Effect of Combined Sulfur and Nitrogen Foliar Supply on Olive Oil Volatile Compounds and Sensory Attributes
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Conditions, Treatments, Sampling, Minerals Concentration Measurement, and Oil Extraction
2.2. HS-SPME GC-FID Analysis of Volatile Compounds
2.3. Sensory Analysis
2.4. Statistical Analysis
3. Results
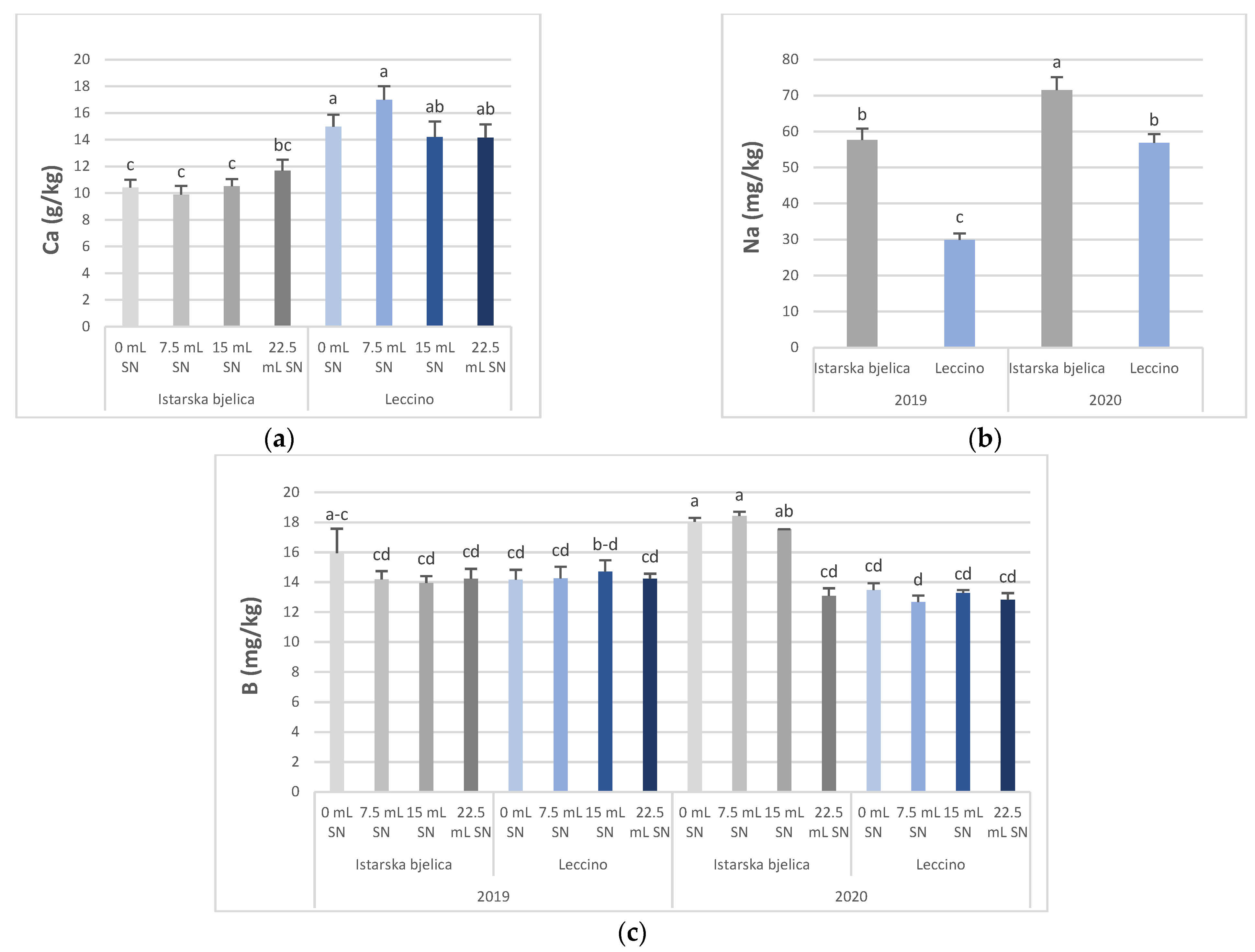
3.1. Concentration of Minerals in Olive Leaves
3.2. VOO Volatile Compounds
3.3. Sensory Analysis
4. Discussion
4.1. Minerals in Olive Leaves
4.2. Volatile Compounds in Virgin Olive Oils
4.3. Sensory Attributes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanouti, K.; Serghini-Caid, H.; Sindic, M.; Wathelet, J.-P.; Bouseta, A.; Elamrani, A. Volatile Compounds, Profiles of Virgin Olive Oils Produced In the Eastern Morocco: Oxidative Stability and Sensory Defects. J. Food Res. 2012, 1, 194. [Google Scholar] [CrossRef][Green Version]
- Kalua, C.M.; Allen, M.S.; Bedgood, D.R.; Bishop, A.G.; Prenzler, P.D.; Robards, K. Olive oil volatile compounds, flavour development and quality: A critical review. Food Chem. 2007, 100, 273–286. [Google Scholar] [CrossRef]
- Šegota, T.; Filipčić, A. Köppen’s classification of climates and the problem of corresponding Croatian terminology. Geoadria 2003, 8, 17–37. [Google Scholar] [CrossRef]
- Şahin, S.; Saeed, N.; Malik, A.; Perez, J.L.; Brockington, J.E. Seasonal Changes of Individual Phenolic Compounds in Leaves of Twenty Olive Cultivars Grown in Texas. J. Agric. Sci. Technol. B J. Agric. Sci. Technol. 2012, 2, 242–247. [Google Scholar]
- Lukić, I.; Krapac, M.; Horvat, I.; Godena, S.; Kosić, U.; Brkić Bubola, K. Three-factor approach for balancing the concentrations of phenols and volatiles in virgin olive oil from a late-ripening olive cultivar. Lwt 2018, 87, 194–202. [Google Scholar] [CrossRef]
- Žanetić, M.; Špika, M.J.; Ožić, M.M.; Bubola, K.B. Comparative study of volatile compounds and sensory characteristics of dalmatian monovarietal virgin olive oils. Plants 2021, 10, 1995. [Google Scholar] [CrossRef] [PubMed]
- Dabbaghi, O.; Tekaya, M.; Flamini, G.; Zouari, I.; El-Gharbi, S.; M’barki, N.; Laabidi, F.; Cheheb, H.; Attia, F.; Aïachi Mezghani, M.; et al. Modification of Phenolic Compounds and Volatile Profiles of Chemlali Variety Olive Oil in Response to Foliar Biofertilization. JAOCS, J. Am. Oil Chem. Soc. 2019, 96, 585–593. [Google Scholar] [CrossRef]
- García-Vico, L.; Belaj, A.; Sánchez-Ortiz, A.; Martínez-Rivas, J.M.; Pérez, A.G.; Sanz, C. Volatile Compound Profiling by HS-SPME/GC-MS-FID of a Core Olive Cultivar Collection as a Tool for Aroma Improvement of Virgin Olive Oil. Molecules 2017, 22, 141. [Google Scholar] [CrossRef] [PubMed]
- Angerosa, F.; D’Alessandro, N.; Basti, C.; Vito, R. Biogeneration of Volatile Compounds in Virgin Olive Oil: Their Evolution in Relation to Malaxation Time. J. Agric. Food Chem. 1998, 46, 2940–2944. [Google Scholar] [CrossRef]
