154 | 0.23 | 0.34 | 0.31 | 0.16 | 0.11 | 0.22 |
| α-Terpinolene | 19.136 | 79.121.136 | 5.87 | 6.02 | 5.23 | 4.99 | 6.12 | 4.57 |
| Myrtenol | 21.222 | 79.108.152 | 0.11 | 0.03 | - | 0.04 | 0.07 | - |
| Bornil acetate | 22.356 | 95.136.196 | 3.44 | 2.09 | 3.48 | 4.55 | 4.01 | 3.12 |
| β-Copaene | 23.118 | 119.161.204 | 0.22 | 0.31 | 0.31 | 0.44 | 0.38 | 0.35 |
| a-Hummulene | 24.936 | 93.121.204 | 2.19 | 2.31 | 2.45 | 3.11 | 3.27 | 3.44 |
| α-Cadinene | 25.224 | 161.189.204 | 0.22 | 0.24 | 0.16 | 0.19 | 0.32 | 0.29 |
| δ-Cadinene | 25.647 | 105.161.204 | 8.29 | 7.93 | 7.71 | 7.17 | 8.03 | 8.56 |
| α-Cadinol | 26.055 | 95.121.222 | 5.63 | 5.15 | 4.94 | 5.9 | 6.18 | 6.57 |
| Trans-caryophyllene | 28.164 | 93.133.204 | 7.88 | 7.92 | 8.44 | 8.12 | 5.66 | 6.32 |
| Area of Origin | Extract | TPC * (mg GAE/g ± SD) | TFC * (mg QE/g ± SD) | TE * mM/g DE ± SD * |
|---|---|---|---|---|
| Cindrel-Iujbea | Buds | 46.77 ± 0.3 | 24.90 ± 0.1 | 115.64 ± 9.1 |
| Needles | 55.53 ± 0.3 | 36.34 ± 0.2 | 124.32 ± 11.3 | |
| Făgăraș-Viștea | Buds | 55.12 ± 0.3 | 37.10 ± 0.2 | 124.21 ± 13.2 |
| Needles | 68.23 ± 0.4 | 26.89 ± 0.2 | 164.93 ± 15.2 | |
| Cozia-Rotunda | Buds | 77.99 ± 0.5 | 54.78 ± 0.3 | 194.54 ± 11.8 |
| Needles | 64.56 ± 0.2 | 45.55 ± 0.2 | 155.71 ± 23.6 |
| Strains | MIC (µL/mL) | ||||||
|---|---|---|---|---|---|---|---|
| CIB | CIN | FVB | FVN | CRB | CRN | Posaconazole | |
| Candida albicans 2231 | 0.5 | 0.25 | 0.5 | 0.25 | 0.5 | 0.25 | 0.125 |
| Cryptococcus neoformans 223 | NE | NE | NE | 1.00 | NE | NE | 0.125 |
| Penicillium chrysogenum | NE | 0.125 | NE | 0.125 | NE | 0.125 | NE |
| Aspergillus flavus 1082 | 0.25 | 0.125 | 0.25 | 0.125 | 0.25 | 0.125 | 0.125 |
| DMSO negative control | NE | NE | NE | NE | NE | NE | NE |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.I.; Lengyel, E.; Apostolescu, F.G.; Soare, L.C.; Botoran, O.R.; Șuțan, N.A. Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania. Horticulturae 2022, 8, 952. https://doi.org/10.3390/horticulturae8100952
Popescu DI, Lengyel E, Apostolescu FG, Soare LC, Botoran OR, Șuțan NA. Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania. Horticulturae. 2022; 8(10):952. https://doi.org/10.3390/horticulturae8100952
Chicago/Turabian StylePopescu, Diana Ionela (Stegarus), Ecaterina Lengyel, Florian George Apostolescu, Liliana Cristina Soare, Oana Romina Botoran, and Nicoleta Anca Șuțan. 2022. "Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania" Horticulturae 8, no. 10: 952. https://doi.org/10.3390/horticulturae8100952
APA StylePopescu, D. I., Lengyel, E., Apostolescu, F. G., Soare, L. C., Botoran, O. R., & Șuțan, N. A. (2022). Volatile Compounds and Antioxidant and Antifungal Activity of Bud and Needle Extracts from Three Populations of Pinus mugo Turra Growing in Romania. Horticulturae, 8(10), 952. https://doi.org/10.3390/horticulturae8100952







