Flowering Phenology of Six Seasonal-Flowering Strawberry Cultivars in a Coordinated European Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Sites, Plant Material, and Cultivation
2.2. Flower Initiation and Development
- Stage 1 = vegetative apex with only leaf primordia;
- Stage 2 = apex round and slightly elevated, apical dome above level of developing stipules;
- Stage 3 = Sepal primordia visible in terminal bud;
- Stage 4 = Secondary flower primordia is visible;
- Stage 5 = Petal primordia visible in terminal bud;
- Stage 6 = Whorls of stamen visible in terminal bud, prolonged sepals cover petals;
- Stage 7 = Carpel primordia visible, central region still undifferentiated;
- Stage 8 = Initiation of carpels complete, anthers still green;
- Stage 9 = All flower parts differentiated, anthers yellow.
- Here, we use floral Stage 2 for the day of the year of flower initiation (DOY-FI). DOY-FI was interpolated using the spline smoothing function.
2.3. Date of Anthesis and Plant Architecture the Next Spring
2.4. Plant Traits and Environmental Factors
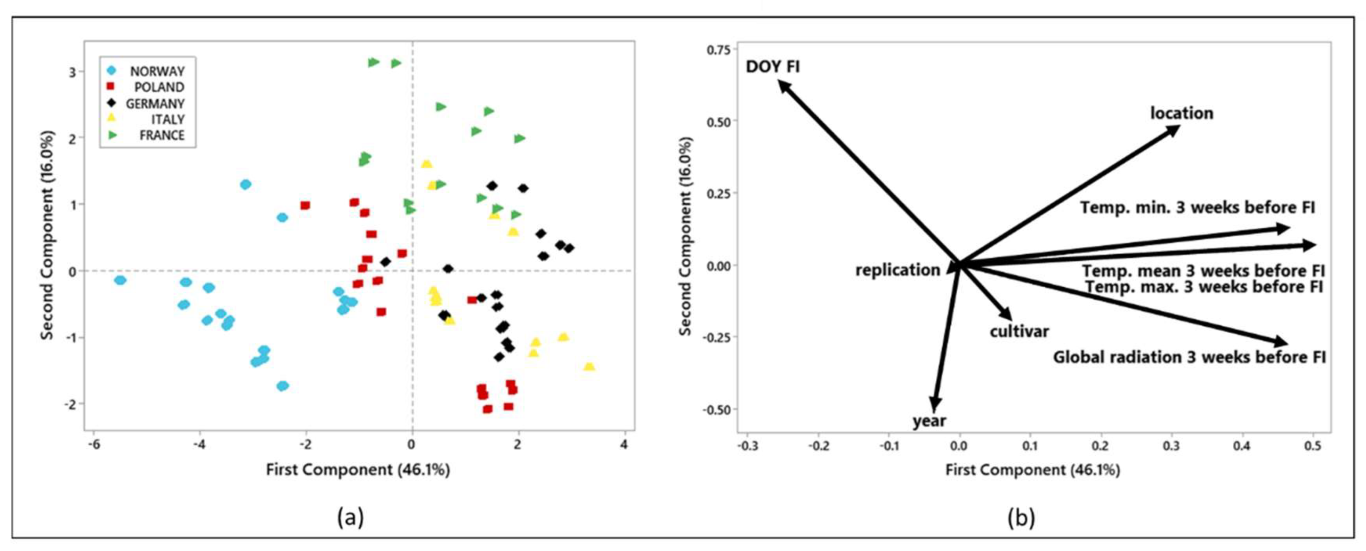
2.5. Statistical Analyses
3. Results
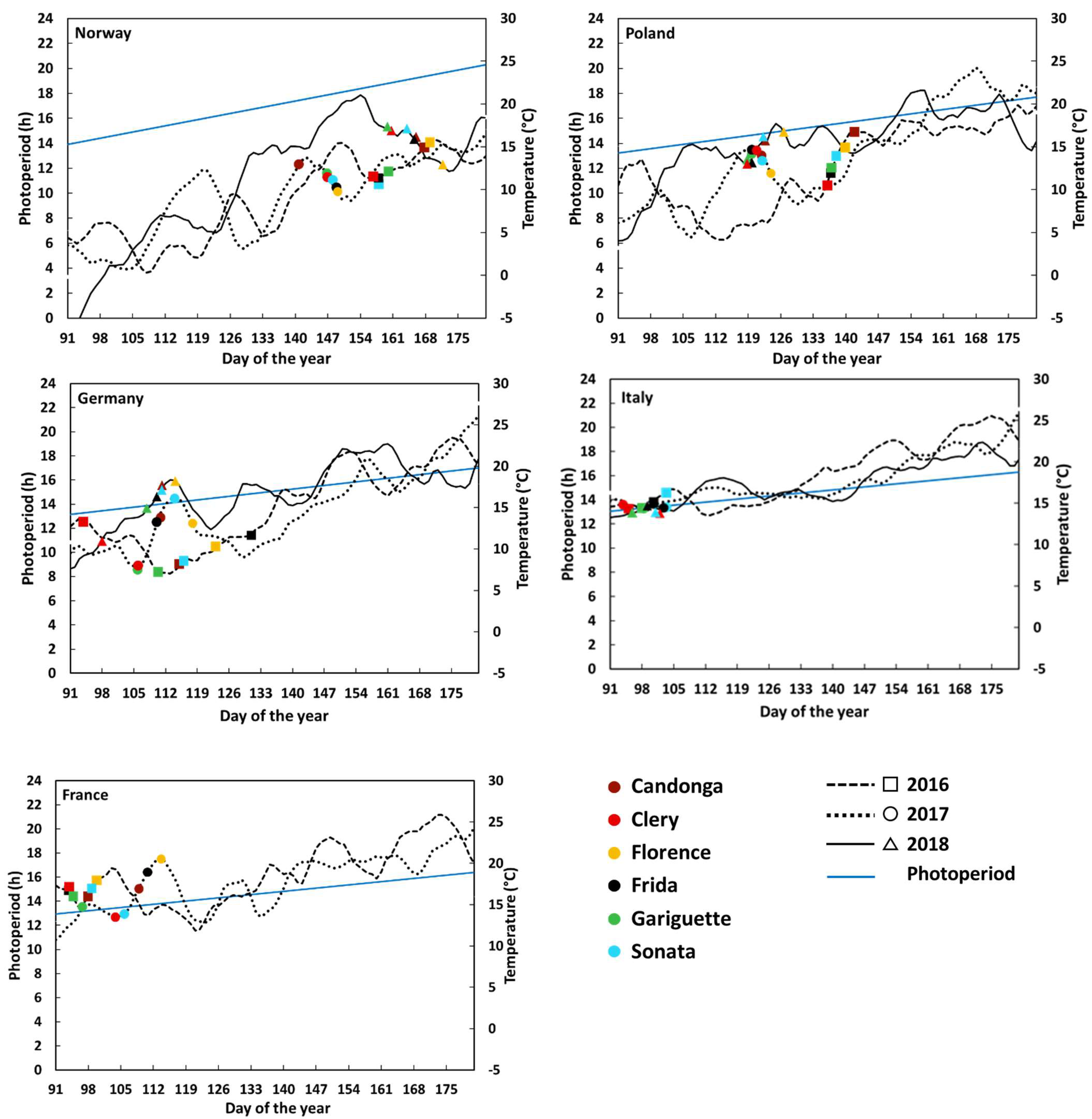
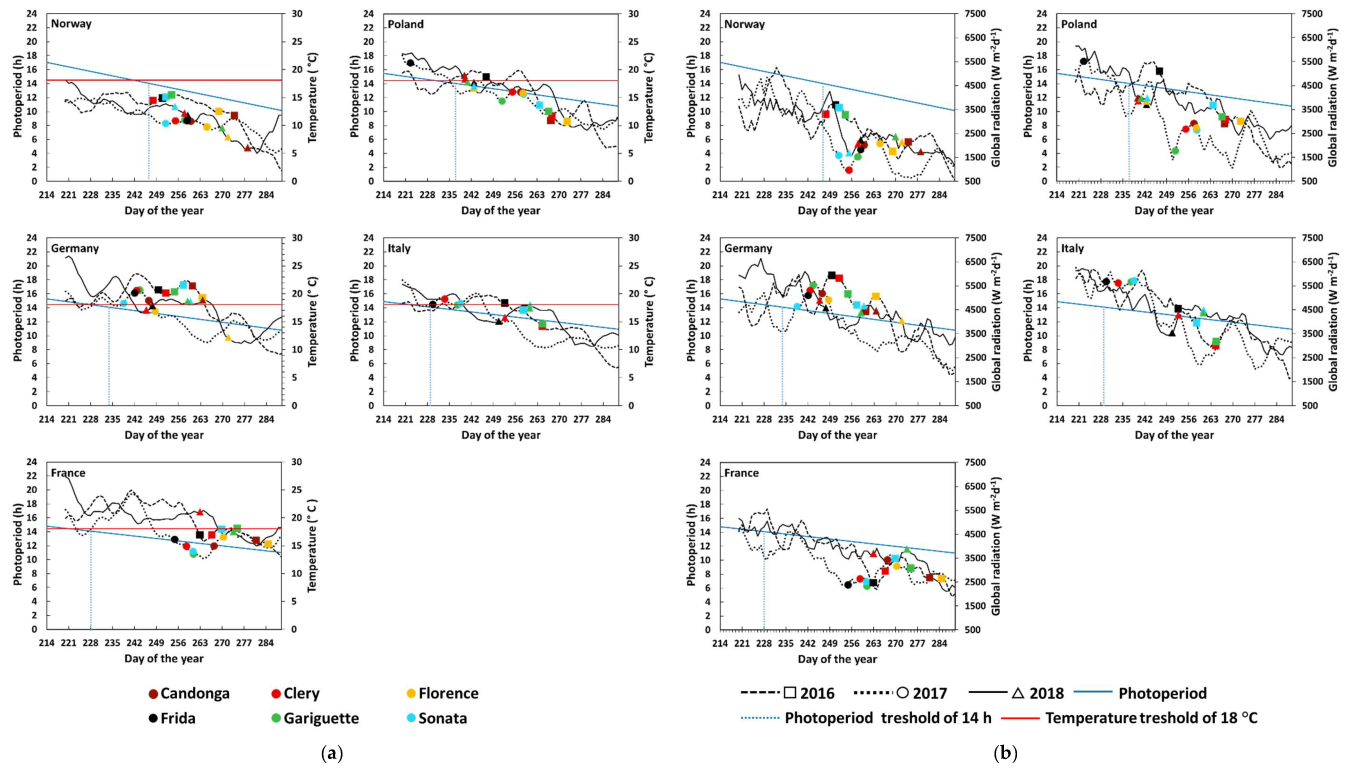
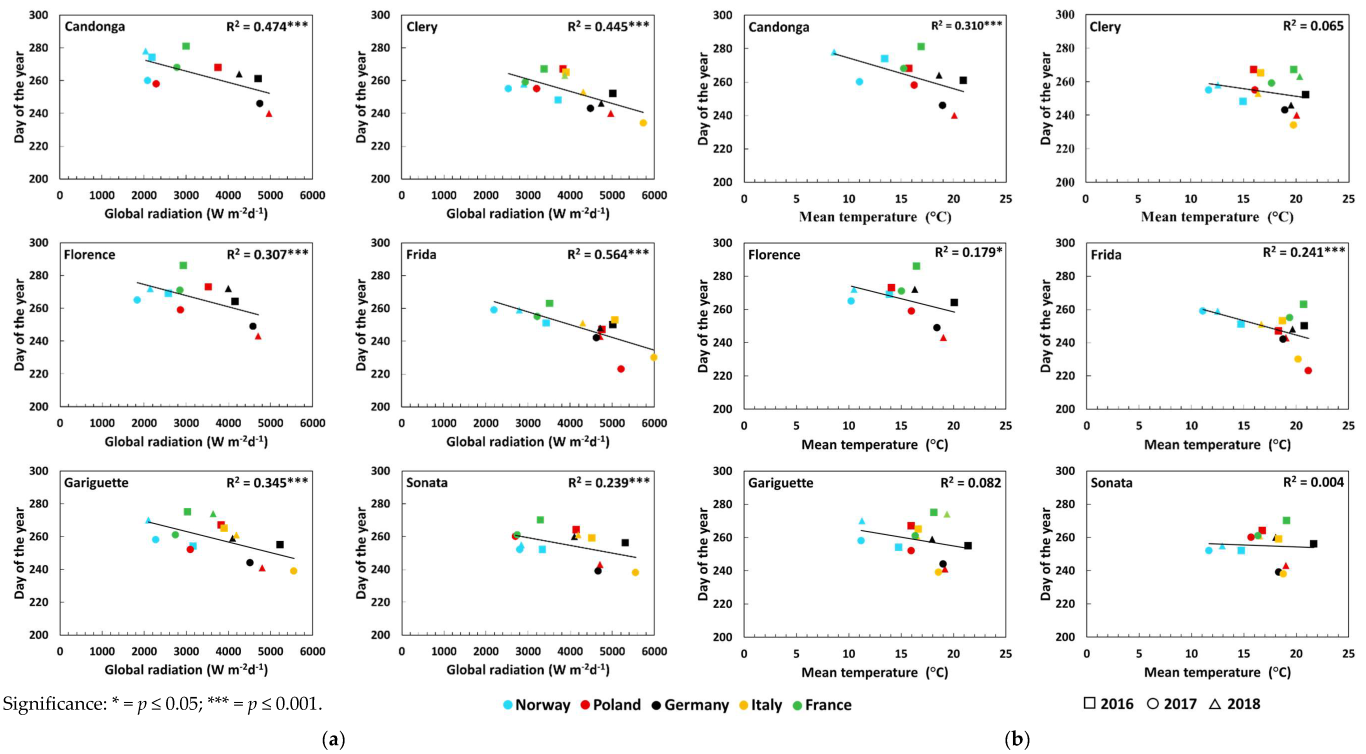
3.1. Flower Initiation
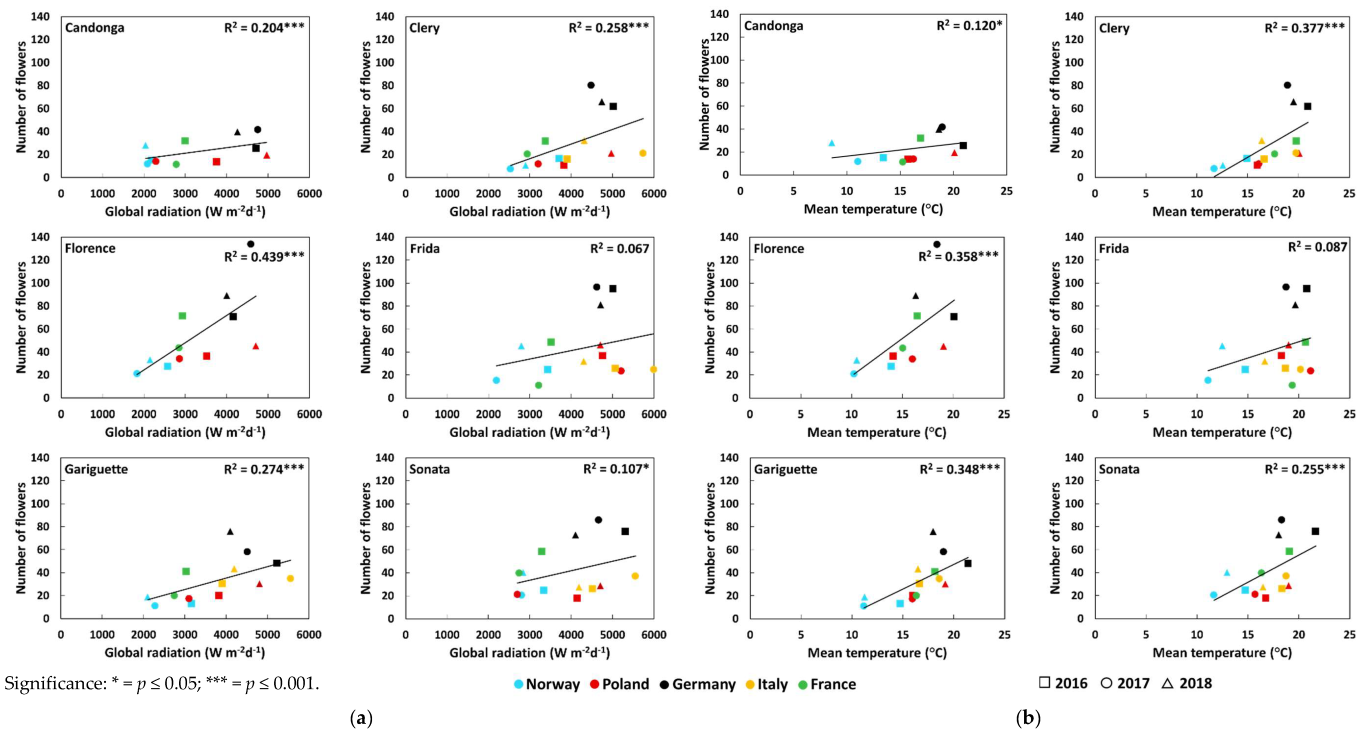
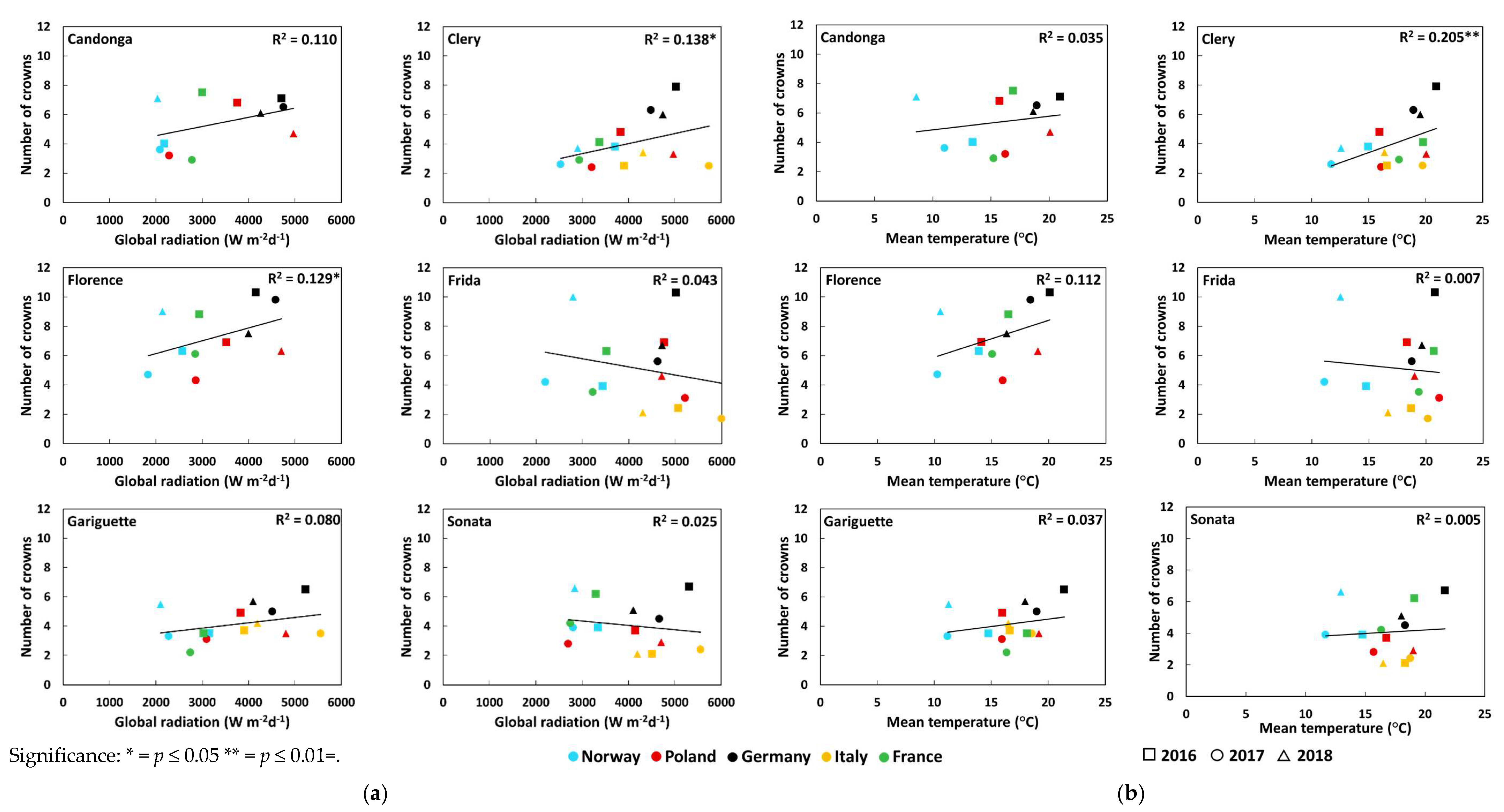
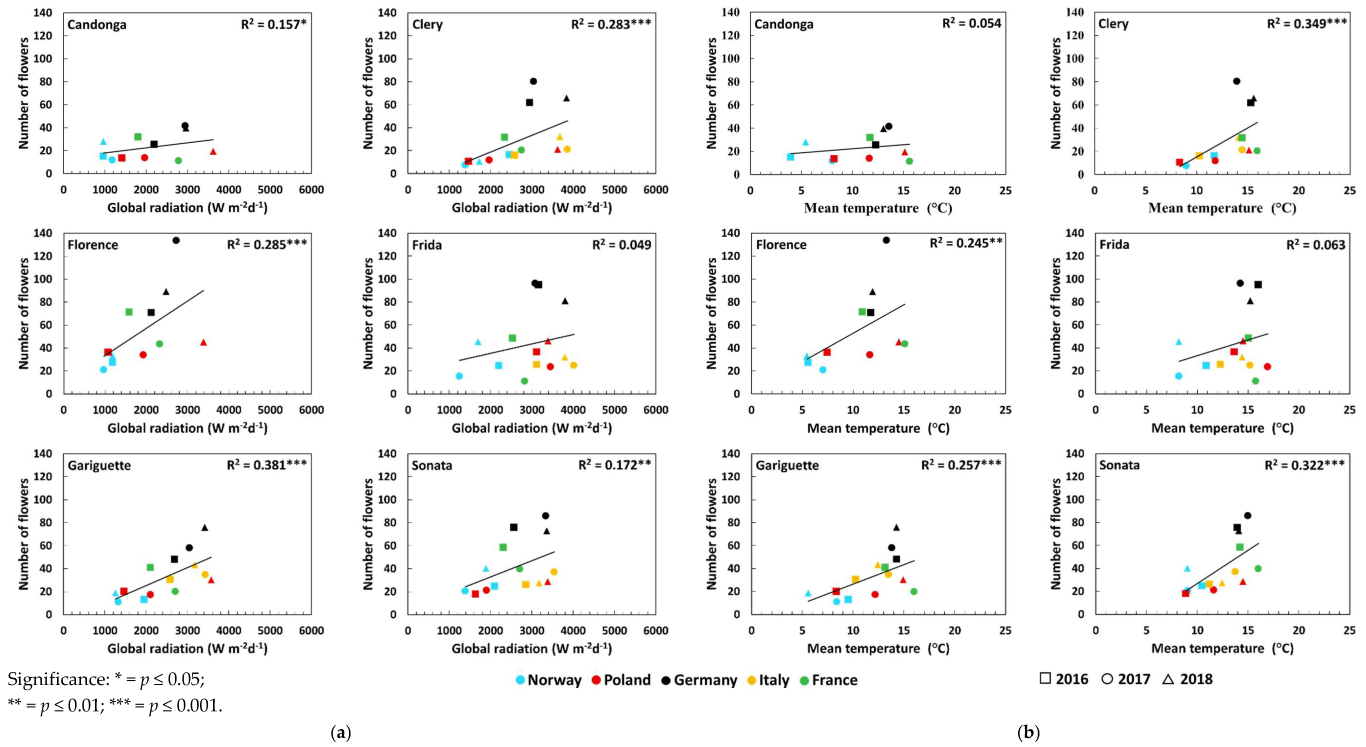
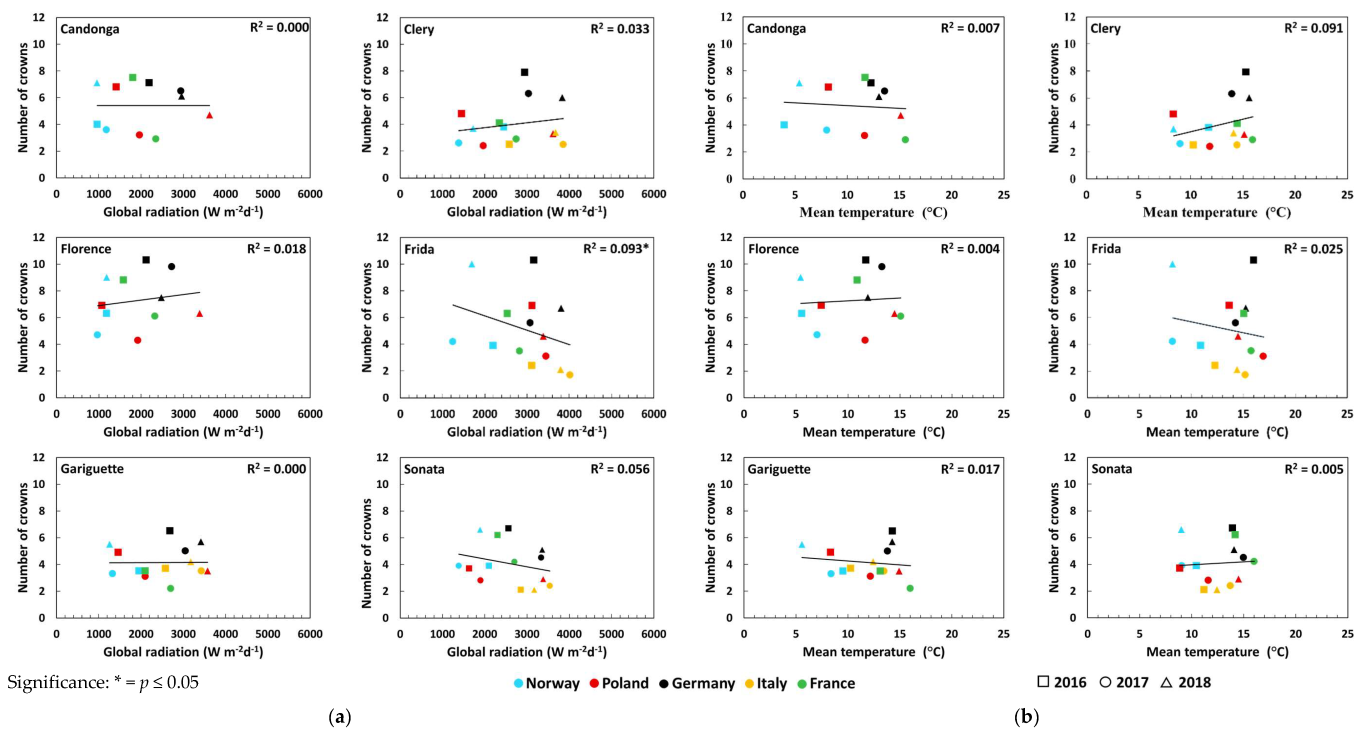
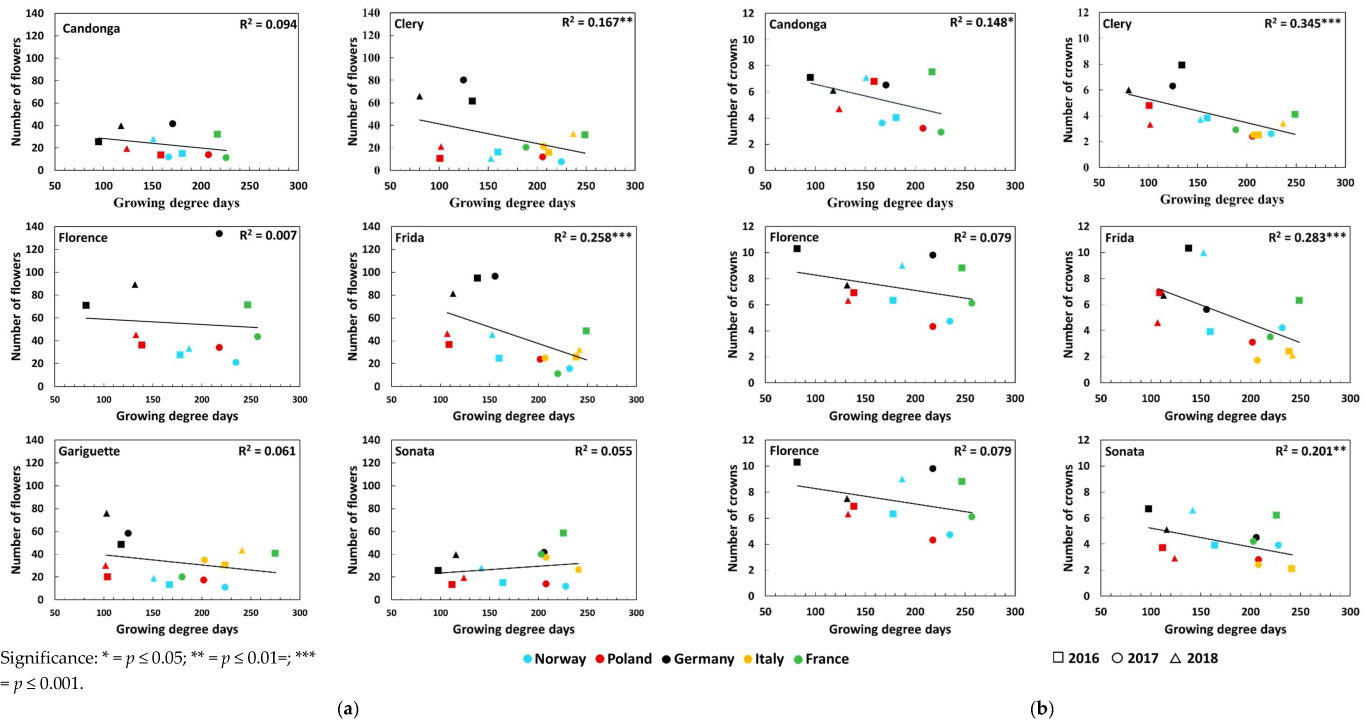
3.2. Number of Crowns and Flowers
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Year | Norway | Poland | Germany | Italy | France | ||
|---|---|---|---|---|---|---|---|
| Period before and after flower | Mean temperature (°C) | 2016 | 9.4 | 12.7 | 15.0 | 13.5 | 17.1 |
| initiation | 2017 | 8.9 | 13.4 | 14.4 | 13.1 | 16.1 | |
| 1 June–30 November | 2018 | 10.8 | 14.0 | 16.1 | 14.3 | 17.7 | |