- Tena, N.; Lazzez, A.; Aparicio-Ruiz, R.; García-González, D.L. Volatile compounds characterizing Tunisian chemlali and chétoui virgin olive oils. J. Agric. Food Chem. 2007, 55, 7852–7858. [Google Scholar] [CrossRef]
- Koprivnjak, O.; Majetić, V.; Bubola, K.B.; Kosić, U. Variability of phenolic and volatile compounds in virgin olive oil from leccino and istarska bjelica cultivars in relation to their fruit mixtures. Food Technol. Biotechnol. 2012, 50, 216–221. [Google Scholar]
- Atta, N.M.M.; Mohamed, E.S.H.A. Effect of foliar fertilization for olive trees on the bioactive compounds, purity and organoleptic attributes of olive oils. Egypt. J. Agric. Res. 2017, 95, 769–785. [Google Scholar] [CrossRef]
- Paul, M.J.; Driscoll, S.P. Sugar repression of photosynthesis: The role of carbohydrates in signalling nitrogen deficiency through source:sink imbalance. Plant Cell Environ. 1997, 20, 110–116. [Google Scholar] [CrossRef]
- Erel, R.; Kerem, Z.; Ben-Gal, A.; Dag, A.; Schwartz, A.; Zipori, I.; Basheer, L.; Yermiyahu, U. Olive (Olea europaea L.) tree nitrogen status is a key factor for olive oil quality. J. Agric. Food Chem. 2013, 61, 11261–11272. [Google Scholar] [CrossRef] [PubMed]
- Erel, R.; Yermiyahu, U.; Van Opstal, J.; Ben-Gal, A.; Schwartz, A.; Dag, A. The importance of olive (Olea europaea L.) tree nutritional status on its productivity. Sci. Hortic. 2013, 159, 8–18. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Bchir, A.; Attia, F.; Cheheb, H.; Daassa, M.; Hammami, M. Enhancement of antioxidants in olive oil by foliar fertilization of olive Trees. JAOCS J. Am. Oil Chem. Soc. 2013, 90, 1377–1386. [Google Scholar] [CrossRef]
- Jones, C.G.; Hartley, S.E. A Protein Competition Model of Phenolic Allocation. Oikos 1999, 86, 27. [Google Scholar] [CrossRef]
- Ahmad, A.; Abdin, M.Z. Effect of sulphur application on lipid, RNA and fatty acid content in developing seeds of rapeseed (Brassica campestris L.). Plant Sci. 2000, 150, 71–76. [Google Scholar] [CrossRef]
- Aulakh, M.S.; Malhi, S.S. Interactions of Nitrogen with Other Nutrients and Water: Effect on Crop Yield and Quality, Nutrient Use Efficiency, Carbon Sequestration, and Environmental Pollution. Adv. Agron. 2005, 86, 341–409. [Google Scholar] [CrossRef]
- Malhi, S.S.; Gill, K.S. Effectiveness of sulphate-S fertilization at different growth stages for yield, seed quality and S uptake of canola. Can. J. Plant Sci. 2002, 82, 665–674. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Anee, T.I.; Fujita, M. Glutathione in plants: Biosynthesis and physiological role in environmental stress tolerance. Physiol. Mol. Biol. Plants 2017, 23, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Romero, N.; Saavedra, J.; Tapia, F.; Sepúlveda, B.; Aparicio, R. Influence of agroclimatic parameters on phenolic and volatile compounds of Chilean virgin olive oils and characterization based on geographical origin, cultivar and ripening stage. J. Sci. Food Agric. 2016, 96, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Bubola, K.B.; Lukić, M.; Novoselić, A.; Krapac, M.; Lukić, I. Olive fruit refrigeration during prolonged storage preserves the quality of virgin olive oil extracted therefrom. Foods 2020, 9, 1445. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Žanetić, M.; Jukić Špika, M.; Lukić, M.; Koprivnjak, O.; Brkić Bubola, K. Complex interactive effects of ripening degree, malaxation duration and temperature on Oblica cv. virgin olive oil phenols, volatiles and sensory quality. Food Chem. 2017, 232, 610–620. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Lukić, M.; Žanetić, M.; Krapac, M.; Godena, S.; Bubola, K.B. Inter-varietal diversity of typical volatile and phenolic profiles of Croatian extra virgin olive oils as revealed by GC-IT-MS and UPLC-DAD analysis. Foods 2019, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Bubola, K.B.; Lukić, M.; Lukić, I.; Koprivnjak, O. Effect of different clarification methods on volatile aroma compound composition of virgin olive oil. Food Technol. Biotechnol. 2019, 57, 503–512. [Google Scholar] [CrossRef] [PubMed]
- da Costa, J.R.O.; Dal Bosco, S.M.; de Souza Ramos, R.C.; Machado, I.C.K.; Garavaglia, J.; Villasclaras, S.S. Determination of volatile compounds responsible for sensory characteristics from Brazilian extra virgin olive oil using HS-SPME/GC-MS direct method. J. Food Sci. 2020, 85, 3764–3775. [Google Scholar] [CrossRef]
- Regni, L.; Proietti, P. Effects of nitrogen foliar fertilization on the vegetative and productive performance of the olive tree and on oil quality. Agriculture 2019, 9, 252. [Google Scholar] [CrossRef]
- Marcelić, Š.; Vidović, N.; Pasković, I.; Lukić, M.; Špika, M.J.; Palčić, I.; Lukić, I.; Petek, M.; Pecina, M.; Herak Ćustić, M.; et al. Combined Sulfur and Nitrogen Foliar Application Increases Extra Virgin Olive Oil Quantity without Affecting Its Nutritional Quality. Horticulturae 2022, 8, 203. [Google Scholar] [CrossRef]
- Vidović, N.; Pasković, I.; Lukić, I.; Žurga, P.; Germek, V.M.; Grozić, K.; Cukrov, M.; Marcelić, Š.; Ban, D.; Talhaoui, N.; et al. Biophenolic profile modulations in olive tissues as affected by manganese nutrition. Plants 2021, 10, 1724. [Google Scholar] [CrossRef]
- Bubola, K.B.; Koprivnjak, O.; Sladonja, B.; Lukić, I. Volatile compounds and sensory profiles of monovarietal virgin olive oil from Buža, Črna and Rosinjola cultivars in Istria (Croatia). Food Technol. Biotechnol. 2012, 50, 192–198. [Google Scholar]
- Fernández-Escobar, R.; Moreno, R.; García-Creus, M. Seasonal changes of mineral nutrients in olive leaves during the alternate-bearing cycle. Sci. Hortic. 1999, 82, 25–45. [Google Scholar] [CrossRef]
- Fernández-Escobar, R. Olive Nutritional Status and Tolerance to Biotic and Abiotic Stresses. Front. Plant Sci. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zheljazkov, V.D.; Cantrell, C.L.; Ebelhar, M.W.; Rowe, D.E.; Coker, C. Productivity, oil content, and oil composition of sweet basil as a function of nitrogen and sulfur fertilization. HortScience 2008, 43, 1415–1422. [Google Scholar] [CrossRef]
