Abstract
Most nashi cultivars require heavy thinning, and this has traditionally been performed by the time and labour-intensive practice of hand thinning. Crop load management is a key cost driver for nashi production, but there are limited cost-effective options available for nashi growers compared to other pome fruit, especially apples and, to a lesser extent, European pears. There is, however, potential to adapt some of the thinning tools and techniques used in apples and European pears to reduce the labour requirements and high cost of thinning in nashi, thus improving industry profitability. Several chemical thinning agents have potential for nashi, and an understanding of the optimal application rates, times and weather conditions for each chemical, as well as the conditions/factors that impact the tree carbon balance, will improve the predictability of chemical thinning. However, it is difficult to target specific flowers/fruitlets within a cluster with chemicals, and the flowers that produce the preferred fruit shape and size are in the middle of the flower cluster. Mechanical thinning during the flowering period with either Darwin or BAUM-style string thinners has potential, particularly as these devices can be used as early as flower emergence. As for chemical thinning, the issue of non-selectivity needs to be addressed; however, the development of mechatronic systems should overcome most problems that occur with the currently available mechanical thinners. Shading at critical times is an avenue that could be explored further to ascertain the critical stage when developing fruit are susceptible to enable the determination of the optimal timing and duration of shading. Targeted pruning and bud thinning during the dormant winter period to reduce the floral bud numbers is a valuable option for the precise placement of fruit in optimal positions and to set up the required number of clusters. This review highlighted several tools/techniques that, with further work, can be incorporated into a systematic approach to crop load management in nashi while reducing the risk and cost.
1. Introduction
Pears belong to the genus Pyrus within the Rosaceae family, subfamily Pomoideae, and are closely related to apple (Malus) and quince (Cydonia). The Pyrus genus can be subdivided into two major groups: Occidental (European) and Oriental (Asian) pears [,]. According to Saito [], there are at least 29 widely recognised primary species within the Pyrus genus. European cultivars all belong to the species P. communis. Cultivated species of Asian pears include the Ussurian pear (P. ussuriensis Maxim), Chinese white pear (P. × bretschneideri Rehder), Chinese sand pear and Japanese pear (P. pyrifolia Nakai) and Xinjiang pear (P. sinkiangensis Yu) [,]. Bao et al. [] suggested that the progenitor of Japanese pears originated from China. Interspecific hybridisation occurs readily between the different Pyrus species [,].
Unlike European pears, which exhibit a pyriform shape, the fruit shape of Asian pears is global or spheroidal []. The other major botanical difference is in the calyx—Asian pears lack the characteristic sepals of European cultivars. The skin may be either bronzed (russeted) or yellow or greenish in colour. The fruit is crisp in texture, juicy with a high sugar content, weak acidity and low aroma [,]. A major physiological difference between the Occidental and Oriental groups is that, unlike European pears, the optimum quality in Asian pears is reached when the fruit is allowed to ripen on the tree [].
Various terms are used for Asian pears: nashi (meaning ‘pear’ in Japanese) is used in Japan, New Zealand, France, Argentina, Brazil and Australia; salad pear, pear-apple and Oriental pear are common names in the United States; and Asian pear is the preferred trade name []. The term nashi will be used for the remainder of this review.
Crop load management is a key cost driver for nashi production, but there are limited options available for nashi growers compared to other pome fruit, especially apples and, to a lesser extent, European pears. As such, the Australian nashi industry is searching for practices/techniques to reduce production costs by reducing the cost of crop load management, thus improving industry profitability. Therefore, this review examines both current and potential tools/techniques to enable a systematic approach to managing crop load in nashi while reducing the risk and cost.
2. Phenological Stages in Nashi
An understanding of the different growth or developmental stages is important for the correct timing of many orchard management practices. Plant growth is highly responsive to day length and climate, and deciduous fruit trees are particularly influenced by winter chill and temperature; hence, application of the BBCH scale, a system for uniform coding of phenologically similar growth stages of all mono- and dicotyledonous plant species [], is a useful tool to ensure that management practices are undertaken at the appropriate times. The general BBCH scale considers 10 principal growth stages numbered from 0 to 9, with secondary growth stages defining short developmental steps characteristic of the respective plant species, which are passed successively during the respective principal growth stage []. These secondary stages are also coded using the numbers 0–9; hence, the combination of numbers for the principal and the secondary stages results in a two-digit code. If two or more principal growth stages occur in parallel, they are indicated by use of a diagonal stroke (example 10/54).
Not all primary stages are applicable to all species; for example, nashi phenology shows eight of the ten principal stages: bud (0), leaf development (1), shoot development (3), inflorescence emergence (5), flowering (6), fruit development (7), fruit maturity (8) and senescence (9) []. Further details of the BBCH scale as it relates to the bud, flower and fruit development of nashi are provided in Table 1.

Table 1.
Principle phenological codes for bud, flower and fruit development for nashi according to the BBCH scale, as identified by Martinez-Nicolás et al. [].
3. Flowering and Fruit Set
Pears flower on spurs, short shoots and one-year-old wood, with flower initiation occurring in the spring–summer of the preceding season. The initiation of spur and terminal shoot buds begins 3–6 weeks after bloom, while axillary buds on first-year wood (extension shoots) are formed later in the season []. Anything that interrupts or reduces the amount of photosynthates produced through photosynthesis will reduce the quality of the developing buds. There are multiple factors that can reduce the available assimilates and, thus, bud quality [,,]. The major factors are listed below:
- excessive vegetative growth;
- low light or shading of buds;
- poor leaf quality caused by inadequate nutrition, irrigation or disease/insect attack;
- competition for assimilates as a result of heavy crop loads;
- trees shutting down as a result of high temperatures during bud development.
Nashi have an indeterminate inflorescence with racemes of 8–10 flowers [], unlike the determinate inflorescence found in apples and quince. Flowers open in progression acropetally from the side-lateral to the terminal. The time of flowering varies with cultivar but is dependent on local climatic conditions and the degree of chill received over winter. Figure 1 illustrates the average flowering times for many nashi cultivars in Orange, NSW, Australia.
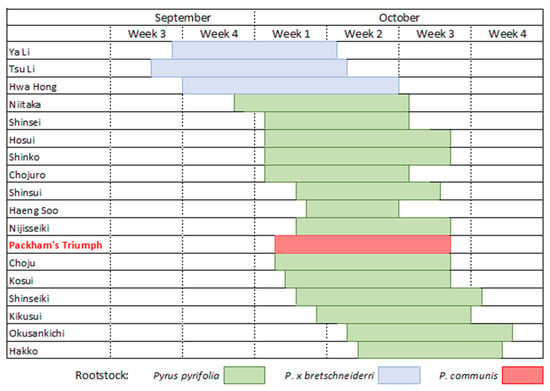
Figure 1.
Flowering times for nashi in Orange, NSW, in relation to the European pear cultivar ‘Packhams’ Triumph’ (adapted from NSW DPI, https://www.dpi.nsw.gov.au/agriculture/horticulture/pomes/other/nashi (accessed on 26 August 2021)).
3.1. Role of Dormancy (Rest) Breakers
Deciduous fruit trees require the accumulation of chill hours over the dormant winter period for synchronous bud break to occur. Unlike the marked seasonal transitions observed in most Northern Hemisphere temperate growing regions, the climate across the Australian temperate regions where nashi is grown is characterised by gradual transitions between seasons and occasionally warm winters, often resulting in insufficient chill accumulation and causing physiological damage to trees, leading to non-synchronous bud burst and extended flowering []. With a warming climate, many production regions worldwide may become marginal in terms of achieving sufficient chill exposure for high-chill cultivars [,].
The application of chemical dormancy-breaking agents has become a routine practice in many regions with insufficient winter chill in order to manipulate bud break, thus synchronising floral bud break and ensuring a commercially viable flowering and fruit set [,]. The most commonly used dormancy breaker in apples is hydrogen cyanamide (HC), and although it is not registered for pears, it is often applied in regions that lack sufficient winter chill for synchronous bud break. Mineral oil (MO) can be combined with hydrogen cyanamide to reduce the dose rate []. Other bud break agents include vegetable oils (VO), methyl esters of fatty acids such as WaikenTM and the fertiliser adjuvant Erger® (Table 2).

Table 2.
Dormancy-breaking products available in Australia. Note that none of these chemicals have label recommendations for pears or nashi.
Studying the use of HC to improve flowering and fruit set in several nashi cultivars (‘Hosui’, ‘Kosui’, ‘Shinsui’, ‘Nijisseiki’ and ‘Shinseiki’) grown in New Zealand, Klinac et al. [] reported an advancement in the onset of flowering and a reduction in the length of the flowering period in all cultivars, but a greater response was observed in the later flowering cultivars ‘Nijisseiki’ and ‘Shinseiki’. They also reported that an application of 3% HC at 30–50 days before natural flowering substantially improved the overlap of the pollinator ‘Shinseiki’ with the flowering periods of other cultivars.
In Brazil, Faoro and Nakasu [] noted that application of HC and MO is required to ensure adequate bud break in ‘Hosui, ‘Kosui’ and ‘Nijisseiki’, while Abreu et al. [] reported that HC and HC+MO were more effective than Erger® in promoting bud break of ‘Hosui’. In studies evaluating the effect of vegetable (sunflower) and mineral oils on bud break, yield and enzymatic activity in the cultivar ‘Hosui’, Botelho et al. [] found that treatment with 4% MO plus 2% VO was as effective as the standard HC treatment in releasing bud dormancy. In trees treated with MO and VO, they reported a reduction in bud activity of the catalase enzymes, indicating the mode of action is through oxidative stress, similar to the action of HC, and concluded that the application of 4% MO plus 2% VO was likely to be a more sustainable alternative for inducing bud break in ‘Hosui’ trees due to the reduction in cost and environmental impact. Multiple techniques, including MO+HC, branch girdling and bending, have been used to promote bud break in Brazil, and flowering of nashi may occur 10–15 days earlier than normal, depending on the timing of the MO and HC treatments [].
According to Honjo et al. [], ‘Kosui’ requires around 800 chill hours to break endodormancy, while other cultivars require more than 1600 h. Faoro and Nakasu [] noted that ‘Kosui’ is well-adapted to warmer areas in Brazil where the chill accumulation is over 400 h, but the high-chill cultivar ‘Nijisseiki’ has poor bloom and reduced shoot growth in areas with chill accumulation between 500 and 700 h. This concurs with the findings of Petri and Herter [] that increasing chilling up to 1440 h resulted in a higher percentage of bud break and flower number per bud for ‘Nijisseiki’. Once sufficient chill hours have accumulated, the application of dormancy breakers has no effect [].
3.2. Fruit Set
Fruit set is the developmental stage marking the transition of a flower (ovary) into a young fruit (BBCH scale 71 []).
Fruit set is dependent on pollen being transported from the anthers to the stigma (pollination), followed by pollen germination and growth of the pollen tubes through the pistil and, finally, fertilisation of the ovules. The inability to complete any of these processes will prevent fruit set from occurring. The germination and growth rate of pollen are dictated by temperature [], and pollen does not germinate when temperatures are below 10 °C []. The stigma is only receptive to pollen for a short time period, and Williams [] reported that the stronger the flowers, the longer they remain receptive, noting also that the ovules of strong flowers remained capable of fertilisation for almost twice as long as those of normal flowers. Thus, the effective pollination period (EPP) is limited by the period of stigma receptivity, the rate of pollen tube growth and ovule longevity [,]. A good description on parameters determining the EPP, i.e., stigmatic receptivity, pollen tube kinetics and ovule longevity, was provided by Sanzol and Herrero []. The EPP can be modified by temperature, flower quality and chemical application []. Frequent rain and/or high humidity during the flowering period are not conducive to good fruit set [].
Rohitha and Klinac [] listed several factors that can influence pollination, fruit set and yield in apple and pear cultivars, including distance from the polliniser and pollen availability, plant nitrogen status, water on the stigmas and the overlapping of flowering between compatible cultivars. Yoneyama [] also noted that pollen attachment is reduced when the sigma is dry and advised that strong, dry winds can prevent full expansion of nashi flowers, leading to loss of viability.
3.3. Pollen Compatibility
Most nashi cultivars exhibit typical gametophytic self-incompatibility and, hence, are self-incompatible [], so cross-pollination with another compatible cultivar is required for good fruit set. As a single multiallelic locus, the S-locus, controls gametophytic self-incompatibility, cross-compatible pollinisers are required that bear different S-haplotypes. Cross-pollination with pollen bearing an S-haplotype different from either of the S-haplotypes of the pistil will achieve stable fruit set []; however, two cultivars with one overlapping S-loci (Figure 2) are semi-compatible [].
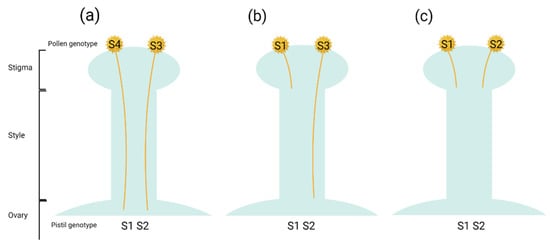
Figure 2.
Self-incompatibility reactions: (a) cross-compatible, (b) semi-compatible and (c) cross-incompatible (Source: created in Biorender.com).
S-genotypes have been determined for a number of nashi cultivars; the only self-compatible cultivar described so far has been ‘Osa-Nijisseiki’, a mutant derived from ‘Nijisseiki’ []. According to Rohitha and Klinac [], ‘Nijisseiki’ and, to a lesser extent, ‘Kosui’ are partially self-compatible. Beutel [] indicated that ‘Nijisseiki’ and ‘Shinseiki’ will set good crops when planted alone or in large single-cultivar blocks.
The S-genotypes of the cultivars grown in Australia are shown in Table 3, and the Japanese recommendations for pollinators are shown in Table 4. In California, the flowering period of the European cultivar ‘Bartlett’ (‘Williams’) also overlaps that of ‘Chojuro’, Nijisseiki’ and ‘Shinseiki’ and is an effective pollinator for these cultivars []. Johnson [] also noted that the European pear cultivar ‘Packham’s Triumph’ may be a more effective pollinator of nashi under Australian conditions, suggesting that the cross-compatibility of both ‘Packham’s Triumph’ and ‘Beurre Bosc’ be examined relative to the nashi cultivars for which there are reasonable blossom overlaps.