| Global radiation (W m−2d−1) | 2016 | 2515 | 3031 | 3420 | 3734 | 3280 | |
| 2016 | 2451 | 2853 | 3181 | 3901 | 2941 | ||
| 2018 | 2819 | 3404 | 3901 | 3850 | 3461 | ||
| Precipitation (mm) | 2016 | 303 | 320 | 171 | n.a. | 202 | |
| 2017 | 400 | 401 | 286 | n.a. | 267 | ||
| 2018 | 237 | 360 | 100 | n.a. | 247 | ||
| Period before anthesis | Mean temperature (°C) | 2017 | 7.0 | 11.2 | 13,8 | 17.2 | 17.6 |
| 1 March–30 June | 2018 | 7.3 | 12.1 | 14.0 | 16.4 | 16,2 | |
| 2019 | 7.0 | 12.3 | 13.4 | 17.1 | * | ||
| Global radiation (W m−2d−1) | 2017- | 3729 | 4124 | 4851 | 4924 | 4014 | |
| 2019 | 4556 | 4778 | 4751 | 4399 | 3645 | ||
| 2019 | 3788 | 4739 | 4860 | 4450 | * | ||
| Precipitation (mm) | 2017 | 172 | 280 | 143 | n.a. | 289 | |
| 2018 | 136 | 123 | 117 | n.a. | 394 | ||
| 2019 | 280 | 136 | 158 | n.a. | - |
| Location | Candonga | Clery | Florence | Frida | Gariguette | Sonata | Location Mean |
|---|---|---|---|---|---|---|---|