- Wong, A.D.; Swiader, J.M.; Juvik, J.A. Nitrogen and sulfur fertilization influences aromatic flavor components in Shrunken2 sweet corn kernels. J. Am. Soc. Hortic. Sci. 1995, 120, 771–777. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Daniels, R.; Schnug, E. Influence of sulfur fertilization on floral scent patterns of crops in full bloom. Landbauforsch. Volkenrode 2010, 60, 45–50. [Google Scholar]
- Shaker, M.A.; Azza, A.A. Relationship between volatile compounds of olive oil and sensory attributes. Int. Food Res. J. 2013, 20, 197–204. [Google Scholar]
- Kiralan, M.; Ozkan, G.; Koyluoglu, F.; Ugurlu, H.A.; Bayrak, A.; Kiritsakis, A. Effect of cultivation area and climatic conditions on volatiles of virgin olive oil. Eur. J. Lipid Sci. Technol. 2012, 114, 552–557. [Google Scholar] [CrossRef]
- Ancín-Azpilicueta, C.; Nieto-Rojo, R.; Gómez-Cordón, J. Effect of foliar urea fertilisation on volatile compounds in Tempranillo wine. J. Sci. Food Agric. 2013, 93, 1481–1485. [Google Scholar] [CrossRef]
- Abdeljelil, Z.B.; Tekaya, M.; Mechri, B.; Flamini, G.; Hammami, M. Changes in volatiles of olive tree Olea europaea according to season and foliar fertilization. Int. J. Agric. Biol. 2017, 19, 1633–1639. [Google Scholar] [CrossRef]
- da Silva, M.D.R.G.; Costa Freitas, A.M.B.; Cabrita, M.J.; Garci, R. Olive Oil Composition: Volatile Compounds. In Olive Oil-Constituents, Quality, Health Properties and Bioconversions; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Mulinacci, N. Virgin olive oil volatile compounds: Composition, sensory characteristics, analytical approaches, quality control, and authentication. J. Agric. Food Chem. 2021, 69, 2013–2040. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Esposto, S.; Lodolini, E.; Selvaggini, R.; Taticchi, A.; Urbani, S.; Montedoro, G.; Serravalle, M.; Gucci, R. Irrigation effects on quality, phenolic composition, and selected volatiles of virgin olive oils cv. leccino. J. Agric. Food Chem. 2007, 55, 6609–6618. [Google Scholar] [CrossRef] [PubMed]
- Gucci, R.; Servili, M.; Esposto, S.; Selvaggini, R. Oil quality of Olive cv. “Leccino” grown under irrigated or dry-farmed conditions. Acta Hortic. 2004, 664, 297–302. [Google Scholar] [CrossRef]
- Runcio, A.; Sorgonà, L.; Mincione, A.; Santacaterina, S.; Poiana, M. Volatile compounds of virgin olive oil obtained from Italian cultivars grown in Calabria. Effect of processing methods, cultivar, stone removal, and antracnose attack. Food Chem. 2008, 106, 735–740. [Google Scholar] [CrossRef]
- Bell, S.J.; Henschke, P.A. Implications of nitrogen nutrition for grapes, fermentation and wine. Aust. J. Grape Wine Res. 2005, 11, 242–295. [Google Scholar] [CrossRef]
- Caporaso, N. Virgin Olive Oils: Environmental Conditions, Agronomical Factors and Processing Technology Affecting the Chemistry of Flavor Profile. J. Food Chem. Nanotechnol. 2016, 2, 21–31. [Google Scholar] [CrossRef]
- Oğraş, Ş.Ş.; Kaban, G.; Kaya, M. Volatile compounds of olive oils from different geographic regions in Turkey. Int. J. Food Prop. 2018, 21, 1833–1843. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europaea L. Cv. Cornicabra) with regard to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef] [PubMed]