Table 3.
Incompatibility groups and S-genotypes of nashi cultivars grown in Australia (adapted from Halász and Hegedűs []).

Table 4.
Pollinator combinations for nashi recommended by MAFF Japan (summarised from Johnson []).
3.4. Seed Number and Fruit Quality
There is a strong relationship between seed set and fruit shape and weight. Most pome fruit contain five carpels, but nashi fruit can have between four and eight carpels, each carpel with the capacity to carry two seeds [,]. Several authors have reported a significant correlation in nashi between the number of fully developed seeds per fruit and fruit weight at harvest [,,]. Rohitha and Klinac [] found that even seed distribution around the fruit core was related to fruit symmetry, and based on the levels of seed set observed in four cultivars, they concluded that considerable potential exists for raising the quality of nashi fruit by optimising pollination and seed set. Johnson [] also noted that perfect fruit shape is enhanced by fully pollinated flowers. Seed number can also have an impact on fruit sugar content; according to Yoneyama [], when more than six seeds are present, an extra seed can increase the sugar content by 0.1–0.2%. The development of fruit without fertilisation, or parthenocarpy, is common in pears [], but cross-pollinated fruit with seeds tend to be larger and more uniform in shape than parthenocarpic fruit or fruit with few seeds [].
Pyrus species are pollinated by insects, but in Japan, cross-pollination is routinely carried out by hand [,], while, in most other countries, cross-pollination is reliant on honeybees and other pollinating insects. Nectar is produced by flowers to attract pollinators; however, the sugar content of pear nectar is low (10–15 °Brix) compared to that in many other fruit tree species []. In studies on bee flower visits in São Joaquim, Faoro and Orth [] reported that pollinating insects were only attracted to pear flowers by their pollen, as nectar secretion was insignificant. Ruderal plants in the inter-row with high nectar sugar content, such as white clover and dandelion, attracted more honeybees than the pear tree flowers []. Goodwin [] recommended that hives should have a high brood/bee ratio, as pollen foragers are likely to be better pollinators than nectar foragers due to the low volume and sugar content of the nectar.
Ambient temperatures have a strong impact on bee activity, with temperatures between 15 and 26 °C being optimal for bee foraging activity [] (cited by Faoro and Orth []) and a lack of activity below 10 °C []. Studies by Gallo et al. [], cited by Faoro and Orth [], demonstrated the positive relationship between honeybee activity and temperature, recording 14.5 flowers visited per minute at 23 °C while, at 14 °C, visits were reduced to 3.1 flowers per minute. Faoro and Orth [] noted the necessity of installing large numbers of honeybee hives to ensure adequate pollination. In Australia, two to five hives per hectare are recommended for pollination, with eight hives per hectare common in New Zealand [].
3.5. Bud Abortion
Floral bud disorders resulting in abortion and/or disintegration of buds when touched were described by Kingston et al. [] and Trevisan et al. []. According to Kingston et al. [], the collective term for these disorders is budjump, and the problem is widespread in New Zealand, with higher incidences reported in the growing regions of the north island than in the south island. Budjump has also been reported in Brazil in areas with insufficient chilling hours [,]. Although there were no reports of this disorder in Japan or California prior to the 1990s [], erratic flowering and bud loss have been observed in Japan’s warmer regions since 2009 [].
The incidence of budjump has been reported to be more severe in cv. ‘Hosui’ than in ‘Kosui’, ‘Shinsui’, ‘Nijisseiki’ or ‘Shinseiki’ []. Nakasu et al. [] found higher levels of bud abortion when high temperatures were followed by a sudden temperature decrease; however, the physiological stage of the flower bud at which abortion occurs is unknown. Bud loss has been reported to be more prevalent on young trees and on young wood from older trees, with the least bud loss at branch tips and on spur buds from older wood []. Following studies examining ‘Kosui’ and ‘Niitaka’ at five locations over a six-year period, Ito et al. [] concluded that warmer autumn–winter temperatures may prevent the acquisition of freezing tolerance, disturb endodormancy progression and disrupt floral organ development, thereby causing flowering disorder. The risk of occurrence of flowering disorder and bud abortion is likely to be higher in high-chill cultivars than in low- or mid-chill cultivars and at lower latitudes compared with higher latitudes [].
4. Commercial Production of Nashi
In Japan, nashi accounts for over 87% of the total planted area allocated to pears [], and the leading cultivars are ‘Kosui’, ‘Hosui’, ‘Nijisseiki’, ‘Niitaka’, ‘Akizuki’, ‘Nansui’, ‘Shinko’ and ‘Gold Nijisseiki’, a black spot-resistant cultivar. Production in Argentina is centred in the Patagonia region, and while multiple cultivars have been introduced, plantings have reduced to three cultivars—‘Shinseiki’, ‘Nijisseiki’ and ‘Hosui’ []. In Brazil, the main cultivars are ‘Hosui’, ‘Kosui’ and ‘Nijisseiki’ []. The commercially important cultivars in New Zealand include ‘Shinsui’, ‘Kosui’, ‘Hosui’ and ‘Nijisseiki’ []. Klinac et al. [] also listed ‘Shinseiki’ as one of the main cultivars for New Zealand. It is difficult to obtain production figures and areas planted with nashi, as most countries report the total pear production, which includes European pears.
Nashi has been produced commercially in Australia for the fresh market for around 35 years, but the industry has remained small, with 1100 tonnes produced in 2020/2021 (production value of AUD 4.5 million), down from 2370 tonnes in 2017/2018 with a production value of AUD 7.0 million [].
There are several cultivars grown in Australia, but the main cultivar is the green smooth skin ‘Nijisseiki’, accounting for 80% of nashi plantings. ‘Nijisseiki’ is extremely precocious, flowering profusely and setting heavily in all seasons; it also has a sensitive skin and is prone to russet. Australian growers report that, while many nashi cultivars have between 5–6 flowers per cluster, ‘Nijisseiki’ commonly sets 10–12 and, at times, up to 15 flowers per cluster, with only 2–3 producing good fruit shape. After finding that, during the early stages of fruit development, the number of leaves per unit branch length for ‘Nijisseiki’ was nearly twice that for other cultivars tested (average of 151 vs. 84 per m, respectively), Buwalda et al. [] suggested that the ability of ‘Nijisseiki’ to sustain very high fruit loads is probably related to the high leaf density. Following a study of nashi production in Japan, Johnson [] recommended that, for successful production in Australia, research into effective thinning would be needed to ensure acceptable fruit size, noting that the large fruit size obtained in Japan was substantially a response to heavy crop thinning practices performed by hand.
5. Crop Load Management
Crop load management is a vital cultural practice in many perennial fruit tree crops that produce an excess of fruit. Optimal crop loads are normally achieved through the removal of excess flowers/fruitlets from the tree (termed crop regulation or thinning) or by the prevention of flower initiation. Bound [] and Webster [] described several methods by which this reduction in flowers/fruitlets can be accomplished:
- hand thinning;
- use of plant bioregulators (PBRs) to prevent fertilisation at flowering or cause abscission of flowers/fruitlets;
- photosynthetic inhibition through shading;
- physical removal by use of mechanical devices;
- cultural practices such as pruning.
The inverse relationship between vegetative growth and flowering is well-recognised, along with the importance of maintaining a balance between canopy volume and fruiting [,]. A high level of photosynthate supply is required for developing fruit []. Thinning excess flowers/fruit helps to balance the fruit:shoot ratio, leading to an increase in assimilates for both reproductive and vegetative sinks [], which results in an increase in fruit size and improved fruit appearance and intrinsic quality, all of which lead to higher crop values [,].
Nashi tend to set very heavily (Figure 3a) and require thinning to achieve large fruit size; thinning is normally done by hand. In Japan, hand thinning is undertaken from as early as 14 days and up to 40 days after petal fall, when it is evident which fruitlets will abort and uneven shape is evident []. According to Jackson and Palmer [], nashi growers in Japan thin at 1.3-cm fruitlet diameter and again 6–8 weeks later, with the fruit in the centre of the cluster normally retained. Johnson [] also noted that fruit from the first few flowers opening in the cluster tend to be flat, and fruit from the third or fourth flowers are retained. Australian growers have advised that fruit in positions 2–5 are normally of acceptable shape, but the fruit from the first opening flower tend to be elongated (Figure 3b), while the later opening flowers produce smaller fruit.
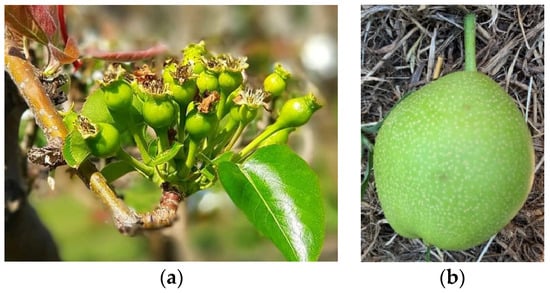
Figure 3.
(a) Typical fruit set in ‘Nijisseiki’. Photo credit: Steven Singh, Seeka Australia Pty Ltd. (b) Elongated fruit shape from the first opening flower in the cluster. Photo credit: Sally Bound, TIA.
Differences in fruit size are recognisable two weeks after pollination []; while fruit thinning can commence as soon as non-fertilised flowers have dropped, a more practical timing is 20–30 days after pollination. To achieve adequate fruit size, 20–30 well-developed leaves are required for each retained fruit []. Yoneyama [] also stressed the importance of adequate leaf area, particularly in ‘Nijisseiki’ and ‘Chojuro’, which have high flower bud numbers. Based on observations in Japan, Jackson and Palmer [] suggested that, for trees planted at 2 × 5-m spacing, about 250 fruit per tree should be left.
In apples and European pears, the crop load is related back to the number of blossom clusters or to the tree size using the following two formulae [], but in nashi, the crop load is commonly measured as the number of fruit m−2 canopy area.
Fruit per 100 blossom clusters = # fruit/# blossom clusters × 100
Fruit number cm−2 trunk cross-sectional area (TCSA) = # fruit/TCSA
Current management methods for nashi in Australia to ensure optimum crop loading can include spur pruning, bud thinning, flower removal, post flowering hand thinning (normally done twice) and, finally, early harvest of poor-quality fruit []. The thinning process starts during winter when trees are still dormant by removing poorly placed spurs and reducing buds back to 1–2 per spur, positioning the retained buds to optimise light and allowing fruits to hang cleanly (Figure 4). This is followed by flower thinning within the clusters and then fruitlet thinning back to one or two fruit per cluster (Figure 5).

Figure 4.
Bud thinning in nashi. Photo credit: Steven Singh, Seeka Australia Pty Ltd.