| Norway | 271 | 254 | 269 | 256 | 261 | 253 | 260.5 B |
| Poland | 255 | 254 | 258 | 238 | 253 | 256 | 252.4 A |
| Germany | 257 | 247 | 262 | 248 | 253 | 252 | 252.8 A |
| Italy | - | 251 | - | 245 | 255 | 253 | 250.8 A |
| France | 275 | 263 | 278 | 259 | 270 | 265 | 268.2 C |
| Cultivar mean | 263.5 cd | 253.7 ab | 265.7 d | 248.2 a | 258.4 bc | 255.0 b | |
| Probability level of significance (ANOVA) | |||||||
| Source of variation | |||||||
| Cultivar (A) | ≤0.001 | ||||||
| Location (B) | ≤0.001 | ||||||
| A×B | ns | ||||||

References
- Darrow, G.M.; Waldo, G.F. Responses of Strawberry Varieties and Species to Duration of the Light Period; Bulletin Tech No. 1488-2016-124781; U.S. Department of Agriculture: Washington, DC, USA, 1934; pp. 1–31.
- Guttridge, C.G. Fragaria x ananassa. In Handbook of Flowering; Halevy, A.H., Ed.; CRC Press: Boca Raton, FL, USA, 1985; Volume IV, pp. 16–33. [Google Scholar]
- Heide, O.M.; Stavang, J.A.; Sønsteby, A. Physiology and genetics of flowering in cultivated and wild strawberries—A review. J. Hortic. Sci. Biotech. 2013, 88, 1–18. [Google Scholar] [CrossRef]
- Ito, H.; Saito, T. Studies on the flower formation in the strawberry plants I. Effects of temperature and photoperiod on the flower formation. Tohoku J. Agric. Res. 1962, 13, 191–203. [Google Scholar]
- Heide, O.M. Photoperiod and temperature interactions in growth and flowering of strawberry. Physiol. Plant. 1977, 40, 21–26. [Google Scholar] [CrossRef]
- Verheul, M.; Sønsteby, A.; Grimstad, S.O. Influence of day and night temperature on flowering of Fragaria x ananassa Duch. cvs. Korona and Elsanta. Sci. Hortic. 2007, 112, 200–206. [Google Scholar] [CrossRef]
- Jonkers, H. On the flower formation, the dormancy and the early forcing of strawberries. Mede. Landbowhgesch. Wageningen 1965, 65, 1–59. Available online: https://edepot.wur.nl/187404 (accessed on 15 June 2022).