- Allalout, A.; Krichène, D.; Methenni, K.; Taamalli, A.; Daoud, D.; Zarrouk, M. Behavior of super-intensive spanish and greek olive cultivars grown in northern tunisia. J. Food Biochem. 2011, 35, 27–43. [Google Scholar] [CrossRef]
- Tous, J.; Romero, A. Cultivar and Location Effects on Olive Oil Quality in Catalonia, Spain. Acta Horticulturae 1994, 356, 323–326. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Pedò, S.; Gigliotti, C.; Bassi, D.; Serraiocco, A. Effects of Seasonal Weather Variability on Olive Oil Composition in Northern Italy. Acta Hortic. 2008, 791, 769–776. [Google Scholar] [CrossRef]
- De Santis, D.; Frangipane, M.T. Sensory Perceptions of Virgin Olive Oil: New Panel Evaluation Method and the Chemical Compounds Responsible. Nat. Sci. 2015, 07, 132–142. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationships with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Yan, J.; Alewijn, M.; van Ruth, S.M. From extra virgin olive oil to refined products: Intensity and Balance Shifts of the Volatile Compounds versus Odor. Molecules 2020, 25, 2469. [Google Scholar] [CrossRef]
- Caruso, G.; Gucci, R.; Urbani, S.; Esposto, S.; Taticchi, A.; Di Maio, I.; Selvaggini, R.; Servili, M. Effect of different irrigation volumes during fruit development on quality of virgin olive oil of cv. Frantoio. Agric. Water Manag. 2014, 134, 94–103. [Google Scholar] [CrossRef]
- Tura, D.; Failla, O.; Bassi, D.; Pedò, S.; Serraiocco, A. Environmental and seasonal influence on virgin olive (Olea europaea L.) oil volatiles in northern Italy. Sci. Hortic. 2009, 122, 385–392. [Google Scholar] [CrossRef]
- Špika, M.J.; Perica, S.; Žanetić, M.; Škevin, D. Virgin olive oil phenols, fatty acid composition and sensory profile: Can cultivar overpower environmental and ripening effect? Antioxidants 2021, 10, 689. [Google Scholar] [CrossRef] [PubMed]


| Source of Variation | g/kg | mg/kg | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phosphorus | Potassium | Calcium | Magnesium | Sodium | Iron | Manganese | Copper | Boron | Molybdenum | Silicon | |
| Treatment (T) | |||||||||||
| 0 mL SN | 2.74 ± 0.17 | 11.12 ± 0.51 | 12.69 ± 0.79 | 0.88 ± 0.03 a | 58.09 ± 5.17 | 28.63 ± 2.01 | 43.84 ± 2.26 a | 18.84 ± 1.75 | 15.41 ± 0.61 a | 1.14 ± 0.10 | 141.93 ± 10.02 |
| 7.5 mL SN | 2.41 ± 0.14 | 11.02 ± 0.33 | 13.43 ± 1.09 | 0.88 ± 0.04 a | 52.70 ± 4.85 | 28.35 ± 2.55 | 38.40 ± 2.52 b | 16.85 ± 0.91 | 14.88 ± 0.60 a | 1.04 ± 0.09 | 139.43 ± 12.82 |
| 15 mL SN | 2.78 ± 0.15 | 11.79 ± 0.52 | 12.36 ± 0.78 | 0.88 ± 0.02 a | 52.98 ± 4.40 | 28.17 ± 2.11 | 39.15 ± 1.96 a,b | 18.29 ± 1.15 | 14.88 ± 0.46 a | 1.11 ± 0.11 | 145.87 ± 11.37 |
| 22.5 mL SN | 2.67 ± 0.18 | 11.70 ± 0.49 | 12.92 ± 0.70 | 0.75 ± 0.04 b | 52.17 ± 4.72 | 26.59 ± 1.65 | 38.48 ± 2.69 b | 18.13 ± 0.98 | 13.59 ± 0.28 b | 1.04 ± 0.09 | 137.79 ± 10.23 |
| p-value | n.s. | n.s. | n.s. | * | n.s. | n.s. | * | n.s. | ** | n.s. | n.s. |
| Cultivar (Cv.) | |||||||||||