Figure 5.
‘Nijisseiki’ cluster before (a) and after (b) thinning. Photo credit: Steven Singh, Seeka Australia Pty Ltd.
Yoneyama [] discussed bud thinning (disbudding) in the context of the removal of unwanted buds and the elimination of water shoots, noting that bud thinning is important to avoid nutrients and resources being taken by unwanted buds. Accessory flower buds can also be a problem, as these buds are formed more than a month after the main flowers in the cluster and open late, producing low-quality fruit. In some cultivars such as ‘Sansui’, accessory flower buds can be 30–50% of the total flower bud numbers [].
5.1. Rootstock and Training System
Discussing the impact of rootstocks on yield efficiency and fruit size, Einhorn [] argued that rootstocks are “a critical factor affecting the precocity, efficiency and productivity of pear trees”. The main rootstocks used for nashi are seedlings of P. betulaefolia, P. bretschneideri, P. calleryana, P. communis, P. pyrifolia (serotina) and P. ussuriensis [,]. As well as their effects on tree size/vigour, productivity, yield consistency and fruit quality, rootstocks differ in their effects on drought and cold resistance and adaptability to different soils and climatic conditions.
One of the main rootstocks used in Australia is P. calleryana selection ‘D6’, which is reported by growers to have good vigour, particularly in lighter soils []. In medium- to high-density plantings aimed at achieving high early production, vigorous rootstocks can cause management problems due to overcrowding and shading [], and as ‘D6’ is a vigorous rootstock it is less suitable for intensive plantings []. The semi-dwarfing rootstocks ‘BP1’ (P. communis selection from South Africa) and ‘BM 2000’ (Australian origin, a result of open pollination of likely parents ‘Williams’ and ‘Packham’) are used in Western Australia, but growers have reported that ‘Nijisseiki’ does not perform well on these less vigorous rootstocks []. ‘Quince A’ is also used on ‘Pappel’; the calmer rootstock has been observed to give improved set, but russet is increased. Johnson [] suggested that P. pyrifolia seedlings would be an appropriate choice but noted that a source of reasonably pure seed would be required to avoid variation resulting from hybridisation. In New Zealand rootstock trials, ‘Hosui’ scions grown on ‘Quince A’ with ‘Buerre Hardy’ interstems were reported to be 60% smaller than ‘Hosui’ on more vigorous seedling stocks, plus they yielded more fruit per unit canopy volume [].
In a 10-year study of European pear cultivars on several rootstocks (D6, BP1, BM2000 and Quince A) in the Australian Goulburn Valley region, Hankin [] reported D6 had a high yield for all three scion cultivars examined but increased the limb extension growth and water shoots in the trees, potentially causing an increase in skin marks and lower pack-outs. The increased growth led to increased labour costs at pruning to control vigour. In terms of the cumulative yield efficiency, Hankin [] concluded that the best rootstocks were BP1 for ’Packham’, BM2000 and ‘Williams’ and Quince A for ‘Corella’ but also noted that the rootstocks that performed well in terms of yield efficiency were quite different to those that performed well in terms of yield. This study highlights the importance of rootstock evaluation trials for nashi cultivars.
There is limited information available on training systems for nashi, but training systems have been shown to have an impact on fruit size in some cultivars. At any given crop load, fruit size has been reported to be significantly higher for ‘Hosui’ trees on T-trellis than on Y-trellis; however, for ‘Shinsui’, ‘Kosui’ and ‘Nijisseiki’, the training systems had no effect on the relationship between fruit size and crop load [].
5.2. Timing of Crop Load Management
For fruit to reach its optimum potential, an adequate supply of carbohydrates is required for the developing fruit. During flowering and fruit set in early spring, carbohydrates are supplied from tree reserves; after development of a sufficient leaf area, the assimilates produced by both spur and extension leaves are translocated to the developing fruitlets []. Vegetative growth and roots compete with developing fruit for carbohydrates, so to avoid wastage of carbohydrate resources while maximising benefits in fruit size and quality, crop regulation practices should be undertaken early in the season before the completion of cell division []; this ensures that tree resources are directed into fruit that will remain on the tree until harvest rather than into fruit that will be thinned prior to harvest; delaying the removal of excess flowers/fruitlets reduces the potential for fruit to reach optimal size and firmness.
Pears have three natural fruit drops []. The first drop of unfertilised flowers occurs soon after petal fall and usually goes unnoticed. The second drop, known as ‘June’ drop in the Northern Hemisphere, or ‘December’ drop in the Southern Hemisphere, occurs 6–8 weeks after bloom and is often the largest, particularly in trees with heavy crop loads; the third drop occurs preharvest []. Trees with small crop loads tend to have a very small or no second drop []. The natural shedding that occurs in the first two drops is insufficient to achieve optimum crop loads, fruit size or quality or to prevent biennial bearing [,].
5.3. Economics of Crop Load Management
The value of managing crop load is in attaining consistent production across the life of the orchard, protecting the trees from damage that may result from excessive crop loads and optimising fruit size and quality []. The optimum crop load for individual trees is related to tree size and structure, but the cropping potential varies between cultivars. Local conditions, tree vigour, training, and nutrition also play a part in determining the optimum crop load []. Excessive crop loads may produce high yields, but fruit size and quality (shape, sugar content and firmness) are compromised, resulting in high reject rates and low returns to the grower; conversely, if the crop load is too low, yields can be reduced, and excess vegetative growth occurs [].
Jones et al. [] outlined the risks involved in thinning, noting that under-thinning can be more economically damaging than overthinning, as it results in small poor-quality fruit and biennial bearing in prone cultivars. In the majority of thinning research to date, little attention has been paid to the economic consequences; hence, relatively little work has been done to identify optimal crop load targets following thinning or to compare distinct thinning treatments on an economic basis [].
Hand thinning of buds/flowers/fruitlets is labour-intensive, and according to Bound and Mitchell [], in the 1990s these thinning methods were costing Australian nashi growers up to AUD 5000 per ha and the industry approximately AUD 1 million annually. The current cost estimates for thinning and budding are >AUD 10,000 per ha [], which is substantially higher than for many other tree fruit industries. With budding and thinning the key cost drivers for nashi production in Australia, the industry is looking for ways to reduce costs and, thus, improve viability to enable increased expansion.
6. Tools/Techniques for Managing Crop Load
There has been considerable worldwide research on crop load management in apples (Malus domestica), resulting in a range of recommendations in many countries. The available information for managing crop load in European pears (P. communis) is increasing [], but there is a lack of information available for nashi.
Most nashi cultivars require heavy thinning, and this has traditionally been done by hand, a time- and labour-intensive practice. There is potential to adapt some of the thinning tools and techniques used in other perennial fruit tree species, particularly closely related apples and European pears, to reduce the labour requirements and high cost of thinning in nashi. Valuable information on thinning European pears can be found in the review by Bound [], and the literature relating specifically to nashi is summarised in the remainder of this paper with references to other crops where applicable or where tools/techniques have not yet been examined in nashi.
6.1. Hand Thinning
One of the most accurate methods of reducing excessive crop loads is hand thinning of either the flowers or fruitlets. In practice, flower thinning is difficult to achieve accurately, as it is not known which flowers will set fruit, and if the retained flowers have not been pollinated they will eventually abscise [], so, in apples and European pears, hand thinning normally commences later in the season once the danger of spring frosts is over and growers can see what has set on the trees [,].
While hand thinning of fruitlets can optimise fruit distribution on the tree, facilitating precision crop loading [], it is a high-cost strategy, as noted previously. Since it is labour-intensive, it is also difficult for large commercial orchards to complete hand thinning before the end of the cell division period, and as cell numbers within the fruit are determined prior to hand thinning, limits have already been placed on fruit size [,]. While most studies on the impact of time of thinning on fruit weight have been conducted on apples, several European pear studies have also shown similar results. Meland [] reported that later thinning reduced fruit size and sugar content in five cultivars studied, while Schmidt [] recommended adjusting the crop load of ‘Bartlett’ early in the season to avoid wasting of resources and to ultimately produce larger, better-quality fruit.
The relationship between fruit size during the growing season and size at harvest is well-known; small fruit will never catch up in size to larger fruit [,]; hence, the basis for hand thinning should be fruit size rather than spacing. In nashi, the benefits of early thinning were noted by Burge et al. [], who reported a 17% increase in fruit size following flower thinning but no increase in trees thinned 26 days after full bloom (dAFB). However, McArtney and Wells [] found that hand thinning as late as 56 dAFB, leaving one fruitlet at each fruiting site, increased fruit weight of ‘Nijisseiki’ and ‘Hosui’ and increased return bloom in ‘Hosui’.
Following blossom removal at the first and second or sixth and seventh floral positions in ‘Hanareum’ and ‘Niikata’ five days before full bloom (dBFB), Lee et al. [] reported an increase in fruit weight ranging from 10 to 16% and improved fruit quality, leading them to recommend targeting of the sixth and seventh positions from the basal part of the flower cluster as a practical thinning method for these two cultivars.
Thinning the cultivars ‘Shinsui’, Hosui’, ‘Kosui’ and ‘Nijisseiki’ to a maximum of 1 or 3 fruit per cluster at two different times, Buwalda et al. [] reported a negative linear relationship between fruit weight at harvest and the number of fruit retained. They also found a reduction in fruit weight following thinning around 60 days after 50% bloom compared to earlier thinning prior to 30 days after 50% bloom and suggested this was probably a result of limited resources being diluted amongst a larger number of fruit during late cell division and early cell wall development. Identifying desirable fruit shape is one of the limitations to early thinning, so, in practice, thinning should be conducted straight after the first natural drop of non- or poorly pollinated flowers [].
A summary of studies on hand-thinning in nashi is provided in Table 5.

Table 5.
Summary of findings on the impact of hand thinning of nashi.
6.2. Chemical Thinning
Chemical thinning is standard industry practice in the apple industry and is becoming more accepted for crop load management in European pears [] but is rarely used for managing crop loads in nashi. Chemical thinning involves the application of caustic or synthetic hormonal PBRs during the bloom and/or post-bloom periods; this is normally followed up with hand thinning after the June/December drop to optimise fruit numbers and remove damaged and misshapen fruit []. Chemical thinning is extremely weather-dependent, and there are numerous other interacting factors affecting the degree of thinning, including rootstock; tree age, vigour and health; blossom density; pollination; choice of chemical and application method and conditions [,]. Hence, optimal crop load management with chemicals can be difficult, as responses to chemical thinning can be unpredictable. In both apples and European pears, the sensitivity to thinning chemicals varies considerably between cultivars [,,,,]. Menzies [] also noted that pears are more difficult to thin with chemicals than apples under Australian conditions.
Thinning chemicals can be classified as either blossom (primary) or post-bloom (secondary) [] and work in one of two ways:
- growth regulators that alter tree physiological processes by mimicking plant hormones [,]. PBRs in this category can be effective during the bloom period and/or as post-bloom (fruitlet) thinners.
- blossom desiccants (or blossom burners) are caustic chemicals that desiccate the pistil of the flower, thus preventing fertilisation. However, additional modes of action, such as extra ethylene formed by the injured flower parts, may contribute to abscission []; a transient reduction in leaf area (and, hence, availability of carbohydrates) may indirectly cause drop of very young fruit []. In general, desiccants are only effective prior to fertilisation, so if fruit set has been achieved prior to spray application, they are ineffective. More than one application of a desiccant can be applied during the flowering period to target specific flowers []. Desiccants tend to be less dependent on weather conditions for their effectiveness compared with thinners in the growth regulator category but can be reactivated if rewetting occurs soon after application or if humidity is high; this can cause severe burning, damaging buds, fruit and leaves [].
While some chemicals are used worldwide, recommended application times and concentrations often vary between countries and growing regions [], partly because of differences in climate and practices in different growing regions, but sometimes, the degree of uptake of new research and technology between growing regions also plays a part. The availability of chemicals also varies between countries, with some countries deregistering chemicals because of their negative effects on the environment or because of high initial registration or reregistration costs []. A summary of chemical thinning agents used in pome fruit is provided in Table 6.

Table 6.
Chemicals used worldwide for the thinning of pomes (Source: Bound []).
6.2.1. Ammonium Thiosulphate
The desiccating chemical ammonium thiosulphate (ATS) has been successfully used to reduce crop load in both apple and European pear [,]. Australian trials over a two-year period showed that ATS was also effective as a blossom thinner of the nashi cultivar ‘Nijisseiki’ []; this study demonstrated that, while applying ATS (768 g/L ammonium thiosulphate) at 20% bloom had some thinning effect, applications at a rate of 2% ATS at either 50% or 80% bloom produced the most consistent thinning across the two crop load variables measured: number of fruit cm-2 trunk TCSA and number of fruit per 100 blossom clusters. Fruit size was improved by 1.0, 1.5 and 2.0% applications at 50 or 80% bloom. This contrasts somewhat with the findings of Bound and Mitchell [], who reported that 80% bloom application of ATS was too late for effective thinning on the European pear cultivar ‘Packham’s Triumph’.
When applying blossom desiccants, the application time is critical, as desiccants work by desiccating the style and stigma of the flower, thus preventing pollination or growth of the pollen tube []. In apples, the early opening flowers produce the largest fruit, so these are allowed to set fruit, and ATS is applied from 20% bloom with the aim of removing the remaining flowers; often, two or three applications are made, depending on the length of the flowering period []. However, as the current recommendation for nashi is to target the third to fifth flowers in the cluster, a different approach to timing is required for effective thinning of nashi.
Leaf burning in ‘Nijisseiki’ was observed by Bound and Mitchell [] at application rates of 2% ATS; however, the damage had no effect on fruit size. In the European pear cultivar ‘Packham’s Triumph’, Bound and Mitchell [] found that concentrations of 1.0–1.5% prevented fruit set without causing unacceptable phytotoxicity. The efficacy and phytotoxicity of ATS are dependent on temperature, humidity and cultivar []. Bound and Mitchell [] suggested that the use of multiple applications during the flowering period may result in better thinning at lower chemical rates; however, this has not been examined yet in nashi.
Table 7 summarises the research undertaken with ATS as a chemical thinner for nashi.