- Konsin, M.; Voipio, I.; Palonen, P. Influence of photoperiod and duration of short-day treatment on vegetative growth and flowering of strawberry (Fragaria x ananassa Duch.). J. Hortic. Sci. Biotech. 2001, 76, 77–82. [Google Scholar] [CrossRef]
- Lieten, F. The effect of nutrition prior to and during flower differentiation on phyllody and plant performance of short day strawberry ‘Elsanta’. Acta Hortic. 2002, 567, 345–348. [Google Scholar] [CrossRef]
- Sønsteby, A.; Opstad, N.; Myrheim, U.; Heide, O.M. Interaction of short day and timing of nitrogen fertilization on growth and flowering of ‘Korona’ strawberry (Fragaria x ananassa Duch.). Sci. Hortic. 2009, 123, 204–209. [Google Scholar] [CrossRef]
- Lang, A. Physiology of Flower Initiation. In Encyclopedia Plant Physiology; Ruhland, W., Ed.; Springer: Berlin, Germany, 1965; Volume XV/1, pp. 1380–1536. [Google Scholar]
- Wang, R.; Eguchi, M.; Gui, Y.; Iwasaki, Y. Evaluating the effect of light intensity on flower development uniformity in strawberry (Fragaria x ananassa) under early induction conditions in forcing culture. HortScience 2020, 55, 670–675. [Google Scholar] [CrossRef]
- Taylor, D.R.; Atkey, P.T.; Wickenden, M.F.; Crisp, C.M. A morphological study of flower initiation and development in strawberry (Fragaria x ananassa) using cryo-scanning electron microscopy. Ann. Appl. Biol. 1997, 130, 141–152. [Google Scholar] [CrossRef]
- Sønsteby, A.; Heide, O.M. Temperature responses, flowering and fruit yield of the June-bearing strawberry cultivars Florence, Frida and Korona. Sci. Hortic. 2008, 119, 49–54. [Google Scholar] [CrossRef]
- Takeda, F.; Glenn, D.M.; Callahan, A.; Slovin, J.; Stutte, G.W. Delaying flowering in shortday strawberry transplants with photo selective nets. Inter. Fruit. Sci. 2010, 10, 134–142. [Google Scholar] [CrossRef]
- Yoshida, Y.; Ozaki, E.; Murakami, K.; Goto, T. Flower induction in June-bearing strawberry by intermittent low temperature storage. J. Jpn. Soc. Hort. Sci. 2012, 81, 670–675. [Google Scholar] [CrossRef]
- Bernier, G.; Havelange, A.; Houssa, C.; Petitjean, A.; Lejeune, P. Physiological signals that induce flowering. Plant Cell. 1993, 5, 1147–1155. [Google Scholar] [CrossRef]
- Eshghi, S.; Tafazoli, E.; Dokhani, S.; Rahemi, M.; Emam, Y. Changes in carbohydrate contents in shoot tips, leaves, and roots of strawberry (Fragaria x ananassa Duch.) during flower-bud differentiation. Sci. Hortic. 2007, 113, 255–260. [Google Scholar] [CrossRef]
- Rivero, R.; Sønsteby, A.; Heide, O.M.; Solhaug, K.A.; Remberg, S.F. Growth analysis of the everbearing strawberry ‘Delizzimo’ under controlled temperature and photoperiod conditions. CABI Agric. Biosci. 2022, 3, 1–15. [Google Scholar] [CrossRef]
- Opstad, N.; Sønsteby, A.; Myrheim, U.; Heide, O.M. Seasonal timing of floral initiation in strawberry: Effects of cultivar and geographic location. Sci. Hortic. 2011, 129, 127–134. [Google Scholar] [CrossRef]
- Le Mière, P.; Hadley, P.; Darby, J.; Battey, N.H. The effect of thermal environment, planting date and crown size on growth, development and yield of Fragaria × ananassa Duch. cv. Elsanta. J. Hortic. Sci. Biotech. 1998, 73, 786–795. [Google Scholar] [CrossRef]
- Døving, A.; Måge, F. Prediction of the strawberry season in Norway. Acta Agric. Scand. Sect. B Plant Soil Sci. 2001, 51, 28–34. [Google Scholar] [CrossRef]
- Woznicki, T.L.; Heide, O.M.; Sønsteby, A.; Måge, F.; Remberg, S.F. Climate warming enhances flower formation, earliness of blooming and fruit size in plum (Prunus domestica L.) in the cool Nordic environment. Sci. Hortic. 2019, 257, 108750. [Google Scholar] [CrossRef]
- Krüger, E.; Schmidt, G.; Brückner, U. Scheduling strawberry irrigation based upon tensiometer measurements and a climatic water balance model. Sci. Hortic. 1999, 81, 409–424. [Google Scholar] [CrossRef]
| NIBIO Norway | INHORT Poland | HGU Germany | Sant’Orsola Italy | INVENIO France | |