| Istarska bjelica | 2.71 ± 0.11 | 10.51 ± 0.26 a | 10.62 ± 0.34 b | 0.83 ± 0.03 | 64.60 ± 2.68 a | 29.34 ± 1.61 | 33.43 ± 1.05 b | 20.96 ± 0.82 a | 15.68 ± 0.42a | 0.96 ± 0.06 b | 152.26 ± 6.47 a |
| Leccino | 2.59 ± 0.12 | 12.30 ± 0.31 b | 15.08 ± 0.53 a | 0.86 ± 0.02 | 43.37 ± 2.86 b | 26.53 ± 1.27 | 16.50 ± 1.39 a | 15.09 ± 0.54 b | 13.70 ± 0.21 b | 1.20± 0.07 a | 130.25 ± 8.46 b |
| p-value | n.s. | *** | *** | n.s. | *** | n.s. | *** | *** | *** | ** | ** |
| Year (Y) | |||||||||||
| 2019 | 2.38 ± 0.11 b | 12.66 ± 0.25 a | 11.64 ± 0.56 b | 0.86 ± 0.03 | 43.78 ± 3.09 b | 22.66 ± 0.93 b | 35.91 ± 1.46 b | 16.79 ± 1.05 b | 14.46 ± 0.28 | 0.88± 0.06 b | 111.93 ± 6.97 b |
| 2020 | 2.92 ± 0.09 a | 10.15 ± 0.24 b | 14.07 ± 0.55 a | 0.83 ± 0.02 | 64.19 ± 2.52 a | 33.21 ± 1.29 a | 44.03 ± 1.60 a | 19.26 ± 0.57 a | 14.92 ± 0.45 | 1.28 ± 0.05 a | 170.58 ± 4.15 a |
| p-value | *** | *** | *** | n.s. | *** | *** | *** | ** | n.s. | *** | *** |
| T × Cv. | n.s. | n.s. | * | n.s. | n.s. | n.s. | n.s. | n.s. | ** | n.s. | n.s. |
| T × Y | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| Cv. × Y | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | n.s. | *** | n.s. | n.s. |
| T × Cv. × Y | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. |
| Aldehydes (mg/kg) | Alcohols (mg/kg) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Source of Variation | 3MB | HAL | Z2PAL * | E2PAL | E3HAL * | Z3HAL * | Z2HAL * | E2HAL | OAL | E,EHAL * | E,ZHAL * | E2POL | HOL | E3HOL | Z3HOL | E2HOL | Z2HOL |
| Treatment (T) | |||||||||||||||||
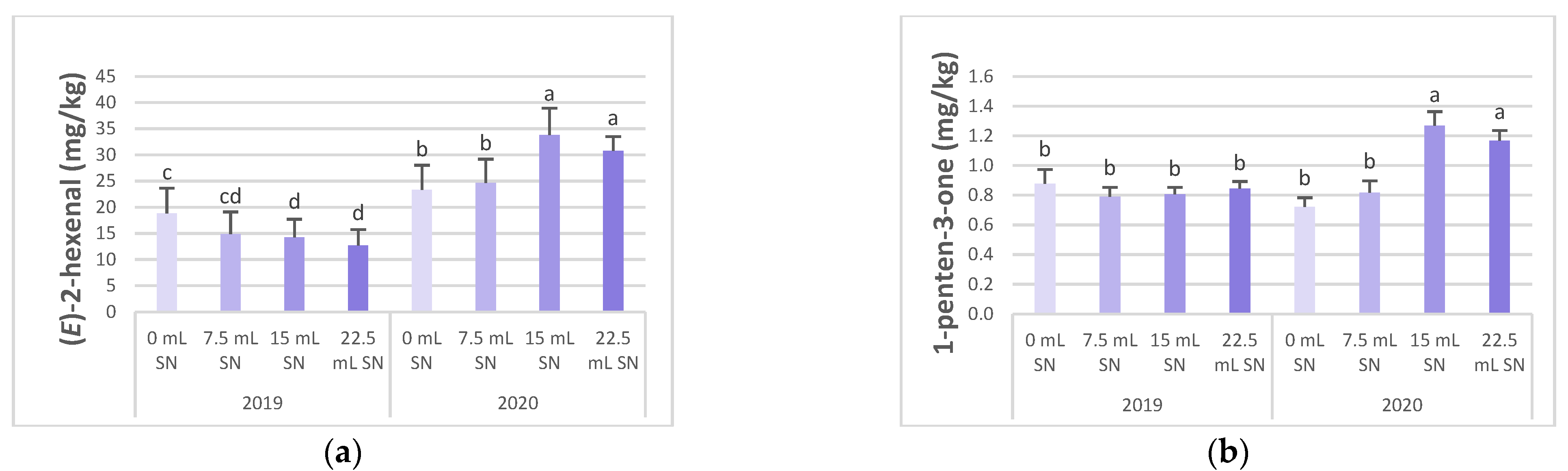
| 0 mL SN | 0.05 ab | 0.25b | 0.03 a | 0.04 b | 0.09 a | 1.00 a | 0.23 a,b | 21.12 b | 0.04 a | 0.04 | 0.05a | 0.03c | 0.02 | <LOD | 0.30a | 0.18 | <LODb |
| 7.5 mL SN | 0.04 b | 0.25b | 0.02 b | 0.05 b | 0.07 b | 0.82 a,b | 0.19 c | 19.81 b | 0.03 b | 0.04 | 0.04ab | 0.04bc | 0.09 | <LOD | 0.23ab | 0.40 | <LODb |
| 15 mL SN | 0.06 a | 0.42a | 0.02 b | 0.06 a | 0.09 a,b | 0.72 b | 0.25 a | 24.03 a | <LOD c | 0.04 | 0.04ab | 0.05b | 0.03 | <LOD | 0.34a | 0.23 | 0.06a |
| 22.5 mL SN | 0.05 a,b | 0.42a | 0.03 a | 0.06 a | 0.08 a,b | 0.73 b | 0.21 b,c | 21.78 a,b | <LOD c | 0.04 | 0.03b | 0.06a | 0.03 | <LOD | 0.13b | 0.14 | 0.04a |
| p-value | ** | *** | *** | *** | *** | *** | *** | *** | *** | n.s. | ** | *** | n.s. | * | *** | n.s. | *** |
| Cultivar (Cv.) | |||||||||||||||||
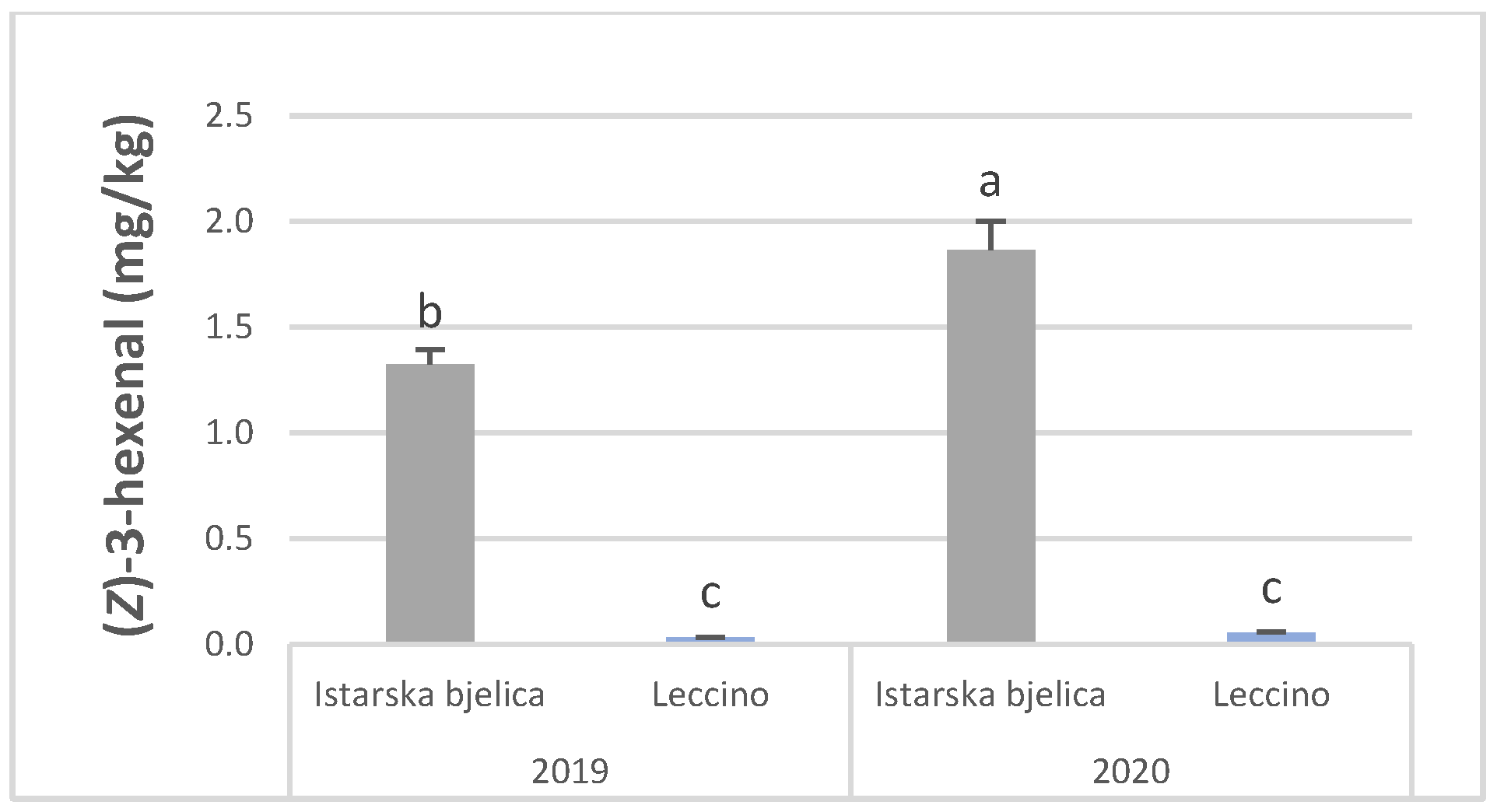
| Istarska bjelica | 0.02 b | 0.35 | 0.03 a | 0.05 | 0.11 a | 1.60 a | 0.24 a | 11.23b | 0.04 a | 0.08 a | 0.07 a | 0.04 b | 0.03 | <LOD | 0.42 a | 0.22 | 0.05 a |
| Leccino | 0.08 a | 0.32 | 0.02 b | 0.05 | 0.05 b | 0.04 b | 0.19 b | 32.14a | <LOD b | <LOD b | 0.01 b | 0.05 a | 0.06 | <LOD | 0.08 b | 0.25 | <LOD b |
| p-value | *** | n.s. | *** | n.s. | *** | *** | *** | *** | *** | *** | *** | * | n.s. | *** | *** | n.s. | *** |
| Year (Y) | |||||||||||||||||
| 2019 | 0.04 b | 0.12b | 0.04 a | 0.05 | 0.07 b | 0.68 b | 0.16 b | 15.20b | 0.04 a | 0.04 | 0.08 a | 0.04 b | 0.06 | <LOD | 0.14 b | 0.23 | <LOD b |