Table 7.
Summary of the findings on the impact of ammonium thiosulphate (ATS) as a chemical thinning agent for nashi.
6.2.2. Lime Sulphur
Lime sulphur (LS) is commonly used by organic apple growers as a blossom thinner [], as it has a desiccating effect, thus preventing fertilisation from occurring. Although there are no reported studies on the application of LS for thinning nashi, there are some reports of its use in European pears.
In Norwegian studies on the European pear cultivars ‘Amanlis’ and ‘Moltke’, Meland and Gjerde [] reported that full bloom application of 5% LS thinned adequately, and Garriz et al. [] concluded that 7% LS applied at 30% bloom was an effective practice for thinning and enhancing fruit quality in ‘Abbé Fetel’ pears in Argentina, However, in studies on ‘Williams’ pears in Argentina and the USA, Dussi et al. [] reported a lack of effect on fruit set following the application of 10% LS or 8000 mg L−1 sulphur at 80% full bloom. Lime sulphur has also been reported to affect photosynthesis, with an additive effect following multiple sprays reducing the photosynthetic rate up to 50% [].
6.2.3. Ethephon
Ethephon (2-chloroethyl phosphonic acid) has been successfully used for several decades as both a blossom and post-bloom thinning agent in apples [,]. It acts by artificially raising ethylene levels, resulting in flower/fruitlet abscission. Multiple studies on the use of ethephon at both flowering and post-bloom have yielded variable results for thinning European pear cultivars. Many of these reported studies are described by Bound [], who suggested that the inconsistency in results observed between the different studies may be the result of a range of individual and interacting factors, including application method and chemical coverage, differences between cultivars in ethephon sensitivity, tree vigour and blossom density. It was also noted that weather conditions, not only at the time of application but also before and after, can impact the degree of absorption, and this is often not reported.
In New Zealand trials on nashi, the application of 600 mg L−1 ethephon applied 9 dBFB has been shown to reduce fruit set in ‘Hosui’, but 300 mg L−1 had no thinning effect []. These authors also reported that Kim et al. [] found a greater thinning response to ethephon applied 14 and 21 dAFB than earlier applications at FB or 7 dAFB on ‘Chojuro’. Working with ‘Nijisseiki’ and ‘Hosui’, McArtney and Wells [] found that 400 mg L−1 ethephon applied 15 dAFB reduced fruit set of ‘Nijisseiki’ by 37% and ‘Hosui’ by 15%, with an average of one ‘Nijisseiki’ fruitlet per cluster being removed. In follow-up work examining a range of concentrations from 0 to 800 mg L−1, fruit set was reduced in proportion to concentration, with a 62% reduction at 800 mg L−1. Reginato and Rojas [] reported that FB applications of 100 and 200 mg L−1 ethephon had a good thinning effect on the cultivar ‘Hosui’, but 400 mg L−1 overthinned. Discussing their results in the context of other studies, McArtney and Wells [] noted that Hong et al. [] reported effective thinning of ‘Shinsui’ and ‘Hosui’ with ethephon concentrations of 200 and 400 mg L−1 applied 15 dAFB, while higher concentrations caused excessive thinning; they also noted that Kim et al. [] observed overthinning of ‘Kosui’ and ‘Okusankichi’ with 400 mg L−1 ethephon applied 14 dAFB while two other cultivars, ‘Chojuro’ and ‘Niitaka’, were thinned efficiently. In an initial study with ethephon on ‘Shinko’ and ‘Hosui’, Prunty and Marini [] reported that application of 678 mg L−1 at 9-mm fruitlet diameter resulted in a 70% reduction in fruit set; a follow-up study on ‘Shinko’ the following year found a linear decline in fruit set per 100 blossom clusters with increasing ethephon concentration from 0 to 678 mg L−1; however, a confounding factor in this study was that all treatments contained carbaryl and superior oil. In ‘Hosui’ grafted on P. betulaefolia rootstock, the maximum ethephon response was reached at 200 mg L−1, with no further increase at higher concentrations [].
McArtney and Wells [] reported that ethephon reduced the mean fruit weight of ‘Hosui’ at harvest by 34 g (21%), but ‘Nijisseiki’ was unaffected. Examining a range of concentrations in a second study on ‘Nijisseiki’, McArtney and Wells [] found reduced fruit weight with increasing ethephon concentration. Kim et al. [] also observed reduced fruit growth and size when examining the thinning effect of ethephon in the cultivars ‘Chojuro’, ‘Kosui’, Niitaka’ and ‘Imamuraaki’; a similar effect was observed on ‘Hosui’ in Chile by Reginato and Rojas [], who concluded that ethephon could inhibit fruit growth. In a New Zealand study with ‘Hosui’, Burge et al. [] found that the application of 300 or 600 mg L−1 ethephon at 9 dBFB reduced crop load, but there was no effect on mean fruit weight.
A reduction in flesh firmness was observed in ‘Nijisseiki’ with increasing ethephon concentration, but fruit soluble solids content and seed number increased []; however, Kim et al. [] saw no effect on soluble solids, fruit firmness or total acidity in ‘Chojuro’, ‘Kosui’, Niitaka’ and ‘Imamuraaki’ following application of 400 mg L−1 ethephon at 14 dAFB. A fruit-flattening effect was observed by Reginato and Rojas [] in ‘Hosui’ following FB application of ethephon. McArtney and Wells [] observed an increase in the incidence of the fruit disorder flesh spot decay after 12 weeks storage in proportion to the ethephon concentration. A 480% increase in calyx disorder following ethephon applications of 600 mg L−1 was observed in ‘Hosui’ by Burge et al. []. McArtney and Wells [] reported differing effects of ethephon on return bloom between cultivars, with increased return bloom of ‘Nijisseiki’ but not ‘Hosui’.
Table 8 summarises the research for ethephon as a chemical thinning agent on nashi.

Table 8.
Summary of the findings on the impact of ethephon as a chemical thinning agent for nashi.
6.2.4. NAA
Naphthalene acetic acid (NAA) and naphthalene acetamide (NAAm/NAD) are commonly used for thinning in apples, and in Australia, NAA is recommended as a blossom spray between FB and 7 dAFB, as applications later than 7 dAFB have been associated with pygmy fruit production []. However, in many countries, NAA and NAAm are applied as post-bloom thinners at petal fall or later [,]. According to Webster [], NAA causes a temporary check in tree growth that can depress fruit size.
In studies on the nashi cv. ‘Nijisseiki’, McArtney and Wells [] reported no effect on fruit set or weight following application of 7.5 mg L−1 NAA at 15 dAFB but did observe a reduction in fruit flesh firmness. No thinning effect was observed in ‘Hosui’ by Burge et al. [] following application of 7.5 and 15 mg L−1 at 14 dAFB, and Prunty and Marini [] also observed a lack of thinning effect with 8 mg L−1 NAA applied at 9-mm fruitlet diameter to the cultivars ‘Hosui’ and ‘Shinko’.
In Chile, Reginato and Rojas [] applied NAA to ‘Hosui’ at three concentrations (5, 10 and 20 mg L−1) and three application times (balloon stage, petal fall (PF) and 10 days after petal fall (dAPF)) and reported that the effect of NAA in reducing fruit set was proportional to the concentration; a greater thinning effect was observed with the earlier applications. Fruit weight in this study was dependent on fruit load after final fruit set.
Results of thinning studies on NAA as a chemical thinning agent are summarised in Table 9.

Table 9.
Summary of the findings on the impact of NAA as a chemical thinning agent for nashi.
6.2.5. 6-Benzyladenine
The synthetic cytokinin 6-benzyladenine (BA) (N-(phenylmethyl)-1H-purine-6-amine) is an effective post-bloom thinner for apples [,], and a discussion on its efficacy as a post-bloom thinner for European pears can be found in the review by Bound [].
In a preliminary study of BA on ‘Nijisseiki’ in the late 1990s in the Australian state of Victoria, Bound and Mitchell (unpublished) examined a range of concentrations (50, 75, 100, 125, 150, 175 and 200 mg L−1) and application times (5, 8, 11, 14, 17, 20, 23 and 26 dAFB) but observed no thinning effects and concluded that the lack of response may have been due to low blossom density in the trial trees (average of 1.52 blossom clusters cm-2 TCSA), as trees with more intense bloom are easier to thin because of increased competition for resources and, thus, increased stress [].
In contrast to the results observed in the preliminary study described above, Ward et al. [] noted that BA delivers yields and fruit sizes comparable to hand thinning and is now used by many nashi growers in the US state of New Jersey. Ward et al. [] reported that 200–250 mg L−1 was effective in reducing fruit set and crop load, as well as the amount of follow-up hand thinning, across multiple cultivars studied; the cost of hand thinning was reduced by up to 50%, saving growers up to USD 2000 per acre.
The time of application for BA is based on fruit size. In apples, the recommended size is 7–10-mm diameter of the king fruitlets [,], which normally occurs 10–25 dAFB. Ward et al. [] recommended a fruit size of approximately one-half inch (12.5 mm) for nashi but noted that, in practical terms, fruit size should be one-third to two-thirds of an inch in diameter (9–16 mm). Temperature is also critical to ensure the efficacy of BA []. The recommendation provided by Ward et al. [] for applying BA to nashi in the US state of New Jersey is temperatures in the range of 72–82 °F (22–28 °C), but Bound et al. [] noted that temperatures needed to be in excess of 15 °C on the day of application for efficacy on apples. The Australian label recommendation is predicted daily maximum of greater than 15 °C with application during a warming trend []. Ward et al. [] warned that applying BA at temperatures above 85 °F (30 °C) can result in overthinning.
The Canadian label (Table 10) noted that applications should be made in the morning or evening when conditions are best for slow drying (cooler temperatures and higher humidity) in order to ensure adequate absorption of the product. Ward et al. [] noted that the efficacy of BA varies with environmental conditions following application, indicating that the amount of thinning increases during the three to five days after application when there is less sun and higher temperatures, particularly at night. There are slight differences in the label recommendations across countries; these differences are summarised in Table 10 below, and Table 11 summarises the findings on the impact of 6-benzyladenine (BA) as a chemical thinning agent for nashi.

Table 10.
Label recommendations for 6-benzyladenine (BA) as a post-bloom thinner in pome fruits.

Table 11.
Summary of the findings on the impact of 6-benzyladenine (BA) as a chemical thinning agent for nashi.
6.2.6. Carbaryl
The carbamate insecticide carbaryl (1-naphthyl (N)-methyl carbamate) is successfully used as a fruitlet thinner in apples but is not effective on European pears [,,]. Studies by Burge et al. [] and Prunty and Marini [] also found that it is ineffective as a thinner of nashi.
Carbaryl is a persistent pesticide that is toxic to bees and mammals [] and has been found in groundwater [], making it an undesirable chemical for further study. It has now been withdrawn from use in many European countries []. A summary of the findings on the effect of carbaryl as a thinner in nashi is provided in Table 12.

Table 12.
Summary of the findings on the impact of carbaryl as a chemical thinning agent for nashi.
6.2.7. Abscisic Acid (ABA)
Abscisic acid (ABA) is a naturally occurring plant hormone that is involved in the regulation of stomatal opening and closing, enabling plants to close stomata to reduce water loss under stressful conditions []. Stomatal closure induces carbohydrate stress due to a decline in leaf photosynthesis [,], which can lead to fruit abscission; hence, ABA has potential as a chemical thinning agent.
Several studies have been undertaken with ABA in European pear cultivars. Greene [] demonstrated a quadratic dose response in ‘Bartlett’ pear from 50–500 mg L−1 applied at 10-mm fruitlet diameter, with 250 mg L−1 producing the same response as 500 mg L−1, and while significant thinning was observed at bloom, PF and 10-mm fruitlet diameter, effectiveness increased at the later development stages. Other authors have reported inconsistent results between regions and years [,]. Arrington et al. [] reported that, within one day of ABA application, the net photosynthesis (Pn) of leaves was reduced 75–90% but gradually returned to 80% of control levels within 7 days, fully recovering by 14 days. This supports the conclusion of Greene [] that ABA has the potential to influence the carbohydrate status within a plant by closing stomates, thus reducing photosynthesis during the time the stomates are closed.
There are conflicting reports on the impact of ABA on fruit weight and other quality parameters: Greene [] reported increased fruit weight, flesh firmness and soluble solids in ‘Bartlett’, while Arrington et al. [] found that weight was increased but fruit firmness, total soluble solids (TSS) content and titratable acidity were unaffected. Cline et al. [] reported some improvement in fruit size of ‘Cold SnapTM’ and ‘Bosc’ but observed a decrease in yield and crop value.
Leaf yellowing, sometimes coupled with defoliation, has been reported by some authors following application of ABA at rates of 250–500 mg L−1 [,,], but Fernandes [] saw no negative effects on leaves or fruit following application of 300 mg L−1 ABA.
A potential interaction between ABA and environmental factors was suggested by Arrington et al. [], with rewetting and cloudy conditions in the days following application potentially contributing to phytotoxic effects by enhancing the ABA uptake. As the degree of sensitivity to chemicals differs between cultivars, the cultivar may also influence the response to ABA.
6.2.8. Metamitron
The triazinone herbicide metamitron is a relatively new post-bloom thinner used on apples, and more recently, pears have been added to the label (Brevis®, 150 g kg−1 metamitron). The mode of action was described by Elsysy et al. [] as temporarily inhibiting photosynthesis through PSII inhibition via electron transport blockage, which reduces the maximum potential quantum efficiency of PSII (Fv/Fm). In apples, observing a negative linear response between metamitron concentration and fruit set, McArtney et al. [] found that Fv/Fm declined two days after foliar application, remaining suppressed for as long as 11 days. Elsysy et al. [] reported the inhibition of photosynthesis for a duration of two to three weeks, although longer persistence was observed in two trials.
Several authors have reported thinning effects on European pears following the application of metamitron as a post-bloom spray. Increased thinning across three trial sites was observed by Maas and van der Steeg [] with increasing concentrations from 175–700 mg L−1 applied at the 10–12-mm fruitlet stage; desirable levels of thinning were also observed with single or repeated applications of 175–350 mg L−1 metamitron at 8–12-mm fruitlet diameter. A linear reduction in photosynthesis and fruit set with increasing metamitron rates (150–600 mg L−1) was reported by Elsysy et al. [] in cv. ‘Bartlett’. Different responses reported across different trials may be due to cultivar and climatic differences [].
The thinning efficacy of metamitron is influenced by the time of application. In studies on cv. ‘Bartlett’, an application at ~7 mm had little effect on fruit abscission, while significant thinning was observed between the 10- and 13-mm fruitlet stages []. Maas and van der Steeg [] also found that metamitron was more effective when applied at 10–12-mm fruitlet diameter than at 6–8 mm. At the smaller fruitlet sizes, leaves are just beginning to expand, so there is minimal leaf area for chemical absorption []; hence, the lack of response is likely due to insufficient metamitron uptake.
Well-pollinated trees have been reported to require higher doses of metamitron than poorly pollinated trees []. According to Maas and van der Steeg [], the presence of seeds enhances the sink activity of the fruit for assimilates, which means that it is more difficult to promote their abscission by photosynthetic inhibition. Seeds also produce growth regulators, and fruit without seeds are more prone to abscise than fruit with seeds [].
Following the proposal by Botton et al. [] that a critical threshold level of carbohydrates within the fruit cortex triggers the activation of the fruit abscission zone, McArtney et al. [] suggested that tree carbohydrate balance at the time of application, daily level of carbon assimilation and allocation of assimilated carbohydrates between competing sinks can all influence the efficacy of metamitron as a fruit thinner.
6.2.9. Potential New Chemical Thinning Technologies
Multiple substances have been assessed as potential chemical thinning agents for pome fruit, but very few have produced consistent results with minimal or no phytotoxicity [,,]. The cost of new chemical development and/or a lack of proprietary exclusivity have been the cause of non-commercialisation of several chemicals that have shown good efficacy—for example, acetic acid []. Several chemicals that have shown potential but require further development are discussed below.
5-Aminolevulinic Acid
The efficacy of the natural amino acid 5-aminolevulinic acid (ALA) as a pear thinner was demonstrated by An et al. []. ALA is present in living cells of microbes, plants and animals [,]; it acts as an essential biosynthetic precursor for all organic heterocyclic tetrapyrrole molecules, including vitamin B12, chlorophyll and heme [].
The mechanism by which ALA thins is the inhibition of pollen germination and tube growth via Ca2+ efflux by activating Ca2+-ATPase [], thus preventing fertilisation. Following several studies, An et al. [] recommended that the application of 100 mg L−1 ALA at 50–75% bloom was the most effective for thinning pears. As a nontoxic biodegradable amino acid present in living cells, ALA has considerable potential as a chemical thinning agent, as it is likely to meet modern environmental and food quality guidelines.
1-Aminocyclopropane-1-Carboxylic Acid
The precursor to ethylene metabolism, 1-aminocyclopropane-1-carboxylic acid (ACC) has shown some promising results as a potential chemical thinning agent for apples and peaches [,]. Studies with ACC on European pears have also been positive. Theron et al. [] reported that the application of 300 mg L−1 at 8–10-mm fruitlet diameter resulted in a 50% reduction in crop load and an increase in fruit weight in ‘Forelle’, while Cline et al. [] found that the same rate of 300 mg L−1 reduced crop load of ‘Bosc’ but observed no thinning effect for ‘Cold Snap™’. Costa et al. [] suggested that the physiological mechanism of ACC action deserves further investigation and recommended further studies to optimize ACC concentration, time of application and possible interactions with other thinning agents, such as ABA and metamitron.
The United States Environmental Protection Agency (EPA) has granted an exemption from the requirement of a tolerance for residues of ACC in or on apples and stone fruit when used in accordance with good agricultural practices, effective 28 June 2021 [].
Polysorbates
Studies with polysorbates 20, 60 and 80 [E432, polyoxyethylene (20) sorbitan monolaurate, Tween 20; E435, polyoxyethylene (20) sorbitan monostearate and E433, polyoxyethylene (20) sorbitan monooleate, Tween 80, respectively] on apple have demonstrated that they have potential as post-bloom thinning agents; these substances are emulsifiers used as additives in the food industry, classified as GRAS (generally recognized as safe) components.
Undertaking a range of studies over several years on four apple cultivars, Stopar and Hladnik [] found a weak thinning effect with 5 mL L−1 polysorbates when applied at PF and 9-mm fruitlet diameter, but adding a third application at 14-mm fruitlet diameter caused a significant cumulative thinning effect, with most of the thinning attributed to the last application. Further work showed that double applications at fruitlet diameters of 12 and 18, or 18 and 20 mm resulted in a significant thinning effect. They did, however, find a russeting effect on one cultivar, ‘Golden Delicious’. They concluded that these polysorbates were efficient thinning agents for all cultivars when applied twice in a later thinning window of fruitlet diameter above 9 mm and noted that, with some additional research, effective polysorbate thinning programs could be developed for cultivars that are not too sensitive to fruit russet.
Potassium Bicarbonate and Calcium Polysulphide
Potassium bicarbonate (KHCO3) was included in the studies by Stopar and Hladnik [] on a range of apple cultivars. They reported that a double application of 8, 12 or 15 g L−1 at first flower and FB thinned ‘Gala’ and ‘Elstar’ apples effectively but increased russet in ‘Gala’.
A FB application of 19 g L−1 calcium polysulphide (CaSx) on the apple cv. ‘Elstar’ was found to be as effective as 15 g L−1 KHCO3 and 10 g L−1 ATS []. However, further studies of these substances are required to confirm their efficacy and impact on fruit skin finish and other quality parameters.
6.2.10. Opportunities for Chemical Thinning in Nashi
While chemical thinning is likely to provide nashi growers with a means of reducing hand thinning costs, studies need to be undertaken to determine the optimal rates and application times for each chemical plus the potential of retaining fruit in the centre of the cluster. The most likely chemical candidates are ATS, BA, metamitron and possibly NAA. The newer chemicals described above, ALA, ACC, polysorbates and potassium bicarbonate, are also worth investigating further.
The use of PBRs as a thinning tool should be considered as part of a larger portfolio of options that are integrated into a whole sustainable systematic program approach for controlling vigour and improving cropping []. The action of chemical thinning agents is related to cultivar, physiological state of the tree and blossom intensity, but meteorological conditions at application also play a major role. Following application of chemical thinning agents, a higher level of fruit abscission is observed with weather conditions that favour high carbohydrate demands but low supply (i.e., when trees are in carbon deficit), particularly low light levels and elevated temperature after treatment [,]. Knowledge of these factors/conditions affecting the tree carbon balance can be used to optimise thinning outcomes (Table 13).