|---|---|---|---|---|---|
| Latitude | 60°40′ N | 51°95′ N | 49°59′ N | 46°4′ N | 44°85′ N |
| Altitude (m a.s.l.) | 262 | 252 | 95 | 925 | 145 |
| Yearly mean temperature (°C) a | 5.0 | 7.9 | 9.9 | 11.3 | 12.9 |
| Type of cultivation | Open field | Open field | Open field | Plastic tunnel | Plastic tunnel |
| Soil type | Loam | Pseudopodsol with light clay | Sandy loam | Soil-less culture | Soil-less culture |
| Regression Coefficients (R2) | |||||||
|---|---|---|---|---|---|---|---|
| ‘Candonga’ | ‘Clery’ | ‘Florence’ | ‘Frida’ | ‘Gariguette’ | ‘Sonata’ | ||
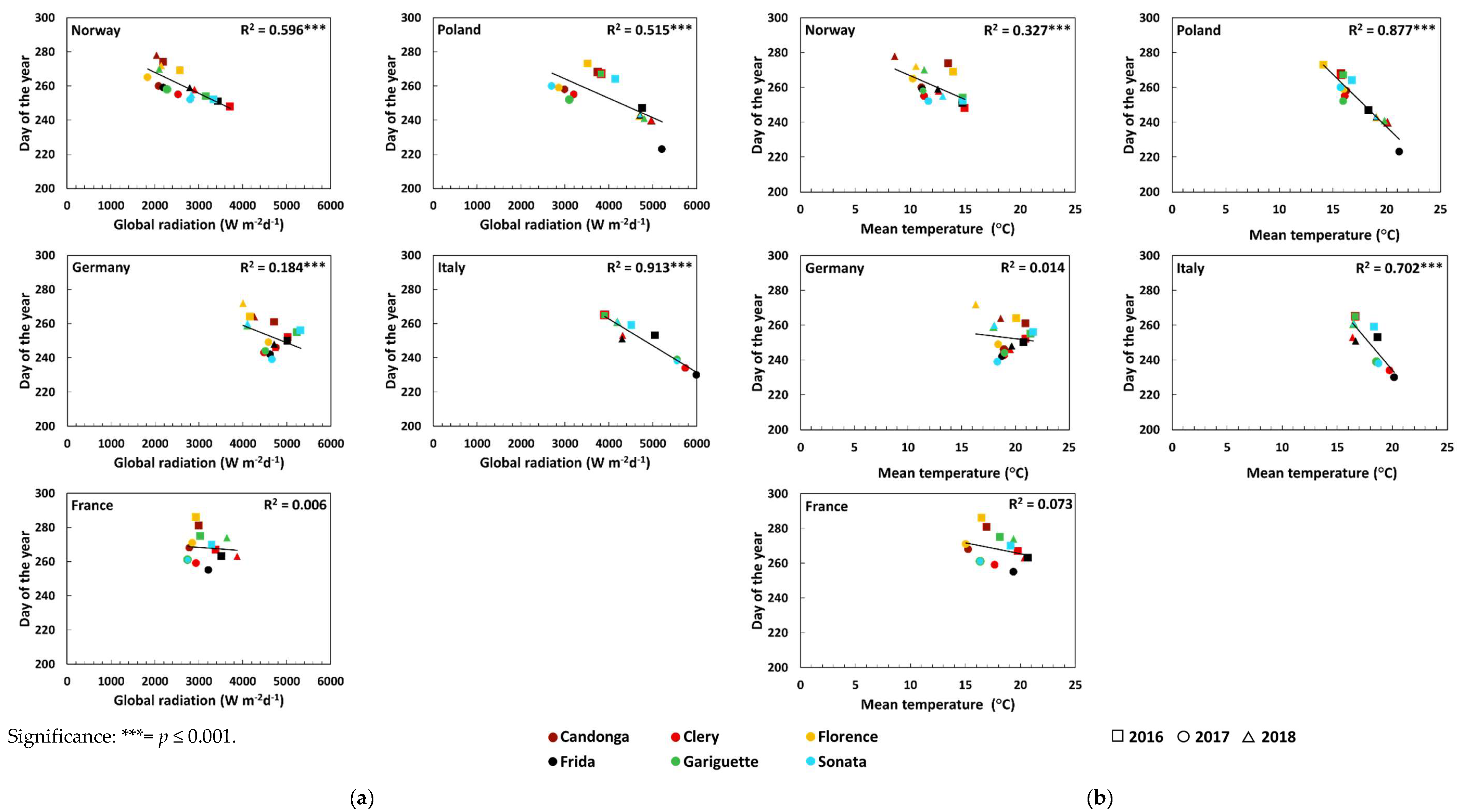
| Global radiation (Wm−2d−1) | Open-field cultivation | 0.50 *** ↓ | 0.33 ** ↓ | 0.33 ** ↓ | 0.56 *** ↓ | 0.32 ** ↓ | 0.09 |
| Soil-less cultivation | - | 0.75 *** ↓ | - | 0.72 *** ↓ | 0.62 *** ↓ | 0.66 *** ↓ | |
| Temperature (°C) | Open-field cultivation | 0.46 *** ↓ | 0.30 ** ↓ | 0.36 *** ↓ | 0.56 *** ↓ | 0.37 *** ↓ | 0.02 |
| Soil-less cultivation | - | 0.02 | - | 0.00 | 0.00 | 0.04 | |
| Location | Year | Number of Crowns per Plant | Number of Flowers per Plant | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Norway | Cand. | Cler. | Flor. | Frid. | Gari. | Sona. | Cand. | Cler. | Flor. | Frid. | Gari. | Sona. | |||
| 2017 | 4.0 | 3.8 | 6.3 | 3.9 | 3.5 | 3.9 | 14.9 | 16.2 | 27.3 | 24.4 | 13.0 | 24.7 | |||
| 2018 | 3.6 | 2.6 | 4.7 | 4.2 | 3.3 | 3.9 | 11.7 | 7.5 | 20.9 | 15.3 | 10.1 | 20.5 | |||
| 2019 | 7.1 | 3.7 | 9.0 | 10.0 | 5.5 | 6.6 | 27.9 | 10.4 | 33.1 | 45.3 | 18.8 | 40.1 | |||
| Mean | 4.9 | 3.4 | 6.7 | 6.0 | 4.1 | 1.8 | 5.0 CD | 18.2 | 11.4 | 27.1 | 28.4 | 14.2 | 28.4 | 21.3 A | |
| Poland | 2017 | 6.8 | 4.8 | 6.9 | 6.9 | 4.9 | 3.7 | 13.5 | 10.5 | 36.1 | 36.5 | 19.9 | 17.9 | ||
| 2018 | 3.2 | 2.4 | 4.3 | 3.1 | 3.1 | 2.8 | 13.8 | 11.7 | 33.9 | 23.5 | 17.2 | 21.1 | |||
| 2019 | 4.7 | 3.3 | 6.3 | 4.6 | 3.5 | 2.9 | 19.4 | 21.0 | 45.1 | 46.1 | 30.1 | 28.6 | |||
| Mean | 4.9 | 3.5 | 5.8 | 4.9 | 3.8 | 3.1 | 4.4 B | 15.6 | 14.4 | 38.8 | 35.4 | 22.4 | 22.3 | 24.8 AB | |
| Germany | 2017 | 7.1 | 7.9 | 10.3 | 10.3 | 6.5 | 6.7 | 25.4 | 61.6 | 70.7 | 94.9 | 48.1 | 75.6 | ||
| 2018 | 6.5 | 6.3 | 9.8 | 5.6 | 5.0 | 4.5 | 41.5 | 80.2 | 133.6 | 96.3 | 58.1 | 85.9 | |||
| 2019 | 6.1 | 6.0 | 7.5 | 6.7 | 6.7 | 5.1 | 39.6 | 65.8 | 89.1 | 81.1 | 75.9 | 72.8 | |||
| Mean | 6.6 | 6.7 | 9.2 | 7.5 | 5.7 | 5.4 | 6.9 D | 35.5 | 69.2 | 97.8 | 90.8 | 60.7 | 78.1 | 72.0 D | |
| Italy | 2017 | - | 2.5 | - | 2.4 | 3.7 | 2.1 | - | 15.8 | - | 25.5 | 30.4 | 26.0 | ||
| 2018 | - | 2.5 | - | 1.7 | 3.5 | 2.4 | - | 21.1 | - | 24.7 | 34.7 | 37.1 | |||
| 2019 | - | 3.4 | - | 2.1 | 4.2 | 2.1 | - | 32.3 | - | 31.9 | 43.3 | 27.3 | |||
| Mean | - | 2.8 | - | 2.1 | 3.8 | 2.2 | 2.7 A | - | 23.0 | - | 27.3 | 36.1 | 30.1 | 29.2 B | |
| France | 2017 | 7.5 | 4.1 | 8.8 | 6.3 | 3.5 | 6.2 | 31.9 | 31.5 | 71.3 | 48.5 | 40.1 | 58.5 | ||
| 2018 | 2.9 | 2.9 | 6.1 | 3.5 | 2.2 | 4.2 | 11.2 | 20.3 | 43.4 | 11.0 | 19.9 | 39.7 | |||
| 2019 | - | - | - | - | - | - | - | - | - | - | - | ||||
| Mean | 5.2 | 3.5 | 7.4 | 4.9 | 2.8 | 5.2 | 4.9 C | 21.6 | 25.9 | 57.3 | 29.8 | 30.3 | 49.1 | 35.7 C | |
| Cultivar mean | 5.4 b | 4.0 a | 7.3 c | 5.1 b | 4.2 a | 4.1 a | 22.8 a | 28.9 b | 60.0 d | 43.2 c | 32.9 b | 41.1 c | |||
| Probability level of significance (ANOVA) | |||||||||||||||
| Source of variation | |||||||||||||||
| Location (A) | <0.001 | <0.001 | |||||||||||||
| Cultivar (B) | <0.001 | <0.001 | |||||||||||||
| Year © | <0.001 | <0.001 | |||||||||||||
| A × B | <0.001 | <0.001 | |||||||||||||
| A × C | <0.001 | <0.001 | |||||||||||||
| B × C | 0.088 | 0.007 | |||||||||||||
| A × B × C | <0.001 | <0.001 | |||||||||||||
| Regression coefficient (R2) | |||||||
|---|---|---|---|---|---|---|---|
| Norway | Poland | Germany | Italy | France | |||
| Three weeks before FI | Number of flowers | Global radiation (Wm−2d−1) | 0.01 | 0.11 * ↑ | 0.01 | 0.00 | 0.05 |