| 2020 | 0.06 a | 0.55a | 0.01 b | 0.05 | 0.09 a | 0.96 a | 0.28 a | 28.17a | 0.00 b | 0.04 | <LODb | 0.05 a | 0.04 | <LOD | 0.36 a | 0.25 | 0.05 a |
| p-value | *** | *** | *** | n.s. | *** | *** | *** | *** | *** | n.s. | *** | *** | n.s. | * | *** | n.s. | *** |
| T×Cv. | * | ** | *** | n.s. | * | * | n.s. | *** | *** | n.s. | * | *** | n.s. | n.s. | *** | n.s. | *** |
| T×Y | *** | *** | * | *** | ** | n.s. | *** | *** | *** | ** | ** | *** | n.s. | n.s. | ** | n.s. | *** |
| Cv.×Y | *** | * | n.s. | *** | n.s. | *** | *** | n.s. | *** | * | *** | *** | n.s. | *** | *** | * | *** |
| T × Cv.×Y | *** | ** | * | n.s. | n.s. | n.s. | n.s. | n.s. | *** | ** | * | *** | n.s. | * | * | n.s. | *** |
| Carboxylic Acid (mg/kg) | Ketones (mg/kg) | Unsaturated Hydrocarbons (mg/kg) | Other (mg/kg) | ||||||||||||||
| Source of Variation | AA * | 3PO | PEN3O | 3E 1,5 OEN * | DECAI * | DECAII * | DECAIII * | Z2P + Z3H | |||||||||
| Treatment (T) | |||||||||||||||||
| 0 mL SN | 0.15 | 0.08 a | 0.80 b | 0.60 | 0.28 | 0.37 | 0.04 b | 0.75 b | |||||||||
| 7.5 mL SN | 0.15 | 0.06 b,c | 0.81 b | 0.58 | 0.26 | 0.33 | 0.13 a | 0.75 b | |||||||||
| 15 mL SN | 0.14 | 0.08 a,b | 1.04 a | 0.65 | 0.28 | 0.38 | 0.03 b,c | 1.37 a | |||||||||
| 22.5 mL SN | 0.13 | 0.06 c | 1.01 a | 0.66 | 0.30 | 0.39 | 0.01 c | 1.26 a | |||||||||
| p-value | n.s. | *** | *** | n.s. | n.s. | n.s. | *** | *** | |||||||||
| Cultivar (Cv.) | |||||||||||||||||
| Istarska bjelica | 0.14 | 0.09 a | 1.04 a | 0.59 b | 0.26 b | 0.31 b | 0.03 b | 1.38 a | |||||||||
| Leccino | 0.14 | 0.05 b | 0.79 b | 0.65 a | 0.30 a | 0.43 a | 0.08 a | 0.69 b | |||||||||
| p-value | n.s. | *** | *** | * | ** | *** | *** | *** | |||||||||
| Year (Y) | |||||||||||||||||
| 2019 | 0.14 | 0.04 b | 0.83 b | 0.53 b | 0.26 b | 0.32 b | 0.02 b | 0.99 b | |||||||||
| 2020 | 0.14 | 0.10 a | 1.00 a | 0.71 a | 0.30 a | 0.41 a | 0.09 a | 1.08 a | |||||||||
| p-value | n.s. | *** | *** | *** | ** | *** | *** | * | |||||||||
| T×Cv. | * | ** | n.s. | n.s. | n.s. | * | *** | *** | |||||||||
| T×Y | *** | *** | *** | *** | *** | *** | *** | *** | |||||||||
| Cv.×Y | n.s. | *** | n.s. | * | n.s. | n.s. | *** | n.s. | |||||||||
| T×Cv.×Y | *** | n.s. | n.s. | n.s. | n.s. | n.s. | *** | * | |||||||||
| Source of Variation | Olive Fruity | Green Grass/ Leaves | Apple | Tomato | Green Almond | Aromatic Herbs | Chicory | Green Banana | Green Coffee Beans | Bitter | Pungent | Astring | Complex | Harmon | Persist | Overall |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment (T) | ||||||||||||||||
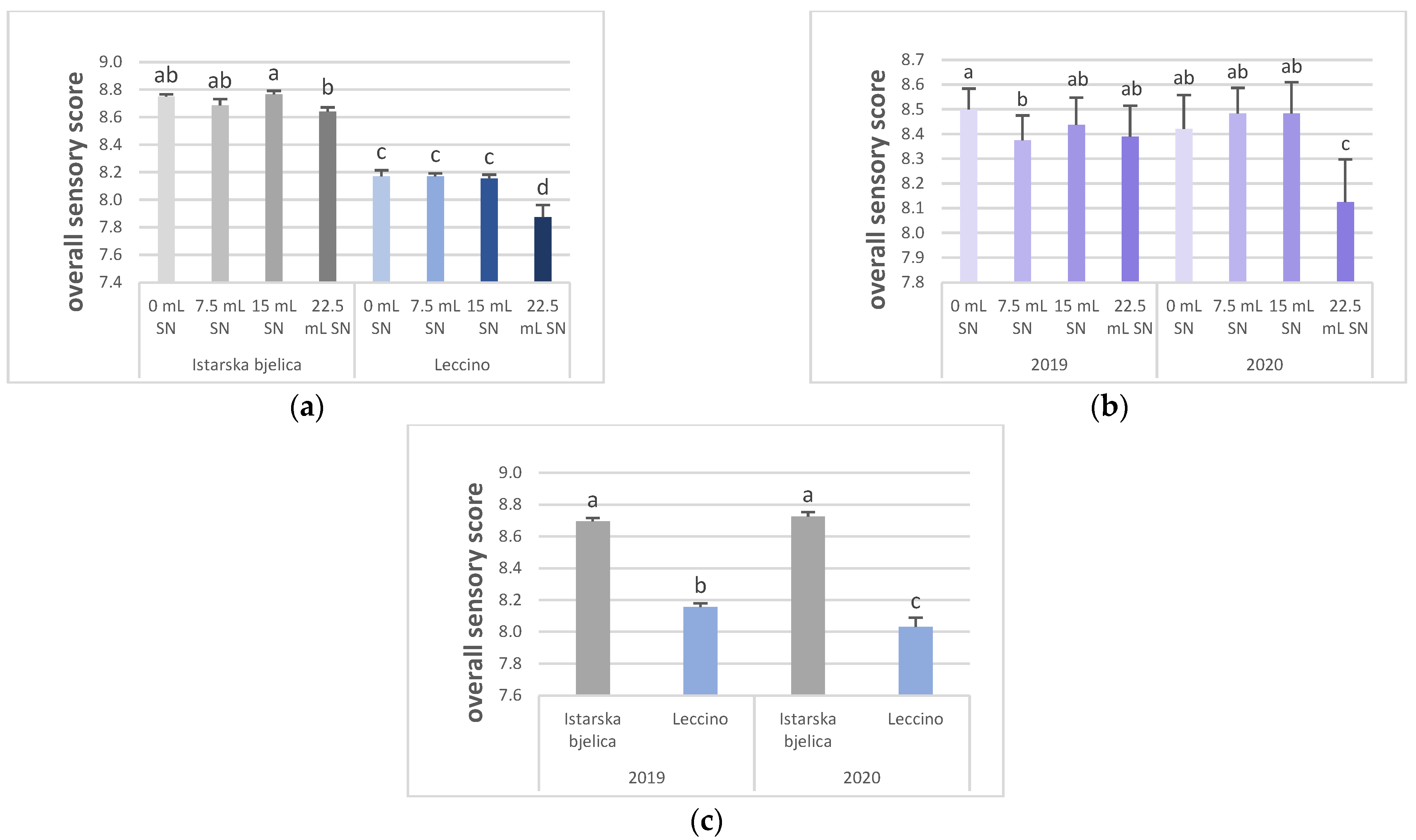
| 0 mL SN | 6.26 a | 4.10 a | 1.69 a | 0.83 b | 2.39 a | 2.23 b | 2.48 c | 2.91 a | 1.43 | 5.95 c | 6.43 c | 1.71 c | 8.63 a,b | 8.72 a | 8.84 b,c | 8.46 a |
| 7.5 mL SN | 6.14 b | 4.02 a,c | 1.54 b | 0.72 b | 2.22 b | 2.52 a | 2.98 a | 3.06a | 1.43 | 6.30 a | 6.78 a | 2.24 a,b | 8.69 a,b | 8.41 b,c | 9.09 a | 8.43 a |
| 15 mL SN | 6.13 b | 3.80 b | 1.37 c | 0.69 b | 2.18 b | 2.38 a | 2.69 b | 2.94 a | 1.34 | 6.06 b,c | 6.57 b | 2.14 b | 8.75 a | 8.53 b | 8.94 a,b | 8.46 a |