Table 13.
Factors affecting the tree carbon balance during fruit development. Source: Bound [].
6.3. Mechanical Thinning
Mechanical thinning provides an environmentally friendly means of reducing crop load, and a range of mechanical devices have been trialled in different tree crops with varying degrees of success. Mechanical thinning can provide considerable savings in labour costs associated with hand thinning; based on 20 ha and 10 years depreciation of the mechanical thinner, Seehuber et al. [] reported the cost of mechanical thinning was half that of hand thinning. While mechanical thinning is applicable to both flowers and developing fruit, it can cause considerable damage to trees and, when used for fruitlet thinning tends to remove the larger fruit, leaving the smaller, less desirable fruit []. Jacobus de Villiers [] and Wouters [] described a range of mechanical systems, including trunk and limb shakers, spiked drum shakers, rope curtains, water jet thinning, hot air blowers and string thinners.
Several disadvantages of trunk shakers, noted by Lopes et al. [], include excessive thinning, reduction in marketable grade fruit, irregular thinning patterns—particularly near the top of the tree, loss of larger fruitlets and significant leaf removal, which can negatively affect fruit growth. Trunk and limb shakers have been successfully used in stone fruit but are not recommended for pome fruit, as fruit is easily bruised []. Spiked drum shakers tend to create an uneven fruit distribution by removing more fruits from the outside of the canopy than the inside [].
The Darwin string thinner developed by an organic apple grower in Germany and the BAUM device developed by the German University of Bonn have both shown potential on several fruit species [,]. The Darwin thinner uses flexible strings/cords rotating around a vertical spindle, and the thinning intensity is adjusted by changing the rotational speed of the spindle, the speed of the tractor or the arrangement of the cords. The BAUM device has three horizontal rotors on a 3-m vertical spindle []. The rotors can be set independently of each other and swung individually out of the tree row, thus providing flexibility for selective thinning of one side of a tree row and different canopy sections (lower or higher and inner or outer part of the tree) []. The BAUM device enables precise control over the number of flowers removed by choosing between a selection of brush type, rotor speed, rotor position and tractor speed and is able to remove peripheral flowers, as well as flowers in the centre of the tree close to the trunk where fruit is normally of lower quality due to shading []. Examples of successful rotor and tractor speeds for thinning in European pears have been provided by Bound [].
Most mechanical thinning studies have been undertaken on peach and other stone fruit, with very few studies in pome fruit []. Timing for use of the different mechanical devices varies between the bloom and fruitlet stages of growth: the flower stage is most suited for the rope curtain, Darwin string thinner, BAUM string thinner and compressed air pulses, while limb/trunk and spiked drum shakers are most suited to the fruitlet stage. String thinners are able to reduce the time required for hand thinning by up to 50% [] and are probably the most feasible mechanised thinning solution in terms of thinning efficacy, speed and ability to control damage [,,].
The ideal tree architecture for successful mechanical thinning is a two-dimensional hedgerow-type system. Voluminous three-dimensional canopies impede machine access to blossom clusters, particularly in the centre of the canopy []. Suitable tree training methods include spindle, solaxe, vertical axis and central leader [,]. Fruit morphology can also be important in the success of mechanical thinning. The long flexible peduncles of the European pear cv. ‘Packham’s Triumph’ have been reported to be a major limitation in mechanical thinning [], while the steep upright long peduncles of cvs. ‘Conference’ and ‘Alexander Lucas’ were partly attributed to successful mechanical thinning [].
As spur leaf development occurs during the flowering period in pome fruit, this can be challenging for flower thinning with mechanical thinners, as spur leaves are important for fruit set, providing photosynthates to developing fruit early in the season []. A loss of greater than 75% of the leaf surface has been reported to reduce both fruit set and the quality in apple [].
A drawback of string thinners is that they can provide an entry point for diseases, such as fire blight (Erwinia amylovora) and canker (Nectria gallingea), via damaged leaves and bark []. An increase of 380% in fire blight infection of apple trees was reported by Ngugi and Schupp [] following thinning with a Darwin string thinner.
A limitation of both the Darwin and BAUM units is their inability to accommodate the requirements of individual trees, although Bound [] suggested that, in this respect, they are no different to the current chemical thinning practices where orchard blocks are treated as one unit, each tree receiving the same amount of chemical. A start to overcoming this limitation is the development of a vision system for real-time determination of flower density combined with a decision support tool to calculate optimum thinning intensity based on current flower density and a mechanical thinning unit controlled in real time []. A commercial system is now available—the Darwin SmaArt Camera System (Fruit-Tec, Markdorf, Germany)—that detects the blossom density of individual trees, passing the data to an onboard computer that calculates the optimum spindle speed and controls the thinning unit [].
Despite the drawbacks of the current commercially available string thinners, there are still advantages to mechanical thinning in that it is not weather-dependent, and thinning can be undertaken early in the flowering period as soon as flowers can be identified on the tree; additionally, the thinning effect is evident immediately after treatment. For crop load management, this technology is suitable for organic orchards, as well as providing a low environmental impact method for conventional orchards.
Mechatronic systems are also under development to overcome the issues of non-selectivity and tree damage; Wouters [] developed a novel mechatronic device offering a high degree of selectivity with minimal tree damage by using a sensor capable of detecting floral buds and pulses of compressed air to remove buds. The removal of floral buds at their natural attachment point means that there is little damage to the tree. Based on the measured floral bud distribution, mechatronic systems such as this can provide precision thinning, and cost analysis has indicated that pneumatic thinning can be an economically feasible alternative to traditional hand thinning [].
With the move towards mechatronics to overcome the problems of tree damage and non-selectivity in combination with a transition towards two-dimensional tree architecture, there is potential in the future for mechanical thinning to provide an efficient environmentally friendly way of managing crop load.
6.4. Photosynthetic Inhibition through Shading
Limiting carbohydrate supply at critical fruit growth stages through shading has been shown to reduce set and/or result in abscission of fruitlets [,]. The majority of shading studies have been undertaken on apple, but the results should also be applicable to both European pear and nashi, although the time and duration of shading may vary.
According to Byers et al. [], the shading of whole trees from 25–35 dAFB can almost completely de-fruit apple trees, and under natural conditions, apple fruit set can be greatly affected by as little as three days of cloud cover. The timing of shading can be critical, as a total fruit drop has been observed in apples with 100% shading at 28 dAFB, while 100% shading for five days starting at 14 dAFB resulted in an ideal level of fruit set equivalent to hand thinning after the June (December) drop []. Byers et al. [] reported a 7–17% reduction in fruit set following shading with 92% shade cloth for 2–3 days at 14, 21 and 28 dAFB, but 2–3 days of shade at 8, 35 or 42 dAFB had no influence on fruit drop. There is a lack of consistency in the literature in how the timing of shading is reported, as some studies used dAFB while others reported fruit size. Three days of shading the whole tree when fruit were 20-mm diameter caused 98% fruit abscission [], and several authors cited by Greene et al. [] suggested that 8–15-mm fruit size is the critical stage when developing fruit are easily thinned. It has been calculated that 2–3 days of 92% artificial shade is equivalent to 3–4 consecutive days of cloudy periods []. Depending on seasonal conditions, fruit size will vary at similar times each season, so fruit size may be a better indicator of sensitivity to carbohydrate stress.
Wünsche et al. [] observed a reduction in leaf carbon assimilation in apple trees sprayed with the kaolin product Surround®; although the trees were treated mid-summer close to harvest to ameliorate fruit sunburn, kaolin-based sprays may have potential in limiting light availability, thus simulating shading, if applied when trees are sensitive to carbohydrate stress.
As seasonal weather conditions have an influence on photosynthesis and carbohydrate stress, further work is required to determine the optimal timing and period of shading required for each cultivar. Determination of the relationship between fruit size and days after full bloom over several seasons may be useful for consistency of application of shading treatments for each cultivar.
6.5. Thermal Shock
Thermal shock was investigated over a two-year period as a method of preventing fertilisation by arresting pollen tube growth in apple []. A variable-temperature heat gun was used to apply short durations of forced heated air treatments to blossoms 24 h after pollination. The results were variable across the two years, but in the first year, a thermal shock of 56 °C for 2 or 4 s duration inhibited pollen tube growth; in the second year, the conditions were optimal for pollen tube growth, and the lack of effect was attributed to the treatments being applied too late. Excessive injury to spur leaf tissue was observed following treatment for 2 s at ≥84 °C or 4 s at ≥70 °C, but pollen tube growth was reduced or arrested at temperature and duration combinations that caused minimal leaf tissue damage.
Kon et al. [] concluded that a 2-s burst at 56 °C would prevent fertilisation without visible injury to spur leaves and suggested that, in future work, consideration should be given to the use of pollen tube growth models as timing aids for thermal shock. They also noted that the structure of the canopy and distance of the heat source from the target were important considerations, so high-density orchards with narrow tree wall canopies could facilitate the application of thermal shock as a thinning method. Combined with vision systems that are undergoing development to detect blossoms (such as those described by Wouters []), thermal shock may have potential for the selective thinning of nashi blossoms.
6.6. Pruning
While the primary function of pruning is to improve canopy light distribution and control tree vigour, maintaining the balance between vegetative and reproductive activity, it should be considered to be the first stage of any crop load management program [], as it can also be used to manage floral bud numbers. Costa et al. [] stated “…pruning is one of the most important agronomic tools that can finely affect flower bud load in order to facilitate later thinning operations”. Reducing floral bud numbers through dormant winter pruning is an environmentally friendly method of reducing crop load, and importantly, it reduces the competition for assimilates between flowers/fruitlets, thus maximising the benefits in terms of assimilate distribution. Pruning to a specified bud number allows growers to start the process of fruit thinning early to reduce competition among flowers and fruitlets, resulting in increased resources for the remaining fruit and improved fruit size and quality [].
In order to manage pruning appropriately, it is important to understand the growth habit of each cultivar, as there are marked differences in growth habits between nashi cultivars. Beutel [] noted that fruit are borne on spurs on 2–6-year-old wood, with the best sizes being produced from 1- to 3-year-old spurs on wood 1–2 inches (2.5–5 cm) in diameter. The cultivars ‘Hosui’, ‘Kosui’ and ‘Shinsui’ are lateral bearers, while ‘Nijisseiki’ is predominantly a spur bearer with comparatively low vigour. ‘Hosui’ and ‘Kosui’ have also been described as tip bearers with a pronounced tendency to fruit on young wood [].
Australian nashi growers have noted lower fruit set in blocks where the tops of the trees are left unpruned (Figure 6) or where winter pruning is delayed. The mechanism for this reduction in fruit set is likely to be the result of shading reducing the amount of light and, thus, photosynthesis in the canopy. This observation may provide another tool for controlling crop load; however, studies will need to be undertaken to determine the long-term impacts on the trees and fruit quality.