| Temperature (°C) | 0.00 | 0.07 * ↑ | 0.10 * ↓ | 0.05 ↓ | 0.01 ↑ | ||
| Number of crowns | Global radiation (Wm−2d−1) | 0.05 | 0.03 | 0.01 | 0.08 | 0.06 | |
| Temperature (°C) | 0.06 | 0.03 | 0.06 | 0.15 * ↓ | 0.00 | ||
| Five weeks after FI | Number of flowers | Global radiation (Wm−2d−1) | 0.01 | 0.15 ** ↑ | 0.03 | 0.02 | 0.32 *** ↓ |
| Temperature (°C) | 0.01 | 0.10 * ↑ | 0.03 | 0.04 | 0.34 *** ↓ | ||
| Number of crowns | Global radiation (Wm−2d−1) | 0.03 | 0.03 | 0.15 ** ↓ | 0.06 | 0.49 *** ↓ | |
| Temperature (°C) | 0.15 * ↓ | 0.10 * ↓ | 0.02 | 0.05 | 0.53 *** ↓ | ||
| Regression Coefficients | ||||||||
|---|---|---|---|---|---|---|---|---|
| Candonga | Clery | Florence | Frida | Gariguette | Sonata | |||
| Number of flowers | Global radiation (Wm−2d−1) | Open-field cultivation | 0.23 * ↑ | 0.50 *** ↑ | 0.48 ***↑ | 0.21 * ↑ | 0.36 *** ↑ | 0.32 ** ↑ |
| Soil-less cultivation | - | 0.01 | - | 0.01 | 0.10 | 0.17 | ||
| Temperature (°C) | Open-field cultivation | 0.12 | 0.52 *** ↑ | 0.35 *** ↑ | 0.24 * ↑ | 0.37 *** ↑ | 0.34 ** ↑ | |
| Soil-less cultivation | - | 0.02 | - | 0.02 | 0.12 | 0.16 | ||
| Number of crowns | Global radiation (Wm−2d−1) | Open-field cultivation | 0.14 | 0.48 *** ↑ | 0.15* ↑ | 0.00 | 0.11 | 0.04 |
| Soil-less cultivation | - | 0.15 | - | 0.46 ** ↓ | 0.21 | 0.43 ** ↓ | ||
| Temperature (°C) | Open-field cultivation | 0.03 | 0.44 *** ↑ | 0.11 | 0.00 | 0.09 | 0.02 | |
| Soil-less cultivation | - | 0.04 | - | 0.30 * ↑ | 0.02 | 0.06 | ||
| Regression coefficients (R2) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Candonga | Clery | Florence | Frida | Gariguette | Sonata | |||
| Number of flowers | Global radiation (Wm−2d−1) | Open-field cultivation | 0.26 * ↑ | 0.49 *** ↑ | 0.36 *** ↑ | 0.23 * ↑ | 0.50 *** ↑ | 0.41 *** ↑ |
| Soil-less cultivation | - | 0.15 | - | 0.03 | 0.18 | 0.33*↓ | ||
| Temperature (°C) | Open-field cultivation | 0.18 * ↑ | 0.54 *** ↑ | 0.37 *** ↑ | 0.20 * ↑ | 0.42 *** ↑ | 0.45 *** ↑ | |
| Soil-less cultivation | - | 0.01 | - | 0.01 | 0.00 | 0.29 * ↑ | ||
| Number of crowns | Global radiation (Wm−2d−1) | Open-field cultivation | 0.01 | 0.23 * ↑ | 0.04 | 0.00 | 0.02 | 0.02 |
| Soil-less cultivation | - | 0.09 | - | 0.68 *** ↓ | 0.04 | 0.60 *** ↓ | ||
| Temperature (°C) | Open-field cultivation | 0.01 | 0.28 ** ↑ | 0.02 | 0.01 | 0.01 | 0.00 | |
| Soil-less cultivation | - | 0.10 | - | 0.08 | 0.40 * ↓ | 0.34 * ↑ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krüger, E.; Woznicki, T.L.; Heide, O.M.; Kusnierek, K.; Rivero, R.; Masny, A.; Sowik, I.; Brauksiepe, B.; Eimert, K.; Mott, D.; et al. Flowering Phenology of Six Seasonal-Flowering Strawberry Cultivars in a Coordinated European Study. Horticulturae 2022, 8, 933. https://doi.org/10.3390/horticulturae8100933
Krüger E, Woznicki TL, Heide OM, Kusnierek K, Rivero R, Masny A, Sowik I, Brauksiepe B, Eimert K, Mott D, et al. Flowering Phenology of Six Seasonal-Flowering Strawberry Cultivars in a Coordinated European Study. Horticulturae. 2022; 8(10):933. https://doi.org/10.3390/horticulturae8100933
Chicago/Turabian StyleKrüger, Erika, Tomasz L. Woznicki, Ola M. Heide, Krzysztof Kusnierek, Rodmar Rivero, Agnieszka Masny, Iwona Sowik, Bastienne Brauksiepe, Klaus Eimert, Daniela Mott, and et al. 2022. "Flowering Phenology of Six Seasonal-Flowering Strawberry Cultivars in a Coordinated European Study" Horticulturae 8, no. 10: 933. https://doi.org/10.3390/horticulturae8100933
APA StyleKrüger, E., Woznicki, T. L., Heide, O. M., Kusnierek, K., Rivero, R., Masny, A., Sowik, I., Brauksiepe, B., Eimert, K., Mott, D., Savini, G., Demene, M., Guy, K., Petit, A., Denoyes, B., & Sønsteby, A. (2022). Flowering Phenology of Six Seasonal-Flowering Strawberry Cultivars in a Coordinated European Study. Horticulturae, 8(10), 933. https://doi.org/10.3390/horticulturae8100933