| 22.5 mL SN | 5.87 c | 3.31 c | 1.54 b | 1.25 a | 1.92 c | 2.18 b | 2.38 c | 2.57 b | 1.58 | 6.19 a,b | 6.68 a | 2.33 a | 8.56 b | 8.28 c | 8.72 c | 8.26 b |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | n.s. | *** | *** | *** | * | *** | *** | *** |
| Cultivar (Cv.) | ||||||||||||||||
| Istarska bjelica | 6.78 a | 4.57 a | 0.97 b | 0.96 a | 1.53b | 2.92a | 3.55 a | 3.19 a | 2.77 a | 7.19 a | 7.41 a | 2.64 a | 9.20 a | 8.66 a | 9.55 a | 8.71 a |
| Leccino | 5.42 b | 3.04 b | 2.10 a | 0.79 b | 2.83a | 1.73b | 1.72 b | 2.54 b | 0.12 b | 5.06 b | 5.81 b | 1.57 b | 8.11 b | 8.31 b | 8.25 b | 8.09 b |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Year (Y) | ||||||||||||||||
| 2019 | 5.84 b | 3.38 b | 1.04 b | 1.53 a | 1.43b | 1.69b | 1.92 b | 2.45 b | 1.09 b | 6.21 a | 6.78 a | 2.05 b | 8.45 b | 8.48 | 8.84 b | 8.43 a |
| 2020 | 6.36 a | 4.24 a | 2.03 a | 0.22b | 2.92a | 2.96a | 3.35 a | 3.28 a | 1.79 a | 6.04 b | 6.44 b | 2.16 a | 8.86 a | 8.48 | 8.95 a | 8.38 b |
| p-value | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | ** | *** | n.s. | * | * |
| T ×Cv. | *** | *** | *** | *** | ***. | *** | *** | *** | n.s. | *** | *** | n.s. | *** | *** | * | *** |
| T ×Y | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | n.s. | *** | ** | * | *** |
| Cv.×Y | *** | *** | *** | *** | *** | *** | n.s. | *** | *** | *** | *** | *** | *** | ** | *** | *** |
| T ×Cv. ×Y | *** | ** | *** | *** | *** | *** | *** | n.s. | n.s. | * | *** | *** | ** | * | * | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vidović, N.; Pasković, I.; Marcelić, Š.; Lukić, I.; Brkić Bubola, K.; Klisović, D.; Novoselić, A.; Palčić, I.; Polić Pasković, M.; Herak Ćustić, M.; et al. Effect of Combined Sulfur and Nitrogen Foliar Supply on Olive Oil Volatile Compounds and Sensory Attributes. Horticulturae 2022, 8, 912. https://doi.org/10.3390/horticulturae8100912
Vidović N, Pasković I, Marcelić Š, Lukić I, Brkić Bubola K, Klisović D, Novoselić A, Palčić I, Polić Pasković M, Herak Ćustić M, et al. Effect of Combined Sulfur and Nitrogen Foliar Supply on Olive Oil Volatile Compounds and Sensory Attributes. Horticulturae. 2022; 8(10):912. https://doi.org/10.3390/horticulturae8100912
Chicago/Turabian StyleVidović, Nikolina, Igor Pasković, Šime Marcelić, Igor Lukić, Karolina Brkić Bubola, Dora Klisović, Anja Novoselić, Igor Palčić, Marija Polić Pasković, Mirjana Herak Ćustić, and et al. 2022. "Effect of Combined Sulfur and Nitrogen Foliar Supply on Olive Oil Volatile Compounds and Sensory Attributes" Horticulturae 8, no. 10: 912. https://doi.org/10.3390/horticulturae8100912
APA StyleVidović, N., Pasković, I., Marcelić, Š., Lukić, I., Brkić Bubola, K., Klisović, D., Novoselić, A., Palčić, I., Polić Pasković, M., Herak Ćustić, M., Petek, M., Jukić Špika, M., Pecina, M., Pongrac, P., & Goreta Ban, S. (2022). Effect of Combined Sulfur and Nitrogen Foliar Supply on Olive Oil Volatile Compounds and Sensory Attributes. Horticulturae, 8(10), 912. https://doi.org/10.3390/horticulturae8100912