Figure 6.
Unpruned tops in a nashi block. Photo credit: Steven Singh, Seeka Australia Pty Ltd.
Following a 17-year study on natural fruitlet abscission in apple, Lordan et al. [] concluded that apple fruit set and final numbers could be relatively well-modelled by flower density, representing the tree’s physiological history, and a carbohydrate model, representing the early season weather effects; hence, a high number of floral buds results in a high final fruit number despite later chemical thinning []. The number of fruiting buds that remain after pruning influences the fruit thinning requirements, fruit quality, tree vigour and return bloom []. This finding can be used to implement a strategy of precision pruning to reduce the number of flower buds per tree to a predefined number through the removal of shoots/limbs.
The technique of removing individual buds or spurs was introduced by Lauri and Lespinasse [,] following observations of high natural spur extinction in regular bearing cultivars and the production of bourse shoots in the remaining floral structures that flower the following season [,]. By reducing bud density through manual removal of floral buds during late winter or early spring, artificial bud (spur) extinction (ABE/ASE) imitates natural bud extinction and is a precision crop load management technique precisely defining the amount of fruit set on the tree, as well as where the fruit is positioned. ABE has the potential to replace chemical thinning as a crop load management tool in apple [,]. In a comparison of chemical thinning and ABE, Bound [] found that ABE was comparable to the use of chemical thinning programs, but it had the advantage of reduced costs in subsequent years following the initial tree setup. The added advantages of ABE are that it is independent of weather conditions and the risk of the negative impacts of chemical thinners on fruit size, shape and skin finish are removed.
Australian nashi growers already practice spur removal, and some also remove individual buds after initial pruning through to bud burst to reduce the number of buds on the spurs (termed bud flicking) []. The techniques currently used by nashi growers in relation to spur and bud thinning may be able to be refined, but growers are looking to reduce labour costs through the implementation of other tools to manage crop loads.
7. Conclusions
A number of tools/techniques that have potential to be incorporated into a systematic approach for managing crop load in nashi have been highlighted in this review. The keys to optimising fruit size, shape and internal quality are good canopy light interception, which is achieved through pruning, early removal of unwanted flowers and/or fruitlets and good pollination. With further research, many of the tools used in other fruit tree crops can be modified or adapted to suit the physiology of nashi. Early thinning offers distinct advantages in relation to fruit size, firmness and sugar content. Both chemical and mechanical thinning have potential for inclusion in nashi thinning programs, as they offer the advantage of early thinning during the flowering period. Chemical thinning can also extend to post-bloom thinning in situations where the crop load has not been reduced sufficiently after the flowering period. A sequential spray program across both the flowering and post-bloom periods spreads the risk in seasons where it is difficult to find a window with suitable application conditions.
Chemical thinning will most likely offer a reduction in thinning costs by reducing the time and labour required for hand thinning, but the risk of the negative impacts that chemical thinners can have on fruit quality cannot be removed. It is also difficult to target specific flowers or fruitlets within a cluster with chemicals. The benefits of chemical thinning can be maximised through an understanding of the physiological mechanisms involved for each active ingredient, along with knowledge of the optimal application timing and dose rates for each chemical thinning agent. As there are differences between cultivars in the degree of sensitivity to each chemical, rigorous studies will need to be undertaken for each cultivar/chemical combination. The predictability of chemical thinning can be improved with a good knowledge of the conditions that impact the carbon balance of the tree. To make optimal use of the carbon deficit conditions, the development of a nashi-specific carbon balance model that simulates carbohydrate supply and demand would allow for a more precise prediction of thinner response under specific physiological and environmental conditions. Mapping of blossom density in combination with a decision support tool to enable a sprayer to target specific trees and/or sections of trees would allow targeted spray applications, overcoming the problem of non-selectivity and accounting for the thinning requirements of individual trees; however, further work is required in this area.
Mechanical thinning during the flowering period with either Darwin or BAUM-style string thinners has potential, particularly as these devices can be used as early as flower emergence (BBCH plant growth stage 5). As for chemical thinning, the issue of non-selectivity needs to be addressed; however, the development of mechatronic systems should overcome most problems that occur with the currently available mechanical thinners. One drawback of mechanical thinners is that they are most suited to two-dimensional tree architecture, so are unlikely to be successful in orchards with large three-dimensional plantings.
Shading at critical times is an avenue that could be explored further; this can be implemented in several ways: (i) through the use of netting/shade cloth, (ii) the application of kaolin-based sprays or (iii) the delayed pruning of treetops. However, for options (i) and (ii), studies are required for each cultivar to ascertain the critical stage when developing fruit can be easily thinned to enable determination of the length of the period of shading and the optimal timing.
Targeted pruning during the dormant winter period is the obvious first step to reducing floral bud numbers. Bud thinning in the form of spur extinction is a valuable option for the precise placement of fruit in optimal positions and to set up the required number of clusters and can be incorporated into the pruning program. However, further refinements to this technique may improve the outcomes.
Funding
This review was funded by Hort Innovation using the nashi research and development levies and contributions from the Australian government as part of Project NA20000—Cost effective thinning for nashi (desktop evaluation and grower workshops). Hort Innovation is the grower-owned, not-for-profit research and development corporation for Australian horticulture.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Ferradini, N.; Lancioni, H.; Torricelli, R.; Russi, L.; Dalla Ragione, I.; Cardinali, I.; Marconi, G.; Gramaccia, M.; Concezzi, L.; Achilli, A.; et al. Characterization and phylogenetic analysis of ancient Italian landraces of pear. Front. Plant Sci. 2017, 8, 751. [Google Scholar] [CrossRef]
- Quinet, M.; Wesel, J.P. Botany and Taxonomy of Pear. In The Pear Genome. Compendium of Plant Genomes; Korban, S.S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 1–33. [Google Scholar] [CrossRef]
- Saito, T. Advances in Japanese pear breeding in Japan. Rev. Breed. Sci. 2016, 66, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Chen, K.; Zhang, D.; Cao, Y.; Yamamoto, T.; Teng, Y. Genetic diversity and similarity of pear (Pyrus L.) cultivars native to East Asia revealed by SSR (simple sequence repeat) markers. Genet. Resour. Crop Evol. 2007, 54, 959. [Google Scholar] [CrossRef]
- Katayama, H.; Amo, H.; Wuyun, T.; Uematsu, C.; Iketani, H. Genetic structure and diversity of the wild Ussurian pear in East Asia. Breed. Sci. 2016, 66, 90–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wrolstad, R.E.; Lombard, P.B.; Richardson, D.G. Chapter 5: The pear. In Quality and Preservation of Fruits; Eskin, N.A.M., Ed.; CRC Press: Boca Raton, FL, USA, 1991; pp. 67–96. [Google Scholar]
- Jun, W.; Hongsheng, G. The production of Asian pears in China. Acta Hortic. 2002, 587, 71–80. [Google Scholar] [CrossRef]
- Benitez, C.E.; Pensel, N.A. Harvest and postharvest conditions for apples and pears. In Production Practices and Quality Assessment of Food Crops; Dris, R., Jain, S.M., Eds.; Kluwer Academic Publishers: Dordrecht, Germany, 2004; Volume 3, pp. 253–293. [Google Scholar]
- Colavita, G.M.; Curetti, M.; Sosa, M.C.; Vita, L.I. Chapter 2: Pear. In Temperate Fruits: Production, Processing and Marketing; Mandal, D., Wermund, U., Phavaphutanon, L., Cronje, R., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2020; pp. 107–182. [Google Scholar]
- Hack, H.; Bleiholder, H.; Buhr, L.; Meier, U.; Schnock-Fricke, U.; Weber, E.; Witzenberger, A. Einheitliche Codierung der phänologischen Entwicklungsstadien mono- und dikotyler Pflanzen—Erweiterte BBCH-Skala, Allgemein. Nachr. Dtsch. Pflanzenschutzd. 1992, 4, 265–270. [Google Scholar]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Heß, B.; Lancashire, P.D.; Schnock, U.; Stauß, R.; van den Boom, T.; et al. The BBCH system to coding the phenological growth stages of plants—History and publications. J. Kult. 2009, 61, 41–52. [Google Scholar]
- Martínez-Nicolás, J.J.; Legua, P.; Melgarejo, P.; Martínez, R.; Hernández, F. Phenological growth stages of nashi tree (Pyrus pyrifolia): Codification and description according to the BBCH scale. Ann. Appl. Biol. 2016, 168, 255–263. [Google Scholar] [CrossRef]
- Bound, S.A. Understanding crop load management in deciduous fruit trees. In Orchard Plant Protection Guide for Deciduous Fruit in NSW 2020-10; NSW Government Department of Primary Industries: Orange, Australia, 2020; pp. 118–123. ISSN 2200-7520. [Google Scholar]
- Jones, K.M.; Bound, S.A.; Miller, P. Crop Regulation of Pome Fruit in Australia; Tasmanian Institute of Research: Hobart, Australia, 1998; ISBN 1-86295-027-X. [Google Scholar]
- Wertheim, S.J. Developments in the chemical thinning of apple and pear. Plant Growth Regul. 2000, 31, 85–100. [Google Scholar] [CrossRef]
- Close, D.C.; Bound, S.A. Advances in understanding apple tree growth: The manipulation of tree growth and devlopment. In Achieving Sustainable Cultivation of Apples; Evans, K., Ed.; Burleigh Dodds Science Publishing Ltd.: Cambridge, UK, 2017; pp. 1–32. ISBN 978-1-78676-032-6. [Google Scholar]
- Esumi, T.; Tao, R.; Yonemori, K. Comparison of early inflorescence development between Japanese pear (Pyrus pyrifolia Nakai) and quince (Cydonia oblonga Mill.). J. Jpn. Soc. Hortic. Sci. 2007, 76, 210–216. [Google Scholar] [CrossRef][Green Version]
- Thomas, D.; Hayman, P.; James, P. Understanding and Managing the Risks and Opportunities from Climate Change on Cherry Production; Final report DAFF project R1#087; South Australian Research and Development Institute (Sustainable Systems): Adelaide, Australia, 2012. [Google Scholar]
- Luedeling, E.; Girvetz, E.H.; Semenov, M.A.; Brown, P.H. Climate Change Affects Winter Chill for Temperate Fruit and Nut Trees. PLoS ONE 2011, 6, e20155. [Google Scholar] [CrossRef] [PubMed]
- Measham, P.F.; Quentin, A.G.; MacNair, N. Climate, winter chill, and decision-making in sweet cherry production. HortScience 2014, 49, 254–259. [Google Scholar] [CrossRef]
- Bound, S.A.; Jones, K.M. Hydrogen cyanamide impacts on flowering, crop load, and fruit quality of red ‘Fuji’ apple (Malus domestica). N. Z. J. Crop Hortic. Sci. 2004, 32, 227–234. [Google Scholar] [CrossRef]
- Bound, S.A.; Miller, P. Effects of Waiken on flowering and spring growth in apple. Acta Hortic. 2006, 727, 167–174. [Google Scholar] [CrossRef]
- Sagredo, K.X.; Theron, K.I.; Cook, N.C. Effect of mineral oil and hydrogen cyanamide concentration on dormancy breaking in ‘Golden Delicious’ apple trees. S. Afr. J. Plant Soil 2005, 22, 251–256. [Google Scholar] [CrossRef]
- Klinac, D.J.; Rohitha, H.; Pevreal, J.C. Use of hydrogen cyanamide to improve flowering and fruit set in nashi (Pyrus serotina Rehd.). N. Z. J. Crop Hortic. Sci. 1991, 19, 87–94. [Google Scholar] [CrossRef]
- Faoro, I.D.; Nakasu, B.H. Japanese pear growing in Brazil. Acta Hortic. 2002, 287, 97–105. [Google Scholar] [CrossRef]
- Abreu, E.S.; Dini, M.; Carra, B.; Marchi, P.M.; Herter, F.B.; Mello-Farias, P.C. Bud break promoters following different chilling hour accumulation of ‘Hosui’ pear. Acta Hortic. 2021, 1303, 299–304. [Google Scholar] [CrossRef]
- Botelho, R.V.; Biasi, L.A.; Maia, A.J.; Nedilha, L.C.B.M.; Viencz, T. Dormancy release in Asian ‘Hosui’pear trees with the use of vegetable and mineral oils. Acta Hortic. 2021, 1303, 317–323. [Google Scholar] [CrossRef]
- Petri, J.L.; Herter, F. Nashi pear (Pyrus pyrifolia) dormancy under mild temperate climate conditions. Acta Hortic. 2002, 587, 353–361. [Google Scholar] [CrossRef]
- Honjo, H.; Kobayashi, Y.; Watanabe, M.; Fukui, R.; Sugiura, T. Effect of intermittent warm periods on bud break and carbohydrate contents of Japanese pear (Pyrus pyrifolia Nakai) at different endodormancy stages. Acta Hortic. 2002, 587, 397–403. [Google Scholar] [CrossRef]
- Yoneyama, K. Guide Book of Nashi Production in Japan; Kawai, S., Translator; Oregon Asian Pear Council: Dayton, OR, USA, 1989. [Google Scholar]
- Williams, R.R. The Effect of Summer Nitrogen Applications on The Quality of Apple Blossom. J. Hortic. Sci. 1965, 40, 31–41. [Google Scholar] [CrossRef]
- Sanzol, J.; Herrero, M. The “effective pollination period” in fruit trees. Sci. Hortic. 2001, 90, 1–17. [Google Scholar] [CrossRef]
- Rohitha, B.H.; Klinac, D.J. Some observations on the influence of temperature on the germination of pollen on excised nashi (Pyrus serotina Rehder var culta Rehder) flowers. N. Z. J. Crop Hortic. Sci. 1994, 22, 339–342. [Google Scholar] [CrossRef]
- Okada, K.; Tonaka, N.; Takasaki, T. Selection of self-compatible trees by S4sm-haplotype specific marker in Japanese pear. Acta Hortic. 2008, 800, 401–407. [Google Scholar] [CrossRef]
- Zhang, S.; Gu, C. Self-incompatibility in pear. In The Pear Genome; Korban, S.S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 180–200. [Google Scholar]
- Halász, J.; Hegedűs, A. Self incompatibility in pears (Pyrus communis L., Pyrus serotina Rehd. and Pryrus ussuriensis) Review. Int. J. Hortic. Sci. 2006, 12, 87–89. [Google Scholar] [CrossRef]
- Rohitha, B.H.; Klinac, D.J. Relationships between seed set and fruit weight and shape of nashi (Pyrus serotina Rehder var culta Rehder). N. Z. J. Crop Hortic. Sci. 1990, 18, 133–136. [Google Scholar] [CrossRef]
- Beutel, J.A. Asian pear. In Specialty and Minor Crops Handbook, 2nd ed.; Publication 3346; University of California, Division of Agriculture and Natural Resources: Oakland, CA, USA, 1998. [Google Scholar]
- Johnson, J.F. A Study of Asian Pears in Japan; Miscellaneous Bulletin 8; Division of Plant Industries, Department of Agriculture New South Wales: Sydney, Australia, 1983. [Google Scholar]
- Goodwin, M. Pollination of Crops in Australia and New Zealand; Publication No. 12/059; Rural Industries Research and Development Corporation: Hamilton, New Zealand, 2012. [Google Scholar]
- Rohitha, B.H.; Klinac, D.J. Compatibility and copping performance of four Asian pear (Pyrus serotina Rehder var culta Rehder) cultivars as pollenisers for the cultivar ‘Hosui’ in the Waikato region, New Zealand. N. Z. J. Crop Hortic. Sci. 1992, 20, 467–469. [Google Scholar] [CrossRef]
- Faoro, I.D.; Orth, A.I. Parthenocarpy in Japanese pear tree cultivars in South Brazil. Acta Hortic. 2011, 909, 415–422. [Google Scholar] [CrossRef]
- Faoro, I.D.; Orth, A.I. Flower visiting insects during the bloom period of Japanese pear orchards in Brazil. Acta Hortic. 2015, 1094, 275–279. [Google Scholar] [CrossRef]
- Silva, A. Polinização. In O Livro da Pêra Rocha; Silva, A., Alexandre, J., Eds.; Grafilipe: Cadaval, Portugal, 2001; Volume 1, pp. 137–166. [Google Scholar]
- Jackson, J.E. Biology of Apples and Pears; Cambridge University Press: Cambridge, UK, 2005; 488p. [Google Scholar]
- Gallo, D.; Nakano, O.; Silveira Neto, S.; Carvalho, R.P.L.; de Baptista, G.C.; Berti Filho, E.; Parra, J.R.P.; Zucchi, R.A.; Alves, S.B.; Vendramim, J.D.; et al. Entomologia Agrícola; FEALQ: Piracicaba, Brazil, 2002; 920p. [Google Scholar]
- Kingston, C.M.; Klinac, D.J.; van Epenhuijse, C.W. Floral bud disorders of nashi (Pyrus serotina) grown in New Zealand. N. Z. J. Crop Hortic. Sci. 1990, 18, 157–159. [Google Scholar] [CrossRef]
- Trevisan, R.; Chavarria, G.; Herter, F.G.; Gonclaves, E.D.; Rodrigues, A.C.; Verissimo, V.; Pereira, I.D.S. Bud fower thinning on the reduction of abortion in pear (Pyrus pyrifolia) in Pelotas region. Rev. Bras. Frutic. 2005, 27, 504–506. [Google Scholar] [CrossRef]
- Nakasu, B.G.; Herter, F.G.; Leite, D.L.; Raseira, M.C.B. Pear flower bud abortion in southern Brazil. Acta Hortic. 1995, 395, 185–192. [Google Scholar] [CrossRef]
- Ito, A.; Sakaue, T.; Fujimaru, O.; Iwatani, A.; Ikeda, T.; Sakamoto, D.; Sugiura, T.; Moriguchi, T. Comparative phenology of dormant Japanese par (Pyrus pyrifolia) flower buds: A possible cause of ‘flowering disorder’. Tree Physiol. 2018, 38, 825–839. [Google Scholar] [CrossRef] [PubMed]
- Klinac, D.J.; Geddes, B. Incidence and severity of the floral bud disorder “budjump” on nashi (Pyrus serotina) grown in the Waikato region of New Zealand. N. Z. J. Crop Hortic. Sci. 1995, 23, 185–190. [Google Scholar] [CrossRef]
- Buwalda, J.G.; Klinac, D.J.; Meekings, J.S. Effects of time and degree of fruit thinning on fruit size and crop yield at harvest for four nashi (Pyrus serotina Rehd.) cultivars. Sci. Hortic. 1989, 39, 131–141. [Google Scholar] [CrossRef]
- Klinac, D.J.; Geddes, B.; Wright, S. Wood age and floral bud distribution on four nashi (Pyrus serotina) cultivars grown on pergola, Y-frame, and centre leader training systems in the Waikato region of New Zealand. N. Z. J. Crop Hortic. Sci. 1995, 23, 191–197. [Google Scholar] [CrossRef]
- Hort Innovation. 2022. Australian Horticulture Statistics Handbook 2020/21—Fruit. Available online: https://www.horticulture.com.au/contentassets/a68c8934a8bf40b4becdc487bacdb60f/hort-innovation-ahsh-20-21-fruit.pdf (accessed on 29 April 2022).
- Bound, S.A. Managing crop load. In Crop Management and Postharvest Handling of Horticultural Products; Dris, R., Niskanen, R., Jain, M., Eds.; Oxford & IBH Publishing Co. Pvt. Ltd.: New Delhi, India, 2001; Volume 1, pp. 89–109. ISBN 1-57808-140-8. [Google Scholar]
- Webster, T. Current approved thinning strategies for apples and pears and recent thinning research trials in Europe. Compact Fruit Tree 2002, 35, 73–76. [Google Scholar]
- Faust, M. Physiology of Temperate Zone Fruit Trees; John Wiley & Sons: New York, NY, USA, 1989. [Google Scholar]
- Costa, G.; Botton, A.; Vizzotto, G. Fruit thinning: Advances and trends. Hortic. Rev. 2019, 46, 185–226. [Google Scholar]
- Jackson, D.; Palmer, J. Pome Fruits. In Temperate and Subtropical Fruit Production, 3rd ed.; Jackson, D.I., Looney, N.E., Morley-Bunker, M., Eds.; CAB International: Oxfordshire, UK, 2011. [Google Scholar]
- Bennett, R.; Van Popering, J.; Magarey, J.; Magarey, A. (Australian Nashi Growers Association, Bunbartha, Australia); Singh, S. (Seeka Australia, Bunbartha, Australia); Crisera, M. (Fruit Growers Victoria, Mooroopna, Australia). Personal communication, 2021.
- Einhorn, T.C. A review of recent Pyrus, Cydonia and Amelanchier rootstock selections for high-density pear plantings. Acta Hortic. 2021, 1303, 185–196. [Google Scholar] [CrossRef]
- White, A.G.; Trussler, S.; Cassidy, D.C.; Johnstone, N.M. Quince rootstock may control nashi size. Orchard. N. Z. 1989, 62, 21–23. [Google Scholar]
- Hankin, M. Pear Rootstock Trial; Final report for Project AP10016; Horticulture Innovation Australia: Sydney, Australia, 2015. [Google Scholar]
- Bound, S.A. Managing Crop Load in European Pear (Pyrus communis L.)—A Review. Agriculture 2021, 11, 637. [Google Scholar] [CrossRef]
- Davis, K.; Stover, E.; Wirth, F. Economics of fruit thinning: A review focussing on apple and citrus. HortTechnology 2004, 14, 282–289. [Google Scholar] [CrossRef]
- Bound, S.A.; Mitchell, L. Chemical thinning in Nashi. Acta Hortic. 2002, 596, 789–791. [Google Scholar] [CrossRef]
- Meland, M. The effect of handthinning on yield and return bloom of five pear cultivars in a northern climate. Acta Hortic. 1998, 475, 275–281. [Google Scholar] [CrossRef]
- Schmidt, T. Chemical thinning options for Bartlett pears. WSU Tree Fruit. 2020. Available online: http://treefruit.wsu.edu/article/chemical-thinning-options-for-bartlett-pears/#:~:text=That%20means%20that%20the%20only,cytokinins%20benzyladenine%20(BA)%20and%20forchlorfenur (accessed on 11 February 2021).
- Williams, M.W.; Billingsley, H.D.; Batjer, L.P. Early season harvest size prediction of ‘Bartlett’ pears. J. Am. Soc. Hortic. Sci. 1969, 94, 596–598. [Google Scholar] [CrossRef]
- Burge, G.K.; Spence, C.B.; Dobson, B.G. The response of ‘Hosui’ Japanese pear to time of hand thinning and chemical thinning agents. Sci. Hortic. 1991, 45, 245–250. [Google Scholar] [CrossRef]
- McArtney, S.J.; Wells, G.H. Chemical thinning of Asian and European pear with ethephon and NAA. N. Z. J. Crop Hortic. Sci. 1995, 23, 73–84. [Google Scholar] [CrossRef]
- Lee, U.Y.; Kim, Y.K.; Shin, I.S.; Oh, K.S.; Junj, O.K.; Chun, J.P. Comparison of fruit development and quality indices according to blossom thinning on early-season ‘Hanareum’ and mid-season ‘Niitaka’ pears. Korean J. Hortic. Sci. Technol. 2015, 33, 486–491. [Google Scholar] [CrossRef]
- Meland, M.; Gjerde, B. Thinning apples and pears in a nordic climate. I. The effect of NAA, ethephon and lime sulfur on fruit set, yield and return bloom of four pear cultivars. Nor. J. Agric. Sci. 1996, 10, 437–451. [Google Scholar]
- Menzies, R. Chemical thinning of some fruit. In Working Papers, Proceedings of the Australian Fruit Research Conference, Surfers Paradise, Australia, 16–18 October 1973; Commonwealth Scientific and Industrial Research Organization: Canberra, Australia, 1973; pp. 3(c)3–3(c)4. [Google Scholar]
- Schmidt, T.; Auvil, T. Pear crop load management and rootstock field testing. Final project report, Washington Tree Fruit Research Commission. 2011. Available online: https://treefruitresearch.org/report/pear-crop-load-management-and-rootstock-field-testing/ (accessed on 30 July 2020).
- Bonghi, C.; Poja, M.; Tonutti, P.; Ramina, A. Ethephon, NAA, and NAD as chemical thinners of pear fruitlets. Acta Hortic. 2002, 596, 717–722. [Google Scholar] [CrossRef]
- Wertheim, S.J. Chemical control of flower and fruit abscission in apple and pear. Acta Hortic. 1973, 34, 321–331. [Google Scholar] [CrossRef]
- McArtney, S. Effective use of apple blossom thinners. In Proceedings of the Great Lakes Fruit, Vegetable & Farm Market EXPO, Grand Rapids, MI, USA, 5–7 December 2006. [Google Scholar]
- Bound, S.A. Precision crop load management of apple (Malus x domestica Borkh.) without chemicals. Horticulturae 2019, 5, 3. [Google Scholar] [CrossRef]
- Bound, S.A. Reducing Risk and Chemical Usage while Improving Efficiency and Predictability of Thinning Practices; Final report for project AP94043; Horticultural Research and Development Corporation: Gordon, Australia, 1998. [Google Scholar]
- Bound, S.A.; Mitchell, L. The effect of blossom desiccants on crop load of ‘Packham’s Triumph’ pear. Acta Hortic. 2002, 596, 793–796. [Google Scholar] [CrossRef]
- Bound, S.A.; Jones, K.M. Ammonium thiosulphate as a blossom thinner of ‘Delicious’ apple, ‘Winter Cole’ pear and ‘Hunter’ apricot. Aust. J. Exp. Agric. 2004, 44, 931–937. [Google Scholar] [CrossRef]
- Dussi, M.C. Sustainable use of plant bioregulators in pear production. Acta Hortic. 2011, 909, 353–367. [Google Scholar] [CrossRef]
- Garriz, P.I.; Alvarez, H.L.; Colavita, G.M.; Gajdos, M.S. Flower thinning of the pear cultivar ‘Abbé Fetel’ with lime sulphur. HortScience 2004, 39, 792. [Google Scholar] [CrossRef]
- Dussi, M.C.; Giardina, G.; Reeb, P.; Gastiazoro, J. Thinning programs in pears cv. Williams. Acta Hortic. 2008, 800, 119–129. [Google Scholar] [CrossRef]
- Kim, K.Y.; Cho, M.D.; Kim, J.K.; Kim, S.B.; Moon, B.W. Effects of ethephon on fruit thinning in pears (Pyrus pyrifolia Nakai). J. Kor. Soc. Hortic. Sci. 1988, 29, 13. [Google Scholar]
- Reginato, G.; Rojas, A. Survey of chemical fruit thinners for Asian pear cv. Hosui. Acta Hortic. 1998, 475, 265–273. [Google Scholar] [CrossRef]
- Hong, K.H.; Kim, Y.S.; Yiem, M.S.; Kim, J.B.; Kim, W.C. Fruit thinning of oriental pears by ethephon application. Res. Rep. Rural Dev. Adm. Hortic. Korea Repub. 1988, 30, 64–72. [Google Scholar]
- Prunty, R.M.; Marini, R.P. Chemical-thinning Asian pears. HortScience 2000, 35, 438B-438. [Google Scholar] [CrossRef]
- Costa, G. Bioregulators application in pear production. In Proceedings of the 6th Conference Innovation in Fruit Growing, Belgrade, Serbia, 2 February 2017; pp. 37–50. [Google Scholar]
- Bound, S.A.; Jones, K.M.; Koen, T.B.; Oakford, M.J. The thinning effect of benzyladenine on red ‘Fuji’ apple trees. J. Hortic. Sci. 1991, 66, 789–794. [Google Scholar] [CrossRef]
- Bound, S.A.; Jones, K.M.; Graham, B.; Oakford, M.J.; Tichon, M. Modelling the effects of timing and rates of application of Benzyladenine as a secondary thinner of ‘Fuji’ apple after ethephon. J. Hortic. Sci. 1993, 68, 967–973. [Google Scholar] [CrossRef]
- Ward, D.; Cowgill, W.; Meadows, R. Cost-Effective Asian Pear Thinning for Productivity and Fruit Quality; Ag Innovations Series Fact Sheet, Project ONE08-090; Sustainable Agriculture Research and Education Program, US Department of Agriculture: Washington, DC, USA, 2013. [Google Scholar]
- Bound, S.A.; Jones, K.M.; Oakford, M.J. Post-bloom thinning with 6-benzyladenine. Acta Hortic. 1997, 463, 493–499. [Google Scholar] [CrossRef]
- Sumitomo Chemical MaxCel Label. 2017. Available online: https://sumitomo-chem.com.au/sites/default/files/sds-label/maxcel_0217.pdf (accessed on 29 April 2022).
- Ward, D.; Cowgill, W.; Vincent, N.; Magron, R.; Gianfagna, T. Cytokinin for chemical fruit thinning of Asian pears. In Proceedings of the 2009 ASHS Annual Conference, St. Louis, MO, USA, 25–28 July 2009. [Google Scholar]
- Tomlin, C. The Pesticide Manual: A World Compendium, Incorporating the Agrochemicals Handbook, 10th ed.; British Crop Protection Council: Fernham, UK, 1994. [Google Scholar]
- Blanchard, M.G.; Newton, L.A.; Runkle, E.S.; Woodard, D.; Campbell, C.A. Exogenous application of abscisic acid improves the postharvest drought tolerance of several annual bedding plants. Acta Hortic. 2007, 755, 127–132. [Google Scholar] [CrossRef]
- Cornic, G. Drought stress inhibits photosynthesis by decreasing stomatal aperture—not by affecting ATP synthesis. Trends Plant Sci. 2000, 5, 187–188. [Google Scholar] [CrossRef]
- Einhorn, T.C. Regulation of flowering and fruit set of European pear. Acta Hortic. 2020, 1295, 1–12. [Google Scholar] [CrossRef]
- Greene, D.W. Influence of abscisic acid and Benzyladenine on fruit set and fruit quality of ‘Bartlett’ pears. HortScience 2012, 47, 1607–1611. [Google Scholar] [CrossRef]
- Arrington, M.; Pasa, M.S.; Einhorn, T.C. Postbloom thinning response of ‘Bartlett’ pears to abscisic acid. HortScience 2017, 52, 1765–1771. [Google Scholar] [CrossRef]
- Theron, K.I.; Lötze, G.F.A.; Reynolds, J.S. Chemical thinning of ‘Forelle’ pear with s-abscisic acid and 1-aminocyclopropane-1-carboxylic acid. Acta Hortic. 2018, 1206, 13–19. [Google Scholar] [CrossRef]
- Cline, J.A.; Carter, K.; Gunter, A.; Bakker, C.; Green, A.C. Response of Bosc and Cold SnapTM pears to thinning with NAA, 6-BA, ACC and s-ABA. Can. J. Plant Sci. 2018, 98, 830–843. [Google Scholar] [CrossRef]
- Fernandes, C. Preliminary experiment on chemical thinning of ‘Coscia’ pear. Acta Hortic. 2020, 1295, 63–66. [Google Scholar] [CrossRef]
- Elsysy, M.A.; Hubbard, A.; Einhorn, T.C. Postbloom thinning of ‘Bartlett’ pear with metamitron. HortScience 2020, 55, 174–180. [Google Scholar] [CrossRef]
- McArtney, S.; Obermiller, J.; Arellano, C. Comparison of the effects of metamitron on chlorophyll fluorescence and fruit set in apple and peach. HortScience 2012, 47, 509–514. [Google Scholar] [CrossRef]
- Maas, F.M.; van der Steeg, P.A.H. Crop load regulation in ‘Conference’ pears. Acta Hortic. 2011, 909, 369–379. [Google Scholar] [CrossRef]
- Yuda, E.; Matsui, H.; Yukimoto, M.; Nakagawa, S.; Wada, K. Effect of 15β OH gibberellins on the fruit set and development of three pear species. J. Jpn. Soc. Hortic. Sci. 1984, 53, 235–241. [Google Scholar] [CrossRef]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef]
- Schmidt, T.R.; Auvil, T.D.; Hanrahan, I.; Castillo, F.; McFerson, J.R. Crop load management of tree fruits in the Pacific Northwest of USA. Acta Hortic. 2011, 903, 759–765. [Google Scholar] [CrossRef]
- Kon, T.M.; Schupp, J.R. Apple crop load management with special focus on early thinning strategies: A US perspective. Hortic. Rev. 2019, 46, 255–298. [Google Scholar]
- An, Y.; Li, J.; Duan, C.; Liu, L.; Sun, Y.; Cao, R.; Wang, L. 5-Aminolevulinic acid thins pear fruits by inhibiting pollen tube growth via Ca2+ -ATPase-mediated Ca2+ efflux. Front. Plant Sci. 2016, 7, 121. [Google Scholar] [CrossRef]
- Akram, N.A.; Ashraf, M. Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regul. 2013, 32, 663–679. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Guo, X.; Rao, D.; Zhou, W.; Sun, J.; Ma, Y. Efficient bioproduction of 5-aminolevulinic acid, a promising biostimulant and nutrient, from renewable bioresources by engineered Corynebacterium glutamicum. Biotechnol. Biofuels 2020, 13, 41. [Google Scholar] [CrossRef]
- Environmental Protection Agency. 1-Aminocyclopropane-1-Carboxylic Acid (1-ACC); Exemption from the requirement for a tolerance. Environmental Protection Agency, 40 CFR Part 180 [EPA–HQ–OPP–2019–0515; FRL–10021–90]. 2021. Available online: https://www.federalregister.gov/documents/2021/06/28/2021-13681/1-aminocyclopropane-1-carboxylic-acid-1-acc-exemption-from-the-requirement-of-a-tolerance (accessed on 29 January 2022).
- Stopar, M.; Hladnik, J. Polysorbates as potential apple thinning agents. Acta Hortic. 2021, 1327, 685–690. [Google Scholar] [CrossRef]
- Fallahi, E.; Greene, D.W. The impact of blossom and postbloom thinners on fruit set and fruit quality in apples and stone fruits. Acta Hortic. 2010, 884, 179–188. [Google Scholar] [CrossRef]
- Seehuber, C.; Damerow, L.; Kunz, A.; Blanke, M.M. Mechanical thinning of ‘Lucas’ and ‘Conference’ pear improves fruit quality. Acta Hortic. 2015, 1094, 289–295. [Google Scholar] [CrossRef]
- Jacobus de Villiers, M.H. Mechanical and Chemical Thinning of Stone Fruit. Master’s Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2014. Available online: https://core.ac.uk/download/pdf/37436594.pdf (accessed on 6 April 2021).
- Wouters, N. Mechatronics for Efficient Thinning of Pear. Ph.D. Thesis, Katholieke Universiteit Leuven, Leuven, Belgium, 2014. [Google Scholar]
- Lopes, M.; Gaspar, P.D.; Simões, M.P. Current status and future trends of mechanized fruit thinning devices and sensor technology. Int. J. Mech. Mechatron. Eng. 2019, 13, 43–57. [Google Scholar]
- Dennis, F.G. The history of fruit thinning. Plant Growth Regul. 2000, 31, 1–16. [Google Scholar] [CrossRef]
- Damerow, L.; Blanke, M.M. A novel device for precise and selective thinning in fruit crops to improve fruit quality. Acta Hortic. 2009, 824, 275–279. [Google Scholar] [CrossRef]
- Greene, D.; Costa, G. Fruit thinning in pome- and stone-fruit: State of the art. Acta Hortic. 2013, 998, 93–102. [Google Scholar] [CrossRef]
- Bertschinger, L.; Stadler, W.; Stadler, P.; Weibel, F.; Schumacer, R. New methods of environmentally safe regulation of flower and fruit set and of alternate bearing of the apple crop. Acta Hortic. 1998, 466, 65–70. [Google Scholar] [CrossRef]
- Ferree, D.C.; Palmer, J.W. Effect of spur defoliation and ringing during bloom on fruiting, fruit mineral level, and net photosynthesis of ‘Golden Delicious’ apple. J. Am. Soc. Hortic. Sci. 1982, 107, 1182–1186. [Google Scholar] [CrossRef]
- Bound, S.A. The influence of severity and time of foliar damage on yield and fruit quality in apple (Malus domestica Borkh.). Europ. J. Hortic. Sci. 2021, 86, 270–279. [Google Scholar] [CrossRef]
- Ngugi, H.K.; Schupp, J.R. Evaluation of the risk of spreading fire blight in apple orchards with a mechanical string blossom thinner. HortScience 2009, 44, 862–865. [Google Scholar] [CrossRef]
- Gebbers, R.; Pflanz, M.; Zude, M.; Betz, A.; Hille, B.; Mattner, J.; Rachow-Autrum, T.; Ozyurtlu, A.; Schischmanow, A.; Scheele, M.; et al. Optithin—precision fruiticulture by tree-specific mechanical thinning. In Proceedings of the 12th International Conference of Precision Agriculture, Sacramento, CA, USA, 20–24 July 2014. [Google Scholar]
- Kon, T.M.; Schupp, M.A.; Winzler, H.A.; Schupp, J.R. Screening thermal shock as an apple blossom thinning method. II. Pollen tube growth and spur leaf injury in response to temperature and duration of thermal shock. HortScience 2020, 55, 632–636. [Google Scholar] [CrossRef]
- Byers, R.E.; Barden, J.A.; Polomski, R.F.; Young, R.W.; Carbaugh, D.H. Apple Thinning by Photosynthetic Inhibition. J. Am. Soc. Hortic. Sci. 1990, 115, 14–19. [Google Scholar] [CrossRef]
- Greene, D.W.; Schupp, J.R.; Winzler, H.W. Effect of abscisic acid and benzyladenine on fruit set and fruit quality of apples. HortScience 2011, 46, 1–6. [Google Scholar] [CrossRef]
- Byers, R.E.; Carbaugh, D.H.; Presley, C.N.; Wolf, T.K. The influence of low light on apple fruit abscission. J. Am. Soc. Hortic. Sci. 1991, 66, 7–17. [Google Scholar] [CrossRef]
- Wünsche, J.N.; Lombardini, L.; Greer, D.H. ‘Surround’ particle film applications—effects on whole canopy physiology of apple. Acta Hortic. 2004, 636, 565–571. [Google Scholar] [CrossRef]
- Robinson, T.L.; Francescatto, P.; Lordan, J. Advances in precision crop load management of apple. Acta Hortic. 2021, 1314, 133–138. [Google Scholar] [CrossRef]
- Lordan, J.; Reginato, G.H.; Lakso, A.N.; Francescatto, P.; Robinson, T.L. Natural fruitlet abscission as related to apple tree carbon balance estimated with the MaluSim model. Sci. Hortic. 2019, 247, 296–309. [Google Scholar] [CrossRef]
- Lauri, P.E.; Lespinasse, J.M. Apple tree training in France: Current concepts and practical implications. Fruits 1999, 54, 441–449. [Google Scholar]
- Lauri, P.E.; Lespinasse, J.M. Vertical axis and SolAxe systems in France. Acta Hortic. 2000, 513, 287–296. [Google Scholar] [CrossRef]
- Lauri, P.E.; Terouanne, E.; Lespinasse, J.M.; Regnard, J.L.; Kelner, J.J. Genotypic differences in the axillary bud growth and fruiting pattern of apple fruiting branches over several years—An approach to regulation of fruit bearing. Sci. Hortic. 1995, 64, 265–281. [Google Scholar] [CrossRef]
- Lauri, P.E.; Terouanne, E.; Lespinasse, J.M. Relationship between the early development of apple fruiting branches and the regularity of bearing—An approach to the strategies of various cultivars. J. Hortic. Sci. 1997, 72, 519–530. [Google Scholar] [CrossRef]
- Tustin, D.S.; Dayatilake, G.A.; Breen, K.C.; Oliver, M.J. Fruit Set Responses to Changes in Floral Bud Load—A New Concept for Crop Load Regulation. Acta Hortic. 2012, 932, 195–202. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).